Abstract
Mutations in ERCC6 are associated with growth failure, intellectual disability, neurological dysfunction and deterioration, premature aging and photosensitivity. We describe siblings with biallelic ERCC6 mutations (NM_000124.2:c. [543+4delA];[2008C>T]) and brain hypomyelination, microcephaly, cognitive decline, and skill regression but without photosensitivity or progeria. DNA repair assays on cultured skin fibroblasts confirmed a defect of transcription-coupled nucleotide excision repair and increased ultraviolet light sensitivity. This report expands the disease spectrum associated with ERCC6 mutations.
Keywords: Hypomyelination, Developmental Delay, Intellectual Disability, Cockayne Syndrome group B, Deafness, Vision loss
INTRODUCTION
Defects of DNA repair and gene expression that impair neural development and cognition can cause intellectual disability (ID) [Lu et al., 2004]. Mechanisms by which defective DNA repair causes neuronal dysfunction and death include programmed neuronal death [Fishel et al., 2007], neuronal collapse after attempting DNA repair through replication-dependent mechanisms [Kruman et al., 2004; Larsen et al., 2005], and loss of glial trophic interactions due to glial dysfunction [Barzilai, 2011; Lobsiger and Cleveland, 2007]. The mechanisms by which defects of gene expression cause neuronal dysfunction and death are incompletely understood; however, pathologic alterations in expression of genes regulating neural development and synapsis are hypothesized to cause cognitive disorders [Day and Sweatt, 2011; Jakovcevski and Akbarian, 2012; Mehler, 2008; van Bokhoven, 2011; Yoo and Crabtree, 2009].
The Excision-Repair Cross-Complementing Rodent Repair Deficiency, Complementation Group 6 (ERCC6) gene, located on chromosome 10, encodes the Cockayne Syndrome Group B (CSB) protein that participates in DNA repair and gene expression. CSB stably interacts with double-stranded nucleic acids and its ATP-independent complementary strand annealing activity generates double-stranded molecules [Muftuoglu et al., 2006]. CSB is essential for nuclear transcription-coupled nucleotide excision repair (TC-NER), which removes RNA polymerase blocking lesions [Orren et al., 1996]. Interacting with and stimulating elongation by RNA polymerases I (~10-fold) and II (~3-fold) [Balajee et al., 1997; van Gool et al., 1997], the CSB protein plays a role in general nuclear transcription and stimulates RNA polymerase II progression beyond pause sites [Licht et al., 2003].
Loss of functional CSB has been associated with Cockayne Syndrome group B, Cerebro-Oculo-Facio-Skeletal Syndrome 1 (COFS1), De Sanctis-Cacchione Syndrome, and UV-Sensitive Syndrome 1 (UVSS1). Despite variable expressivity, Cockayne Syndrome group B usually presents within the first 2 years of life and is characterized by growth failure, cutaneous photosensitivity, dysmyelination of white matter, cachectic dwarfism, progeroid features, and death at an average age of 12 years [Friedberg and Friedberg, 2006; Jones and Smith, 2006]. COFS1 is the prenatal form of Cockayne syndrome and is characterized by congenital microcephaly, congenital cataracts and/or microphthalmia, arthrogryposis, severe psychomotor developmental delay, height-weight growth delay, and facial dysmorphism [Pena and Shokeir 1974]. De Sanctis-Cacchione Syndrome exhibits findings of xeroderma pigmentosum and neurological disturbances including intellectual disability. Manifesting in early infancy, individuals with De Sanctis-Cacchione Syndrome have skin sensitivity to ultraviolet (UV) light, abnormal skin pigmentation, a high frequency of skin cancers, photophobia, conjunctivitis, ectropion, symblepharon with ulceration and scarring, progressive mental deterioration, cerebellar ataxia, hyporeflexia or areflexia, choreoathetosis and spasticity [Harrison and Shuster 1979]. UVSS1 is characterized by skin sensitivity to ultraviolet (UV) light and abnormal skin pigmentation (solar lentigines) [Itoh et al., 1996]. Neurological symptoms and deterioration are not characteristic of the disease. UVSS1 can be caused by biallelic mutations of ERCC6 as well as by biallelic mutations of UVSSA, which encodes a protein that stabilizes CSB [Horibata et al., 2004; Nakazawa et al., 2012; Schwertman et al., 2012].
Herein we extend the phenotypic spectrum for biallelic ERCC6 mutations to include static hypomyelination, microcephaly, mild postnatal growth restriction and slow regression of skills without evidence of photosensitivity, progeria, or the leukodystrophy classically associated with CSB dysfunction [Laugel, 1993].
