Abstract
Cargo-mediated regulation of vesicular transport has received great attention lately. Rab GTPases, forming the largest branch of the Ras GTPase superfamily, regulate almost every step of vesicle-mediated trafficking. Growing evidence suggests that mutations, aberrant expression, and altered post-translational modifications of Rab GTPases are associated with human diseases. However, their regulatory mechanisms and how they are connected to cargo proteins are still poorly understood. Accumulating data indicate that G protein-coupled receptors (GPCRs) directly associate with Rab GTPases and that these interactions dictate receptor trafficking. Yet, it remained unclear whether the receptors could regulate the targeting and activity of Rab GTPases in various cell compartments. It is only in recent years that experimental studies showed that GPCR signaling and interaction with Rab-associated regulatory proteins modulate the localization and activity of Rab GTPases. This research is revealing novel regulatory mechanisms of these small GTPases and should contribute to the progress in effective drug development. Recently published in the Journal of Cell Science, Lachance et al. present a novel role for ubiquitylation of Rab11a by a β2AR/HACE1 complex in regulating Rab11a activity and β2AR trafficking.
Keywords: G Protein-Coupled Receptors (GPCRs), HACE1, Rab GTPases, Rab11a, ubiquitylation, vesicular trafficking, β2AR
GPCRs and the Regulation of Rab GTPases
More than 60 Rab GTPases, forming the largest branch of Ras-related small GTPases, are involved in almost every step of vesicle-mediated transport.1 Each Rab GTPase has a distinct subcellular localization that correlates with the compartments between which they coordinate transport.2 During the last decade, many studies described the involvement of Rab GTPases in the regulation of GPCR trafficking and signaling. Rab GTPases were shown to be key regulators of GPCR anterograde and retrograde transport (Rab1, Rab2, Rab6, and Rab8),3-6 endocytosis (Rab5),7-11 recycling (Rab4 and Rab11),11-19 and degradation (Rab7).20 We and others established that a direct interaction between a GPCR and a Rab GTPase appears to be necessary in the proper trafficking of the receptor.4,7,12,15,17-19 Rab-mediated vesicular transport is well known to regulate membrane receptor trafficking, but less is understood about whether membrane receptors conversely regulate the Rab-trafficking machinery.21-23 In this regard, an elegant study performed by Seachrist et al. determined that activation of the angiotensin type 1 receptor stimulates the GTP-loading of Rab5a, suggesting that the receptor could act as a potential GEF or might recruit a GEF protein, thereby increasing the level of GTP-bound Rab5a.7 Yudowski et al. subsequently demonstrated that Rab4 was regulated by PKA following activation of the β2-adrenergic receptor (β2AR).24
Post-translational modifications, such as the addition of one or two prenyl groups to the C-terminus is necessary for the initial targeting of Rab GTPases to membranes.25,26 The transfer of a geranylgeranyl moiety from geranylgeranyl pyrophosphate to cysteines in Rab proteins with an -CAAX (where A is an aliphatic residue and X is any amino acid), -XXCC, -XCXC and -CCXX C-terminal is catalyzed by a specific Rab geranylgeranyltransferase (RGGT). Some Rab GTPases containing a -CAAX motif were reported to be processed post-geranylgeranylation by proteolysis and methylation.27 It has long been thought that Rab geranylgeranylation can be influenced by environmental changes to extracellular signals, but supporting evidence is scarce.28,29 Interestingly, our results indicated that overexpression of the β2AR impaired the geranylgeranylation of co-expressed Rab6a, Rab8a, and Rab11a in HEK293 cells, which was reversed by agonist stimulation of the receptor.22 Our data suggested that this was mediated through a direct interaction between the β2AR and RGGT.22 Thus, it seems plausible that a GPCR, through an interaction with RGGT, partakes in the membrane targeting and regulation of its associated Rab GTPases to direct its own trafficking.
