Abstract
Vaccinia virus dissemination relies on the recruitment of the nucleation promoting factor N-WASP underneath cell-associated extracellular virus (CEVs) and subsequent recruitment and activation of the ARP2/3 complex, a major actin nucleator of the host cell. We have recently discovered that, in addition to the N-WASP/ARP2/3 pathway, vaccinia actin-based motility also relies on the small GTPase Rac1 and its downstream effector the formin-type actin nucleator FHOD1. Here we discuss the potential signaling mechanisms supporting the integration of the N-WASP/ARP2/3 and Rac1/FHOD1 pathways. We suggest the existence of a receptor tyrosine kinase family member that would integrate the Src-dependent activation of the N-WASP/ARP2/3 pathway and the GTP exchange factor-dependent activation of the Rac1/FHOD1 pathway.
Keywords: Dissemination, spread from cell to cell, actin-based motility, vaccinia virus, Rac1, FHOD1, N-WASP, ARP2/3 complex
Vaccinia Virus
Vaccinia virus is a large enveloped virus with a double-stranded DNA genome that belongs to the genus Orthopoxvirus in the Poxviridae family of viruses. Orthopoxviruses include animal pathogens such as variola virus, monkeypox virus, and cowpox virus. Variola virus infection causes smallpox and was declared eradicated in 1980 after a worldwide immunization campaign using vaccinia virus as a vaccine strain.1 Vaccinia virus replication in the cytoplasm of an infected cell yields two infectious forms, intracellular mature virus (IMV) and extracellular virus (EV). Viral dissemination is supported by the propagation of IMVs upon cell lysis and by direct cell-to-cell spread of EVs through actin-based motility.2 Here, we discuss the role of the small GTPase Rac1 in vaccinia virus actin-based motility.
Vaccinia Actin-Based Motility: The N-WASP/ARP2/3 Pathway
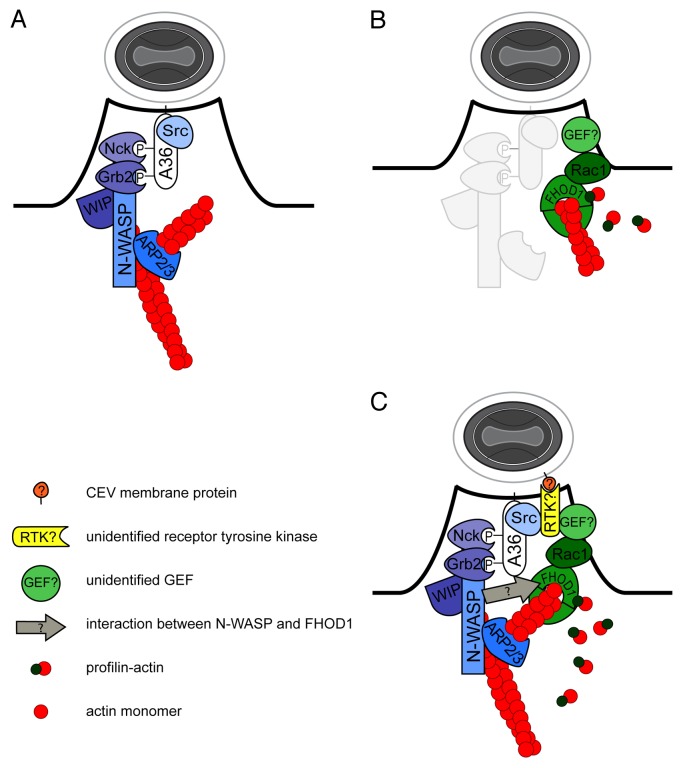
Similar to intracellular pathogens such as Listeria monocytogenes and Shigella flexneri, vaccinia virus achieves motility through manipulation of the ARP2/3 complex, a major actin nucleator of the host cell.3 Upon egress of intracellular enveloped virus through fusion with the plasma membrane, the viral protein A36 is positioned in the plasma membrane underneath the cell-associated extracellular virus (CEV).4 Non-receptor tyrosine kinases of the Src/Abl families phosphorylate A36,5-8 thereby generating docking sites for the adaptor proteins Nck1 and Grb2. Nck1 and Grb2 mediate the recruitment of a complex of the WASP-interacting protein WIP and the nucleation-promoting factor N-WASP, that in turn recruits and activates the ARP2/3 complex9-11(Fig. 1A). ARP2/3-dependent actin nucleation leads to the generation of a branched actin network that supports the formation of virus-tipped membrane protrusions. This results in the release of EVs into the extracellular environment or the propagation to the neighboring cells.2,12,13
Figure 1. (A) Canonical model for vaccinia actin tail formation by the activation of the N-WASP/ARP2/3 pathway. Phosphorylation of the cytoplasmatic domain of A36 underneath CEVs (gray and white oval) by Src and Abl families of non-receptor tyrosine kinases mediates the recruitment of Nck1 and Grb2 adaptor proteins that in turn recruit a complex of WIP and N-WASP to activate the ARP2/3 complex promoting actin tail formation. (B) Vaccinia actin tail formation involves the activation of the Rac1/FHOD1 pathway. The small GTPase Rac1 is activated underneath CEVs by a yet unidentified guanine-nucleotide exchange