CLINICAL REPORTS
Patient 1
Patient 1 was an 8-year-old Caucasian male who presented to the Undiagnosed Diseases Program (UDP) at the National Institutes of Health with brain hypomyelination, difficulty learning new information in school and gross motor skill regression. He was the firstborn child of non-consanguineous healthy parents. The family history was significant for a similarly affected younger brother (Patient 2) and sister (Patient 3). He also had an unaffected younger sister.
He was delivered by cesarean following an uncomplicated pregnancy. He crawled and spoke single words by 12 months and walked by 17 months. He was toilet trained by 3 years of age. He developed nystagmus at age 3 years. Due to clumsiness and falling, he began physical and occupational therapy at age 4 years; however, his condition progressed, and by 8 years, he had a resting tremor, mild truncal titubation, an ataxic gait, and a positive Gowers’ sign. In kindergarten, he had difficulties with complex sentence structure, abstract thinking and memory and was diagnosed with a learning disability. At 8 years and 10 months, the Stanford-Binet Intelligence Scale measured his nonverbal IQ at 100 and his verbal IQ at 83. Also at this time, the Vineland Adaptive Behavior Scales, 2nd edition, showed his receptive language age equivalent was 47 months and his gross motor age equivalent was 33 months (Table I). He had poor manipulation skills, grip strength, and visual motor coordination. By 10 years, he had pigmentary retinopathy and sensorineural hearing loss.
Table I.
Adaptive behavior and intelligence quotients of the three patients (Patients II-1, II-2, II-3) and their unaffected sister (Patient II-4).
| Feature | II-1 | II-2 | II-3 | II-4 |
|---|---|---|---|---|
| Chronological Age | 8 y, 10 m | 7 y, 1 m | 5 y | 2 y |
| Communication SS | 81 | 88 | 85 | 101 |
| Receptive Age Equivalent* | 3 y, 11 m | 4 y, 7 m | 2 y, 6 m | 2 y, 2 m |
| Expressive Age Equivalent* | 7 y | 8 y | 3 y, 6 m | 2 y, 3 m |
| Daily Living SS* | 71 | 76 | 91 | 105 |
| Socialization SS* | 76 | 83 | 105 | 112 |
| Motor SS* | 91 | 96 | ||
| Gross Motor Age Equivalent* | 2 y, 9 m | 2 y, 4 m | 3 y, 5 m | 2 y, 3 m |
| Fine Motor Age Equivalent* | 5 y, 10 m | 5 y, 7 m | 5 y, 1 m | 1 y, 10 m |
| Adaptive Behavior Composite SS* | 74 | 80 | 91 | 104 |
| NVIQ SS | 100 | 90 | 102 | 121** |
| VIQ SS | 83 | 86 | 95 | 132** |
| FSIQ SS | 91 | 82 | 99 | 126 |
Measured using the Vineland Adaptive Behavior Scales, Second Edition.
Estimate, since this test does not provide this exact score
Abbreviations: SS=Standard Score (Mean = 100, Standard Deviation=15); FSIQ, Full Scale Intelligence Quotient; m, month; NVIQ, Nonverbal Intelligence Quotient; VIQ, Verbal Intelligence Quotient; y, year.
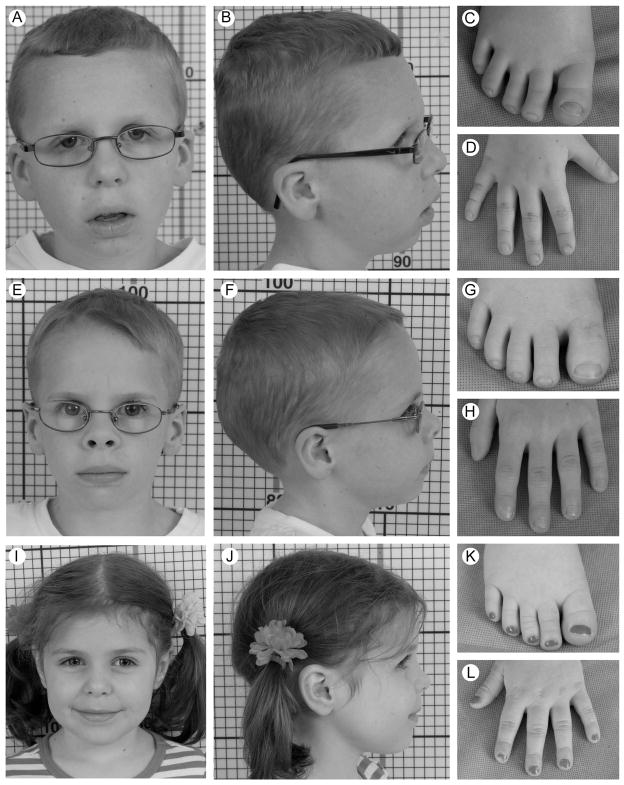
On physical examination at 8 years, his height, weight, and head circumference were all <5th centile. He was mildly dysmorphic with myopathic facies, hooded-eyelids, and straight superior major helices of the ears (Fig 1), and had bilateral horizontal nystagmus, a resting tremor, hypoactive deep tendon reflexes, and difficulty walking.
