Abstract
POLYAMINE OXIDASE 1 (OsPAO1), from rice (Oryza sativa), and POLYAMINE OXIDASE 5 (AtPAO5), from Arabidopsis (Arabidopsis thaliana), are enzymes sharing high identity at the amino acid level and with similar characteristics, such as polyamine specificity and pH preference; furthermore, both proteins localize to the cytosol. A loss-of-function Arabidopsis mutant, Atpao5–2, was hypersensitive to low doses of exogenous thermospermine but this phenotype could be rescued by introduction of the wild-type AtPAO5 gene. Introduction of OsPAO1, under the control of a constitutive promoter, into Atpao5–2 mutants also restored normal thermospermine sensitivity, allowing growth in the presence of low levels of thermospermine, along with a concomitant decrease in thermospermine content in plants. By contrast, introduction of OsPAO3, which encodes a peroxisome-localized polyamine oxidase, into Atpao5–2 plants could not rescue any of the mutant phenotypes in the presence of thermospermine. These results suggest that OsPAO1 is the functional ortholog of AtPAO5.
Keywords: Arabidopsis thaliana, AtPAO5, Oryza sativa, OsPAO1, polyamine oxidase, thermospermine
Polyamines (PAs) are aliphatic amines with low molecular masses. They are essential for growth and/or required for acclimation to environmental changes in plants.1-5 Major plant PAs include putrescine (Put), spermidine (Spd), spermine (Spm), and thermospermine (T-Spm). Copper-containing amine oxidases (CuAOs) and flavin-containing polyamine oxidases (PAOs) play central roles in PA catabolism in plants.6,7
Plant PAOs are classified into 2 groups based on their reaction mode: the first group contains those undergoing the terminal catabolism reaction, in which the carbon at the endo-side of the N4-nitrogen of the free PAs is oxidized; those in the second group undergo the PA back-conversion reaction, in which oxidation occurs on the carbon at the exo-side of the N4-nitrogen of the free PAs.8
The rice (Oryza sativa) genome contains 7 genes encoding PAOs, sequentially termed OsPAO1 to OsPAO7.9 Three of these genes, OsPAO3, OsPAO4, and OsPAO5, were found to be more abundantly expressed, relative to the other 4, in seedlings and during later growth stages. Their gene products, OsPAO3, OsPAO4, and OsPAO5, were found to catalyze PA back-conversion reactions and to localize to peroxisomes.9 The 4th member of this gene family, OsPAO1, was induced in roots, but not in shoots, following exogenous application of Spm or T-Spm. Recombinant OsPAO1 showed a preference for Spm and T-Spm as substrates and back-converted them to Spd but not further to Put.10 The optimal pH for OsPAO1 was pH 6.0 for T-Spm and pH 8.5 for Spm.10 cDNA from the fifth member of the gene family, OsPAO7, was isolated from rice flower organs. Its gene product, OsPAO7, was involved in the terminal catabolism pathway, especially in male organs.11 OsPAO7 also appears to participate in lignin formation in anther walls and pollen grains.11