factor (GEF). Downstream of Rac1, recruitment and activation of FHOD1 promotes vaccinia actin tail formation. Profilin stimulates formin activities of FHOD1. (C) A model for the integration of N-WASP/ARP2/3 and Rac1/FHOD1 pathways. A vaccinia protein exposed on the outer membrane of CEVs engages a yet unidentified receptor tyrosine kinase (RTKs) leading to the activation of Src and subsequent phosphorylation of A36, as well as the recruitment of a GEF for Rac1, thereby integrating the activities of the N-WASP/ARP2/3 and Rac1/FHOD1 pathways. N-WASP interacts with FHOD1 through an unknown mechanism (arrow).
Vaccinia Actin-Based Motility: The Rac1/FHOD1 Pathway
In order to gain insight into the mechanisms controlling vaccinia actin-based motility we developed an RNAi-based screen for host factors required for vaccinia spread from cell to cell. Screening of a siRNA library covering regulators of the actin cytoskeleton led to the identification of the formin FHOD1 as a cellular factor required for vaccinia dissemination. We observed that silencing FHOD1 resulted in fewer CEVs with actin tails and a slower rate of elongation of the formed actin tails.14 Formins exist in an auto-inhibited conformation due to interactions between the N-terminal FH3 domain and the C-terminal DAD domain.15-18 Binding of activated small GTPases of the Rho/Rac/Cdc42 family to the GTPase binding domain (GBD) domain of formins relaxes the auto-inhibited conformation, which contributes to formin activation.19 In the activated form, the FH2 domain of formins is able to nucleate, elongate and cap the plus end of actin filaments.20-26 The elongation activity of formins is stimulated through binding of profilin-actin to the FH1 domain.27,28 We observed that the functional GBD and FH2 domains of FHOD1 were required for both actin tail formation and localization of the formin to vaccinia actin tails. In addition, we found that actin tail formation requires both the FH1 domain of FHOD1 and profilin.14 In previous studies, the GBD domain of FHOD1 was shown to physically interact with the activated form of Rac1 and expression of active Rac1 led to the recruitment of FHOD1 to the plasma membrane.29-31 In vaccinia-infected cells, we observed that GFP-tagged Rac1 was enriched and activated at the plasma membrane surrounding actin tails. In agreement with previous studies, we confirmed a role for Rac1 in the recruitment and activation of FHOD1. First, silencing Rac1 or overexpression of dominant-negative Rac1 mimicked the phenotype of FHOD1 silencing. Second, silencing Rac1 impaired the recruitment of FHOD1 to vaccinia actin tails. Third, the effect of dominant-negative Rac1 on vaccinia actin tail formation could be rescued by overexpression of full-length or constitutively active versions of FHOD1.14 Like other GTP binding proteins, small GTPases of the Rho/Rac/Cdc42 family cycle between a GTP-bound active form and a GDP-bound inactive form. Guanine-nucleotide exchange factors (GEFs) convert the inactive GDP-bound GTPase to an active GTP-bound form. GTPase activating proteins (GAPs) inactivate GTPases by promoting GTP hydrolysis. Dominant-negative versions of small GTPases act by stabilizing the interaction with and sequestering the upstream GEF involved in GTPase activation.32 Thus, the effect of dominant-negative Rac1 overexpression on vaccinia actin tail formation suggested the involvement of a Rac1 GEF in the activation of Rac1 (Fig. 1B). The identity of this putative Rac1 GEF remains unknown.