Figure 1.
Facial features, hands and feet of Patients 1 (Images A–D), 2 (Images E–H) and 3 (Images I–L) at the physical examination at the NIH.
Patient 2
Patient 2 was the 7-year-old younger brother of Patient 1. He presented to the UDP with brain hypomyelination, acquired microcephaly and developmental delay first noted at 22 months.
He was delivered by cesarean following an uncomplicated pregnancy. His growth and development during infancy and early childhood were within the normal range including language, gross and fine motor skills; he was toilet trained by 4 years. At 5 years of age, he was noted to have short attention span and loss of speech and motor skills. Despite physical, occupational and speech therapy, he did not improve and by 7 years, he required ankle-foot orthotics to walk. Vineland Adaptive Behavior Scales, 2nd edition, showed that his gross motor age equivalent was 28 months and his receptive language age equivalent was 55 months. Testing using the Differential Ability Scales, 2nd edition (DAS-II) showed that his full scale IQ was 82 (Table I). By 8 years, he had pigmentary retinal degeneration and sensorineural hearing loss and needed assistance with activities of daily living.
On physical examination at 7 years, his height, weight, and head circumference were <5th centile. He was mildly dysmorphic with a frontal upsweep, slightly broad great toes, and short distal phalanges of the thumbs (Fig 1), and had bilateral nystagmus and wore glasses. In addition, he had a resting tremor, hypoactive deep reflexes, mild titubation, a positive Gowers’ sign and difficulties walking.
Patient 3
Patient 3 was the younger sister of Patients 1 and 2. She was delivered by cesarean following an uncomplicated pregnancy. She stood independently and spoke single words by 12 months and walked independently by 17 months. She attended a special needs preschool and had physical therapy for mild proximal weakness. Vineland Adaptive Behavior Scales, 2nd edition, showed that at age 5 years her receptive language age equivalent was 30 months and her gross motor age equivalent was 41 months. DAS-II testing measured her full scale IQ as 99 (Table I). By 6 years old, she had pigmentary retinal degeneration and sensorineural hearing loss.
On physical exam at 5 years, her height, weight and head circumference were <5th pcentile. She was nondysmorphic except for slightly broad great toes (Figure 1).
Laboratory studies
Patient 1 had a normal karyotype, acylcarnitine profile, plasma amino acid profile and urine guanidinoacetate level. By bacterial artificial chromosome microarray, he did not have a detectable pathogenic microdeletion or microduplication. All 3 patients had normal sterol profiles as well as normal patterns of plasma N-glycan and transferrin glycosylation; levels of fucosylated and under-galactosylated N-linked glycan levels were normal and not indicative of an increased glycan aging index [Vanhooren et al., 2010]. The urine oligosaccharide pattern for the 3 patients, but not the unaffected sibling, showed elevated small fucosylated oligosaccharides and a unique oligosaccharide of unknown clinical significance at m/z 1111.
Imaging studies
All patients had brain MRIs showing brain hypomyelination that was initially interpreted as delayed myelination (Fig 2). Although the patients manifested progressive cognitive, functional and visual decline, subsequent brain MRIs demonstrated static hypomyelination and no evidence of cerebellar atrophy or hypoplasia (Supplementary Figure S1.). The hypomyelination involved the deep and periventricular white matter, the posterior pons and the hilum of the dentate nucleus. Myelination of the cerebellar peduncles, subcortical white matter and the corpus callosum was relatively preserved.
Figure 2.
T2 weighted brain magnetic resonance images of Patients 1 (A–B), 2 (C), and 3 (D). Images for Patient 1 were obtained at age 4 (A) and 7 (B) years, and the images for Patients 2 and 3 were obtained at age 4 years. Consistent with hypomyelination, there is poor differentiation between gray and white matter of all patients. Also, note the development of generalized atrophy and enlarged ventricles for Patient 1 between the ages of 4 (A) and 7 (B) years.
METHODS
Methods and associated references are available in the online version of the paper (Supplemental material).
