Abstract
Mitochondrial AtFtsH4 protease is one of four inner membrane-bound FtsH proteases in Arabidopsis. We found that the loss of AtFtsH4 regulates Arabidopsis development and architecture by mediating the peroxidase-dependent interplay between hydrogen peroxide (H2O2) and auxin homeostasis. These morphological changes were correlated with elevated levels of both hydrogen peroxide and peroxidases, which suggested that ftsh4–4 plant was related to the oxidative stress, and that the architecture was caused by the auxin homeostasis perturbation. This view was supported by the expression levels of several auxin signaling genes and auxin binding and transport genes were decreased significantly in ftsh4–4 plants. Taken together, our data published in the May issue of Molecular Plant suggests a link between the lack of AtFtsH4 protease, oxidative stress,s and auxin homeostasis to regulate plant growth and development. However, the detail molecular mechanisms of AtFtSH4 regulating oxidation stress and auxin homeostasis is unclear. Here, we present evidence that the high level accumulated of H2O2 in ftsh4–4 may correlates with the decreased mitochondrial respiration genes. We also showed that the decreased auxin level and auxin transport may caused by the inhibition of mitochondrial respiratory chain complexes.
Keywords: Arabidopsis thaliana, auxin, FtSH4, hydrogen peroxide, mitochondrial respiratory chain complexes
In Arabidopsis, there are 4 mitochondrial located FtsH proteases which belong to the ATP-dependent metalloprotease family protein. FtSH3 and FtSH10 are considered to be m-AAA proteases,1 while FtSH4 and FtSH11 showed i-AAA protease characteristics.2 The function of plant mitochondrial AAA protease is still very unclear at present but complementation experiments show that the important functions of m-AAA protease in fungi and plants are conserved.3 With the exception of AtFtSH11, the Arabidopsis mitochondrial AAA proteases may be related to the plant oxidative phosphorylation system.4 AtFtSH4 influences the vegetative growth of Arabidopsis late rosette leaves formation under short day conditions, depending on preventing the accumulation of oxidized proteins.5 Numerous studies on the chloroplast FtSH protease have found that chloroplast FtSH proteases act mainly through ROS to control the leaf morphological changes.6 There has, however, been little study into the function of plant mitochondrial FtSH protease. The integrity of the mitochondrial inner membrane depends on a number of membrane localized FtSH/AAA proteases, and these proteases can specifically degrade badly folded or unassembled inner membrane protein.
Using one new T-DNA null mutant we investigated the function of AtFtsH4 in peroxide hydrogen accumulation and plant development. The results indicated that the loss-function of AtFtSH4 significantly affects morphogenesis of Arabidopsis. The ftsh4–4 mutation caused the decrease of free IAA concentration, the perturbation of auxin signaling, and the elevation of H2O2 and peroxidase levels. The dwarfism and increased in axillary branching of the ftsh4–4 mutant could be reversed by expressing the iaaM gene or by knocking down the peroxidase genes PRX34 and PRX33, both of which elevated auxin level in ftsh4–4 mutant. Moreover, the microarray data showed that several auxin transport genes downregulated significantly in the ftsh4–4 mutant. Collectively, our data indicate that the mitochondrial ATP-dependent protease, FtSH4, is a modulator between H2O2 and auxin homeostasis to regulate plant growth and development.
However, though the significant H2O2 accumulation and auxin homeostasis perturbation were caused by the loss-function of AtFtsH4, whether these results are relate to the mitochondrial dysfunction in ftsh4–4 is not clear. It has been reported that the aging ftsh4 under SD conditions accumulated high level of reactive oxygen species (ROS) and carbonylated proteins, and the aging ftsh4 plants was suffered from oxidative stress.5 In the mitochondrial matrix of phenotype displayed ftsh4 plants, elevated electron-dense material, and aggregates of oxidized proteins were accumulated.5 But why the lack of AtFtSH4 can cause the accumulation of ROS and oxidized proteins, and the relationship between AtFtSH4 and the mitochondrial oxidative phosphorylation, was not researched.