Ubiquitylation of Rab GTPases
Monoubiquitylation of K-Ras at Lys147 impedes GAP-mediated GTP hydrolysis and promotes association with specific downstream effectors.30,31 In view of the small number of characterized Rab GAPs and Rab GEFs, and the size of the Rab family, one could speculate that Rab GTPases activation might also be controlled by a posttranslational mechanism such as ubiquitylation. Earlier unpublished work from our laboratory revealed the presence of a Rab11a form that was heavier by ~8 kDa in western blot analysis that was reminiscent of Rab11a ubiquitylation when the β2AR was co-expressed. However, ubiquitylation of Rab GTPases had never been reported before. A key contribution made by Tang et al. that was pivotal in moving our research forward reported that HACE1 (HECT domain and ankyrin repeat containing E3 ubiquitin protein ligase 1) interacts with a subset of Rab GTPases.32 However, the authors did not observe any effect on Rab ubiquitylation. In our latest publication on Rab GTPases, we showed that a β2AR/HACE1 complex promotes Rab11a ubiquitylation on Lys145.21 Our data indicated that this ubiquitylation is involved in activation of Rab11a and in the regulation of β2AR recycling to the plasma membrane. The β2AR/HACE1 complex also potentiated the ubiquitylation of Rab6a and Rab8a, but not of other Rab GTPases that were studied. This work brought to light a new regulatory mechanism of Rab GTPases through their ubiquitylation, with demonstrated functional effects on Rab11a. It also provided evidence that a cargo protein, such as a GPCR, can regulate its own trafficking by inducing the ubiquitylation and activation of a Rab GTPase.
The mechanism by which Rab11a ubiquitylation is involved in its activation remains to be determined. Rab11a ubiquitylation on Lys145 is akin to that of K-Ras on Lys147.30 The latter severely abrogates K-Ras interaction with and the response to GAPs.31 On the other hand, some GEFs for other small GTPases were shown to be E3 ubiquitin ligases.33-36 Further experiments will be necessary to determine whether HACE1 acts as a GEF for Rab11a or whether Rab11a ubiquitylation regulates its interaction with GEFs, GAPs or effectors. Unpublished observations made during our mass spectrometry analyses21 suggest that Rab11a ubiquitylation might increase its interaction with Rab11-FIPs (Rab11-family interacting proteins). The Rab11-FIPs constitute an evolutionarily conserved protein family that act as effector molecules for multiple Rab and Arf (ADP-ribosylation factor) GTPases. FIPs are key players in the regulation of multiple distinct membrane trafficking events.37 Additional work will also be required to characterize the type of ubiquitylation that Rab11a is undergoing in presence of the β2AR/HACE1 complex. Preliminary data suggest that Rab11a can be ubiquitylated on other Lys residues in absence of the β2AR/HACE1 complex. The consequences of these other putative ubiquitylation events on Rab11a activation will be interesting to study. In this line of idea, Baker et al. recently reported that different Ras isoforms are mono-ubiquitylated at distinct sites, with distinct mechanisms of action, but with a common ability to activate the protein.38
