Abstract
Background and Objectives
Serologic screening for the major transfusion transmissible viruses (TTV) is critical to blood safety and has been widely implemented. However, actual performance as measured by proficiency testing has not been well studied in Sub-Saharan Africa. Therefore, we conducted an external quality assessment of laboratories engaged in transfusion screening in the region.
Materials and Methods
Blinded test panels, each comprising 25 serum samples that were pedigreed for HIV, HBsAg, HCV and negative status, were sent to participating laboratories. The panels were tested using the laboratories’ routine donor screening methods and conditions. Sensitivity and specificity were calculated and multivariable analysis was used to compare performance against mode of testing, country and infrastructure.
Results
A total of 12 African countries and 44 laboratories participated in the study. The mean (range) sensitivities for HIV, HBsAg and HCV were 91.9% (14.3-100), 86.7% (42.9-100) and 90.1% (50-100), respectively. Mean specificities for HIV, HBsAg and HCV were 97.7%, 97% and 99.5% respectively. After adjusting for country and infrastructure, rapid tests had significantly lower sensitivity than enzyme immunoassays (EIA) for both HBsAg (p<0.0001) and HCV (p<0.05). Sensitivity also varied by country and selected infrastructure variables.
Conclusion
While specificity was high, sensitivity was more variable and deficient in a substantial number of testing laboratories. These findings underscore the importance of proficiency testing and quality control, particularly in Africa where TTV prevalence is high.
Keywords: Blood transfusion, laboratory proficiency testing, Africa, HIV, Hepatitis B surface antigens, Hepatitis C antibodies
INTRODUCTION
Proficiency testing is critical to ensure that laboratory test results are indeed valid; this is particularly important to blood banking. The importance of an external quality assessment (EQAS) of laboratory performance is evident in the World Health Organization’s (WHO) recommendation that proficiency testing be implemented globally [1]. This has already been adopted in high and middle-income countries where laboratory accreditation is often contingent upon an external evaluation of laboratory performance.
In contrast, there are limited examples of proficiency testing in Africa, particularly related to blood transfusion. Instead, proficiency testing in Africa has largely focused on clinical infectious disease testing such as examination of peripheral blood smears for detection of malaria and other blood-borne parasites, serological testing for HIV, laboratory diagnosis of tuberculosis and staining techniques for identification of bacteria [2-4]. Barriers to wider implementation of proficiency testing in Africa include cost, logistics, a lack of skilled personnel and the required infrastructure to establish systems of external evaluation [5, 6].
Over the past decade, there has been considerable external funding and technical assistance for transfusion services in Africa. Both the President’s Emergency Plan For AIDS Relief (PEPFAR) and the World Health Organization’s (WHO) regional strategy of “Safe Blood by 2012” have been catalytic in this regard. [7] The latter identified key areas of deficiency in blood safety: national oversight and policy, donor recruitment, laboratory testing and appropriate clinical use of blood [8]. In addition both hemovigilance and external quality assessment are key –albeit neglected- elements for the safe functioning of a transfusion service. This is pertinent in Africa, given the high prevalence of the major transfusion transmitted viruses (TTV) [HIV, HBV and HCV] in both the general and blood donor populations.
Following the report of two recent EQAS studies[9, 10] in Francophone Africa, we sought to evaluate test performance at laboratories in Anglophone and Lusophone African countries so as to document and contrast performance across Sub-Saharan Africa (SSA).
MATERIALS AND METHODS
We conducted a cross-sectional assessment of test performance using a convenience sample of laboratories that presently conduct transfusion screening in Africa, using a standardized and blinded test panel. Seventeen countries in SSA were invited to participate in the study. Countries that had participated in the prior Francophone African study were excluded from the new study. We identified national coordinators in each of the countries that agreed to participate, who in turn identified laboratories that conduct in-country transfusion-related screening and were willing to participate in the study.
