Abstract
Event-based prospective memory (PM) tasks require individuals to remember to perform an action when they encounter a specific cue in the environment, and have clear relevance for daily functioning for individuals with HIV. In many everyday tasks, the individual must not only maintain the intent to perform the PM task, but the PM task response also competes with the alternative and more habitual task response. The current study examined whether event-based PM can be improved by slowing down the pace of the task environment. Fifty-seven young adults living with HIV performed an ongoing lexical decision task while simultaneously performing a PM task of monitoring for a specific word (which was focal to the ongoing task of making lexical decisions) or syllable contained in a word (which was nonfocal). Participants were instructed to refrain from making task responses until after a tone was presented, which occurred at varying onsets (0–1600ms) after each stimulus appeared. Improvements in focal and non-focal PM accuracy were observed with response delays of 600ms. Furthermore, the difference in PM accuracy between the low demand focal PM task and the resource demanding non-focal PM task was reduced by half across increasingly longer delays, falling from 31% at 0ms delay to only 14% at 1600ms delay. The degree of ongoing task response slowing for the PM conditions, relative to a control condition that did not have a PM task and made lexical decisions only, also decreased with increased delay. Overall, the evidence indicates that delaying the task responses of younger HIV-infected adults increased the probability that the PM relevant features of task stimuli were adequately assessed prior to the ongoing task response, and by implication that younger HIV infected adults can more adequately achieve PM goals when the pace of the task environment is slowed down.
Keywords: HIV/AIDS, Prospective memory, Executive functions, Response delay, Cue focality
Many activities in our daily lives depend on our ability to defer intended actions and remember to perform them at a certain time or when a specific event is encountered, a neurocognitive function referred to as prospective memory (PM). PM is commonly conceptualized as having two distinct sub-constructs, time- and event-based PM, which are based on the type of cue that triggers the performance of the delayed intention. Time-based PM tasks require individuals to remember to perform an action at a specific time and is thought to rely heavily on resource-demanding monitoring processes (Einstein & McDaniel, 2005). For example, an individual may need to remember to take a medication dose at 9:30pm. Event-based PM tasks, on the other hand, require individuals to remember to perform an action when they encounter an environmental cue, and rely on more on a mixture of resource-demanding processes (Smith, 2003) and automatic processes (McDaniel & Einstein, 2000). For example, an individual may form the intention to refill a prescription on the way home from work when passing the pharmacy. Across time- and event-based cues, PM depends on the integrity of both prefrontal (e.g., Brodmann’s area 10; Burgess, Gonen-Yaacovi, & Volle, 2011) and temporolimbic (e.g., Gordon, Shelton, Bugg, McDaniel, & Head, 2011) systems and has clear relevance for successful daily functioning outcomes in a variety of clinical populations.
To that end, there has been growing interest in the neurocognitive mechanisms and real-world impact of PM in adults living with HIV disease (Carey et al., 2006). Although HIV-infected persons show slightly greater deficits in time-based as compared to event-based PM (Martin et al., 2007; Zogg et al., 2011), the latter are nevertheless prevalent and can adversely affect real-world outcomes (e.g., medication adherence; Woods et al., 2009). In addition, although HIV-associated deficits in event-based PM are generally moderate in magnitude (Carey et al., 2006; Rosenthal et al., 2013; Zogg et al., 2011; cf. Martin et al., 2007), they tend to be exacerbated by increasing the strategic demands of the PM task (Woods et al., 2010; Woods et al., in press). In terms of its neurocognitive correlates, event-based PM deficits in HIV infection are most prevalent among persons with HIV-associated neurocognitive disorders (HAND, i.e., global neurocognitive dysfunction; Zogg et al., 2011) and are related to impairments in retrospective memory, executive functions, and information processing speed (Zogg et al., 2011). Of clinical relevance, event-based PM deficits are independently associated with higher rates of healthcare non-compliance (Zogg et al., 2010), medication non-adherence (Contardo, Black, Beauvais, Dieckhaus, & Rosen, 2009), dependence in instrumental activities of daily living (Woods et al., 2008), and unemployment (Woods et al., 2011) in HIV. As such, there is a need to understand the cognitive mechanisms of event-based PM in HIV in an effort to inform the development of effective interventions to improve PM and thus optimize real-world outcomes for persons living with HIV infection.
With an eye toward that goal, this study draws upon two influential theories of PM, the Multi-Process View (MPV; Einstein & McDaniel, 2005) and the Preparatory Attentional and Memory (PAM) theory (Smith, 2003), to help determine whether event-based PM is amenable to improvement in younger persons living with HIV. Briefly, MPV posits that the cognitive demands of PM retrieval fall along a continuum from automatic to highly strategic that varies according to the specific parameters of the cue, intention, and ongoing task. According to MPV, event-based PM tasks are generally less strategically demanding than time-based tasks; however, there is considerable heterogeneity in the resource demands and difficulty of event-based PM tasks. A scientifically reliable example of such heterogeneity in event-based PM is evident in the distinction between focal and non-focal PM tasks. An event-based PM task is considered “focal” if the cue to respond is central to the cognitive processing of the ongoing task (e.g., remembering to buy stamps during a planned trip to the post-office) and “non-focal” if the ongoing task does not direct one’s attention to the relevant features of a PM target (e.g., remembering to buy stamps when withdrawing cash at the automated teller machine). In the laboratory, the focal/non-focal distinction is often examined using the Einstein and McDaniel (1990) PM experimental paradigm. In this approach, if the PM task is to respond to a specific target word (i.e., cue) that appears during a lexical decision making task, then this constitutes a focal PM task because it is central to the ongoing process of deciding whether letter strings are words or not. In contrast, the PM task of detecting specific target syllables (e.g., “tor”) would be a non-focal PM cue since identifying isolated syllables is not a central aspect of deciding whether a string of letters constitutes a word (i.e., the ongoing task). Although there is some disagreement between MPV and the Preparatory Attentional and Memory theory (Smith, 2003) about the extent to which focal tasks utilize cognitive resources during the ongoing task (Smith, Hunt, McVay, & McConnell, 2007), it is generally agreed that non-focal PM tasks are more cognitively demanding. PAM theory refers to these resource demanding processes as “preparatory attention”. Evidence for the demanding nature of non-focal PM is provided by slower response times (i.e., “costs”) to non-PM target trials (i.e., trials not requiring a PM response; Smith, 2003), and deficits in PM accuracy for populations in which executive control resources are diminished, such as older adults (e.g., Kliegel, Jäger, & Phillips, 2008), Parkinson’s disease (e.g., Foster, McDaniel, Repovs, & Hershey, 2009), and depression (e.g., Li, Loft, Weinborn, & Mayberry, in press).