Possible Dimerization of Rab GTPases
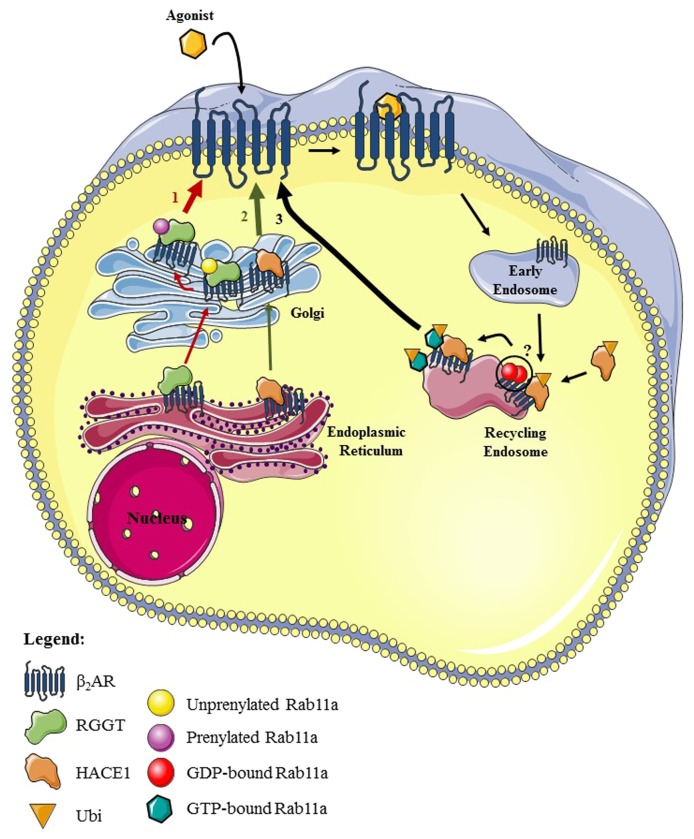
Dimerization of small GTPases has been described about two decades ago. Rho GTPases (Cdc42, Rac2, and RhoA)39 and Ras GTPase40 were reported to dimerize in both GDP- or GTP-bound states. The homophilic interaction of Cdc42 and Rac2, but not RhoA, in the GTP-bound state, caused a significant stimulation of the intrinsic GTPase activity, suggesting that dimerization might play a role in the negative regulation of specific Rho GTPases.39 Membrane localization was demonstrated to allow Ras GTPases to form dimers, which is crucial for its downstream effector activation, Raf-1.40 Arf-1 GTP-bound homodimers were also described to be important in the budding and scission of COP-I vesicles.41,42 Crystallography studies revealed the existence of GDP-bound Rab homodimers (Rab9, Rab11 and Rab27b).43-45 Rab5 dimerization was proposed to occur by yeast two-hybrid analyses as well as by in vitro and in cellulo cross-linking experiments.46 Interestingly, our data suggested that the β2AR, which interacts with the GDP-bound form of Rab11,19 increased the formation of Rab11a dimers in cellulo, as evidenced by the appearance of a ~50 kDa band of the predicted size of a Rab11a dimer in cell lysates and by increased co-immunoprecipitation between differentially epitope-tagged Rab11a in presence of the receptor.21 This was inhibited by co-expression of HACE1. The HACE1/Rab11a interaction was promoted by β2AR co-expression. We thus proposed that the β2AR acts as a scaffold to promote the HACE1 interaction with, ubiquitylation of, and activation of Rab11a. This could possibly result in dissociation of Rab11a dimers from the receptors and interaction of active Rab11a with effectors to regulate β2AR recycling.21 However, we cannot exclude the possibility that the receptor promotes the interaction between Rab11a and another ~25 kDa protein leading to the appearance of a ~50 kDa band in cell lysates, or alternatively that increased differentially tagged Rab11a co-immunoprecipitation is caused by the formation of receptor dimers/oligomers, and that both of these processes would be reduced by the expression of HACE1. More work is needed to fully address whether Rab11a dimerizes in a cellular context. Nevertheless, accumulating data suggest that Rab GTPases also undergo dimerization like other members of the Ras superfamily. A schematic representation of our recent findings is shown in Figure 1.