Panels
The panels were prepared at Institut National de la Transfusion Sanguine (INTS) in Paris, France; each panel comprised 25 samples that included 8 negative samples, 5 HIV (four HIV-1 and one HIV-2), 4 HCV, 5 HBsAg positives (confirmed by neutralization assay) and three mixed samples to mimic co-infections (HCV/HIV, HBsAg /HCV, and one HBsAg/HIV; Appendix Table A). All samples (except S3) were obtained through dilution with a negative sample in order to obtain a range of the antigen or antibody concentrations. Each sample was pedigreed in the French Laboratory Reference with the following enzyme immunoassays (EIAs): Vidas HIV DUO Ultra (BioMérieux, Craponne, France), Genscreen HIV Ag/Ab Ultra (Bio-Rad, Marne la Coquette, France), PRISM HIV (Abbott, Rungis, France), for HIV; ETI MAK4 (Dia Sorin, Saluggia, Italy), PRISM HBsAg (Abbott), for HBsAg; Monolisa HCV Ag/Ab Ultra (Bio-Rad), Monolisa HCV Ab plus v2 Ultra (Bio-Rad), PRISM HCV(Abbott), for HCV. Moreover, positive confirmatory results for HIV and HCV were obtained with WB HIV (HIV blot 2.2, Abbott) and RIBA HCV (Ortho Clinical Diagnostic, Issy, France). The assays were performed in accordance with the manufacturer’s instructions. The panel was distributed in a coded fashion and tubes within each panel were numbered uniquely to allow for blinded testing.
In-country Workflow
The panels were couriered to a major international airport that was logistically closest to the national coordinator. The coordinator was tasked with retrieval and redistribution to the participating laboratories, which were located in different parts of the country. The panels were shipped frozen at a minimum of ≤-20°C with strict attention to maintenance of the cold chain during both international and in-country shipment; this was monitored during the study. One panel was lost during shipment and was not replaced.
Upon receipt at each laboratory, the designated peripheral coordinator communicated the panel number to Blood Systems Research Institute (BSRI). A corresponding data collection sheet was relayed to the peripheral laboratory. with instructions to perform routine testing using standard methods and under conditions normally applied to transfusion samples. After testing, the results were e-mailed back to BSRI using the prescribed data collection form for subsequent analysis. Upon receipt of the results form at BSRI, a questionnaire was relayed to the peripheral coordinator to collect data on the laboratory infrastructure and the mode of testing.
Definitions
For the purposes of the study, we designated the category of Enzyme Immunoassay (EIA) to include those automated or semi-automated assays that were able to detect antibodies (in the case of HIV and HCV) or antigens (in the case of HbsAg). We recognize that some EIAs are indeed chemilumiscent assays rather than true enzyme-based assays. We defined combo tests as automated or semi automated assays, which were able to capture both antibodies and antigens. Rapid tests refer specifically to manual, point of care tests.
We referred to HIV, HBsAg and HCV yet acknowledge that antibody (e.g. Anti-HIV or anti-HCV) or antigen may be targeted, depending on which assay is employed. Specifically, combo tests are able to capture both antigen and antibodies.
Statistical Analysis
Test sensitivity, namely the proportion of true positives that were correctly identified as positive, and test specificity, namely the proportion of true negatives that were correctly identified as negative, were calculated for each virus, by laboratory. There were a total of 7 HIV (4 samples mono-infected with HIV 1, 1 mono-infected with HIV2 and 2 mixed samples), 6 HCV (4 mono-infected and 2 mixed samples) and 7 HBsAg (5 mono-infected and 2 mixed samples) and 8 negative “gold standard” results based upon pedigree testing at our central laboratory. The primary outcome variable in all subsequent bivariate and multivariate analyses was sensitivity expressed as a number from 0 to 1. An initial bivariate analysis compared sensitivity separately by country and by test type using the PROC GENMOD procedure. In order to maintain confidentiality, the countries were assigned random codes that do not correspond to the order of countries in Table 1. For each virus, additional correlations between sensitivity and the following variables were examined using ANOVA (PROC GLM): infrastructure (capabilities to produce blood components), number and type of staff (dedicated versus non-dedicated), percentage of voluntary non-remunerated blood donors (VNRBD), highest qualification of laboratory/center director, total number of refrigerators, year of the newest refrigerator and frequency of electricity blackouts. Finally, for each virus, a separate multivariate model was constructed with PROC GENMOD with sensitivity as the outcome variable. The “stepwise” option for independent variable selection and a p value ≤ 0.1 for retention led to a different set of predictor variables for each model. All analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC).