RESULTS
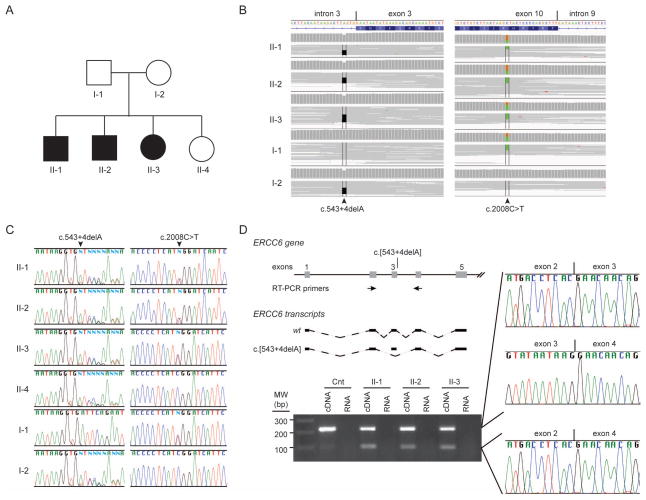
In the absence of biochemical abnormalities or clinical features pathognomic of a known disease, we pursued exome sequencing of the patients and their parents (Fig 3A). Selection of rare, high quality variants segregating with disease identified 279 candidate mutations including a pair of ERCC6 mutations (NM_000124.2: c.[543+4delA];[2008C>T]) (Fig 3B). Intronic mutation NM_000124.2: c.543+4delA was paternally inherited, and NM_00124.2: c.2008C>T was maternally inherited. NM_000124.2: c.2008C>T encodes the missense mutation NP_000115.1: p.R670W, a mutation previously reported in association with Cockayne syndrome [Laugel et al., 2010; Mallery et al., 1998]. To our best knowledge, the NM_000124.2: c.543+4delA, an intronic mutation that alters a conserved nucleotide near the canonical splice donor site of exon 3, has not been reported in association with Cockayne syndrome.
Figure 3.
Identification and characterization of biallelic ERCC6 mutations. A) Pedigree showing unaffected parents (I-1 and I-2), affected children (II-1, II-2 and II-3) and unaffected child (II-4). (B) IGV views of exome short reads showing biallelic ERCC6 mutations: a maternally inherited intronic deletion (left, c.543+4delA) and a paternally inherited point mutation associated previously with Cockayne syndrome (right, c.2008C>T). (C) Sanger traces for PCR amplification products of each ERCC6 mutation site. Note confirmation of the paternally intronic deletion (c.543+4delA) and maternally inherited point mutation (c.2008C>T). (D) ERCC6 RT-PCR amplification products and sequences from mRNA derived from the cultured skin fibroblasts of patient II-1. Note that the smaller PCR has exon 3 deleted and is derived from the splice product of ERCC6 exon 2 to exon 4. The positions of the PCR primers are shown in the diagram.
To test if the intronic mutation NM_00124.2: c.[543+4delA] impeded pre-mRNA splicing, we generated cDNA prepared from RNA isolated from skin fibroblasts cultured from two patients. For each, PCR amplification spanning exon 3 generated two products, one including exon 3 and one deleted for exon 3 (Fig 3D). Deletion of exon 3 encodes the frameshift mutation NP_000115.1:p.Ser142*. Consequently, we hypothesized that the aberrantly spliced mRNA is subject to nonsense-mediated decay; however, qPCR showed nearly equivalent levels of ERCC6 mRNA in skin fibroblasts cultured from the patients and controls (Fig 4A). Approximately 50% of the patients’ ERCC6 mRNA contained exon 3 (Fig 4B).
Figure 4.
Impact of the ERCC6 mutations on ERCC6 expression, cellular response to ultraviolet light (UV) and urinary free glycan excretion. (A) ERCC6 mRNA levels in cultured skin fibroblasts from controls and propositae. (B) ERCC6 mRNA containing exon 3 in cultured skin fibroblasts from the controls and propositae. (C) Immunoblotting results from the skin fibroblasts of the controls and propositae showing expression full length ERCC6 protein. p84 was probed as a loading control. (D–F) Results of DNA repair assays on Patient 1 fibroblasts (C5RO) and fibroblasts from an individual with severe Cockayne syndrome group B (CS267BE). (D) Comparative survival of fibroblasts following exposure to increasing doses of UV light. (E) Unscheduled DNA synthesis showed no defect of global nucleotide excision repair. (F) A 55–90% reduction in transcription recovery following UV irradiation suggested a defect of TC-NER. (G) Urine free glycan pattern of an unaffected sex- and age-matched control. (H) Urine free glycan pattern of Patient II-1 showing elevated small fucosylated oligosaccharides at m/z 692, 1070, 1315 and 1519.