Integration of the N-WASP/ARP2/3 and Rac1/FHOD1 Pathways
In addition to the A36-mediated N-WASP/ARP2/3 pathway (Fig. 1A), our recent studies thus revealed that robust actin-based motility relies on the activation of the Rac1/FHOD1 pathway (Fig. 1B). What mechanisms potentially integrate the activation of these two signaling pathways? Small-GTPases such as Rac1 are notorious for their role in the recruitment of N-WASP/WAVE family members to the plasma membrane.33,34 However, we found that in the context of vaccinia actin tail formation, silencing Rac1 had no effect on the recruitment of N-WASP. By contrast, silencing N-WASP affected the recruitment of FHOD1,14 which may be mediated through the interaction of N-WASP with adaptor proteins, such as WISH35,36(Fig. 1C). Importantly, the recruitment and activation of Rac1 to CEVs did not rely on the viral protein A36.14 This critical result uncovered that, although required for FHOD1 recruitment, the N-WASP/ARP2/3 pathway is likely dispensable for Rac1 recruitment and activation underneath CEVs. It is noteworthy that the signaling events leading to Src activation, which presumably involves the activation of a member of the receptor tyrosine kinase (RTK) family, are unknown. We note that RTKs are well suited to integrate the activities of the N-WASP/ARP2/3 and Rac1/FHOD1 pathways. RTKs not only activate Src,37,38 but also modulate the activity of small GTPases of the Rho/Rac/Cdc42 family through recruitment and activation GTP exchange factors (GEFs) to the plasma membrane.39 In fact, recent data indicate that CEVs recruit the GEF intersectin-1 prior to vaccinia actin tail formation.40 Intersectin-1 regulates the N-WASP/ARP2/3 pathway through activation of Cdc42.10,40 Whether intersectin-1 may function as a GEF for Rac1 remains to be tested. In conclusion, we suggest the existence of RTK(s) that would integrate the activity of the N-WASP/ARP2/3 and Rac1/FHOD1 pathways through the activities of Src and a yet unidentified GEF for Rac1 (Fig. 1C). The identification of the putative GEF(s) and RTK(s), as well as putative viral components engaging outside-in RTK signaling, will unveil the missing link in vaccinia actin-based motility (Fig. 1C).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Moss B. Poxviridae: The Viruses and Their Replication. Philadelphia: Lippincott Williams & Wilkins, 2007. [Google Scholar]
- 2.Roberts KL, Smith GL. Vaccinia virus morphogenesis and dissemination. Trends Microbiol. 2008;16:472–9. doi: 10.1016/j.tim.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Welch MD, Way M. Arp2/3-mediated actin-based motility: a tail of pathogen abuse. Cell Host Microbe. 2013;14:242–55. doi: 10.1016/j.chom.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Eijl H, Hollinshead M, Smith GL. The vaccinia virus A36R protein is a type Ib membrane protein present on intracellular but not extracellular enveloped virus particles. Virology. 2000;271:26–36. doi: 10.1006/viro.2000.0260. [DOI] [PubMed] [Google Scholar]
- 5.Newsome TP, Scaplehorn N, Way M. SRC mediates a switch from microtubule- to actin-based motility of vaccinia virus. Science. 2004;306:124–9. doi: 10.1126/science.1101509. [DOI] [PubMed] [Google Scholar]
- 6.Reeves PM, Bommarius B, Lebeis S, McNulty S, Christensen J, Swimm A, Chahroudi A, Chavan R, Feinberg MB, Veach D, et al. Disabling poxvirus pathogenesis by inhibition of Abl-family tyrosine kinases. Nat Med. 2005;11:731–9. doi: 10.1038/nm1265. [DOI] [PubMed] [Google Scholar]
- 7.Newsome TP, Weisswange I, Frischknecht F, Way M. Abl collaborates with Src family kinases to stimulate actin-based motility of vaccinia virus. Cell Microbiol. 2006;8:233–41. doi: 10.1111/j.1462-5822.2005.00613.x. [DOI] [PubMed] [Google Scholar]
