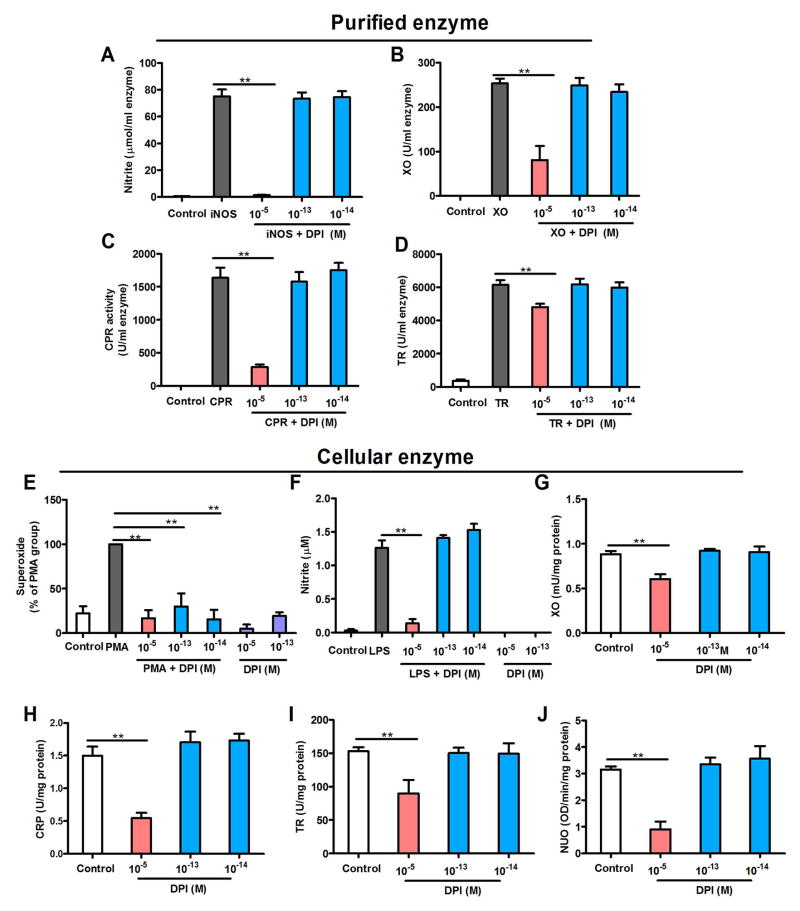
Figure 2.
Subpicomolar DPI displays specificity for NOX2. (A–D) DPI at 10−13 and 10−14 M fails to inhibit commercially purified (A) iNOS (nitrite production as an index), (B) xanthine oxidase, (C) cytochrome P450 reductase and (D) thioredoxin reductase, although DPI at micromolar concentrations decreases these enzyme activities. Data are expressed as the mean ± SEM from three to four experiments performed in duplicate. (E–J) The effects of DPI on the enzymatic activities of NOX2, iNOS, xanthine oxidase, cytochrome P450 reductase, thioredoxin reductase and NADH-ubiquinone oxidoreductase in neuron-glia cultures. (E) Cellular NOX2 activation was induced by PMA in neuron-glia cultures. Superoxide production was used as an index of NOX2 activity. The addition of 10−13 or 10−14 M DPI inhibits NOX2-generated superoxide as efficiently as micromolar concentrations, indicating NOX2 inhibition. Data are expressed as a percentage of the PMA group (mean ± SEM) from three to four experiments performed in duplicate. (F) Cellular iNOS was induced in neuron-glia cultures by incubation with LPS for 12 h. Unlike micromolar concentrations, DPI at 10−13 and 10−14 M fails to reduce the generation of iNOS-generated nitrite. (G–J) DPI at 10−5 M, but not 10−13 and 10−14 M, inhibits xanthine oxidase, cytochrome P450 reductase, thioredoxin reductase and NADH-ubiquinone oxidoreductase in neuron-glia cultures. Data are expressed as the mean ± SEM from three to four experiments performed in duplicate. The results were analyzed using one-way ANOVA, followed by Bonferroni’s post hoc multiple comparison test. **p < 0.01. XO, xanthine oxidase; CPR, cytochrome P450 reductase; TR, thioredoxin reductase (TR); NUO, NADH-ubiquinone oxidoreductase.