In the current study we were interested in examining event-based PM situations in which individuals need to remember to make an atypical behavioural response instead of a more habitual behavioural response. This specific type of event-based PM task is common in everyday life (Dismukes, 2012; Reason, 1990). For example, most people have had the experience of needing to remember to stop at the store when driving home (requiring a left turn at a particular intersection), but instead habitually turn right, as this is the usual route home. Most people have also had the experience of going into a room intending to perform some action and, perhaps distracted, quickly performing a habitual task instead of the intended task. This specific type of event-based PM task is also of clear relevance to younger HIV-infected adults who are faced with the challenges of multiple competing demands in the context of developing independence in everyday functioning (e.g., household management, employment) while simultaneously managing a chronic disease (e.g., attending medical appointments, adhering to medication regimens), often in the context of major psychosocial stressors (e.g., stigma, lower socioeconomic status) and comorbidities (e.g., substance use). For instance, the realization of the intention to engage in safe sex (e.g., condoms) and drug use (e.g., clean needles) behaviors can require the disengagement from habitual, highly engaging and rewarding risk behaviors (Weinborn, Bucks, Stritzke, Leighton, & Woods, 2013).
According to MPV and PAM theory, event-based PM may be improved in this specific situation if the pace at which behavioural responses are made can be slowed down. That is, individuals may be more likely to be able to recognize the situation as requiring an atypical PM response, instead of the more habitual response, if provided more processing time. This has direct relevance to everyday life to the extent that individuals are in control of the speed at which they process events in their daily lives and thus can operate at a slower pace. For example, if you intend to give a message to a friend the next time you see him or her, you potentially have the full length of the encounter with that friend to remember your intention. Or, in the context of HIV disease among younger adults, providing increased organizational efficiencies and psychosocial supports for newly developing household activities and skills may increase resources available to attend to cues for healthcare activities, such as medication taking behaviors.
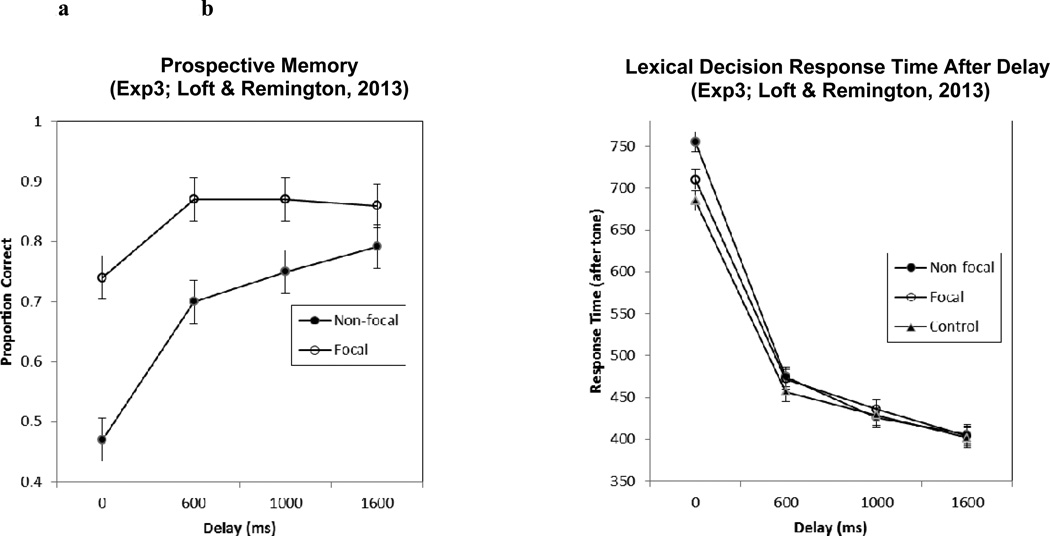
In this study, we evaluate this question by using an experimental delayed response PM paradigm developed by Loft and Remington (2013). This paradigm aims to examine whether difficulties in remembering to perform non-focal PM tasks are due -- at least in part -- to the competing rapid response demands of the more habitual and speeded ongoing task. In an effort to reduce the competing demands, Loft and Remington delayed participant task responses by presenting a tone at varying onsets (0–1600ms) following stimulus presentation that indicated when participants were allowed to make a task response. The Loft and Remington participants were 72 undergraduates (49 females), with a mean age of 18.6 years, from an Australian University. As shown in Figure 1, non-focal PM accuracy was 27% worse than focal PM at the 0ms task response delay (i.e., no task delay), as would be predicted by the MPV and PAM. However, non-focal and focal PM did not differ significantly when task responses were delayed by 1600ms. Loft and Remington argued that delaying task responses increased the probability that the non-focal PM features of stimuli were adequately assessed prior to the ongoing task response. As shown in the right panel of Figure 2, costs to the ongoing task were observed for non-focal, but not focal, PM conditions compared to control conditions (no PM task) at 0ms delay. However, these costs were no longer significant at delays of 600ms or longer, suggesting that the improved PM accuracy with longer delays may be a function of a reduction in the cognitive demands of the PM task. Thus, Loft and Remington demonstrated that slowing down the rate at which one responds to an ongoing task improves PM performance, especially on the more strategically demanding non-focal PM task by allowing for additional time to detect the PM cue.
Figure 1.
Prospective memory performance (Figure 1a; left panel) and response time to the ongoing task (Figure 1b; right panel) as a function of condition and delay in Experiment 3 of the Loft and Remington (2013) study. In this graph and the subsequent graph, error bars represent within-subjects standard error and were calculated following the suggestions by Masson & Loftus (2003; Loftus & Masson, 1994). The 95% confidence intervals are approximately 2 X the illustrated SEs (Masson & Loftus, 2003).