Table 1.
Demographic Data on participating countries and Collection Infrastructure
| Country and Number of Centers/Laboratories | n | % | |
|---|---|---|---|
| Botswana | 2 | 4.5 | |
| Cape Verde | 2 | 4.5 | |
| Ghana | 3 | 6.8 | |
| Kenya | 9 | 20.5 | |
| Lesotho | 1 | 2.3 | |
| Mauritius | 1 | 2.3 | |
| Nigeria | 7 | 15.9 | |
| South Africa | 3 | 6.8 | |
| Tanzania | 7 | 15.9 | |
| Uganda | 6 | 13.6 | |
| Zambia | 2 | 4.5 | |
| Zimbabwe | 1 | 2.3 | |
|
| |||
|
Electricity Outage (n=42)
| |||
| At least Once a year/Never | 12 | 28.6 | |
| At least Once a month | 11 | 26.2 | |
| At least Once a week | 11 | 26.2 | |
| At least Once a day | 8 | 19 | |
|
| |||
|
Infrastructure (n=42)
| |||
| Produce Components incl. Platelets | 32 | 76.2 | |
| Produce RBCs only/ No Components at all | 10 | 23.8 | |
|
| |||
|
Staff Breakdown (n=41)
| |||
| Dedicated | 33 | 80.5 | |
| Rotational/Mixed | 8 | 19.5 | |
|
| |||
|
Qualification of Director of Center (n=40)
| |||
| Doctoral (MD/PhD/equivalent) | 19 | 47.5 | |
| Non-Doctoral | 21 | 52.5 | |
|
| |||
|
% VNRBD*
(n=42)
| |||
| <75% | 11 | 26.2 | |
| 76-99% | 10 | 23.8 | |
| 100% | 21 | 50 | |
|
| |||
|
Number of Refrigerators (n=42)
| |||
| 0-5 | 23 | 60.5 | |
| =>6 | 15 | 39.5 | |
|
| |||
| Other | Mean | Median | Range |
|
| |||
| No of Collections | 385053 | 14531 | 862-595000 |
| No of Collections Tested | 38705 | 15427 | 1313-580000 |
| % VNRBD* | 83.3 | 99.5 | 6-100 |
| Number of Staff | 9.74 | 6.5 | 2-30 |
| No of Refrigerators | 7.9 | 5 | 2-100 |
| Year of Newest Refrigerator | NA | NA | 1999-2011 |
VNRBD: voluntary non-remunerated blood donors
RESULTS
Among the 17 invited countries, a total of 12 countries and 44 screening laboratories participated in the study: Botswana (n=2 laboratories), Cape Verde (n=2), Ghana, (n=3). Kenya (n=9), Lesotho (n=1), Mauritius (n=1), Nigeria (n=7), South Africa (n=3), Tanzania (n=7), Uganda (n=6), Zambia (n=2) and Zimbabwe (n=1) (Figure 1). Reasons for non-participation were not specified.
Figure 1. Map of Countries that have participated in the African Proficiency testing Studies.
Map of Africa that shows Anglophone and Lusophone African countries (dark grey), which participated in the African Proficiency Testing Study (n=12). Countries that participated in the previous proficiency testing study in Francophone Africa are displayed in light grey (n=17). The participating laboratories are indicated with black dots dots (n=44).