- 8.Scaplehorn N, Holmström A, Moreau V, Frischknecht F, Reckmann I, Way M. Grb2 and Nck act cooperatively to promote actin-based motility of vaccinia virus. Curr Biol. 2002;12:740–5. doi: 10.1016/S0960-9822(02)00812-6. [DOI] [PubMed] [Google Scholar]
- 9.Donnelly SK, Weisswange I, Zettl M, Way M. WIP provides an essential link between Nck and N-WASP during Arp2/3-dependent actin polymerization. Curr Biol. 2013;23:999–1006. doi: 10.1016/j.cub.2013.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreau V, Frischknecht F, Reckmann I, Vincentelli R, Rabut G, Stewart D, Way M. A complex of N-WASP and WIP integrates signalling cascades that lead to actin polymerization. Nat Cell Biol. 2000;2:441–8. doi: 10.1038/35017080. [DOI] [PubMed] [Google Scholar]
- 11.Frischknecht F, Moreau V, Röttger S, Gonfloni S, Reckmann I, Superti-Furga G, Way M. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature. 1999;401:926–9. doi: 10.1038/44860. [DOI] [PubMed] [Google Scholar]
- 12.Doceul V, Hollinshead M, van der Linden L, Smith GL. Repulsion of superinfecting virions: a mechanism for rapid virus spread. Science. 2010;327:873–6. doi: 10.1126/science.1183173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horsington J, Lynn H, Turnbull L, Cheng D, Braet F, Diefenbach RJ, Whitchurch CB, Karupiah G, Newsome TP. A36-dependent actin filament nucleation promotes release of vaccinia virus. PLoS Pathog. 2013;9:e1003239. doi: 10.1371/journal.ppat.1003239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez DE, Agaisse H. The formin FHOD1 and the small GTPase Rac1 promote vaccinia virus actin-based motility. J Cell Biol. 2013;202:1075–90. doi: 10.1083/jcb.201303055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alberts AS. Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J Biol Chem. 2001;276:2824–30. doi: 10.1074/jbc.M006205200. [DOI] [PubMed] [Google Scholar]
- 16.Lammers M, Rose R, Scrima A, Wittinghofer A. The regulation of mDia1 by autoinhibition and its release by Rho*GTP. EMBO J. 2005;24:4176–87. doi: 10.1038/sj.emboj.7600879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nezami AG, Poy F, Eck MJ. Structure of the autoinhibitory switch in formin mDia1. Structure. 2006;14:257–63. doi: 10.1016/j.str.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Schönichen A, Alexander M, Gasteier JE, Cuesta FE, Fackler OT, Geyer M. Biochemical characterization of the diaphanous autoregulatory interaction in the formin homology protein FHOD1. J Biol Chem. 2006;281:5084–93. doi: 10.1074/jbc.M509226200. [DOI] [PubMed] [Google Scholar]
- 19.Wallar BJ, Alberts AS. The formins: active scaffolds that remodel the cytoskeleton. Trends Cell Biol. 2003;13:435–46. doi: 10.1016/S0962-8924(03)00153-3. [DOI] [PubMed] [Google Scholar]
- 20.Harris ES, Li F, Higgs HN. The mouse formin, FRLalpha, slows actin filament barbed end elongation, competes with capping protein, accelerates polymerization from monomers, and severs filaments. J Biol Chem. 2004;279:20076–87. doi: 10.1074/jbc.M312718200. [DOI] [PubMed] [Google Scholar]
- 21.Li F, Higgs HN. The mouse Formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr Biol. 2003;13:1335–40. doi: 10.1016/S0960-9822(03)00540-2. [DOI] [PubMed] [Google Scholar]
- 22.Moseley JB, Maiti S, Goode BL. Formin proteins: purification and measurement of effects on actin assembly. Methods Enzymol. 2006;406:215–34. doi: 10.1016/S0076-6879(06)06016-2. [DOI] [PubMed] [Google Scholar]