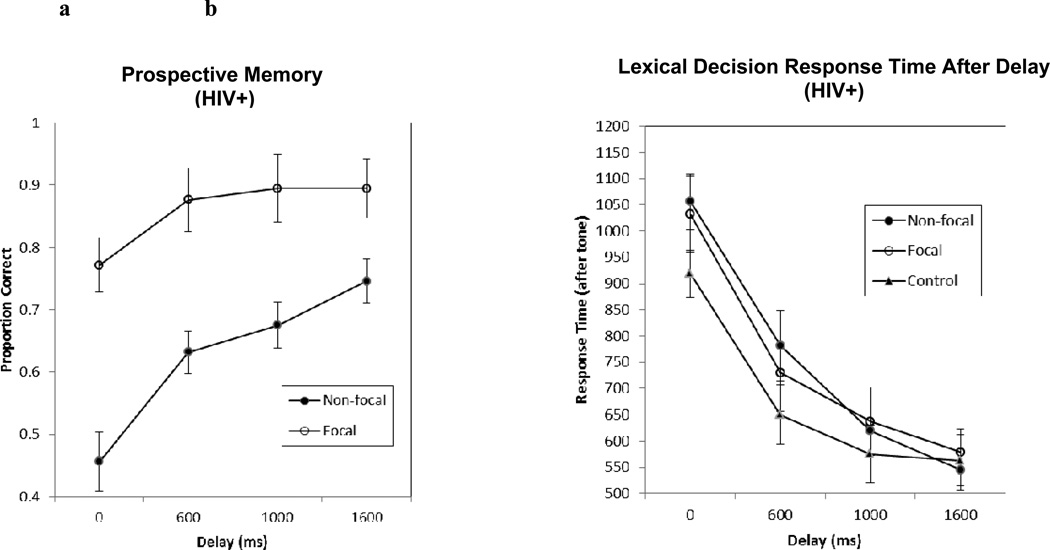
Figure 2.
Prospective memory performance (Figure 2a; left panel) and response time to the ongoing task (Figure 2b; right panel) as a function of condition and delay in the current study.
The current study applied the Loft and Remington (2013) delayed response methodology to determine whether event-based PM can be improved under laboratory conditions in a sample of 57 younger adults infected with HIV. Younger adults living with HIV infection are subject to a host of psychosocial stressors (e.g., stigma) and comorbidities (e.g., substance use; Woods et al., in press) that may influence the availability and utilization of complex cognitive processes required for effective PM. The same PM procedure used in Experiment 3 by Loft and Remington was used in the current study. Participants performed a lexical decision task while being required to make a PM response to a specific word (focal), or any word that contained a target syllable (non-focal). It was certainly not clear at the outset of this experiment whether individuals infected with HIV would be capable of utilizing response delays to improve PM accuracy. Indeed, there are many factors that influence the viability of translating experiments developed on healthy younger adults in cognitive psychology laboratories to real-world clinical populations in neuropsychology who have varying psychosocial backgrounds (e.g., socioeconomic status), neurocognitive impairments, and comorbidities that can influence outcomes (see Delis, Kaplan, & Kramer, 2003 for an elegant review of these challenges). In the case of younger adults with HIV infection, these individuals commonly come from very disadvantaged backgrounds, have high rates of confounding neuropsychiatric conditions (Nichols et al., 2013), and evidence disproportionately higher rates of HIV-associated neurocognitive impairment (Nichols et al., 2013), which can directly affect PM. We would not expect task response delays to improve PM performance if these aforementioned factors increase the likelihood that a large proportion of PM errors made by HIV+ individuals reflect cases of PM goal neglect (Duncan, Emslie, Williams, Johnson, & Freer, 1996), or momentary lapses of attention from the entire task set (Teasdale et al., 1995). That is, our response delays will only benefit PM if the infrequently executed PM task goal is being maintained at some minimum required level—in other words, that participants are actively bearing in mind that the PM task is something to be achieved. If this is the case, we expected to observe an interaction between condition (focal vs. non-focal) and response delay (0ms, 600ms, 1000ms, 1600ms) for PM accuracy and response time costs to the lexical decision task, with a steeper PM improvement and lower costs in non-focal PM (vs. focal PM) across increasingly long response delays.
Method
Participants
This study was approved by the human research protections program at the University of California, San Diego and Wayne State University. The eligible study sample included 60 young adults with HIV infection who were recruited from urban HIV clinics in San Diego (n = 30) and Detroit (n = 30). Inclusion criteria were HIV infection (determined by enzyme linked immunosorbent assay and confirmed by Western blot test) and the ability to provide informed consent on the day of evaluation. We excluded potential subjects with psychotic disorders or neurological conditions known to adversely affect cognition (e.g., seizure disorder, traumatic brain injury with loss of consciousness ≥15 min). Three participants’ PM data were not recorded correctly, leaving 57 individuals with complete data who comprised the final sample. Study participants’ demographic and clinical characteristics are displayed in Table 1.
Table 1.
Characteristics of the 57 Younger HIV+ Study Participants
| Characteristic | HIV+ (N=57) |
|---|---|
| Demographics | |
| Age (years) | 22.6 (1.3) |
| Education (years) | 12.1 (1.3) |
| Estimated verbal IQ (WTAR reading) | 89.9 (13.8) |
| Sex (% male) | 86.0 |
| Ethnicity (%) | |
| Caucasian | 12.3 |
| African-American | 56.1 |
| Hispanic | 29.8 |
| Other | 1.8 |
| Global Severity Index (% elevated)a | 33.3 |
| Somatization | 31.6 |
| Depression | 31.6 |
| Anxiety | 33.3 |
| Substance Use Disorder (%)b | |
| Any | 70.2 |
| Alcohol | 66.7 |
| Marijuana | 56.2 |
| Cocaine | 5.3 |
| Methamphetamine | 17.6 |
| Inhalants | 3.5 |
| Sedatives | 7.0 |
| Opioids | 7.0 |
| Other | 1.8 |
| HIV Disease | |
| HIV-associated neurocognitive disorder (%) | 73.7 |
| Estimated duration of infection (mos.)c | 34 (18, 52) |
| Nadir CD4 T-cell count (cells/µl)c | 288 (235, 423) |
| Current CD4 T-cell count (cells/µl)c | 539 (369, 671) |
| Currently Immunosuppressed (% CD4 < 200) | 5.0 |
| AIDS status (%) | 20.0 |
| Plasma HIV RNA (% detectable) | 50.0 |
| ART Use (%) | 77.2 |
Note.
Based on the Brief Symptom Inventory (BSI; Derogatis, 1993)
Based on meeting at least minimal risk criteria for substance use disorder over the past three months as measured by the Alcohol, Smoking, and Substance Use Involvement Screening Test (ASSIST v. 3.0).
Data are presented as medians and interquartile ranges.
ART = Antiretroviral therapy. WTAR = Wechsler Test of Adult Reading.
