Abstract
Plant hemoglobins (Hbs) have been identified as master regulators in determining the developmental fate of specific cells during maize embryogenesis. Whether an embryogenic cell lives or undergoes programmed cell death (PCD) is modulated by Hbs, through their tight interactions with nitric oxide (NO) and auxin. During maize embryogenesis, Hb-suppressing cells accumulate NO, are depleted of auxin, and are committed to die. We propose that Hbs control cell fate by regulating NO and auxin homeostasis, and that this type of mechanism may influence other hormonal responses modulating plant behavior during development and stress conditions.
Keywords: hemoglobin, nitric oxide, hormone, cell fate, embryogenesis
The de-differentiation and re-differentiation programs of already differentiated cells are the basis of totipotency, a characteristic unique to plants providing extreme flexibility in fate acquisition. The execution of both programs is generally initiated by exogenous and endogenous cues and it is mediated by transcriptional, post-transcriptional, and post-translational mechanisms activating signaling cascades. For example, cell de-differentiation and embryogenic tissue formation in somatic embryogenesis are often triggered by supplementation of auxin, while the re-differentiation step leading to the development of somatic embryos is achieved in an auxin-free environment.1 Other hormones, such as abscisic acid (ABA), cytokinin (CK), gibberellic acid (GA), ethylene (ET), and brassinosteroid (BR), applied individually or in combination, also act as modulators of the de-differentiation/re-differentiation switch.2-6 The most well characterized example of hormone interaction in cell fate specification involves the antagonistic link between auxin and CK, which specify the root stem-cell niches.7 The interaction between the transcription factor WIND1 (wound induced de-differentiation 1) and CK has also been implicated in the cell de-differentiation processes.8
Nitric oxide is a transducer of auxin in activating cell division during embryogenic cell formation,9 and this process might be altered by Hb, an effective scavenger of NO. The expression pattern of Hbs has been widely examined in different systems in relation to hormone or stress responses.10-15 Investigations of how Hb expression patterns contribute to plant development, and in particular embryo formation, have been described recently. Work from our lab revealed that suppression of Arabidopsis Hb2, normally expressed in the explant at the sites where embryogenic tissue originates, increased endogenous auxin (indole-3-acetic acid, IAA) production and favored the formation of somatic embryos.16
Based on the premise that Hbs are effective NO scavengers,17 a more recent study identified maize Hbs as key determinants in the cell death/survival decision during in vitro embryogenesis.18 In a model proposed by the authors, suppression of the 2 maize Hbs, ZmHb1 and ZmHb2, triggers PCD by favoring the accumulation of NO and Zn2+, which through the activation of a mitogen-activated protein kinase cascade leads to the accumulation of ROS in the cells destined to die. While the mode of action of the 2 Hbs is similar, their different localization pattern influences somatic embryogenesis in opposite ways. While ZmHb1 is expressed in many cells of the embryo proper and its suppression results in massive death and embryo abortion, ZmHb2 is mainly localized in the cells anchoring the immature embryos to the embryogenic tissue. Removal of these cells by PCD releases the embryos and favors the continuation of their developmental program.
To fine-tune development and stress responses employing a limited number of mobile growth regulators, plants have developed mechanisms to differentiate cues in adjacent cells elicited by the same growth regulator.17 Toward this end, the close interaction between NO and growth regulators may contribute to this differentiation process.19,20 In the case of auxin, the regulation of hormone transport is a major factor in determining cell fate, i.e., the fate of the cell is determined by the presence or absence of the hormone through its transport in that specific domain. In addition to being a downstream signaling molecule in the auxin response, NO might influence auxin transport, possibly through its regulation by Hbs (Fig. 1).
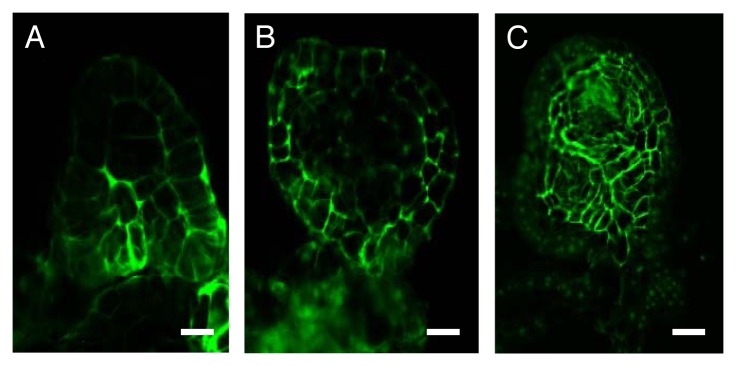
Figure 1. Immunolocalization of ZmPIN1 proteins in immature somatic maize embryos downregulating ZmHb1 or ZmHb2. Compared with WT embryos (A) where ZmPIN1 is mainly localized in the basal domains, in embryos suppressing ZmHb1 (B) the fluorescent signal is restricted to the apical layers of cells. ZmPIN1 is expressed throughout the ZmHb2 suppressing embryos (C). Scale = 30μm.