Furthermore to determine if the missense mutation p.R670W destabilized the CSB protein, we performed immunoblotting of lysates from cultured skin fibroblasts of the patients and an unaffected control. Focusing on the full length CSB protein, not the truncated p.Ser142* protein, the fibroblast lysates from the patient and the unaffected control had similar steady state levels of CSB protein (Fig 4C). This suggests that either the p.R670W protein is stable or that full length ERCC6 mRNA produced by the NM_00124.2: c.[543+4delA] allele contributes more protein than indicated by the preceding qRT-PCR experiment.
Given the mild, but clearly progressive phenotype and the detection of normally spliced mRNA, we tested if fibroblasts from the patients manifest features of CSB protein deficiency. Specifically, we measured global nucleotide excision repair, TC-NER and sensitivity to ultraviolet light in cultured skin fibroblasts from Patient 1. As measured by unscheduled DNA synthesis, the fibroblasts had no defect of global nucleotide excision repair. They had a 55–90% reduction in recovery from transcription arrest following exposure to ultraviolet light and a 2.3 fold increase in sensitivity to ultraviolet light (Fig 4D–F), indicating a defect of TC-NER. However, these findings were milder than usually seen in Cockayne Syndrome group B patients, suggesting the possibility of residual CSB enzyme activity.
Given that each affected child, but not the unaffected sibling, had a urine free glycan pattern showing elevated small fucosylated oligosaccharides (Fig 4G), we hypothesized that these free glycans are a biomarker for loss of CSB activity or a clue to a pathological mechanism for CSB dysfunction. To test this hypothesis, we used MALDI-TOF/TOF to analyze urine from two unrelated patients with ERCC6 mutations and classical Cockayne Syndrome group B as well as cultured skin fibroblasts from the proband. Both unrelated patients had an unusual pattern of urine free glycans enriched with fucosylated species, but the pattern was not identical to that observed in the three affected individuals studied herein (Supplementary Figure S2). Also, the abnormal pattern of fucosylated oligosaccharides was not observed in lysates of cultured fibroblasts from the proband (data not shown). Therefore we conclude that unusual patterns of urine free glycans may be common in individuals with abnormalities of CSB function, but that the variation in the patterns among individuals precludes use as a specific biomarker.
DISCUSSION
We report on three siblings, two boys and one girl, with CSB dysfunction characterized by brain hypomyelination and mid-childhood onset of retinal pigmentary changes, sensorineural hearing loss and loss of motor and cognitive skills. These three siblings carried biallelic ERCC6 mutations, but lacked features characteristic of COFS1, De Sanctis-Cacchione syndrome, UV sensitivity syndrome, and classical Cockayne syndrome [Cockayne, 1936]. Cockayne syndrome type I was originally described with an onset before two years of age and characterized by growth failure, cutaneous photosensitivity, pigmentary retinopathy, and brain MRIs showing leukodystrophy [Boltshauser et al., 1989; Sugita et al., 1992]. Cockayne syndrome type II was defined by early onset and death before 7 years of age, and Cockayne syndrome type III presented with later onset and slower development of symptoms. Recent studies blurred the distinction among these subgroups [Nance and Berry, 1992] and suggested that Cockayne syndrome has a continuous severity spectrum [Laugel, 2013].
Our patients broaden that spectrum. They display many features consistent with CSB dysfunction (Supplementary Table SI), including growth failure and neurological dysfunction (although less severe), pigmentary retinopathy, sensorineural hearing loss, and impaired recovery of RNA synthesis following UV irradiation. Like some individuals with Cockayne syndrome group B, our patients lack cutaneous photosensitivity [Lehmann et al., 1993; Sonmez et al., 2006; Tinsa et al., 2009]. However, none of the reported cases of Cockayne syndrome had features as mild as those we described (Supplementary Table SII). It remains to be determined if they will later develop more characteristic signs and symptoms of Cockayne syndrome, since the rate of disease progression is not predictable [Laugel, 2013].
These patients’ milder phenotype might result from residual expression of wild type CSB protein arising from residual wild type splicing of the paternal allele (c.543+4delA); this is supported by the mild phenotype observed with another splicing mutation within this intron (c.544-1G>A) [Laugel et al., 2010]. Additionally, the primary disease mechanism may not be a prominent defect of TC-NER but rather a defect of CSB function in base excision DNA repair [Licht et al., 2003], mitochondrial activity [Kamenisch et al., 2010], or coordination of a transcriptional response [Brooks et al., 2008; Filippi et al., 2008]. Consistent with this, Revet et al. [2012] found that in Ercc6−/− mice, brain hypomyelination resulted from reduced expression of myelin basic protein.
