Abstract
DELLA proteins are key negative regulators in the phytohormone gibberellin’s (GA) signaling. In addition to this role, the DELLA proteins upregulate the gene expression levels of the positive regulators in GA signaling, such as GA 20-oxidase, GA receptor, and a transcriptional regulator, SCARECROW-LIKE3 (SCL3), which enables the regulation of GA feedback. Since DELLAs lack a known DNA binding domain, other transcription factor(s) that recruit DELLAs to DNA are essential for this regulation. Recently, we showed that the INDETERMINATE DOMAIN family proteins serve as transcriptional scaffolds to exert the transactivation activity of DELLAs. This finding and further analyses regarding the function of SCL3 indicate that the balance of the DELLAs and SCL3 protein levels (both are GRAS proteins) regulates downstream gene expression through IDDs binding to DNA. Here, we review the regulatory system in plants similar to ours and also discuss the interactive network between GRAS and IDD proteins.
Keywords: co-regulator exchange system, downstream of DELLA, feedback of gibberellin signaling, GRAS, IDD
Gibberellin (GA) is a plant hormone that modulates various processes involved in plant growth, organ development, and environmental responses. These include leaf expansion, flowering time, seed germination, stem elongation, and the development of flowers, fruit, and seeds.1 In the past few decades, several protein components involved in GA signaling have been identified. Among these, DELLA proteins are the key negative regulators in GA signaling. GA is perceived by a GA-receptor, GID1, and then elicits the degradation of DELLAs,2-4 resulting in a de-repressed GA signaling state. DELLA proteins are characterized by a DELLA/TVHYNP motif at the N-terminus and a GRAS domain (named after its first 3 members: GA INSENSITIVE [GAI], REPRESSOR of ga1–3 [RGA], and SCARECROW [SCR]) at the C-terminus, placing DELLAs within the GRAS family of transcriptional regulators. The DELLA subfamily is highly conserved among angiosperms, gymnosperms, and ferns, but not in Physcomitrella patens, a model organism for mosses (bryophytes). Arabidopsis has 5 DELLAs, GAI, RGA, RGA-LIKE (RGL)1, RGL2, and RGL3, whereas, rice has one DELLA, SLENDER RICE1.1,5
DELLAs, lacking a known DNA-binding domain, are thought to act as transcriptional regulators in 2 ways.6 To interact with DNA-binding domains of transcription factors and inhibit the expression of their target genes, or to interact with other transcription factors as transcriptional co-activators to promote the expression of downstream genes. In the latter case, the downstream genes include the positive regulators in GA signaling, such as GA biosynthetic enzymes, GA 3-oxidase and GA 20-oxidase, GA receptor GID1, and transcriptional regulators, such as SCL3, which are involved in GA feedback regulation. In this context, the principal question was how DELLAs positively regulate gene expression levels. Recently, we identified certain members of the IDD transcription factor family, AtIDD3, –4, –5, –9, and –10, that can act as transcriptional scaffolds, which mediate between DELLAs and the promoter sequence of the downstream genes.7 They interact with both the GRAS domain of RGA and the promoter sequence of the DELLA-target gene, SCARECROW-LIKE3 (SCL3).8,9 The IDD protein family is characterized by a highly conserved N-terminal domain containing 4 zinc finger motifs that bind to DNA and 2 C-terminal short motifs.10 Although an IDD protein was first isolated as the causal factor for a late-flowering mutant of maize,11 the diverse physiological functions of IDDs have been revealed in Arabidopsis and rice, including the regulation of auxin signaling and flowering time, as well as gravitropic responses.12-16 Our experiments indicated that RGA and the IDDs synergistically upregulate the expression of SCL3. Plants overexpressing AtIDD3 fused with SRDX, a plant specific repression domain, partially mimicked the GA-deficient plant, suggesting that the AtIDD3-SRDX protein causes DELLA’s loss of transactivation activity in the feedback loop of GA signaling.