Recently, Arabidopsis AtPAO5, shown by phylogenic analysis to belong to the same clade as OsPAO1,9 has been characterized by 2 independent research groups.12,13 Ahou et al. (2014) described AtPAO5 as a Spm oxidase/dehydrogenase. They partially purified AtPAO5 from transgenic Arabidopsis plants overexpressing (His)6-tagged AtPAO5 under the CaMV 35S promoter. This partially purified enzyme showed a high preference for Spm, N1-acetyl Spm, and T-Spm as substrates. Based on an analysis of PA content in wild-type plants, transgenic plants, and the Atpao5 loss-of-function mutant, these authors concluded that, even in vivo, AtPAO5 catalyzes Spm, N1-acetyl Spm, and T-Spm oxidation/dehydrogenation.12 By contrast, we concluded that AtPAO5 is a T-Spm oxidase based on the following evidence: 2, allelic, loss-of-function mutants, Atpao5–1 and Atpao5–2 contained 2-fold higher levels of T-Spm than wild-type plants. Both Atpao5 mutants showed a specific hypersensitivity to low doses (5–10 μM) of T-Spm but not to other PAs.13 Introduction of the complete AtPAO5 gene into the Atpao5–2 mutant restored tolerance to exogenous T-Spm; moreover, T-Spm content in the complemented Atpao5–2 lines decreased to wild-type levels.13
In this study, we address whether OsPAO1 is able to complement the growth defects of Atpao5–2 observed in the presence of low levels of T-Spm, and discuss the physiological function of OsPAO1 in rice plants. We used the Protein Structure Prediction Server program (http://140.113.239.111/~ps2/) to deduce the tertiary structures of OsPAO1 and AtPAO5. A comparison of these enzymes showed high levels of similarity between their folded structures across the whole protein (Fig. 1A, OsPAO1, and 1B, AtPAO5). This result is consistent with their similar enzymatic behaviors and with the sub-cellular localizations of OsPAO1 and AtPAO5. By contrast, although OsPAO3 (Fig. 1C), OsPAO4 (Fig. 1D), and OsPAO5 (Fig. 1E), PAO proteins found in the peroxisome, showed high structural similarities to each other, their tertiary structures clearly differed from OsPAO1 and AtPAO5.
Figure 1. Predicted tertiary structures of OsPAO1 (A), AtPAO5 (B), OsPAO3 (C), OsPAO4 (D), and OsPAO5 (E) displayed using the Protein Structure Prediction Server program (http://140.113.239.111/~ps2/).
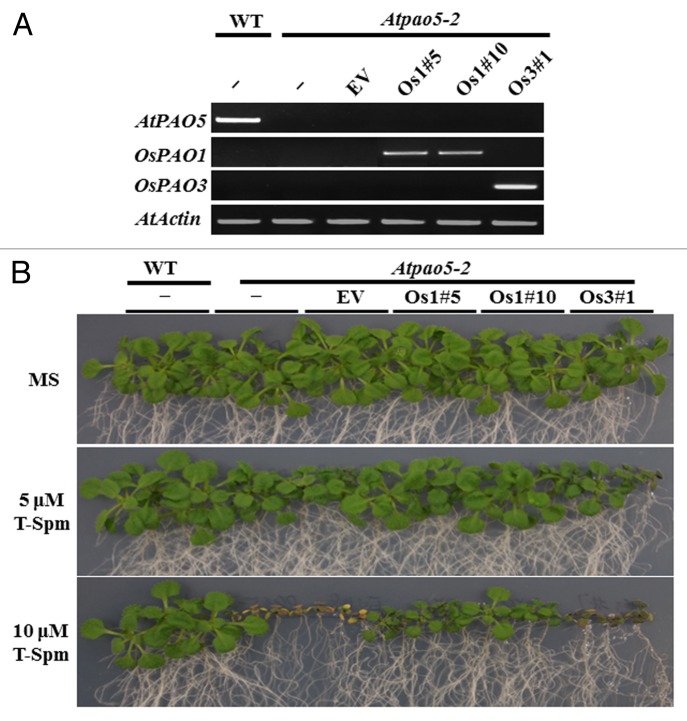
We reported recently that Arabidopsis AtPAO5 is a T-Spm oxidase, and that seedlings of the Atpao5–2 mutant, in which AtPAO5 gene expression is disrupted by a T-DNA insertion, show defective growth of their aerial parts in the presence of low doses of T-Spm.13 Here, we tested whether OsPAO1 open reading frame (ORF), under the control of a constitutive promoter, could complement the growth defects of the Atpao5–2 mutant. As a control, we also tested the ability of full-length OsPAO3 ORF, whose product, OsPAO3, is localized to peroxisomes, to rescue Atpao5–2 mutants. For this purpose, we generated 2 transgenic lines of Atpao5–2 plants containing either OsPAO1 or OsPAO3, as well as a control line of Atpao5–2 transformed with the empty vector (Atpao5–2EV). Expression levels in planta of native AtPAO5 and the rice transgenes were determined using RT-PCR analysis (Fig. 2A).