Transcriptional anomalies could also explain the unusual urine oligosaccharides we observed in individuals with ERCC6 because protein glycosylation requires a balance between production of glycan and protein that is, at least in part, dependent upon gene expression. Consistently, decreased asialo-transferrin and N-glycan abnormalities in the cerebrospinal fluid occur in vanishing white matter (VWM) disease, which is due to mutations in eukaryotic initiation factor 2B, eIF2B [Fogli et al., 2012; Vanderver et al., 2008]. Alternatively, the abnormal urinary oligosaccharides in our patients might merely be byproducts of the disease, as occurs with inflammatory processes, cell death and mitochondrial dysfunction [Ma et al., 2006]. Finally, because changes in protein glycosylation occur with aging [Vanhooren et al., 2010], the urine oligosaccharides might reflect the premature aging of individuals with Cockayne syndrome group B. However, neither the serum nor the fibroblast N-glycan profiles showed evidence of increased aging. Future research into the precise structure of these oligosaccharides will provide more insight into their origins and possible contribution to the pathogenesis of CSB deficiency.
In summary, biallelic mutations in ERCC6 cause a wide phenotypic spectrum. As shown here, the primary clinical manifestations of CSB dysfunction can consist of only brain hypomyelination, mild dysmorphia, and mid-childhood loss of skills. Lastly, the identification of abnormal urine oligosaccharides in five patients with CSB dysfunction raises intriguing questions about their relevance to disease pathogenesis and will require further investigation.
Supplementary Material
Acknowledgments
We thank Dr. Camilo Toro for selection of MRI photographs and Drs. Mariska Davids and Megan Kane for critical review of the manuscript. This work was supported in part by the Common Fund, Office of the Director and the Intramural Research Program of the National Human Genome Research Institute (NIH, Bethesda, Maryland).
Footnotes
Conflict of interest: none.
References
- Balajee AS, May A, Dianov GL, Friedberg EC, Bohr VA. Reduced RNA polymerase II transcription in intact and permeabilized Cockayne syndrome group B cells. Proc Natl Acad Sci U S A. 1997;94(9):4306–4311. doi: 10.1073/pnas.94.9.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai A. The neuro-glial-vascular interrelations in genomic instability symptoms. Mech Ageing Dev. 2011;132(8–9):395–404. doi: 10.1016/j.mad.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Boltshauser E, Yalcinkaya C, Wichmann W, Reutter F, Prader A, Valavanis A. MRI in Cockayne syndrome type I. Neuroradiology. 1989;31(3):276–277. doi: 10.1007/BF00344359. [DOI] [PubMed] [Google Scholar]
- Brooks PJ, Cheng TF, Cooper L. Do all of the neurologic diseases in patients with DNA repair gene mutations result from the accumulation of DNA damage? DNA Repair (Amst) 2008;7(6):834–848. doi: 10.1016/j.dnarep.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne EA. Dwarfism with retinal atrophy and deafness. Archives of disease in childhood. 1936;11(61):1–8. doi: 10.1136/adc.11.61.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. Epigenetic mechanisms in cognition. Neuron. 2011;70(5):813–829. doi: 10.1016/j.neuron.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi S, Latini P, Frontini M, Palitti F, Egly JM, Proietti-De-Santis L. CSB protein is (a direct target of HIF-1 and) a critical mediator of the hypoxic response. EMBO J. 2008;27(19):2545–2556. doi: 10.1038/emboj.2008.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishel ML, Vasko MR, Kelley MR. DNA repair in neurons: so if they don’t divide what’s to repair? Mutat Res. 2007;614(1–2):24–36. doi: 10.1016/j.mrfmmm.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Fogli A, Merle C, Roussel V, Schiffmann R, Ughetto S, Theisen M, Boespflug-Tanguy O. CSF N-glycan profiles to investigate biomarkers in brain developmental disorders: application to leukodystrophies related to eIF2B mutations. PLoS One. 2012;7(8):e42688. doi: 10.1371/journal.pone.0042688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg EC. DNA repair and mutagenesis. xxix. Washington, D.C: ASM Press; 2006. p. 1118. [Google Scholar]