Materials and Procedure
After providing written, informed consent, participants completed the Loft and Remington (2013) experiment as part of a brief neurocognitive evaluation that included the Wechsler Test of Adult Reading (WTAR; Psychological Corporation, 2001) and a neuropsychiatric assessment that included the Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST; World Health Organization, 2002) and the Brief Symptom Inventory (BSI; Derogatis, 1993). HIV disease and treatment variables were extracted from clinic charts.
Neuropsychological Assessment
Participants also completed a relatively brief neurocognitive battery that covered many of the essential domains recommended by the Frascati group (Antinori et al., 2007) for diagnosis of HIV-associated Neurocognitive Disorders (HAND), including: 1) Retrospective learning and memory as measured by the Hopkins Verbal Learning Test – Revised (HVLT-R; Brandt & Benedict, 2001) and Brief Visuospatial Memory Test – Revised (BVMT-R; Benedict, 1997); 2) Executive functions as measured by Tower of London – Drexel Version (Culbertson & Zillmer, 2001), Trailmaking Test Part B (Army Individual Test Battery, 1944; Heaton, Miller, Taylor, & Grant, 2004), and the Color Word Interference Test from the Delis–Kaplan Executive Function System (DKEFS; Delis, Kaplan, & Kramer, 2001); 3) Information processing speed as measured by Trailmaking Test Part A (Heaton et al., 2004), Symbol Digit Modalities Test (SDMT; Smith, 1982); and 4) Motor skills as measured by the Grooved Pegboard Test (Heaton et al., 2004; Klove, 1963). All tests were administered and scored according to the publication manuals. Raw scores were converted to demographically-adjusted T-scores using the best available normative standards correcting for age, education, gender, and ethnicity, as available and appropriate (referenced above). In order to classify subjects with HAND we used procedures that are well established and validated in neuroAIDS (Carey et al., 2004). Specifically, we converted T-scores to deficit scores (range 0 – 5), which were then averaged to generate a global deficit score (GDS) for which a standard cutpoint of .5 was used to determine HAND.
Prospective Memory Experiment
We used the same PM procedure as Experiment 3 by Loft and Remington (2013). A pool of 576 medium-typicality words (occurring 20–50 times per million) was randomly selected from the 1994 issues of the Sydney Morning Herald word database (Dennis, 1995). Of these, 276 were used as words. The remaining 300 were converted to non-words by randomly replacing every vowel in each word. For the study herein, a small number of items from the original Loft and Remington study were replaced as some of the Australian English words were not applicable for American English-speaking participants.
From this pool of words and non-words, three lists were created (A, B, and C), with each containing 92 words and 100 non-words (serving as non-PM items). Three cue syllables (i.e., fer, tor, and ver) were selected to create three lists of PM cue words. Twenty-four medium frequency words with one of the syllables were then selected from the TSMH database (Dennis, 1995) as cues. In Set A, the cue syllable was fer (i.e., feral, ferry, offering, prefer, refer, referee, suffer, transfer). In Set B, the cue syllable was tor (i.e., victory, actor, investor, editor, historic, factory, operator, monitor). In Set C, the cue syllable was ver (i.e., coverage, verdict, poverty, deliver, clever, forever, version, recover).
The presentation of words and non-words within each list was random, except that PM cues were presented in a random position between trials 5–25, 26–50, 51–75, 76–100, 101– 125, 126–150, 151–175, and 176–200. Under focal conditions, participants were presented with one cue from the relevant set eight times, with each of the cues from each PM cue list used equally as often as a focal cue across participants. Under non-focal conditions, participants were presented with one syllable cue word in each allocated cue trial position. Overall, participants under non-focal conditions were presented with all eight cues from their designated PM cue list. Under control conditions, participants were also presented with each of eight cues in the allocated cue trial positions, but these exemplars held no special significance, as participants were not instructed to attend to them during this baseline task.
The order of condition presentation (control, focal, and non-focal) and lists (A, B, and C) was counterbalanced across condition. Four delays (0ms, 600ms, 1,000ms, and 1,600ms) were randomly assigned to non-cue trials, and each tone delay was presented equally often. The tone delay presented on cue trials was manipulated; for the first four presentations of cues, each tone was presented once in a random order, and in the next four presentations of cues, each tone was presented once again in a random order. The assignment of cues to trial positions and the assignment of tones to cues were yoked across the three within-subject conditions.
Procedure
Each trial contained two displays. The first was a focus point “+”, displayed in white on a black background at the centre of the screen for 250ms. The focus point was then replaced by a string of letters. A 500-Hz tone was then played 0ms, 600ms, 1,000ms or 1,600ms after the letter string presentation. Participants were instructed to decide whether letter strings were English words or non-words by pressing one of two keys (“F” for a word, “J” for a non-word). Participants were told to respond as quickly and as accurately as possible after hearing the tone that followed the letter string presentation and the letter strings remained on the screen until a valid response was made. If responses were made before the tone, a message was displayed reminding participants to wait for the tone. A block of 24 practice trials was completed before the three blocks of experimental trials. The primary outcome measures from the lexical decision task were accuracy (i.e., proportion of items correctly identified as words and non-words) and response time (i.e., time from the tone presentation to the response)
Under control conditions, participants performed the lexical decision task only. Under PM conditions, participants were instructed that the experimenter had a secondary interest in their ability to remember to perform actions in the future. Specifically, under focal conditions, participants were instructed to press “9” whenever a specific word was presented. Under non-focal conditions, participants were instructed to press “9” whenever a word with a PM cue syllable was presented. Participants completed a three-minute distractor puzzle before beginning each block.
Results
Prospective Remembering
PM accuracy was defined as the proportion of PM cue trials that received a “9” response on the cue trial or the two trials that followed. Consistent with Loft and Remington (2013), late PM responses that we made on the ongoing task trial following the ongoing task trial in which the PM cue was presented, were rare (a total of two late responses were made). The proportion of false alarms (pressing the “9” key on a non-cue trial spaced at least three trials from a cue trial) was low, but significantly greater for non-focal conditions (M = .01, SD = .02) compared to focal conditions (M = .003, SD = .01), t(56) = 2.42, p = .02, d = .43. There were a total of five cue trials across participants to which a response was made before the tone. We excluded these trials from the PM analyses because they reflected cases where the full delay was not utilized by the participant.