Infrastructure and tests performed (Table 1)
Seventy one percent of participating laboratories reported some level of interruption of electricity. Seventy six percent of blood centers were able to produce blood components. Twenty four percent transfused RBCs only or, alternatively, whole blood only. An average of 50% of blood was collected from VNRBD (range 6%-100%); the remainder was collected from family replacement donors. No paid donation was reported. Forty seven percent of laboratories had a medical director with a medical and/or a doctoral level education (MD or PhD). The median number of collections was 14,531 units per year (range 862-595,000 units). The median number of collections tested annually at the participating centers was 15,427 (range 1,313-580,000).
The number of laboratories that performed rapid testing was as follows: 3 (6.98%) for HIV, 2 (4.88%) for HBsAg, and 2 (4.88%) for HCV (Appendix Table B). The number of laboratories performing combo (Ag/Ab) testing was 35 (81.40%) for HIV and 6 (14.63%) for HCV. Only three of the participating laboratories performed nucleic acid testing (NAT) routinely; only the serological findings are reported in this manuscript.
Proficiency Testing
The mean sensitivity for HIV, HBsAg and HCV was 91.9%, 86.7% and 90.1%, respectively. Sensitivity of 100% was attained for HIV, HBsAg, and HCV in 29 (66%), 24 (55%), and 28 (64%) laboratories (Figure 2). The mean specificity for HIV, HBsAg and HCV was 97.7%, 97% and 99.5%, respectively. Specificity of 100% was attained for HIV, HBsAg and HCV and HIV in 34 (77%), 31 (70%) and 41 (93%) laboratories (Figure 2).
Figure 2.
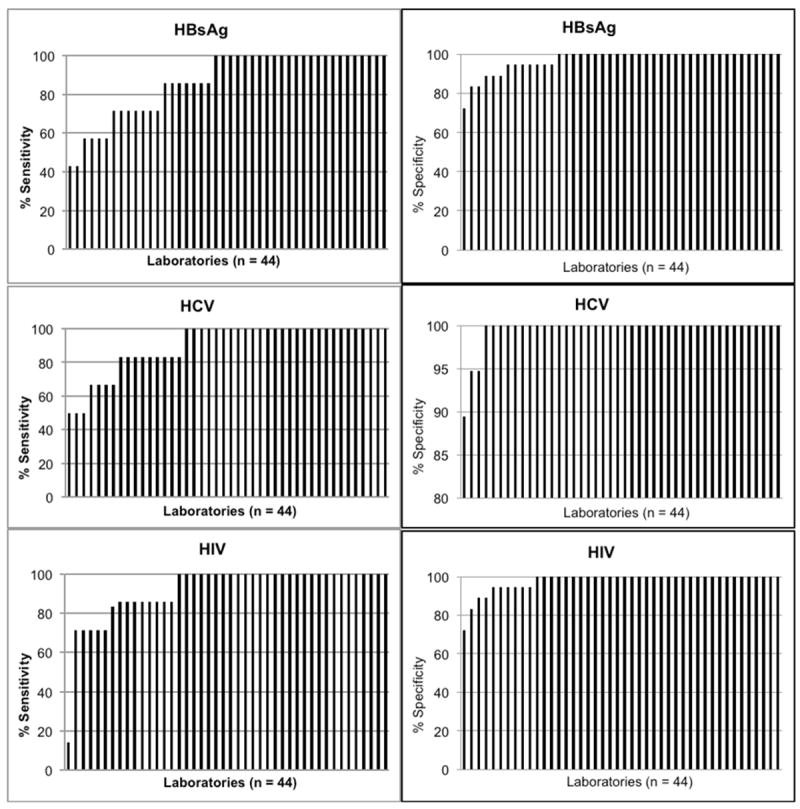
Sensitivity & Specificity by laboratory for HBsAg, HCV & HIV. In each graph, laboratories are sorted in order of increasing sensitivity or specificity.