Additionally, we focused on the function of the SCL3 protein. SCL3, like the DELLAs, possesses a GRAS domain at its C-terminus and belongs to the GRAS family.17 Based on the sequence similarity between DELLAs and SCL3, we hypothesized that SCL3 can also interact with IDDs. The interaction between SCL3 and the IDDs was expected to inhibit DELLA-IDD interactions based on previous genetic studies that showed SCL3 functions antagonistically to the DELLAs.9,18 In fact, we demonstrated the interaction between SCL3 and IDD proteins, and we also revealed that RGA and SCL3 competitively interact with AtIDD3. Furthermore, we demonstrated that the competitive relationship reflects the transcriptional regulation of their downstream genes, such as SCL3.
In the conclusion of our recent paper, we proposed that DELLAs, SCL3, and IDDs constitute a “co-activator/co-repressor exchange regulation system” to fine-tune GA feedback regulation (Fig. 1). In this model, DELLAs act as co-activators binding to the promoter of the downstream genes, including SCL3, through the transcriptional scaffolds, IDDs. After increasing SCL3 transcription, accumulated SCL3 protein acts as a co-repressor, leading to an increased level of the SCL3-IDD complex, and then, it reduces the expression of downstream genes, including itself. Such a regulatory system, in which a transcription factor regulates positively or negatively the downstream genes depending on its interactions with co-activators or co-repressors, respectively, is well studied in animals;19 however, it has been rarely reported in plants. To our knowledge, Arabidopsis is proposed to have a similar system for regulating flowering time and for the development of the inflorescence meristem. FLOWERING LOCUS T (FT), a key positive regulator that induces flowering, and its homolog TERMINAL FLOWER1 (TFL1), which antagonizes FT’s function, seem to act as mediators to recruit co-activators and co-repressors, respectively, through interactions with the DNA-binding complex, which consists of 14-3-3 and FLOWERING LOCUS D (FD).20 When flowering is induced, FT moves from leaves to the shoot apical meristem and then excludes TFL1 from the 14-3-3-FD complex. This results in the formation of the FT-14-3-3-FD transactivation complex that promotes the expression of downstream genes.20 Considering this, the co-regulator exchange system may be not limited to the GA signaling pathway but may be widely used in other signaling pathways in plants.
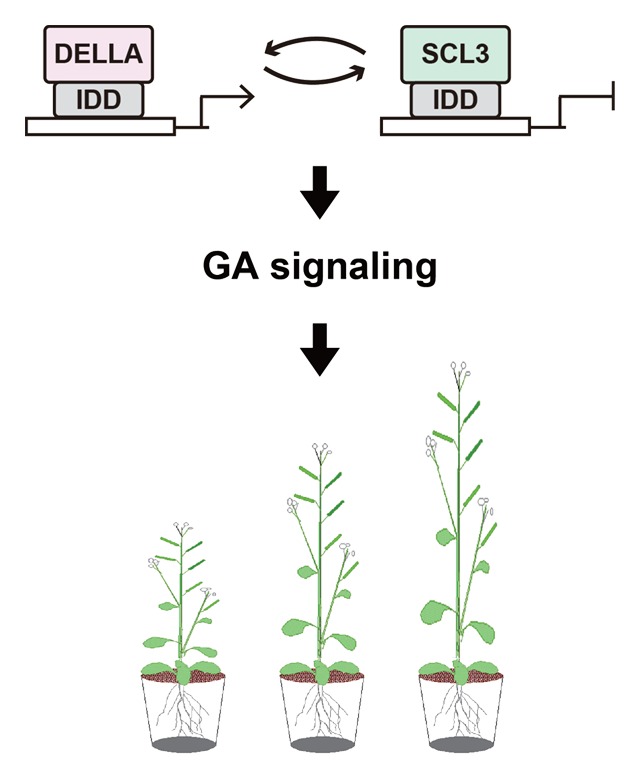
Figure 1. The co-activator/co-repressor exchange system, consisting of DELLA, SCARECROW-LIKE3 (SCL3), and INDETERMINATE DOMAIN (IDD) proteins, regulates the feedback loop in gibberellin signaling.