A 2 (Condition: focal vs. non-focal) × 4 (Delay: 0ms, 600ms, 1000ms, 1600ms) repeated-measures ANOVA was conducted on the PM accuracy presented in Figure 2a. There was an effect of condition (F(1,56) = 31.11, p <.001, ηp2 = .36) and delay (F(3,213) = 16.75, p < .001, ηp2 = .23). These main effects were qualified by a significant interaction between condition and delay (F(3,168) = 5.61, p =.046, ηp2 = .05), such that the detrimental effect of the non-focal condition on PM accuracy was decreased with increasing delay. However, planned contrasts indicated higher focal PM accuracy than non-focal PM accuracy at 0ms delay (.77 vs. .46), t(56) = 6.01, p < .001, d = .93, at 600ms delay (.88 vs. .63), t(56) = 4.27, p < .001, d = .76, at 1000ms delay (89% vs. 68%), t(56) = 3.73, p < .001, d = .61, and at 1600ms delay (89% vs. 75%), t(56) = 2.89, p = .005, d = .48. Thus, although differences in PM as a function of cue focality were significantly reduced with increased delay, the difference between focal and non-focal PM accuracy remained significant, even at the longest (1600ms) delay. Follow up contrasts revealed that both non-focal (18% improvement from 0ms baseline, t(56) = 3.82, p <. 001) and focal (10% improvement from 0ms baseline, t(56) = 2.57, p = .01) PM were improved by a 600ms delay compared to the baseline no delay trials. However, as indicated in Figure 2a, focal PM accuracy did not further improve beyond 600ms+ delays (1000ms: t(56)= 2.60, p = 0.012; 1600ms: t(56)= 2.03, p = 0.047), whereas non-focal PM accuracy continued to improve with 600ms+ delays (1000ms: t(56)= 3.97, p < 0.001; 1600ms: t(56)= 5.44, p < 0.001).
Ongoing Task Performance
For the lexical decision task analyses, we excluded PM cue trials and PM false alarms, as well as the four trials that followed cues. Analyses of response times were based on word trials only (Loft & Remington, 2013). We excluded incorrect lexical decisions, and trials in which responses were made before the tone. Following the recommendations made by Ratcliff (1993), and the associated response time data trimming procedures used in prior PM research (e.g., Einstein et al. 2005; Loft & Humphreys, 2012; Marsh, Hicks, & Cook, 2005), we also excluded trials with response times greater than three standard deviations from a participant’s grand mean for that delay (Loft & Remington, 2013).
A 3 (Condition: focal, non-focal, control) × 4 (Delay: 0ms, 600ms, 1000ms, 1600ms) repeated-measures ANOVA was conducted on lexical decision accuracy and response time. There was no effect of condition (F(2, 112) = 1.25, p = .29) and no significant interaction (F(6, 336) = 1.41, p = .21) for lexical decision accuracy. There was a significant effect of delay (F(3, 168) = 3.81,. p = .01, ηp2 = .06), with lexical decision accuracy increasing with increased delay (M = 89%, 91%, 90%, 91%, for 0ms, 600ms, 1000ms, and 1600ms, respectively). This small (2%) benefit of increased delay to lexical decision accuracy likely reflects a lexical decision speed-accuracy trade-off, whereby slowing lexical decision responses improved lexical decision accuracy (see Wagenmakers, Ratliff, Gomez, & McKoon, 2008).
Response times to word trials across the four delay conditions are presented in Figure 2b. Results revealed main effects of condition (F(2, 112) = 3.55, p = .03, ηp2 = .06) and delay (F(3, 168) = 113.08, , p <.001, ηp2 = .67). These main effects were qualified by a marginally significant interaction between condition and delay (F(6, 336) = 2.13, p = .05, ηp2 = .04). As illustrated in Figure 2b, this interaction between condition and delay indicates that the increased response times for the focal PM and non-focal PM conditions, relative to the control condition, decreased with increased delay. We compared response times under non-focal conditions to those under control conditions, and focal conditions to control conditions, to examine whether costs were significant at each delay. These planned contrasts indicated that response times at 0ms delay were slower under non-focal compared to control conditions (1056ms vs. 919 ms, respectively), t(56) = 3.81, p < .001, d = .37, and under focal compared to control conditions (1032ms vs. 919ms, respectively), t(56) = 2.66, p = .01, d = .25. At 600ms delay, response times were slower for non-focal compared to control conditions (783ms vs. 650ms, respectively), t(56) = 2.81, p = .01, d = .28, and were marginally slower under focal compared to control conditions (730ms vs. 650ms), t(56) = 1.95, p = .06. There were no differences in response time at 1000ms delay between non-focal and control conditions (619ms vs. 575ms), or between focal and control conditions (637ms vs. 575ms) (ps >.10). Similarly, there were no differences in response time at 1600ms delay between non-focal and control conditions (545ms vs. 563ms), or between focal and control conditions, (579ms vs. 563ms) (ps >.10). Thus, costs to ongoing tasks (i.e., increased response time for PM conditions compared to control conditions) was observed at 0ms delay and 600ms delay, but not at 1000ms delay or at 1600ms delay, when participants were under both non-focal and focal PM conditions.
Discussion
HIV-associated deficits in PM are common and adversely impact health outcomes. We focused on a particular type of event-based PM task situation where individuals need to remember to substitute an atypical (PM) action instead of a more habitual (speeded) ongoing task response. Results from the present study represent an important first step in determining how PM may be enhanced under these laboratory conditions. Specifically, we observed that younger HIV-infected adults were capable of utilizing externally imposed response delays to improve PM performance and to eliminate costs (i.e., slowed response times) to ongoing tasks. That is, we found that although HIV+ participants overall made more PM errors under the non-focal condition, their accuracy increased when they were instructed to delay task responses, consistent with what has been observed in healthy younger adults (Loft & Remington, 2013). Specifically, the difference in PM accuracy between the low (i.e., focal) versus the high (i.e., non-focal) strategic demand conditions decreased by half across increasingly longer delays, falling from 31% at 0ms delay to only 14% at 1600ms delay. It is likely that delaying task responses increased the probability that the non-focal PM features of the stimuli were adequately assessed prior to an ongoing task response. Crucially, this finding suggests that PM task goals were maintained to at least some degree by HIV participants on a trial-by trial basis, the realization of which was greatly enhanced by providing cognitive supports (i.e., slowing down the response time requirements of the ongoing task). The present findings converge with a recent study from Iudicello and colleagues (2012), which showed that the provision of structured semantic cues improved HIV-associated deficits in verbal fluency, presumably as a function of reducing demands on speeded strategic search and retrieval processes. In contrast to Loft and Remington (2013), however, in the current study there remained a modest effect of cue focality on PM error even at the 1600ms delay, which suggests that the 1600ms response delay did not completely resolve the competing cognitive demands of the ongoing task. It remains to be determined whether even longer delays (>2000ms), perhaps along with corresponding instructions on effective cue detection strategies, would further improve non-focal PM performance in HIV to such a degree that it could be as accurate as focal PM.