Figure 2. Complementation with OsPAO1, but not OsPAO3, rescues growth arrest in Atpao5–2 induced by T-Spm. (A) Expression of transgenes in Atpao5–2 plants. Gene expression levels in Atpao5–2 plants, transformed with the empty vector (EV), OsPAO1 or OsPAO3, were analyzed using RT-PCR. (B) Growth phenotypes of wild-type, Atpao5–2, and the Atpao5–2EV plants on 0.5 × MS medium (top), or on 0.5 × MS medium containing 5 μM T-Spm (middle) or 10 μM T-Spm (bottom). WT: Col-0; EV: Atpao5–2EV; Os1#5 and Os1#10: independent transgenic Atpao5–2 lines containing CaMV35S::OsPAO1; Os3#1: Atpao5–2 transgenic line containing CaMV35S::OsPAO3. Photographs were taken 20 d after sowing.
No differences in appearance were observed between wild-type plants, Atpao5–2EV controls, and transgenic Atpao5–2 lines when plants were grown on medium without T-spm (Fig. 2B, top photograph). As expected, growth of Atpao5–2 and Atpao5–2EV plants was arrested in a dose-dependent manner when T-Spm was added to the growth medium (Fig. 2B, middle and bottom photographs). Two independent transgenic Atpao5–2 lines expressing OsPAO1 (Os1#5 and Os1#10) were able to grow in the presence of T-spm (Fig. 2B), but a line expressing OsPAO3 (Os3#1) continued to show arrested growth under such conditions (Fig. 2B). We conclude, therefore, that OsPAO1, but not OsPAO3, can complement the growth defect of Atpao5–2 in the presence of low doses of T-Spm. All the transgenic lines showed normal growth phenotypes on media containing the polyamines Put (Fig. 3B), Spd (Fig. 3C), or Spm (Fig. 3D), even at high concentrations, indicating specificity of PAO function.
Figure 3. Growth phenotypes of wild-type, Atpao5–2 and transgenic Atpao5–2 lines on media containing either no PA (A), 1 mM Put (B), 500 μM Spd (C), or 100 μM Spm (D). WT: Col-0; EV, Atpao5–2EV; Os1#5 and Os1#10: independent transgenic Atpao5–2 lines containing CaMV35S::OsPAO1; Os3#1: Atpao5–2 transgenic line containing CaMV35S::OsPAO3. Photographs were taken 20 d after sowing.
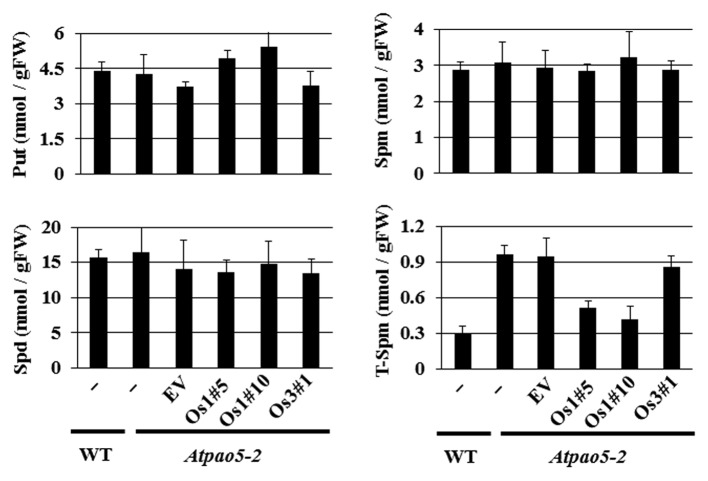
We analyzed the PA content of wild-type, Atpao5–2, Atpao5–2-EV, and Atpao5–2 transgenic plants expressing either OsPAO1 or OsPAO3 grown under physiological conditions. As shown in Figure 4, no major differences in levels of Put (Fig. 4A), Spd (Fig. 4B), or Spm (Fig. 4C) were observed in any of these lines, whereas T-Spm levels were about 3-fold higher in the Atpao5–2 and Atpao5–2EV lines, and also in Atpao5–2 plants expressing OsPAO3; these same lines showed defective growth in the presence of T-Spm (Fig. 4D). Atpao5–2 mutants expressing OsPAO1 had higher levels of T-Spm than wild-type plants but their T-Spm content was approximately 50% lower than that found in the Atpao5–2 and Atpao5–2EV lines (Fig. 4D). All our results suggest that OsPAO1, like AtPAO5, functions as a T-Spm oxidase in plants; however, a definitive demonstration of its function in rice plants (O. sativa) would require gene disruption or silencing in that species.