Cell fate specification by auxin during embryogenesis
The generation of the whole plant from a single cell requires the spatial and temporal coordination of cell division, differentiation, and death processes, that are the manifestation of fate acquisition. During the initial phases of embryogenesis, auxin-mediated signaling plays a crucial role in cell fate determination.21,22 After the first apical division of the zygote, the embryonic pattern is largely established and maintained by polar auxin transport (PAT) mediated by several PIN members.23 Among the developmental processes influenced by auxin are the basal-cell lineage specification of the embryo and the differentiation of the suspensor.22 The ontogeny, development, and dismantling by PCD of the suspensor are the best studied examples of the influence exercised by a plant growth regulator, i.e., auxin, on the fate of a whole organ.24 Moreover, the antagonistic interaction between auxin and CK during early embryogenesis is also critical for the specification of the root stem-cell niche.7 The participation of a more complex hormone network, involving ABA and JA, acting in conjunction with auxin and CK in the specification of the embryonic poles cannot be excluded.25
Involvement of Hbs in the auxin-mediated cell fate determination
Auxin occupies a central role in the fate specification of embryogenic cells.26 The identification of early master regulators influencing the auxin response is, therefore, essential to understand cell behavior. Synergistic effects of auxin and NO have been documented in several systems and are not only limited to the establishment of the embryonic body plan, but also to the formation of adventitious roots and root nodules.9,19 Speculations on the interaction between auxin and the NO-scavenging Hbs have been proposed,17 and evidence that modifying the expression of a specific Hb modulates several auxin-mediated morphogenic events in Arabidopsis has been demonstrated.16
Suppression of the Arabidopsis Hb2 stimulates auxin production in specific cells through a process initiated by an elevation in NO which downregulates the transcription factor MYC2, an auxin biosynthetic inhibitor.27 In addition to repressing auxin levels, MYC2 is a master regulator of several other growth regulators, including jasmonic acid (JA), salicylic acid (SA), GA, and ABA.28 In reference to these observations, unpublished data from our lab indicates that the changes in Arabidopsis embryogenic cell fate are the result of an elevation in JA, in the same cells where Hb2 is suppressed and auxin levels elevated. Furthermore, the results with the monocot maize system demonstrate that the sites of expression of ZmHb1 and ZmHb2 are instrumental factors in determining the developmental fate, either survival or death, of specific embryogenic cells.18
The distinct Hb-initiated regulatory mechanisms in either Arabidopsis, with respect to cell-specific auxin enrichment,16 or maize, with respect to the cell lineage-specific survival/death decision,18 reveal differences in Hb action between monocots and dicots. While suppression of Hb increases auxin level in Arabidopsis,16 it decreases it in maize where cells committed to die by PCD can be visualized by a reduced or absent auxin signal (Fig. 2). The induction of PCD following depletion of auxin has also been shown in other systems.29 Despite these different responses operating among species, it is apparent that expression (or repression) of a specific Hb influences cell fate by modulating hormone synthesis and possibly response in a process modulated by NO. Thus we proposed that in the maize embryogenic system cells suppressing ZmHb1 or ZmHb2 have increasing levels of NO, a reduced auxin content, and are destined to die by PCD. However, the dynamics of the Hb-regulated cell fate switch may require other intermediates, as previous studies from our lab revealed that suppression of Hb increases ethylene production in maize embryogenic suspension cultures,30 and overexpression of Hb1 and Hb2 favors the in vitro formation of shoots in Arabidopsis by enhancing cytokinin sensitivity.31 In addition, as implied by the diversity of putative cis-regulatory elements in the maize Hb promoters,32 and in the participation of Hbs in pathogen plant interaction,33 future efforts should be focused on establishing the factors responsible for the specific localization of different Hbs in distinct domains of the same tissue, and the involvement of Hbs in diverse developmental and stress-related responses through the modulation of NO and its interaction with plant growth regulators.