It is interesting to note that although both non-focal and focal PM performance showed considerable improvement with the 600ms delay, such initial improvements in focal PM accuracy were not appreciated at longer delays as they were with non-focal PM. One possibility is that by virtue of its relatively lesser strategic demands, focal PM essentially “normalized” with the modest cognitive support provided by the 600ms delay. Indeed, the focal PM accuracy of this HIV cohort at longer delays was in the range of 90%, which is broadly on par with the healthy young, well-educated Australian sample in the Loft and Remington (2013) experiment. It is unlikely that providing delays longer than 1600ms would further improve focal PM performance in HIV, given this accuracy plateau. The approximate 11% error rate for focal PM at delays greater than 600ms are unlikely to have resulted from to the competing rapid response demands of the ongoing task, but more likely reflect cases where HIV individuals truly ‘forgot’ the PM task requirements or suffered moments of inattention from the entire task set (Duncan et al., 1996; Teasdale et al., 1995), or where HIV individuals were not wholly accurate in their ability to monitor and detect PM cues, which is also apparent in younger healthy samples (Loft & Remington, 2013).
It is nevertheless crucial that we take great care when comparing the current data to that of Loft and Remington (2013) data given the fact that we did not have a matched HIV seronegative comparison group in the current study. That is, the participants in the Loft and Remington study may have differed on characteristics other than HIV status, such as education, premorbid IQ, and confounding factors such as substance use disorders. This notwithstanding, it is notable that lexical decision response times were substantially elevated, and substantially more variable, in the current study compared to Loft and Remington and a host of other prior research using lexical decision making as an ongoing task among healthy adults (e.g., Loft & Humphreys, 2012; Smith, 2003). Lexical decision accuracy was also not at high ceiling within the HIV+ sample (M = .90), which could be a function of relatively low premorbid verbal IQ (mean = 89) as measured by the WTAR. In addition, costs to both focal and non-focal PM tasks persisted when a 600ms delay response delay period was provided to HIV+ participants. It remains to be determined whether these findings generalize to other aspects of the HIV epidemic (e.g., older adults) and PM (e.g., time-based PM, semi-naturalistic tasks).
Of secondary interest, these data also provide unique insights into the cognitive architecture of HIV-associated event-based PM deficits in younger adults. Although we employed the Loft and Remington (2013) delayed response methodology, these data may be interpreted comparably to the traditional Einstein and McDaniel (1990) event-based PM paradigm because participants could not predict which delay length would occur on a particular trial. As such, the conditions when there was no delay (0ms) are equivalent to past PM research examining PM focality effects (e.g., Loft & Humphreys, 2012). At 0ms delay, we found poorer non-focal PM compared to focal PM for our HIV+ participants. This finding fits the assumptions of the MPV model and PAM theory that non-focal PM tasks are more resource-demanding than focal PM tasks (Einstein & McDaniel, 2005; Smith, 2003), which may therefore be particularly vulnerable to disruption in the setting of HIV disease. However, in contrast to past research with healthy participants, we found significant costs (+113ms) to ongoing tasks under focal conditions compared to control conditions at no delay (0ms), and these costs were comparable to the non-focal condition when compared to the control condition at no delay (0ms) (+137ms). As illustrated in Figure 2b, with increased delay the response time costs to the ongoing task compared to the control condition decreased at similar rates for the non-focal and focal PM condition. Thus overall, we found no evidence of a difference in costs as a function of cue focality in our young HIV+ sample. This suggests that our younger HIV+ adults were not able to automatically retrieve the PM action when focal targets were processed, and thus that even the less-demanding focal PM retrieval may require greater strategic resources relative to what is required for healthy participants. At the same time, the very fact that costs were observed under PM conditions indicates that younger HIV+ adults have the capacity to allocate preparatory attentional processes (Smith, 2003) or other types of strategic monitoring (Einstein & McDaniel, 2005) to PM task requirements, as seen in healthy participants (Loft & Remington, 2013). Thus, this pattern of PM performance coalesces with prior clinical studies showing that HIV-associated deficits in event-based PM are driven by strategic rather than automatic processes, including executively-demanding aspects of encoding the cue-intention pairing (Woods et al., 2010) and cue monitoring (Doyle et al., 2013); given that these executive functions are at risk for impairment following HIV infection, degradation of such strategic processes may be the mechanism by which PM impacts real-world outcomes like antiretroviral adherence (e.g., Contardo et al., 2009).
There are several unique aspects of the study sample that warrant interpretive consideration. First and foremost, the relative youth of the cohort may influence the generalizability of these data to other subpopulations of the HIV epidemic, as this group was comprised of a high proportion of males and ethnic minorities with relatively short durations of HIV infection, nearly two-thirds of whom had detectable viral loads in plasma. Indeed there are very few studies of neurocognitive and everyday functioning outcomes in this highly vulnerable population who may experience tremendous psychosocial burdens (e.g., stigma), high neuropsychiatric comorbidity rates (e.g., substance abuse, which was 70% in the current sample), and HIV-associated neurocognitive disorders (HAND). Indeed a recent multi-site study by Nichols et al. (2013) showed that two-thirds of younger HIV+ infected persons experience HAND, especially those with lower CD4 counts and comorbid alcohol use disorders. A limitation of this study was the absence of data on important psychosocial factors (e.g., stigma, sexual orientation, and socioeconomic status) and our relatively cursory measurement of neuropsychiatric comorbidities (e.g., mood and substance use) that are clearly relevant to the daily functioning of younger HIV-infected adults. Examination of the role of important these psychosocial and neuropsychiatric factors that may be particularly influential to younger adults living with HIV infection on PM (and their moderating influence on PM’s relationship with everyday functioning outcomes) will be essential to developing effective neurocognitive interventions, particularly as this group is faced with the myriad challenges of transitioning to adulthood and functional independence.