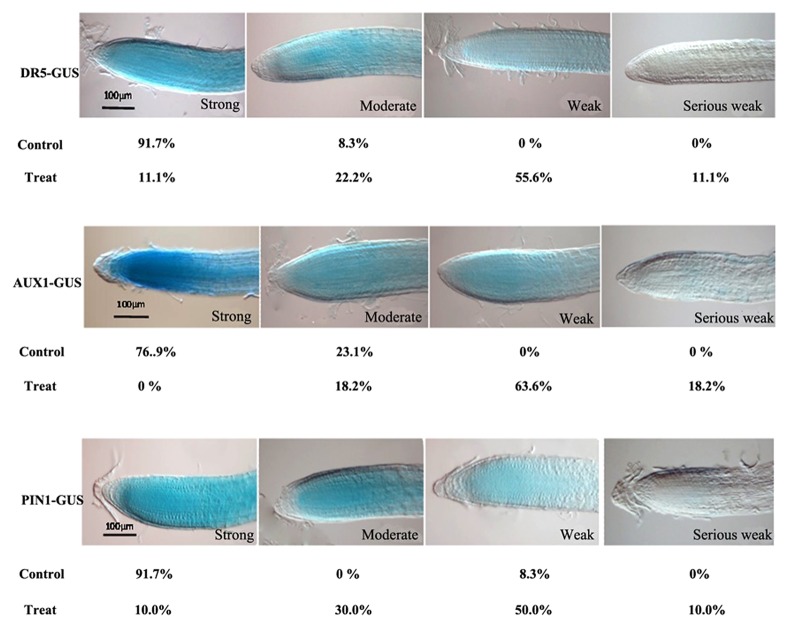
In this communication, we show additional data to support the function of AtFtsH4 in regulating the expressions of mitochondrial respiratory chain complexes genes and auxin transportation. First, the auxin transportation in the ftsh4–4 mutant was tested to elucidate whether the auxin transportation is also disturbed by the accumulation. In the 12-d-old seedlings in long day conditions, the H2O2 accumulation was soared in the ftsh4–4 mutant tested by the DAB staining. As consequently, the expression of auxin efflux carrier PIN1 was perturbed in the ftsh4–4 seedlings, including in the cotyledons, hypocotyls, root, and root apical (Fig. 1). These results further implied that the disturbed auxin signaling is relate to the H2O2 accumulation, and confirmed our results in the Molecular Plant paper. Currently, the generation of ROS is considered resulting from the respiratory activity of mitochondria, mainly from the Complex I, Complex III activities, and the respiratory chain complexes V which responsible for ATP generation through the process of oxidative phosphorylation (OxPhos).7,8 The mitochondrial respiratory chain is consisted of 5 protein complexes named as Complex I (NADH dehydrogenase), Complex II (succinate dehydrogenase), Complex III (cytochrome c reductase), Complex IV (cytochrome c oxidase), and Complex V (ATP synthase).9 We have detected a series number of these complexes genes, and found that CI76 in the Complex I,10 CYC1–1 in the Complex III,11 COX6B in the Complex IV,12 and ATP6 in the Complex V11 were downregulated significantly by the knock out of AtFtSH4 under the long day condition (Fig. 2). These results indicated that the mitochondrial respiratory chain complexes may be controlled by the AtFtSH4. In order to demonstrate whether the auxin homeostasis could be perturbed by the dysfunction of respiratory chain in Arabidopsis, the seedlings were treated by the inhibitors of mitochondrial respiratory chain complexes. After treated by the rotenone (inhibitor for Complex I), cyazofamid (inhibitor for Complex III), and NaN3 (inhibitor for Complex IV and V) synchronously, the expressions of auxin responsive marker gene DR5, auxin influx carrier AUX1, and auxin efflux carrier PIN1 were downregulated or inhibited significantly (Fig. 3). These results were identified that in the ftsh4–4 mutant. And these results combined with the results that in Figure 2 implied that AtFtSH4 affect the ROS accumulation may through control the integrity of mitochondrial respiratory chain complexes.
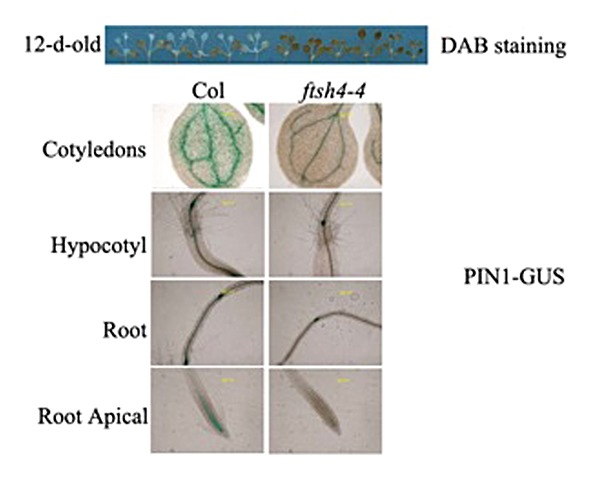
Figure 1. Soared hydrogen peroxide perturbs the auxin transportation in ftsh4–4 mutant. GUS staining for 6 h.