- 23.Moseley JB, Sagot I, Manning AL, Xu Y, Eck MJ, Pellman D, Goode BL. A conserved mechanism for Bni1- and mDia1-induced actin assembly and dual regulation of Bni1 by Bud6 and profilin. Mol Biol Cell. 2004;15:896–907. doi: 10.1091/mbc.E03-08-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otomo T, Tomchick DR, Otomo C, Panchal SC, Machius M, Rosen MK. Structural basis of actin filament nucleation and processive capping by a formin homology 2 domain. Nature. 2005;433:488–94. doi: 10.1038/nature03251. [DOI] [PubMed] [Google Scholar]
- 25.Pruyne D, Evangelista M, Yang C, Bi E, Zigmond S, Bretscher A, Boone C. Role of formins in actin assembly: nucleation and barbed-end association. Science. 2002;297:612–5. doi: 10.1126/science.1072309. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol. 1999;1:136–43. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- 27.Kovar DR, Harris ES, Mahaffy R, Higgs HN, Pollard TD. Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell. 2006;124:423–35. doi: 10.1016/j.cell.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 28.Romero S, Le Clainche C, Didry D, Egile C, Pantaloni D, Carlier MF. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell. 2004;119:419–29. doi: 10.1016/j.cell.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 29.Gasteier JE, Madrid R, Krautkrämer E, Schröder S, Muranyi W, Benichou S, Fackler OT. Activation of the Rac-binding partner FHOD1 induces actin stress fibers via a ROCK-dependent mechanism. J Biol Chem. 2003;278:38902–12. doi: 10.1074/jbc.M306229200. [DOI] [PubMed] [Google Scholar]
- 30.Schulte A, Stolp B, Schönichen A, Pylypenko O, Rak A, Fackler OT, Geyer M. The human formin FHOD1 contains a bipartite structure of FH3 and GTPase-binding domains required for activation. Structure. 2008;16:1313–23. doi: 10.1016/j.str.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Westendorf JJ. The formin/diaphanous-related protein, FHOS, interacts with Rac1 and activates transcription from the serum response element. J Biol Chem. 2001;276:46453–9. doi: 10.1074/jbc.M105162200. [DOI] [PubMed] [Google Scholar]
- 32.Feig LA. Tools of the trade: use of dominant-inhibitory mutants of Ras-family GTPases. Nat Cell Biol. 1999;1:E25–7. doi: 10.1038/10018. [DOI] [PubMed] [Google Scholar]
- 33.Bompard G, Caron E. Regulation of WASP/WAVE proteins: making a long story short. J Cell Biol. 2004;166:957–62. doi: 10.1083/jcb.200403127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–65. doi: 10.1016/S0092-8674(03)00120-X. [DOI] [PubMed] [Google Scholar]
- 35.Fukuoka M, Suetsugu S, Miki H, Fukami K, Endo T, Takenawa T. A novel neural Wiskott-Aldrich syndrome protein (N-WASP) binding protein, WISH, induces Arp2/3 complex activation independent of Cdc42. J Cell Biol. 2001;152:471–82. doi: 10.1083/jcb.152.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westendorf JJ, Koka S. Identification of FHOD1-binding proteins and mechanisms of FHOD1-regulated actin dynamics. J Cell Biochem. 2004;92:29–41. doi: 10.1002/jcb.20031. [DOI] [PubMed] [Google Scholar]
- 37.Bromann PA, Korkaya H, Courtneidge SA. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene. 2004;23:7957–68. doi: 10.1038/sj.onc.1208079. [DOI] [PubMed] [Google Scholar]
- 38.Frame MC, Fincham VJ, Carragher NO, Wyke JA. v-Src’s hold over actin and cell adhesions. Nat Rev Mol Cell Biol. 2002;3:233–45. doi: 10.1038/nrm779. [DOI] [PubMed] [Google Scholar]
- 39.Schiller MR. Coupling receptor tyrosine kinases to Rho GTPases--GEFs what’s the link. Cell Signal. 2006;18:1834–43. doi: 10.1016/j.cellsig.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 40.Humphries AC, Donnelly SK, Way M. Cdc42 and the Rho GEF intersectin-1 collaborate with Nck to promote N-WASP-dependent actin polymerisation. J Cell Sci. 2014;127:673–85. doi: 10.1242/jcs.141366. [DOI] [PubMed] [Google Scholar]