In summary, this experiment demonstrated that brief response delays to the ongoing task can improve event-based PM performance in younger adults living with HIV disease. The notable improvements in PM using this response delay methodology may have implications for designing cognitive rehabilitation interventions for persons living with HIV disease. This is important because clinicians managing individuals with HIV-associated Neurocognitive Disorders (HAND) are presently without effective pharmacological and cognitive interventions (see Weber, Blackstone, & Woods, 2013). To that end, these data suggest that interventions such as Goal Management Training (Levine et al., 2000), in which individuals with brain injury are provided with prompts that disrupt their ongoing daily activities (similar to a response delay) and refocus their attention on intended goals (i.e., STOP! and take a deep breath and plan before beginning a new task), may be effective for improving functional outcomes such as medication adherence in HIV. Similarly, Time Pressure Management (Winkins, Van Heugten, Wade, & Fasotti, 2009) has been effective in teaching individuals to anticipate speeded tasks that will require attentional resources beyond their capacity, and assist them in pre-structuring plans to execute those tasks in order to reduce errors due to mental slowness. In practical terms, to the extent that HIV-infected persons are in control of the speed at which they perform events in their daily lives and thus can operate at a slower pace, they may appreciate an improvement in everyday event-based PM. Lastly, encouraging use of focal (i.e., task-related) cues, such as a pill bottle as an adherence reminder, may be effective in reducing real world PM errors in the context of HAND.
Acknowledgments
This research was supported by NIH grants R01DA034497 (SNK, SPW), R01MH073419 (SPW), T32DA31098 (SPW), F31-DA034510, F31-DA035708, and P30MH062512, as well as and Discovery Grant DP12010311 (SL) from the Australian Research Council.
References
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Army Individual Test Battery. Manual of directions and scoring. Washington, DC: War Department, Adjutant General’s Office; 1944. [Google Scholar]
- Benedict RHB. Brief Visuospatial Memory Test-Revised. Lutz, FL: Psychological Assessment Resources; 1997. [Google Scholar]
- Brandt J, Benedict RHB. Hopkins Verbal Learning Test—Revised. Professional manual. Lutz, FL: Psychological Assessment Resources, Inc; 2001. [Google Scholar]
- Burgess PW, Gonen-Yaacovi G, Volle E. Functional neuroimaging studies of prospective memory: What have we learnt so far? Neuropsychologia. 2011;49:2246–2257. doi: 10.1016/j.neuropsychologia.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I The HNRC Group. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. Journal of Clinical and Experimental Neuropsychology. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Heaton RK, Grant I The HNRC Group. Prospective memory in HIV-1 infection. Journal of Clinical and Experimental Neuropsychology. 2006;28:536–548. doi: 10.1080/13803390590949494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contardo C, Black AC, Beauvais J, Dieckhaus K, Rosen MI. Relationship of prospective memory to neuropsychological function and antiretroviral adherence. Archives of Clinical Neuropsychology. 2009;24:547–554. doi: 10.1093/arclin/acp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson WC, Zillmer EA. The Tower of London DX (TOL-DX) manual. North Tonawanda, NY: Multi-Health Systems; 2001. [Google Scholar]
- Delis D, Kaplan EB, Kramer J. The Delis–Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Dennis S. The Sydney Morning Herald word database. Noetica: Open Forum, 1(4) 1995 Retrieved from http://psy.uq.edu.au/CogPsych/Noetica. [Google Scholar]
- Derogatis LR. The Brief Symptom Inventory. Pearson; 1993. [Google Scholar]
- Dismukes RK. Prospective memory in workplace and everyday settings. Current Directions in Psychological Science. 2012;21:215–220. [Google Scholar]
- Doyle K, Loft S, Morgan EE, Weber E, Cushman C, Johnston E The HNRP Group. Prospective memory in HIV-associated neurocognitive disorders (HAND): The neuropsychological dynamics of time monitoring. Journal of Clinical and Experimental Neuropsychology. 2013;35:359–372. doi: 10.1080/13803395.2013.776010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Emslie H, Williams P, Johnson, Freer C. Intelligence and the frontal lobe: The organization of goal-directed behaviour. Cognitive Psychology. 1996;30:257–303. doi: 10.1006/cogp.1996.0008. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA. Normal aging and prospective memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1990;16:717–726. doi: 10.1037//0278-7393.16.4.717. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA. Prospective memory: Multiple retrieval processes. Current Directions in Psychological Science. 2005;14:286–290. [Google Scholar]
- Einstein GO, McDaniel MA, Thomas R, Mayfield S, Shank H, Morrisette N, Breneiser J. Multiple processes in prospective memory retrieval: Factors determining monitoring versus spontaneous retrieval. Journal of Experimental Psychology: General. 2005;134:327–342. doi: 10.1037/0096-3445.134.3.327. [DOI] [PubMed] [Google Scholar]
- Foster ER, McDaniel MA, Repovs G, Hershey T. Prospective memory in Parkinson disease across laboratory and self-reported everyday performance. Neuropsychology. 2009;23:347–358. doi: 10.1037/a0014692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon BA, Shelton JT, Bugg JM, McDaniel MA, Head D. Structural correlates of prospective memory. Neuropsychologia. 2011;49:3795–3800. doi: 10.1016/j.neuropsychologia.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Odessa, FL: Psychological Assessment Resources, Inc; 2004. [Google Scholar]
- Iudicello JE, Kellogg EJ, Weber E, Smith C, Grant I, Drane DL The HNRP Group. Semantic cueing improves category verbal fluency in persons living with HIV infection. Journal of Neuropsychiatry and Clinical Neurosciences. 2012;24:183–190. doi: 10.1176/appi.neuropsych.11100301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliegel M, Jäger T, Phillips LH. Adult age differences in event-based prospective memory: A meta-analysis on the role of focal versus non-focal cues. Psychology and Aging. 2008;23:203–208. doi: 10.1037/0882-7974.23.1.203. [DOI] [PubMed] [Google Scholar]
- Kløve H. Clinical neuropsychology. In: Forster FM, editor. The Medical Clinics of North America. New York: Saunders; 1963. [PubMed] [Google Scholar]
- Levine B, Robertson IH, Clare L, Carter G, Hong J, Wilson BA, Stuss DT. Rehabilitation of executive functioning: an experimental-clinical validation of goal management training. Journal of the International Neuropsychological Society. 2000;6:299–312. doi: 10.1017/s1355617700633052. [DOI] [PubMed] [Google Scholar]