Figure 1. Simplified schematic representation of the molecular mechanisms used by the β2AR to control its own trafficking through Rab11a-associated machinery. (1) The interaction between the β2AR and RGGT promotes the receptor maturation through the ER and Golgi and allows for modulation of Rab11a prenylation.22 (2) HACE1, an E3 ubiquitin ligase known to interact with a subset of Rab GTPases,32 associates with the β2AR to favor export of the receptor through a mechanism independent of its ligase activity that remains to be determined.21 (3) A β2AR/HACE1 complex promotes Rab11a ubiquitylation, which leads to Rab11a GTP-loading and possibly triggers the dissociation of putative Rab11a GDP-bound homodimers. This would in turn cause activation of effectors thereby enhancing β2AR recycling.21
Future Directions
Numerous questions remain to be addressed. To cite a few, it will be interesting to study whether other Rab GTPases are ubiquitylated, the residues targeted, the resulting functional effects and the specificity of the E3 ligases involved. Do other GPCRs following different trafficking itineraries or diverse receptor families behave like the β2AR on different Rab GTPases? How does ubiquitylation of a Rab GTPase lead to its activation? Does it modulate the interaction between the Rab protein and GEFs, GAPs or effectors? Do Rab GTPases dimerize and what is the role of this dimerization? While many studies focused on the characterization of the Rab binding sites on GPCRs, the Rab domains involved in these interactions remain unexplored. In addition to the G boxes, Switch I and II regions, Rab GTPases possess other domains known as the Rab Family (RabF) and Rab Subfamily specifics (RabSF) motifs. Interestingly, RabF motifs surround both Switch regions, and have been shown to play a significant role in Rab GTPase activity.47 Knowing the GPCR binding sites on Rab GTPases will deepen our understanding of the molecular mechanisms employed by these receptors to control the activity of these small GTPases. Identifying whether other proteins of the Rab molecular machinery also associate with GPCRs, like we have shown for RGGT,22 will also be highly relevant.
Conclusions
The observations described above begin to unveil how a membrane receptor through signaling and interaction with Rab GTPases and their associated machinery can regulate its own vesicular trafficking. Emerging roles for Rab GTPases and their accessory proteins have been reported in human disease, as nicely reviewed in.48-50 However, these aspects are still underexplored therapeutically.50 Understanding how Rab GTPases and their accessory proteins are regulated should be the subject of intense research and an essential foundation for effective drug targeting.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research (grant number MOP-184095) and a Chercheur Senior salary award from the Fonds de la Recherche en Santé du Québec to J.L.P. J.L.P. is the recipient of the André-Lussier Research Chair. V.L. received a studentship from the FRQS during part of this work.
References
- 1.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–17. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 2.Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–49. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong C, Wu G. Regulation of anterograde transport of adrenergic and angiotensin II receptors by Rab2 and Rab6 GTPases. Cell Signal. 2007;19:2388–99. doi: 10.1016/j.cellsig.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammad MM, Kuang YQ, Morse A, Dupré DJ. Rab1 interacts directly with the β2-adrenergic receptor to regulate receptor anterograde trafficking. Biol Chem. 2012;393:541–6. doi: 10.1515/hsz-2011-0284. [DOI] [PubMed] [Google Scholar]