In the bivariate model, when evaluating the sensitivity of detection by mode of testing test (combo [Ag/Ab] or rapid testing as compared to the use of EIA), the sensitivity was lower with rapid tests than with EIA for both HIV (P=0.007) and HBsAg (p=0.001)(Table 2). There was no significant difference in sensitivity between HIV and HCV combo (Ag/Ab) testing compared to EIA. Evaluation of sensitivity by country demonstrated, significantly reduced sensitivity in countries #3 and #8 for HBsAg, #7 and #8 for HCV and #3 and #10 for HIV.
Table 2.
Bivariate Model of Sensitivity for Detection HBsAg, HCV and HIV by Mode of Testing using an EIA reference. The reference category for mode of testing is EIA; a negative parameter indicates worse sensitivity and a positive one indicates better sensitivity.
| Virus and Mode of Testing1 | Parameter Estimate | 95% CI | p-value |
|---|---|---|---|
|
| |||
| HBV | |||
| Rapid test | -0.47 | (-0.66, -0.27) | <0.001 |
|
| |||
| HCV | |||
|
|
|||
| Ag/Ab Combo test | 0.08 | (-0.06, 0.21) | 0.248 |
|
|
|||
| Rapid test | -0.06 | (-0.28, 0.16) | 0.586 |
|
| |||
| HIV | |||
|
|
|||
| Ag/Ab Combo test | -0.01 | (-0.14, 0.12) | 0.894 |
|
|
|||
| Rapid test | -0.28 | (-0.48, -0.08) | 0.007 |
In the multivariate model (Table 3), sensitivity was significantly lower for HBsAg and HCV using rapid testing as compared to EIA. In contrast, the detection of HIV using rapid testing was not statistically different from EIA. There was lower sensitivity for HCV and higher sensitivity for HIV using combo (Ag/Ab) testing as compared to EIA yet these did not reach statistical significance. In the multivariate analysis, country #8 continued to show a reduced sensitivity for both HBsAg and HCV; only country #7 displayed reduced sensitivity for HIV after controlling for mode of testing and infrastructure.
Table 3.
Multivariate model of Sensitivity for the Detection of HBsAg, HCV and HIV*. For each variable, the reference category is indicated in the footnotes; a negative parameter indicates worse sensitivity and a positive one better sensitivity. Variables included in each model differed; those not shown were not significantly associated with sensitivity.
| HBsAg
| |||
|---|---|---|---|
| Variable | Parameter | 95% CI | p value |
|
| |||
| Test categorya | |||
|
|
|||
| Rapid test | -0.52 | (-0.73, -0.30) | <0.0001 |
|
| |||
| Countryb | |||
|
|
|||
| Country #8 | -0.20 | (-0.34, -0.05) | 0.0077 |
|
| |||
| Infrastructurec | |||
|
|
|||
| Produces components including Platelets | -0.10 | (-0.25, 0.05) | 0.1821 |
|
| |||
| % Voluntary Donorsd | |||
|
|
|||
| 76%-99% | 0.03 | (-0.08, 0.14) | 0.5687 |
|
|
|||
| 100% | 0.11 | (0.09, 0.20) | 0.0335 |
|
| |||
|
HCV
| |||
| Test categorya | |||
|
|
|||
| Ag/Ab Combo test | -0.19 | (-0.38, 0.01) | 0.0619 |
|
|
|||
| Rapid test | -0.30 | (-0.50, -0.09) | 0.0047 |
|
| |||
| Countryb | |||
|
|
|||
| Country #8 | -0.33 | (-0.49, -0.16) | 0.0001 |
|
| |||
| Qualification of Directore | |||
|
|
|||
| Doctoral | 0.15 | (0.04, 0.25) | 0.0060 |
|
| |||
| Staff Breakdownf | |||
|
|
|||
| Dedicated | 0.12 | (0.03, 0.21) | 0.0089 |
|
| |||
| Number of staffg | |||
|
|
|||
| 6-10 | 0.08 | (-0.00, 0.16) | 0.0586 |
|
|
|||
| >10 | 0.05 | (-0.07, 0.17) | 0.4016 |
|
| |||
| % Voluntary Donorsd | |||
|
|
|||
| 76%-99% | -0.09 | (-0.19, 0.01) | 0.0710 |
|
|
|||
| 100% | -0.03 | (-0.12, 0.07) | 0.5737 |
|
| |||
|
HIV
| |||
| Test categorya | |||
|
|
|||
| Ag/Ab Combo test | 0.06 | (-0.14, 0.27) | 0.5508 |
|
|
|||
| Rapid test | -0.09 | (-0.34, 0.16) | 0.4610 |
|
| |||
| Countryb | |||
|
|
|||
| Country #7 | 0.22 | (-0.02, 0.46) | 0.0727 |
|
| |||
| Infrastructurec | |||
|
|
|||
| Produces components including Platelets | 0.28 | (0.12, 0.44) | 0.0005 |
|
| |||