Figure 4. Analysis of polyamine levels in wild-type and transgenic Atpao5–2 plants under standard growth conditions. (A) Put; (B) Spd; (C) Spm; (D) T-Spm. Asterisks indicate significant differences from Atpao5–2 plants (the Student t-test; **P < 0.01).
Materials and Methods
Plant materials and growth conditions
Arabidopsis thaliana (L.) Heynh. wild-type plants (Columbia-0 (Col-0) accession) and the T-DNA insertion line Atpao5–2 (SALK_053110)13 were used in this study. All seeds were surface-sterilized by treating with 70% ethanol for 1 min, then with 1% sodium hypochloride and 0.1% Tween-20 for 15 min, followed by extensive washing with sterile distilled water. Sterilized seeds were placed onto vermiculite or on 0.5 × Murashige and Skoog (MS) medium containing 1.5% agar and 1% sucrose (pH 5.6). Growth conditions were 22 °C with a 14 h light/10 h dark photocycle.
Chemicals
Putrescine (Put), spermidine (Spd), and spermine (Spm) were purchased from Nacalai-Tesque Ltd. Thermospermine (T-Spm) was chemically synthesized, as described previously.14 All other analytical grade chemicals were obtained from Sigma-Aldrich Corp, Wako Pure Chemical Industries Ltd, and Nacalai-Tesque Ltd.
Generation of transgenic lines of Arabidopsis
To transform Atpao5–2 plants, fragments encompassing the coding regions of the OsPAO1 and OsPAO3 cDNAs were amplified using PCR with the primer pairs listed in Table S1. The OsPAO1 ORF fragment was double-digested with XbaI and SacI, and the OsPAO3 ORF fragment with SalI and XhoI, prior to subcloning into the corresponding sites of the pPZP2Ha3(+) vector,15 to yield pPZP2Ha3(+)-OsPAO1 and pPZP2Ha3(+)-OsPAO3, respectively.
These constructs were introduced into Agrobacterium tumefaciens strain GV3101 by freeze-thaw method,16 and the Agrobacterium transformants used to transfect Atpao5–2 plants via the floral dip method.17 T1 seeds collected from dipped plants were grown on MS agar medium containing 25 mg/mL hygromycin (hyg) and 50 mg/mL carbenicillin to select primary transformants. T2 seeds, obtained from self-fertilization of primary transformants, were surface-sterilized and grown on hyg-containing plates; those showing a 3:1 (resistant: sensitive) segregation ratio were selected to produce the homozygous (hygR/hygR) T3 lines used in this study. As a control for the effects of transformation, we also generated a line of Atpao5–2 plants containing the empty binary vector pPZP2Ha3(+) (Atpao5–2EV).
RT-PCR analysis
Total RNA was extracted from whole aerial parts of 2-wk-old Arabidopsis seedlings using Sepasol-RNA I Super (Nacalai-Tesque), according to the manufacturer’s instructions. First-strand cDNA was synthesized using ReverTra Ace (Toyobo Co Ltd) and oligo-dT primers. RT-PCR analysis was performed, as described previously,18 using a cDNA template and the primers listed in Table S1. Constitutively expressed AtActin (accession number, At3g18780) was used as an internal control for the analysis.