We found physical and genetic relationships between the GRAS proteins, DELLAs and SCL3, and the IDD family proteins, AtIDD3, –4, –5, –9, and –10. Previous research on GRAS and IDD proteins also found interactions between them. GRAS family members, SCR and SHOOT-ROOT (SHR), and IDD family members, AtIDD3 and AtIDD10, regulate each other’s expression levels and can form complexes to regulate root development.21,22 The interaction between SHR and AtIDD4 has been reported,23 as has the interaction between DELLAs and AtIDD1, which regulates seed dormancy.24 These results, and ours, illustrate the importance of the emerging common theme, the interaction and transcription network between GRAS and IDD proteins that is likely involved in multiple signaling pathways and many aspects of physiological events in plants.24
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science (JSPS) through the World-Leading Researchers (M.U.-T.), and the Japanese Ministry of Education, Culture, Sports, Science, and Technology Program for Leading Graduate Schools “Integrative Graduate Education and Research in Green Natural Sciences” (H.Y.).
Glossary
Abbreviations:
- FD
FLOWERING LOCUS D
- FT
FLOWERING LOCUS T
- GA
gibberellin
- GAI
GA INSENSITIVE
- GID1
GIBBERELLIN INSENSITIVE DWARF1
- IDD
INDETERMINATE DOMAIN
- RGA
REPRESSOR of ga1-3
- RGL
RGA-LIKE
- SCL3
SCARECROW-LIKE3
- SCR
SCARECROW
- SHR
SHOOT-ROOT
- TFL1
TERMINAL FLOWER1
References
- 1.Itoh H, Ueguchi-Tanaka M, Matsuoka M. Molecular biology of gibberellins signaling in higher plants. Int Rev Cell Mol Biol. 2008;268:191–221. doi: 10.1016/S1937-6448(08)00806-X. [DOI] [PubMed] [Google Scholar]
- 2.Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, Chow TY, Hsing YI, Kitano H, Yamaguchi I, et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature. 2005;437:693–8. doi: 10.1038/nature04028. [DOI] [PubMed] [Google Scholar]
- 3.Nakajima M, Shimada A, Takashi Y, Kim YC, Park SH, Ueguchi-Tanaka M, Suzuki H, Katoh E, Iuchi S, Kobayashi M, et al. Identification and characterization of Arabidopsis gibberellin receptors. Plant J. 2006;46:880–9. doi: 10.1111/j.1365-313X.2006.02748.x. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths J, Murase K, Rieu I, Zentella R, Zhang ZL, Powers SJ, Gong F, Phillips AL, Hedden P, Sun TP, et al. Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell. 2006;18:3399–414. doi: 10.1105/tpc.106.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun TP. The molecular mechanism and evolution of the GA-GID1-DELLA signaling module in plants. Curr Biol. 2011;21:R338–45. doi: 10.1016/j.cub.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 6.Davière JM, Achard P. Gibberellin signaling in plants. Development. 2013;140:1147–51. doi: 10.1242/dev.087650. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida H, Hirano K, Sato T, Mitsuda N, Nomoto M, Maeo K, Koketsu E, Mitani R, Kawamura M, Ishiguro S, et al. DELLA protein functions as a transcriptional activator through the DNA binding of the indeterminate domain family proteins. Proc Natl Acad Sci U S A. 2014;111:7861–6. doi: 10.1073/pnas.1321669111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zentella R, Zhang ZL, Park M, Thomas SG, Endo A, Murase K, Fleet CM, Jikumaru Y, Nambara E, Kamiya Y, et al. Global analysis of della direct targets in early gibberellin signaling in Arabidopsis. Plant Cell. 2007;19:3037–57. doi: 10.1105/tpc.107.054999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang ZL, Ogawa M, Fleet CM, Zentella R, Hu J, Heo JO, Lim J, Kamiya Y, Yamaguchi S, Sun TP. Scarecrow-like 3 promotes gibberellin signaling by antagonizing master growth repressor DELLA in Arabidopsis. Proc Natl Acad Sci U S A. 2011;108:2160–5. doi: 10.1073/pnas.1012232108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colasanti J, Tremblay R, Wong AY, Coneva V, Kozaki A, Mable BK. The maize INDETERMINATE1 flowering time regulator defines a highly conserved zinc finger protein family in higher plants. BMC Genomics. 2006;7:158. doi: 10.1186/1471-2164-7-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colasanti J, Yuan Z, Sundaresan V. The indeterminate gene encodes a zinc finger protein and regulates a leaf-generated signal required for the transition to flowering in maize. Cell. 1998;93:593–603. doi: 10.1016/S0092-8674(00)81188-5. [DOI] [PubMed] [Google Scholar]