- Harrison PV, Shuster S. De Sanctis-Cacchione syndrome. Arch Dermatol. 1979;115(6):676. doi: 10.1001/archderm.1979.04010060002006. [DOI] [PubMed] [Google Scholar]
- Horibata K, Iwamoto Y, Kuraoka I, Jaspers NG, Kurimasa A, Oshimura M, Ichihashi M, Tanaka K. Complete absence of Cockayne syndrome group B gene product gives rise to UV-sensitive syndrome but not Cockayne syndrome. Proc Natl Acad Sci U S A. 2004;101(43):15410–15415. doi: 10.1073/pnas.0404587101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Yamaizumi M, Ichihashi M, Hiro-Oka M, Matsui T, Matsuno M, Ono T. Clinical characteristics of three patients with UVs syndrome, a photosensitive disorder with defective DNA repair. Br J Dermatol. 1996;134(6):1147–1150. [PubMed] [Google Scholar]
- Jakovcevski M, Akbarian S. Epigenetic mechanisms in neurological disease. Nat Med. 2012;18(8):1194–1204. doi: 10.1038/nm.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Smith’s recognizable patterns of human malformation. xviii. Philadelphia: Elsevier Saunders; 2006. p. 954. [Google Scholar]
- Kamenisch Y, Fousteri M, Knoch J, von Thaler AK, Fehrenbacher B, Kato H, Becker T, Dolle ME, Kuiper R, Majora M, Schaller M, van der Horst GT, van Steeg H, Rocken M, Rapaport D, Krutmann J, Mullenders LH, Berneburg M. Proteins of nucleotide and base excision repair pathways interact in mitochondria to protect from loss of subcutaneous fat, a hallmark of aging. J Exp Med. 2010;207(2):379–390. doi: 10.1084/jem.20091834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruman II, Wersto RP, Cardozo-Pelaez F, Smilenov L, Chan SL, Chrest FJ, Emokpae R, Jr, Gorospe M, Mattson MP. Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron. 2004;41(4):549–561. doi: 10.1016/s0896-6273(04)00017-0. [DOI] [PubMed] [Google Scholar]
- Larsen NB, Rasmussen M, Rasmussen LJ. Nuclear and mitochondrial DNA repair: similar pathways? Mitochondrion. 2005;5(2):89–108. doi: 10.1016/j.mito.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Laugel V. Cockayne Syndrome. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, editors. GeneReviews. Seattle (WA): 1993. [PubMed] [Google Scholar]
- Laugel V. Cockayne syndrome: the expanding clinical and mutational spectrum. Mech Ageing Dev. 2013;134(5–6):161–170. doi: 10.1016/j.mad.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Laugel V, Dalloz C, Durand M, Sauvanaud F, Kristensen U, Vincent MC, Pasquier L, Odent S, Cormier-Daire V, Gener B, Tobias ES, Tolmie JL, Martin-Coignard D, Drouin-Garraud V, Heron D, Journel H, Raffo E, Vigneron J, Lyonnet S, Murday V, Gubser-Mercati D, Funalot B, Brueton L, Sanchez Del Pozo J, Munoz E, Gennery AR, Salih M, Noruzinia M, Prescott K, Ramos L, Stark Z, Fieggen K, Chabrol B, Sarda P, Edery P, Bloch-Zupan A, Fawcett H, Pham D, Egly JM, Lehmann AR, Sarasin A, Dollfus H. Mutation update for the CSB/ERCC6 and CSA/ERCC8 genes involved in Cockayne syndrome. Hum Mutat. 2010;31(2):113–126. doi: 10.1002/humu.21154. [DOI] [PubMed] [Google Scholar]
- Lehmann AR, Thompson AF, Harcourt SA, Stefanini M, Norris PG. Cockayne’s syndrome: correlation of clinical features with cellular sensitivity of RNA synthesis to UV irradiation. J Med Genet. 1993;30(8):679–682. doi: 10.1136/jmg.30.8.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht CL, Stevnsner T, Bohr VA. Cockayne syndrome group B cellular and biochemical functions. Am J Hum Genet. 2003;73(6):1217–1239. doi: 10.1086/380399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobsiger CS, Cleveland DW. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci. 2007;10(11):1355–1360. doi: 10.1038/nn1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429(6994):883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Ma B, Simala-Grant JL, Taylor DE. Fucosylation in prokaryotes and eukaryotes. Glycobiology. 2006;16(12):158R–184R. doi: 10.1093/glycob/cwl040. [DOI] [PubMed] [Google Scholar]
- Mallery DL, Tanganelli B, Colella S, Steingrimsdottir H, van Gool AJ, Troelstra C, Stefanini M, Lehmann AR. Molecular analysis of mutations in the CSB (ERCC6) gene in patients with Cockayne syndrome. Am J Hum Genet. 1998;62(1):77–85. doi: 10.1086/301686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler MF. Epigenetics and the nervous system. Ann Neurol. 2008;64(6):602–617. doi: 10.1002/ana.21595. [DOI] [PubMed] [Google Scholar]