High-performance liquid chromatography (HPLC) analysis of polyamine levels
The basic protocol has been described previously.19 In brief, plant samples (0.3–0.5 g per sample) were collected and stored at −80 °C until use. Samples were pulverized under liquid nitrogen using a pestle and mortar; 5 volumes (2.5 mL per 0.5 g plant sample) of 5% (v/v) cold perchloric acid (PCA) were added to the powder. The mixture was transferred to a plastic tube and kept on ice for 1 h. After centrifugation at 15,000 × g for 30 min at 4 °C, the supernatant was filtered using a filter syringe (pore size: 0.2 μm). Next, 1 mL of 2 N NaOH was added to 1.5 mL of plant extract and the mixture was vortexed before 10 µL of benzoyl chloride was added. The sample was mixed, incubated at room temperature for 20 min, and then 2 mL of saturated NaCl solution was added. Following the addition of 2 mL diethyl ether, samples were again mixed vigorously and then centrifuged at 3,000 × g for 10 min at 4 °C for phase-separation. Finally, a 1.5 mL aliquot of the organic solvent phase was evaporated and the residue resuspended in 50 µL of methanol.
Levels of the benzoylated PAs in each sample were analyzed using a programmable Hewlett Packard series 1100 liquid chromatograph with a reverse-phase column (4.6 × 250 mm, TSK-GEL ODS-80Ts, TOSOH) and detected at 254 nm. One cycle of the run lasted a total of 60 min at a flow rate of 1 mL/min at 30 °C; the detailed breakdown of each run was as follows: 42% acetonitrile for 25 min for PA separation, an increase to 100% acetonitrile over 3 min, then 100% acetonitrile for 20 min for washing, a decrease to 42% acetonitrile over 3 min, and finally 42% acetonitrile for 9 min.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr M Yano, Dr M Teranishi, and Dr J Hidema for providing plasmids and bacterial strains. We also thank the Arabidopsis Biological Resource Center, Ohio State University for providing Atpao5–2 mutant seeds. This study was supported by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT) to T.K. (21380063 and 26·04081) and to D.W.K. (25·5682, Grant-in-Aid for Young Scientists) and grants from The Saito Gratitude Foundation and The Japan Science Society (the Sasagawa Scientific Research Grant) to DWK. TL was financially supported by the China Scholarship Council.
Glossary
Abbreviations:
- CaMV
Cauliflower mosaic virus
- CuAOs
copper-containing amine oxidases
- ORF
open reading frame
- PA
polyamine
- PAO
polyamine oxidase
- Put
putrescine
- Spd
spermadine
- Spm
spermine
- T-Spm
thermospermine
References
- 1.Alcázar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C, Carrasco P, Tiburcio AF. Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta. 2010;231:1237–49. doi: 10.1007/s00425-010-1130-0. [DOI] [PubMed] [Google Scholar]
- 2.Handa AK, Mattoo AK. Differential and functional interactions emphasize the multiple roles of polyamines in plants. Plant Physiol Biochem. 2010;48:540–6. doi: 10.1016/j.plaphy.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Kusano T, Berberich T, Tateda C, Takahashi Y. Polyamines: essential factors for growth and survival. Planta. 2008;228:367–81. doi: 10.1007/s00425-008-0772-7. [DOI] [PubMed] [Google Scholar]
- 4.Takano A, Kakehi J, Takahashi T. Thermospermine is not a minor polyamine in the plant kingdom. Plant Cell Physiol. 2012;53:606–16. doi: 10.1093/pcp/pcs019. [DOI] [PubMed] [Google Scholar]
- 5.Tiburcio AF, Altabella T, Bitrián M, Alcázar R. The roles of polyamines during the lifespan of plants: from development to stress. Planta. 2014 doi: 10.1007/s00425-014-2055-9. In press. [DOI] [PubMed] [Google Scholar]