- 12.Morita MT, Sakaguchi K, Kiyose S, Taira K, Kato T, Nakamura M, Tasaka M. A C2H2-type zinc finger protein, SGR5, is involved in early events of gravitropism in Arabidopsis inflorescence stems. Plant J. 2006;47:619–28. doi: 10.1111/j.1365-313X.2006.02807.x. [DOI] [PubMed] [Google Scholar]
- 13.Tanimoto M, Tremblay R, Colasanti J. Altered gravitropic response, amyloplast sedimentation and circumnutation in the Arabidopsis shoot gravitropism 5 mutant are associated with reduced starch levels. Plant Mol Biol. 2008;67:57–69. doi: 10.1007/s11103-008-9301-0. [DOI] [PubMed] [Google Scholar]
- 14.Seo PJ, Ryu J, Kang SK, Park CM. Modulation of sugar metabolism by an INDETERMINATE DOMAIN transcription factor contributes to photoperiodic flowering in Arabidopsis. Plant J. 2011;65:418–29. doi: 10.1111/j.1365-313X.2010.04432.x. [DOI] [PubMed] [Google Scholar]
- 15.Cui D, Zhao J, Jing Y, Fan M, Liu J, Wang Z, Xin W, Hu Y. The arabidopsis IDD14, IDD15, and IDD16 cooperatively regulate lateral organ morphogenesis and gravitropism by promoting auxin biosynthesis and transport. PLoS Genet. 2013;9:e1003759. doi: 10.1371/journal.pgen.1003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu X, Tang D, Li M, Wang K, Cheng Z. Loose Plant Architecture1, an INDETERMINATE DOMAIN protein involved in shoot gravitropism, regulates plant architecture in rice. Plant Physiol. 2013;161:317–29. doi: 10.1104/pp.112.208496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun X, Jones WT, Rikkerink EH. GRAS proteins: the versatile roles of intrinsically disordered proteins in plant signalling. Biochem J. 2012;442:1–12. doi: 10.1042/BJ20111766. [DOI] [PubMed] [Google Scholar]
- 18.Heo JO, Chang KS, Kim IA, Lee MH, Lee SA, Song SK, Lee MM, Lim J. Funneling of gibberellin signaling by the GRAS transcription regulator scarecrow-like 3 in the Arabidopsis root. Proc Natl Acad Sci U S A. 2011;108:2166–71. doi: 10.1073/pnas.1012215108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–28. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- 20.Hanano S, Goto K. Arabidopsis TERMINAL FLOWER1 is involved in the regulation of flowering time and inflorescence development through transcriptional repression. Plant Cell. 2011;23:3172–84. doi: 10.1105/tpc.111.088641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welch D, Hassan H, Blilou I, Immink R, Heidstra R, Scheres B. Arabidopsis JACKDAW and MAGPIE zinc finger proteins delimit asymmetric cell division and stabilize tissue boundaries by restricting SHORT-ROOT action. Genes Dev. 2007;21:2196–204. doi: 10.1101/gad.440307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogasawara H, Kaimi R, Colasanti J, Kozaki A. Activity of transcription factor JACKDAW is essential for SHR/SCR-dependent activation of SCARECROW and MAGPIE and is modulated by reciprocal interactions with MAGPIE, SCARECROW and SHORT ROOT. Plant Mol Biol. 2011;77:489–99. doi: 10.1007/s11103-011-9826-5. [DOI] [PubMed] [Google Scholar]
- 23.Koizumi K, Gallagher KL. Identification of SHRUBBY, a SHORT-ROOT and SCARECROW interacting protein that controls root growth and radial patterning. Development. 2013;140:1292–300. doi: 10.1242/dev.090761. [DOI] [PubMed] [Google Scholar]
- 24.Feurtado JA, Huang D, Wicki-Stordeur L, Hemstock LE, Potentier MS, Tsang EW, Cutler AJ. The Arabidopsis C2H2 zinc finger INDETERMINATE DOMAIN1/ENHYDROUS promotes the transition to germination by regulating light and hormonal signaling during seed maturation. Plant Cell. 2011;23:1772–94. doi: 10.1105/tpc.111.085134. [DOI] [PMC free article] [PubMed] [Google Scholar]