- Muftuoglu M, Sharma S, Thorslund T, Stevnsner T, Soerensen MM, Brosh RM, Jr, Bohr VA. Cockayne syndrome group B protein has novel strand annealing and exchange activities. Nucleic Acids Res. 2006;34(1):295–304. doi: 10.1093/nar/gkj410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa Y, Sasaki K, Mitsutake N, Matsuse M, Shimada M, Nardo T, Takahashi Y, Ohyama K, Ito K, Mishima H, Nomura M, Kinoshita A, Ono S, Takenaka K, Masuyama R, Kudo T, Slor H, Utani A, Tateishi S, Yamashita S, Stefanini M, Lehmann AR, Yoshiura K, Ogi T. Mutations in UVSSA cause UV-sensitive syndrome and impair RNA polymerase IIo processing in transcription-coupled nucleotide-excision repair. Nat Genet. 2012;44(5):586–592. doi: 10.1038/ng.2229. [DOI] [PubMed] [Google Scholar]
- Nance MA, Berry SA. Cockayne syndrome: review of 140 cases. Am J Med Genet. 1992;42(1):68–84. doi: 10.1002/ajmg.1320420115. [DOI] [PubMed] [Google Scholar]
- Orren DK, Dianov GL, Bohr VA. The human CSB (ERCC6) gene corrects the transcription-coupled repair defect in the CHO cell mutant UV61. Nucleic Acids Res. 1996;24(17):3317–3322. doi: 10.1093/nar/24.17.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena SD, Shokeir MH. Autosomal recessive cerebro-oculo-facio-skeletal (COFS) syndrome. Clin Genet. 1974;5(4):285–293. doi: 10.1111/j.1399-0004.1974.tb01695.x. [DOI] [PubMed] [Google Scholar]
- Revet I, Feeney L, Tang AA, Huang EJ, Cleaver JE. Dysmyelination not demyelination causes neurological symptoms in preweaned mice in a murine model of Cockayne syndrome. Proc Natl Acad Sci U S A. 2012;109(12):4627–4632. doi: 10.1073/pnas.1202621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwertman P, Lagarou A, Dekkers DH, Raams A, van der Hoek AC, Laffeber C, Hoeijmakers JH, Demmers JA, Fousteri M, Vermeulen W, Marteijn JA. UV-sensitive syndrome protein UVSSA recruits USP7 to regulate transcription-coupled repair. Nat Genet. 2012;44(5):598–602. doi: 10.1038/ng.2230. [DOI] [PubMed] [Google Scholar]
- Sonmez FM, Celep F, Ugur SA, Tolun A. Severe form of Cockayne syndrome with varying clinical presentation and no photosensitivity in a family. J Child Neurol. 2006;21(4):333–337. doi: 10.1177/08830738060210041601. [DOI] [PubMed] [Google Scholar]
- Sugita K, Takanashi J, Ishii M, Niimi H. Comparison of MRI white matter changes with neuropsychologic impairment in Cockayne syndrome. Pediatr Neurol. 1992;8(4):295–298. doi: 10.1016/0887-8994(92)90369-a. [DOI] [PubMed] [Google Scholar]
- Tinsa F, Bellalah M, Brini I, Bousnina D, Lehmann A, Boussetta K, Bousnina S. Infantile onset of Cockayne syndrome without photosensitivity in a Tunisian girl. Tunis Med. 2009;87(12):877–879. [PubMed] [Google Scholar]
- van Bokhoven H. Genetic and epigenetic networks in intellectual disabilities. Annu Rev Genet. 2011;45:81–104. doi: 10.1146/annurev-genet-110410-132512. [DOI] [PubMed] [Google Scholar]
- van Gool AJ, Citterio E, Rademakers S, van Os R, Vermeulen W, Constantinou A, Egly JM, Bootsma D, Hoeijmakers JH. The Cockayne syndrome B protein, involved in transcription-coupled DNA repair, resides in an RNA polymerase II-containing complex. EMBO J. 1997;16(19):5955–5965. doi: 10.1093/emboj/16.19.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderver A, Hathout Y, Maletkovic J, Gordon ES, Mintz M, Timmons M, Hoffman EP, Horzinski L, Niel F, Fogli A, Boespflug-Tanguy O, Schiffmann R. Sensitivity and specificity of decreased CSF asialotransferrin for eIF2B-related disorder. Neurology. 2008;70(23):2226–2232. doi: 10.1212/01.wnl.0000313857.54398.0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhooren V, Dewaele S, Libert C, Engelborghs S, De Deyn PP, Toussaint O, Debacq-Chainiaux F, Poulain M, Glupczynski Y, Franceschi C, Jaspers K, van der Pluijm I, Hoeijmakers J, Chen CC. Serum N-glycan profile shift during human ageing. Exp Gerontol. 2010;45(10):738–743. doi: 10.1016/j.exger.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Yoo AS, Crabtree GR. ATP-dependent chromatin remodeling in neural development. Curr Opin Neurobiol. 2009;19(2):120–126. doi: 10.1016/j.conb.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.