- Li R, Loft S, Weinborn M, Mayberry M. Event-based prospective memory deficits in individuals with high depressive symptomatology: Problems controlling attentional resources? Journal of Clinical and Experimental Neuropsychology. doi: 10.1080/13803395.2014.918090. (in press). [DOI] [PubMed] [Google Scholar]
- Loft S, Humphreys MS. Enhanced recognition of words previously presented in a task with non-focal prospective memory requirements. Psychonomic Bulletin & Review. 2012;19:1142–1147. doi: 10.3758/s13423-012-0303-1. [DOI] [PubMed] [Google Scholar]
- Loft S, Remington RW. Brief delays in responding reduce focality effects in event-based prospective memory. The Quarterly Journal of Experimental Psychology. 2013;66:1432–1447. doi: 10.1080/17470218.2012.750677. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Masson ME. Using confidence intervals in within-subject designs. Psychonomic Bulletin & Review. 1994;1:476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Marsh RL, Hicks JL, Cook GI. On the relationship between effort toward an ongoing task and cue detection in event-based prospective memory. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2005;31:68–75. doi: 10.1037/0278-7393.31.1.68. [DOI] [PubMed] [Google Scholar]
- Martin EM, Nixon H, Pitrack DL, Weddington W, Rains NA, Nunnally G, Bechara A. Characteristics of prospective memory deficits in HIV-seropositive substance-dependent individuals: Preliminary observations. Journal of Clinical and Experimental Neuropsychology. 2007;29:496–504. doi: 10.1080/13803390600800970. [DOI] [PubMed] [Google Scholar]
- Masson ME, Loftus GR. Using confidence intervals for graphically based data interpretation. Canadian Journal of Experimental Psychology. 2003;57:203–220. doi: 10.1037/h0087426. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO. Strategic and automatic processes in prospective memory retrieval: A multiprocess framework. Applied Cognitive Psychology. 2000;14:S127–S144. [Google Scholar]
- Nichols SL, Bethel J, Garvie PA, Patton DE, Thornton S, Kapogiannis BG, Woods SP. Neurocognitive functioning in antiretroviral therapy-naïve youth with behaviorally acquired human immunodeficiency virus. Journal of Adolescent Health. 2013;53:763–771. doi: 10.1016/j.jadohealth.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychological Corporation. Wechsler Test of Adult Reading. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- Ratcliff R. Methods for dealing with reaction time outliers. Psychological Bulletin. 1993;114:510–532. doi: 10.1037/0033-2909.114.3.510. [DOI] [PubMed] [Google Scholar]
- Reason J. Human error. New York: Cambridge University Press; 1990. [Google Scholar]
- Rosenthal LS, Skolasky RL, Moxley RT, 4th, Roosa HV, Selnes OA, Eschman A, Sacktor N. A novel computerized functional assessment for human immunodeficiency virus-associated neurocognitive disorder. Journal of NeuroVirology. 2013;19:432–441. doi: 10.1007/s13365-013-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. Symbol Digit Modalities Test manual. Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- Smith RE. The cost of remembering to remember in event-based prospective memory: Investigating the capacity demands of delayed intention performance. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:347–361. doi: 10.1037/0278-7393.29.3.347. [DOI] [PubMed] [Google Scholar]
- Smith RE, Hunt RR, McVay JC, McConnell MD. The cost of event-based prospective memory: Salient target events. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2007;33:734–746. doi: 10.1037/0278-7393.33.4.734. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Dritschel BH, Taylor MJ, Proctor L, Lloyd CA, Nimmo-Smith I, Baddeley AD. Stimulus-independent thought depends on central executive resources. Memory & Cognition. 1995;23:551–559. doi: 10.3758/bf03197257. [DOI] [PubMed] [Google Scholar]
- Wagenmakers EJ, Ratcliff R, Gomez P, McKoon G. A diffusion model account of criterion shifts in the lexical decision task. Journal of Memory and Language. 2008;58:140–159. doi: 10.1016/j.jml.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E, Blackstone K, Woods SP. Cognitive neurorehabilitation of HIV-associated neurocognitive disorders: A qualitative review and call to action. Neuropsychology Review. 2013;23:81–98. doi: 10.1007/s11065-013-9225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinborn M, Bucks R, Stritzke W, Leighton A, Woods SP. Time-Based prospective memory predicts engagement in risk behaviors amongst substance users: Results from clinical and nonclinical samples. Journal of the International Neuropsychological Society. 2013;19:284–294. doi: 10.1017/S1355617712001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO ASSIST Working Group. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction. 2002;97:1183–1194. doi: 10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- Winkens I, Van Heugten CM, Wade DT, Fasotti L. Training patients in Time Pressure Management, a cognitive strategy for mental slowness. Clinical Rehabilitation. 2009;23:79–90. doi: 10.1177/0269215508097855. [DOI] [PubMed] [Google Scholar]
- Woods SP, Dawson MS, Weber E, Gibson S, Grant I, Atkinson JH The HNRC Group. Timing is everything: Antiretroviral non-adherence is associated with impairment in time-based prospective memory. Journal of the International Neuropsychological Society. 2009;15:42–52. doi: 10.1017/S1355617708090012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Dawson MS, Weber E, Grant I The HNRC Group. The semantic relatedness of cue-intention pairings influences event-based prospective memory failures in older adults with HIV infection. Journal of Clinical and Experimental Neuropsychology. 2010;32:398–407. doi: 10.1080/13803390903130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Doyle KL, Morgan EE, Naar-King S, Outlaw AY, Nichols SL, Loft S. Journal of the International Neuropsychological Society. doi: 10.1017/S1355617714000435. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Iudicello JE, Moran LM, Carey CL, Dawson MS, Grant I The HNRC Group. HIV-associated prospective memory impairment increases risk of dependence in everyday functioning. Neuropsychology. 2008;22:110–107. doi: 10.1037/0894-4105.22.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Weber E, Weisz B, Twamley EW, Grant I the HNRP Group. Prospective memory deficits are associated with unemployment in persons living with HIV infection. Rehabilitation Psychology. 2011;56:77–84. doi: 10.1037/a0022753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zogg JB, Woods SP, Weber E, Doyle K, Grant I The HNRP Group. Are time- and event-based prospective memory comparably affected in HIV infection? Archives of Clinical Neuropsychology. 2011;26:250–259. doi: 10.1093/arclin/acr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zogg JB, Woods SP, Weber E, Iudicello JE, Dawson MS, Grant I The HNRC Group. HIV-associated prospective memory impairment in the laboratory predicts failures on a semi-naturalistic measure of health care compliance. The Clinical Neuropsychologist. 2010;24:945–962. doi: 10.1080/13854046.2010.501343. [DOI] [PMC free article] [PubMed] [Google Scholar]