- 5.Dong C, Yang L, Zhang X, Gu H, Lam ML, Claycomb WC, Xia H, Wu G. Rab8 interacts with distinct motifs in alpha2B- and beta2-adrenergic receptors and differentially modulates their transport. J Biol Chem. 2010;285:20369–80. doi: 10.1074/jbc.M109.081521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang G, Wu G. Small GTPase regulation of GPCR anterograde trafficking. Trends Pharmacol Sci. 2012;33:28–34. doi: 10.1016/j.tips.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seachrist JL, Laporte SA, Dale LB, Babwah AV, Caron MG, Anborgh PH, Ferguson SS. Rab5 association with the angiotensin II type 1A receptor promotes Rab5 GTP binding and vesicular fusion. J Biol Chem. 2002;277:679–85. doi: 10.1074/jbc.M109022200. [DOI] [PubMed] [Google Scholar]
- 8.Schmidlin F, Dery O, DeFea KO, Slice L, Patierno S, Sternini C, Grady EF, Bunnett NW. Dynamin and Rab5a-dependent trafficking and signaling of the neurokinin 1 receptor. J Biol Chem. 2001;276:25427–37. doi: 10.1074/jbc.M101688200. [DOI] [PubMed] [Google Scholar]
- 9.Murph MM, Scaccia LA, Volpicelli LA, Radhakrishna H. Agonist-induced endocytosis of lysophosphatidic acid-coupled LPA1/EDG-2 receptors via a dynamin2- and Rab5-dependent pathway. J Cell Sci. 2003;116:1969–80. doi: 10.1242/jcs.00397. [DOI] [PubMed] [Google Scholar]
- 10.Moore RH, Sadovnikoff N, Hoffenberg S, Liu S, Woodford P, Angelides K, Trial JA, Carsrud ND, Dickey BF, Knoll BJ. Ligand-stimulated beta 2-adrenergic receptor internalization via the constitutive endocytic pathway into rab5-containing endosomes. J Cell Sci. 1995;108:2983–91. doi: 10.1242/jcs.108.9.2983. [DOI] [PubMed] [Google Scholar]
- 11.Grimsey NL, Goodfellow CE, Dragunow M, Glass M. Cannabinoid receptor 2 undergoes Rab5-mediated internalization and recycles via a Rab11-dependent pathway. Biochim Biophys Acta. 2011;1813:1554–60. doi: 10.1016/j.bbamcr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Esseltine JL, Dale LB, Ferguson SS. Rab GTPases bind at a common site within the angiotensin II type I receptor carboxyl-terminal tail: evidence that Rab4 regulates receptor phosphorylation, desensitization, and resensitization. Mol Pharmacol. 2011;79:175–84. doi: 10.1124/mol.110.068379. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Li HF, Felder RA, Periasamy A, Jose PA. Rab4 and Rab11 coordinately regulate the recycling of angiotensin II type I receptor as demonstrated by fluorescence resonance energy transfer microscopy. J Biomed Opt. 2008;13:031206. doi: 10.1117/1.2943286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner LA, Hajjhussein H, Frederick-Dyer KC, Bahouth SW. Rab11a and its binding partners regulate the recycling of the ß1-adrenergic receptor. Cell Signal. 2011;23:46–57. doi: 10.1016/j.cellsig.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamelin E, Thériault C, Laroche G, Parent JL. The intracellular trafficking of the G protein-coupled receptor TPbeta depends on a direct interaction with Rab11. J Biol Chem. 2005;280:36195–205. doi: 10.1074/jbc.M503438200. [DOI] [PubMed] [Google Scholar]
- 16.Moore RH, Millman EE, Alpizar-Foster E, Dai W, Knoll BJ. Rab11 regulates the recycling and lysosome targeting of beta2-adrenergic receptors. J Cell Sci. 2004;117:3107–17. doi: 10.1242/jcs.01168. [DOI] [PubMed] [Google Scholar]
- 17.Volpicelli LA, Lah JJ, Fang G, Goldenring JR, Levey AI. Rab11a and myosin Vb regulate recycling of the M4 muscarinic acetylcholine receptor. J Neurosci. 2002;22:9776–84. doi: 10.1523/JNEUROSCI.22-22-09776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wikström K, Reid HM, Hill M, English KA, O’Keeffe MB, Kimbembe CC, Kinsella BT. Recycling of the human prostacyclin receptor is regulated through a direct interaction with Rab11a GTPase. Cell Signal. 2008;20:2332–46. doi: 10.1016/j.cellsig.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Parent A, Hamelin E, Germain P, Parent JL. Rab11 regulates the recycling of the beta2-adrenergic receptor through a direct interaction. Biochem J. 2009;418:163–72. doi: 10.1042/BJ20080867. [DOI] [PubMed] [Google Scholar]