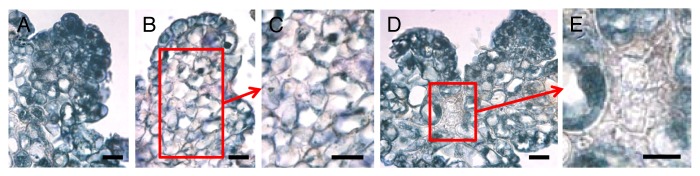
Figure 2. Immunolocalization of the auxin IAA in immature somatic maize embryos downregulating ZmHb1 or ZmHb2. Compared with WT (A) where IAA is present through the whole embryo, in embryos suppressing ZmHb1 (B) the signal is restricted to the apical layers of cells. Sub-apical cells are depleted of IAA (C). In embryos downregulating ZmHb2 (D) IAA localizes in all the embryonic cells with the exclusion of “anchor cells” connecting the immature embryos (E). Cells depleted of IAA are most likely those suppressing the respective ZmHbs and destined to die (compared with Figure 1 and 3 in Huang et al., 2014). Scale = 30μm.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Thorpe TA, Stasolla C. Somatic embryogenesis. Current Trends in the Embryology of Angiosperms. Bhojwani SS, Soh WY, eds. Dordrecht: Kluwer Academic Publisher, 2001:279-336. [Google Scholar]
- 2.Chugh A, Khurana P. Gene expression during somatic embryogenesis: recent advances. Curr Sci. 2002;83:715–30. [Google Scholar]
- 3.Thibaud-Nissen F, Shealy RT, Khanna A, Vodkin LO. Clustering of microarray data reveals transcript patterns associated with somatic embryogenesis in soybean. Plant Physiol. 2003;132:118–36. doi: 10.1104/pp.103.019968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feher A, Pasternak TP, Dudits D. Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult. 2003;74:201–28. doi: 10.1023/A:1024033216561. [DOI] [Google Scholar]
- 5.Zeng F, Zhang X, Zhu L, Tu L, Guo X, Nie Y. Isolation and characterization of genes associated to cotton somatic embryogenesis by suppression subtractive hybridization and macroarray. Plant Mol Biol. 2006;60:167–83. doi: 10.1007/s11103-005-3381-x. [DOI] [PubMed] [Google Scholar]
- 6.Karlova R, Boeren S, Russinova E, Aker J, Vervoort J, de Vries S. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 protein complex includes BRASSINOSTEROID-INSENSITIVE1. Plant Cell. 2006;18:626–38. doi: 10.1105/tpc.105.039412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Müller B, Sheen J. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature. 2008;453:1094–7. doi: 10.1038/nature06943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwase A, Mitsuda N, Koyama T, Hiratsu K, Kojima M, Arai T, Inoue Y, Seki M, Sakakibara H, Sugimoto K, et al. The AP2/ERF transcription factor WIND1 controls cell dedifferentiation in Arabidopsis. Curr Biol. 2011;21:508–14. doi: 10.1016/j.cub.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Otvös K, Pasternak TP, Miskolczi P, Domoki M, Dorjgotov D, Szucs A, Bottka S, Dudits D, Fehér A. Nitric oxide is required for, and promotes auxin-mediated activation of, cell division and embryogenic cell formation but does not influence cell cycle progression in alfalfa cell cultures. Plant J. 2005;43:849–60. doi: 10.1111/j.1365-313X.2005.02494.x. [DOI] [PubMed] [Google Scholar]
- 10.Dordas C, Hasinoff BB, Igamberdiev AU, Manac’h N, Rivoal J, Hill RD. Expression of a stress-induced hemoglobin affects NO levels produced by alfalfa root cultures under hypoxic stress. Plant J. 2003;35:763–70. doi: 10.1046/j.1365-313X.2003.01846.x. [DOI] [PubMed] [Google Scholar]
- 11.Smagghe BJ, Blervacq AS, Blassiau C, Decottignies JP, Jacquot JP, Hargrove MS, Hilbert JL. Immunolocalization of non-symbiotic hemoglobins during somatic embryogenesis in chicory.PlantSign. Behav. 2007;2:43–9. doi: 10.4161/psb.2.1.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parent C, Berger A, Folzer H, Dat J, Crevècoeur M, Badot PM, Capelli N. A novel nonsymbiotic hemoglobin from oak: cellular and tissue specificity of gene expression. New Phytol. 2008;177:142–54. doi: 10.1111/j.1469-8137.2007.02250.x. [DOI] [PubMed] [Google Scholar]
- 13.Bustos-Sanmamed P, Tovar-Méndez A, Crespi M, Sato S, Tabata S, Becana M. Regulation of nonsymbiotic and truncated hemoglobin genes of Lotus japonicus in plant organs and in response to nitric oxide and hormones. New Phytol. 2011;189:765–76. doi: 10.1111/j.1469-8137.2010.03527.x. [DOI] [PubMed] [Google Scholar]
- 14.Leiva-Eriksson N, Pin PA, Kraft T, Dohm JC, Minoche AE, Himmelbauer H, Bülow L. Differential expression patterns of non-symbiotic hemoglobins in sugar beet (Beta vulgaris ssp. vulgaris) Plant Cell Physiol. 2014;55:834–44. doi: 10.1093/pcp/pcu027. [DOI] [PubMed] [Google Scholar]