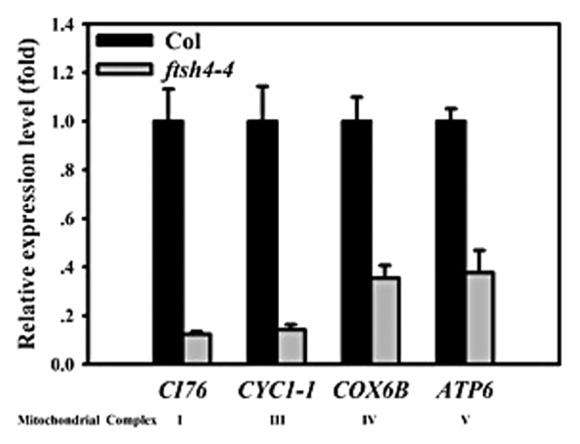
Figure 2. Mitochondrial respiratory chain complexes genes were downregulated in ftsh4–4 mutant. CI76, At5g37510; CYC1–1, At3g27240; COX6B, At4g37830; ATP6, AtMG00410. 21-d-old seedlings were used for RNA extraction and gene expression detection.
Figure 3. Dysfunction of mitochondrial respiratory chain complexes destroyed the auxin homeostasis. Control and Treat, seedlings were treated without or with 1.25 mM rotenone, 0.1 mM cyazofamid, and 1 mM NaN3, synchronously. All kinds of seeds germinated in the MS plates for 5 d, and then transferred to the plates with or without inhibitors for further more 3 d. After treatment, the seedlings were staining by GUS solution overnight.
Taken together, our results elucidate the relationship between AtFtsH4 gene, oxidative stress, and auxin homeostasis. Our results indicate that the loss-function of AtFtsH4 has impaired the mitochondrial oxidative phosphorylation system. And this deficiency my caused by instability of respiratory complexes. The impaired oxidative phosphorylation system may cause a soared accumulation of ROS and oxidized proteins to perturb the function of mitochondrial. It is well known that ROS has the ability to impair the auxin signaling, and then caused the change of Arabidopsis architecture. Recent studies have shown that elevated ROS-induced oxidative stress results in a severe auxin deficiency phenotypes, referred to as stress-induced morphological responses, in Arabidopsis seedlings with perturbation in auxin levels, and/or distribution.13-17 Redox signaling pathways play important roles in modulating the plant development to adapt to their growth environment.18 Auxin homeostasis could be altered by H2O2 induced changes to PINOID gene expression, which affects polar auxin transport.13 In addition to the influence on auxin homeostasis through the regulation of enzymes involved in auxin biosynthesis and conjugation,19,20 oxidative degradation of auxin through H2O2-dependent peroxidases occurs as well.19,21 However, the molecular basis of mitochondrial genes or proteins playing the ROS and auxin interaction need more evidence to elucidate. Diverse experiments performed on mutants deficient in mitochondrial i-AAA and m-AAA proteases indicate that these membrane embedded proteases are crucial components of the defense against mitochondrial oxidative protein damage at least in Arabidopsis and mammals. AtFtSH4 may degrade carbonylated membrane proteins and protect mitochondrial membrane proteins against oxidation, like the chloroplast FtsH proteases degrade oxidatively damaged proteins.22 Furthermore, our results have proved that mitochondrial membrane are likely associated with instability of the mitochondrial respiratory chain complexes leading to their dysfunction which results in an increase in ROS production and progression of oxidative damage, and AtFtSH4 may play important roles in this biological process. However, the exact relations between mitochondrial respiratory chain complexes and AtFtSH4 and auxin homeostasis require further investigations.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (31370350, 31271471).