- 20.Dale LB, Seachrist JL, Babwah AV, Ferguson SS. Regulation of angiotensin II type 1A receptor intracellular retention, degradation, and recycling by Rab5, Rab7, and Rab11 GTPases. J Biol Chem. 2004;279:13110–8. doi: 10.1074/jbc.M313333200. [DOI] [PubMed] [Google Scholar]
- 21.Lachance V, Degrandmaison J, Marois S, Robitaille M, Génier S, Nadeau S, Angers S, Parent JL. Ubiquitylation and activation of a Rab GTPase is promoted by a β₂AR-HACE1 complex. J Cell Sci. 2014;127:111–23. doi: 10.1242/jcs.132944. [DOI] [PubMed] [Google Scholar]
- 22.Lachance V, Cartier A, Génier S, Munger S, Germain P, Labrecque P, Parent JL. Regulation of β2-adrenergic receptor maturation and anterograde trafficking by an interaction with Rab geranylgeranyltransferase: modulation of Rab geranylgeranylation by the receptor. J Biol Chem. 2011;286:40802–13. doi: 10.1074/jbc.M111.267815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esseltine JL, Ferguson SS. Regulation of G protein-coupled receptor trafficking and signaling by Rab GTPases. Small GTPases. 2013;4:132–5. doi: 10.4161/sgtp.24304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yudowski GA, Puthenveedu MA, Henry AG, von Zastrow M. Cargo-mediated regulation of a rapid Rab4-dependent recycling pathway. Mol Biol Cell. 2009;20:2774–84. doi: 10.1091/mbc.E08-08-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brighouse A, Dacks JB, Field MC. Rab protein evolution and the history of the eukaryotic endomembrane system. Cell Mol Life Sci. 2010;67:3449–65. doi: 10.1007/s00018-010-0436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casey PJ, Seabra MC. Protein prenyltransferases. J Biol Chem. 1996;271:5289–92. doi: 10.1074/jbc.271.10.5289. [DOI] [PubMed] [Google Scholar]
- 27.Leung KF, Baron R, Ali BR, Magee AI, Seabra MC. Rab GTPases containing a CAAX motif are processed post-geranylgeranylation by proteolysis and methylation. J Biol Chem. 2007;282:1487–97. doi: 10.1074/jbc.M605557200. [DOI] [PubMed] [Google Scholar]
- 28.Leung KF, Baron R, Seabra MC. Thematic review series: lipid posttranslational modifications. geranylgeranylation of Rab GTPases. J Lipid Res. 2006;47:467–75. doi: 10.1194/jlr.R500017-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Goalstone ML, Leitner JW, Berhanu P, Sharma PM, Olefsky JM, Draznin B. Insulin signals to prenyltransferases via the Shc branch of intracellular signaling. J Biol Chem. 2001;276:12805–12. doi: 10.1074/jbc.M009443200. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki AT, Carracedo A, Locasale JW, Anastasiou D, Takeuchi K, Kahoud ER, Haviv S, Asara JM, Pandolfi PP, Cantley LC. Ubiquitination of K-Ras enhances activation and facilitates binding to select downstream effectors. Sci Signal. 2011;4:ra13. doi: 10.1126/scisignal.2001518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker R, Lewis SM, Sasaki AT, Wilkerson EM, Locasale JW, Cantley LC, Kuhlman B, Dohlman HG, Campbell SL. Site-specific monoubiquitination activates Ras by impeding GTPase-activating protein function. Nat Struct Mol Biol. 2013;20:46–52. doi: 10.1038/nsmb.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang D, Xiang Y, De Renzis S, Rink J, Zheng G, Zerial M, Wang Y. The ubiquitin ligase HACE1 regulates Golgi membrane dynamics during the cell cycle. Nat Commun. 2011;2:501. doi: 10.1038/ncomms1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barr F, Lambright DG. Rab GEFs and GAPs. Curr Opin Cell Biol. 2010;22:461–70. doi: 10.1016/j.ceb.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosa JL, Casaroli-Marano RP, Buckler AJ, Vilaró S, Barbacid M. p619, a giant protein related to the chromosome condensation regulator RCC1, stimulates guanine nucleotide exchange on ARF1 and Rab proteins. EMBO J. 1996;15:4262–73. [PMC free article] [PubMed] [Google Scholar]