| Number of staffg | |||
|
|
|||
| 6-10 | 0.06 | (-0.03, 0.15) | 0.1670 |
|
|
|||
| >10 | 0.04 | (-0.08, 0.15) | 0.5494 |
|
| |||
| % Voluntary Donorsd | |||
|
|
|||
| 76%-99% | 0.03 | (-0.07, 0.14) | 0.5484 |
|
|
|||
| 100% | 0.05 | (-0.07, 0.16) | 0.4157 |
Reference Category
EIA
Country #0
Produces RBCs only/No components
<75% Voluntary donors
Non-doctoral qualification
Rotational
2-5 staff
Other significant findings in the multivariate model include an increased sensitivity for the detection of HBsAg in laboratories that reported 100% VNRBD. There was also an increased sensitivity for detection of HCV where the laboratory director had a doctoral degree or if the laboratory employed a dedicated rather than rotational staff. Lastly, laboratories that reported the ability to produce platelets rather than whole blood or red cells alone, were shown to have a significantly increased sensitivity for HIV
DISCUSSION
These results suggest that the sensitivity of operational TTV testing is deficient in a significant number of laboratories engaged in transfusion screening in SSA. Of the 44 laboratories that were surveyed, approximately 40% demonstrated some level of deficiency in detection of at least one of the three major TTVs (HIV, HBV and HCV). The mode of testing, country in which the laboratories were located and certain aspects of infrastructure were all shown to have an effect on sensitivity for detection of TTVs. The use of rapid tests in particular correlated with poor sensitivity of detection as compared to EIAs or combo Ag/Ab assays, even after controlling for country and infrastructure. Our study also showed that despite the logistical challenges, EQAS is important and feasible in under-resourced settings.
Deficiencies in sensitivity and specificity represent independent blood safety hazards. Our major focus was that of sensitivity where a deficiency poses risk of an infectious unit entering the blood supply. In contrast, although a deficiency in specificity poses less of an immediate risk to patients, it incurs wastage through unnecessary disposal of non-infectious units and deferral of eligible blood donors. Sensitivity of detection was impacted by three key variables: the mode of testing, the country in which the laboratories were located and the infrastructure at the index laboratory. Infrastructure was evaluated using several surrogate measures such as the ability to produce components other than red cells or whole blood, the staffing, the level of qualification of the laboratory director and the proportion of blood that was collected from VNRBD.
Although the use of rapid testing was shown to affect detection of TTVs adversely, rapid tests are often employed out of necessity rather than choice and have an important role in areas with limited infrastructure. Importantly, rapid tests have demonstrated good efficacy when operated correctly [11, 12]. This has been exemplified in voluntary testing and counseling centers (VCT) where rapid testing offers a critical access point to prevention and treatment, particularly in remote areas [13-15]. Furthermore, the study was designed as a means to identify potential areas of deficiency, rather than to establish cause. There are multiple reasons that impact performance of rapid tests such as the storage conditions, environment (e.g. heat and humidity), input volumes, incubation time, operator training and interpretation of the test results that warrant investigation [16]. Furthermore, deficiencies in quality assurance with point of care testing are common and may contribute to suboptimal performance. This is particularly problematic in Africa where the transfusion service may not control the procurement of the test kits and suppliers vary between consignments.