- 6.Cona A, Rea G, Angelini R, Federico R, Tavladoraki P. Functions of amine oxidases in plant development and defence. Trends Plant Sci. 2006;11:80–8. doi: 10.1016/j.tplants.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Planas-Portell J, Gallart M, Tiburcio AF, Altabella T. Copper-containing amine oxidases contribute to terminal polyamine oxidation in peroxisomes and apoplast of Arabidopsis thaliana. BMC Plant Biol. 2013;13:109. doi: 10.1186/1471-2229-13-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angelini R, Cona A, Federico R, Fincato P, Tavladoraki P, Tisi A. Plant amine oxidases “on the move”: an update. Plant Physiol Biochem. 2010;48:560–4. doi: 10.1016/j.plaphy.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Ono Y, Kim DW, Watanabe K, Sasaki A, Niitsu M, Berberich T, Kusano T, Takahashi Y. Constitutively and highly expressed Oryza sativa polyamine oxidases localize in peroxisomes and catalyze polyamine back conversion. Amino Acids. 2012;42:867–76. doi: 10.1007/s00726-011-1002-3. [DOI] [PubMed] [Google Scholar]
- 10.Liu T, Kim DW, Niitsu M, Berberich T, Kusano T. Oryza sativa polyamine oxidase 1 back-converts tetraamines, spermine and thermospermine, to spermidine. Plant Cell Rep. 2014;33:143–51. doi: 10.1007/s00299-013-1518-y. [DOI] [PubMed] [Google Scholar]
- 11.Liu T, Kim DW, Niitsu M, Maeda S, Watanabe M, Kamio Y, Berberich T, Kusano T. Polyamine oxidase 7 is a terminal catabolism-type enzyme in Oryza sativa and is specifically expressed in anthers. Plant Cell Physiol. 2014;55:1110–22. doi: 10.1093/pcp/pcu047. [DOI] [PubMed] [Google Scholar]
- 12.Ahou A, Martignago D, Alabdallah O, Tavazza R, Stano P, Macone A, Pivato M, Masi A, Rambla JL, Vera-Sirera F, et al. A plant spermine oxidase/dehydrogenase regulated by the proteasome and polyamines. J Exp Bot. 2014;65:1585–603. doi: 10.1093/jxb/eru016. [DOI] [PubMed] [Google Scholar]
- 13.Kim DW, Watanabe K, Murayama C, Izawa S, Niitsu M, Michael AJ, Berberich T, Kusano T. Polyamine oxidase 5 regulates Arabidopsis thaliana growth through a thermospermine oxidase activity. Plant Physiol. 2014 doi: 10.1104/pp.114.242610. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niitsu M, Samejima K. Syntheses of a series of linear pentaamines with three and four methylene chain intervals. Chem Pharm Bull (Tokyo) 1986;34:1032–8. doi: 10.1248/cpb.34.1032. [DOI] [Google Scholar]
- 15.Fuse T, Sasaki T, Yano M. Ti-plasmid vectors useful for functional analysis of rice genes. Plant Biotechnol. 2001;18:219–22. doi: 10.5511/plantbiotechnology.18.219. [DOI] [Google Scholar]
- 16.Weigel D, Glazebrook J. Transformation of agrobacterium using the freeze-thaw method. CSH Protoc. 2006;2006:1031–6. doi: 10.1101/pdb.prot4666. [DOI] [PubMed] [Google Scholar]
- 17.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–43. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhu X, Thalor SK, Takahashi Y, Berberich T, Kusano T. An inhibitory effect of the sequence-conserved upstream open-reading frame on the translation of the main open-reading frame of HsfB1 transcripts in Arabidopsis. Plant Cell Environ. 2012;35:2014–30. doi: 10.1111/j.1365-3040.2012.02533.x. [DOI] [PubMed] [Google Scholar]
- 19.Naka Y, Watanabe K, Sagor GHM, Niitsu M, Pillai MA, Kusano T, Takahashi Y. Quantitative analysis of plant polyamines including thermospermine during growth and salinity stress. Plant Physiol Biochem. 2010;48:527–33. doi: 10.1016/j.plaphy.2010.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.