- 35.Swaminathan G, Tsygankov AY. The Cbl family proteins: ring leaders in regulation of cell signaling. J Cell Physiol. 2006;209:21–43. doi: 10.1002/jcp.20694. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Lubkov V, Taylor LJ, Bar-Sagi D. Feedback regulation of Ras signaling by Rabex-5-mediated ubiquitination. Curr Biol. 2010;20:1372–7. doi: 10.1016/j.cub.2010.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horgan CP, McCaffrey MW. The dynamic Rab11-FIPs. Biochem Soc Trans. 2009;37:1032–6. doi: 10.1042/BST0371032. [DOI] [PubMed] [Google Scholar]
- 38.Baker R, Wilkerson EM, Sumita K, Isom DG, Sasaki AT, Dohlman HG, Campbell SL. Differences in the regulation of K-Ras and H-Ras isoforms by monoubiquitination. J Biol Chem. 2013;288:36856–62. doi: 10.1074/jbc.C113.525691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang B, Zheng Y. Negative regulation of Rho family GTPases Cdc42 and Rac2 by homodimer formation. J Biol Chem. 1998;273:25728–33. doi: 10.1074/jbc.273.40.25728. [DOI] [PubMed] [Google Scholar]
- 40.Inouye K, Mizutani S, Koide H, Kaziro Y. Formation of the Ras dimer is essential for Raf-1 activation. J Biol Chem. 2000;275:3737–40. doi: 10.1074/jbc.275.6.3737. [DOI] [PubMed] [Google Scholar]
- 41.Beck R, Sun Z, Adolf F, Rutz C, Bassler J, Wild K, Sinning I, Hurt E, Brügger B, Béthune J, et al. Membrane curvature induced by Arf1-GTP is essential for vesicle formation. Proc Natl Acad Sci U S A. 2008;105:11731–6. doi: 10.1073/pnas.0805182105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beck R, Prinz S, Diestelkötter-Bachert P, Röhling S, Adolf F, Hoehner K, Welsch S, Ronchi P, Brügger B, Briggs JA, et al. Coatomer and dimeric ADP ribosylation factor 1 promote distinct steps in membrane scission. J Cell Biol. 2011;194:765–77. doi: 10.1083/jcb.201011027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chavas LM, Torii S, Kamikubo H, Kawasaki M, Ihara K, Kato R, Kataoka M, Izumi T, Wakatsuki S. Structure of the small GTPase Rab27b shows an unexpected swapped dimer. Acta Crystallogr D Biol Crystallogr. 2007;63:769–79. doi: 10.1107/S0907444907019725. [DOI] [PubMed] [Google Scholar]
- 44.Pasqualato S, Senic-Matuglia F, Renault L, Goud B, Salamero J, Cherfils J. The structural GDP/GTP cycle of Rab11 reveals a novel interface involved in the dynamics of recycling endosomes. J Biol Chem. 2004;279:11480–8. doi: 10.1074/jbc.M310558200. [DOI] [PubMed] [Google Scholar]
- 45.Wittmann JG, Rudolph MG. Crystal structure of Rab9 complexed to GDP reveals a dimer with an active conformation of switch II. FEBS Lett. 2004;568:23–9. doi: 10.1016/j.febslet.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Daitoku H, Isida J, Fujiwara K, Nakajima T, Fukamizu A. Dimerization of small GTPase Rab5. Int J Mol Med. 2001;8:397–404. doi: 10.3892/ijmm.8.4.397. [DOI] [PubMed] [Google Scholar]
- 47.Pereira-Leal JB, Seabra MC. The mammalian Rab family of small GTPases: definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J Mol Biol. 2000;301:1077–87. doi: 10.1006/jmbi.2000.4010. [DOI] [PubMed] [Google Scholar]
- 48.Li G. Rab GTPases, membrane trafficking and diseases. Curr Drug Targets. 2011;12:1188–93. doi: 10.2174/138945011795906561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelly EE, Horgan CP, Goud B, McCaffrey MW. The Rab family of proteins: 25 years on. Biochem Soc Trans. 2012;40:1337–47. doi: 10.1042/BST20120203. [DOI] [PubMed] [Google Scholar]
- 50.Agola JO, Jim PA, Ward HH, Basuray S, Wandinger-Ness A. Rab GTPases as regulators of endocytosis, targets of disease and therapeutic opportunities. Clin Genet. 2011;80:305–18. doi: 10.1111/j.1399-0004.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]