References
- 1.Janska H, Piechota J, Kwasniak M. ATP-dependent proteases in biogenesis and maintenance of plant mitochondria. Biochim Biophys Acta. 2010;1797:1071–5. doi: 10.1016/j.bbabio.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 2.Urantowka A, Knorpp C, Olczak T, Kolodziejczak M, Janska H. Plant mitochondria contain at least two i-AAA-like complexes. Plant Mol Biol. 2005;59:239–52. doi: 10.1007/s11103-005-8766-3. [DOI] [PubMed] [Google Scholar]
- 3.Kolodziejczak M, Kolaczkowska A, Szczesny B, Urantowka A, Knorpp C, Kieleczawa J, Janska H. A higher plant mitochondrial homologue of the yeast m-AAA protease. Molecular cloning, localization, and putative function. J Biol Chem. 2002;277:43792–8. doi: 10.1074/jbc.M203831200. [DOI] [PubMed] [Google Scholar]
- 4.Kolodziejczak M, Gibala M, Urantowka A, Janska H. The significance of Arabidopsis AAA protease for activity and assembly/stability of mitochondrial OXPHOS complexes. Physiol Plant. 2007;129:135–42. doi: 10.1111/j.1399-3054.2006.00835.x. [DOI] [Google Scholar]
- 5.Gibala M, Kicia M, Sakamoto W, Gola EM, Kubrakiewicz J, Smakowska E, Janska H. The lack of mitochondrial AtFtsH4 protease alters Arabidopsis leaf morphology at the late stage of rosette development under short-day photoperiod. Plant J. 2009;59:685–99. doi: 10.1111/j.1365-313X.2009.03907.x. [DOI] [PubMed] [Google Scholar]
- 6.Kato Y, Miura E, Ido K, Ifuku K, Sakamoto W. The variegated mutants lacking chloroplastic FtsHs are defective in D1 degradation and accumulate reactive oxygen species. Plant Physiol. 2009;151:1790–801. doi: 10.1104/pp.109.146589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moller IM. Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:561–91. doi: 10.1146/annurev.arplant.52.1.561. [DOI] [PubMed] [Google Scholar]
- 8.Rhoads DM, Umbach AL, Subbaiah CC, Siedow JN. Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 2006;141:357–66. doi: 10.1104/pp.106.079129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babcock GT, Wikström M. Oxygen activation and the conservation of energy in cell respiration. Nature. 1992;356:301–9. doi: 10.1038/356301a0. [DOI] [PubMed] [Google Scholar]
- 10.Klodmann J, Sunderhaus S, Nimtz M, Jänsch L, Braun HP. Internal architecture of mitochondrial complex I from Arabidopsis thaliana. Plant Cell. 2010;22:797–810. doi: 10.1105/tpc.109.073726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer EH, Taylor NL, Millar AH. Resolving and identifying protein components of plant mitochondrial respiratory complexes using three dimensions of gel electrophoresis. J Proteome Res. 2008;7:786–94. doi: 10.1021/pr700595p. [DOI] [PubMed] [Google Scholar]
- 12.Millar AH, Eubel H, Jänsch L, Kruft V, Heazlewood JL, Braun HP. Mitochondrial cytochrome c oxidase and succinate dehydrogenase complexes contain plant specific subunits. Plant Mol Biol. 2004;56:77–90. doi: 10.1007/s11103-004-2316-2. [DOI] [PubMed] [Google Scholar]
- 13.Pasternak T, Potters G, Caubergs R, Jansen MA. Complementary interactions between oxidative stress and auxins control plant growth responses at plant, organ, and cellular level. J Exp Bot. 2005;56:1991–2001. doi: 10.1093/jxb/eri196. [DOI] [PubMed] [Google Scholar]
- 14.Blomster T, Salojärvi J, Sipari N, Brosché M, Ahlfors R, Keinänen M, Overmyer K, Kangasjärvi J. Apoplastic reactive oxygen species transiently decrease auxin signaling and cause stress-induced morphogenic response in Arabidopsis. Plant Physiol. 2011;157:1866–83. doi: 10.1104/pp.111.181883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leyser O. The fall and rise of apical dominance. Curr Opin Genet Dev. 2005;15:468–71. doi: 10.1016/j.gde.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Potters G, Pasternak TP, Guisez Y, Jansen MA. Different stresses, similar morphogenic responses: integrating a plethora of pathways. Plant Cell Environ. 2009;32:158–69. doi: 10.1111/j.1365-3040.2008.01908.x. [DOI] [PubMed] [Google Scholar]
- 17.Tognetti VB, Mühlenbock P, Van Breusegem F. Stress homeostasis - the redox and auxin perspective. Plant Cell Environ. 2012;35:321–33. doi: 10.1111/j.1365-3040.2011.02324.x. [DOI] [PubMed] [Google Scholar]
- 18.Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17:1866–75. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ljung K, Hull AK, Kowalczyk M, Marchant A, Celenza J, Cohen JD, Sandberg G. Biosynthesis, conjugation, catabolism and homeostasis of indole-3-acetic acid in Arabidopsis thaliana. Plant Mol Biol. 2002;49:249–72. doi: 10.1023/A:1015298812300. [DOI] [PubMed] [Google Scholar]
- 20.Woodward AW, Bartel B. Auxin: regulation, action, and interaction. Ann Bot. 2005;95:707–35. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gazarian IG, Lagrimini LM, Mellon FA, Naldrett MJ, Ashby GA, Thorneley RN. Identification of skatolyl hydroperoxide and its role in the peroxidase-catalysed oxidation of indol-3-yl acetic acid. Biochem J. 1998;333:223–32. doi: 10.1042/bj3330223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindahl M, Spetea C, Hundal T, Oppenheim AB, Adam Z, Andersson B. The thylakoid FtsH protease plays a role in the light-induced turnover of the photosystem II D1 protein. Plant Cell. 2000;12:419–31. doi: 10.1105/tpc.12.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]