- 15.Dumont E, Jokipii-Lukkari S, Parkash V, Vuosku J, Sundström R, Nymalm Y, Sutela S, Taskinen K, Kallio PT, Salminen TA, et al. Evolution, three-dimensional model and localization of truncated hemoglobin PttTrHb of hybrid aspen. PLoS One. 2014;9:e88573. doi: 10.1371/journal.pone.0088573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elhiti M, Hebelstrup KH, Wang A, Li C, Cui Y, Hill RD, Stasolla C. Function of type-2 Arabidopsis hemoglobin in the auxin-mediated formation of embryogenic cells during morphogenesis. Plant J. 2013;74:946–58. doi: 10.1111/tpj.12181. [DOI] [PubMed] [Google Scholar]
- 17.Hill RD. Non-symbiotic haemoglobins - What's happening beyond nitric oxide scavenging? AoB Plants 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang S, Hill RD, Wally OS, Dionisio G, Ayele BT, Jami SK, Stasolla C. Hemoglobin control of cell survival/death decision regulates in vitro plant embryogenesis. Plant Physiol. 2014;165:810–25. doi: 10.1104/pp.114.239335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freschi L. Nitric oxide and phytohormone interactions: current status and perspectives. Front Plant Sci. 2013;4:398. doi: 10.3389/fpls.2013.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu M, Lamattina L, Spoel SH, Loake GJ. Nitric oxide function in plant biology: a redox cue in deconvolution. New Phytol. 2014;202:1142–56. doi: 10.1111/nph.12739. [DOI] [PubMed] [Google Scholar]
- 21.Jenik PD, Gillmor CS, Lukowitz W. Embryonic patterning in Arabidopsis thaliana. Annu Rev Cell Dev Biol. 2007;23:207–36. doi: 10.1146/annurev.cellbio.22.011105.102609. [DOI] [PubMed] [Google Scholar]
- 22.Möller B, Weijers D. Auxin control of embryo patterning. Cold Spring Harb Perspect Biol. 2009;1:a001545. doi: 10.1101/cshperspect.a001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michniewicz M, Zago MK, Abas L, Weijers D, Schweighofer A, Meskiene I, Heisler MG, Ohno C, Zhang J, Huang F, et al. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell. 2007;130:1044–56. doi: 10.1016/j.cell.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 24.Kawashima T, Goldberg RB. The suspensor: not just suspending the embryo. Trends Plant Sci. 2010;15:23–30. doi: 10.1016/j.tplants.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Vanstraelen M, Benková E. Hormonal interactions in the regulation of plant development. Annu Rev Cell Dev Biol. 2012;28:463–87. doi: 10.1146/annurev-cellbio-101011-155741. [DOI] [PubMed] [Google Scholar]
- 26.Rademacher EH, Lokerse AS, Schlereth A, Llavata-Peris CI, Bayer M, Kientz M, Freire Rios A, Borst JW, Lukowitz W, Jürgens G, et al. Different auxin response machineries control distinct cell fates in the early plant embryo. Dev Cell. 2012;22:211–22. doi: 10.1016/j.devcel.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Dombrecht B, Xue GP, Sprague SJ, Kirkegaard JA, Ross JJ, Reid JB, Fitt GP, Sewelam N, Schenk PM, Manners JM, et al. MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell. 2007;19:2225–45. doi: 10.1105/tpc.106.048017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kazan K, Manners JM. MYC2: the master in action. Mol Plant. 2013;6:686–703. doi: 10.1093/mp/sss128. [DOI] [PubMed] [Google Scholar]
- 29.Xia QZ, Zhang XL, Nie YC, Guo XP. [Withdrawal of exogenous auxin induces programmed cell death of cotton embryogenic suspension cultures] Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao. 2005;31:78–84. [PubMed] [Google Scholar]
- 30.Manac’h-Little N, Igamberdiev AU, Hill RD. Hemoglobin expression affects ethylene production in maize cell cultures. Plant Physiol Biochem. 2005;43:485–9. doi: 10.1016/j.plaphy.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Elhiti M, Hebelstrup KH, Hill RD, Stasolla C. Manipulation of hemoglobin expression affects Arabidopsis shoot organogenesis. Plant Physiol Biochem. 2011;49:1108–16. doi: 10.1016/j.plaphy.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Hill RD, Huang S, Stasolla C. Hemoglobins, programmed cell death and somatic embryogenesis. Plant Sci. 2013;211:35–41. doi: 10.1016/j.plantsci.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Mur LA, Sivakumaran A, Mandon J, Cristescu SM, Harren FJ, Hebelstrup KH. Haemoglobin modulates salicylate and jasmonate/ethylene-mediated resistance mechanisms against pathogens. J Exp Bot. 2012;63:4375–87. doi: 10.1093/jxb/ers116. [DOI] [PMC free article] [PubMed] [Google Scholar]