Nonetheless, the comparatively poor performance of rapid testing was consistent with that reported in two studies in Francophone Africa [9, 10]. The first, a pilot study of six laboratories reported significantly lower performance for rapid testing as compared to EIA[10]. An expanded follow-up study, which used similar methods to our study, evaluated fifty-one laboratories representing 17 countries, demonstrated respective sensitivity and specificity of detection of 81.4% and 99.6% for HIV, 75.6% and 94.5% for HBsAg and 80.0% and 98.1% for HCV. In contrast, the reported sensitivities for rapid testing were 72.4% for HIV, 47.4% for HBsAg and 63.7% for HCV [9].
Even after controlling for modality of testing and infrastructure, a minority of participating countries still maintained significant deficiencies for both HBsAg and HCV. This suggests a systemic problem across all laboratories in those countries that might require an assessment and intervention at the national level. The HIV sensitivity was not shown to be significantly affected by country, which may be ascribed to comparatively greater investment in HIV testing as part of a broader HIV prevention strategy. For example, the WHO program of “Safe Blood by 2012” targeted universal blood screening for HIV, and PEPFAR, an HIV focused program, has been prominent in supporting blood safety in SSA.
The data on transfusion infrastructure offers further insight into blood banking capacity in SSA and attests to the diversity in size and concomitant level of infrastructure. Of note, over two thirds of laboratories reported electricity outage at least once per month and almost a fifth reported daily interruptions. Notably, there was significantly increased sensitivity for the detection of HCV in laboratories that had a director with a doctoral degree or a staff of dedicated rather than rotating technologists; this likely reflects general human capacity in those laboratories. We also found that laboratories that produced components (specifically platelets) as opposed to whole blood alone had a significantly increased sensitivity for HIV. Indeed, the ability to produce components, a surrogate of blood center infrastructure, was the only variable that was shown to have a significant effect on sensitivity of detection for HIV.
The major strength of the study was its focus on a neglected area of public health in Africa. Proficiency testing specific to blood transfusion is lacking, despite the high prevalence of TTVs in Africa [17-19]. There is recognition that viral marker screening is critical to mitigation of TTV risk; however, in the absence of quality assurance, such screening offers false assurance. While we had expected greater reservation to participate in this study, the regional transfusion services (with few exceptions) offered strong support. A secondary gain of the study has been the establishment of a regional transfusion-focused research network to conduct both a follow-up EQAS as well as to support independent transfusion-related research. This could serve to influence, positively, clinical practice and blood safety in the future.
There are several limitations of the study. First, the observed step-like drop off in sensitivity and specificity (Figure 2) is due to the small number of positive samples in a given panel. Thus, a single error incurs a relatively large decrease in sensitivity, potentially misrepresenting the true performance characteristics at a given laboratory. This limitation is shared with other proficiency testing studies using pedigreed panels of modest size, and may be offset by a positive bias from laboratory awareness that an EQAS panel is being tested. Second, the preparation of the samples is another potential limitation. Specifically, some of the samples were diluted and may approximate low level infection rather than samples that might be more typically encountered in donors with unrecognized infection i.e. moderate level antibody or HBsAg. However, low titer samples are encountered in daily practice and still pose risk of TTI. Third, the low number of participating laboratories that used rapid testing limits the generalizability of our findings, despite being consistent with previous studies [9, 10]. Fourth, due to our use of a convenience sample of both countries and laboratories within each country, selection bias could have led to either over- or underestimation of performance. Specifically, five countries elected not to participate; reasons for non-participation were not communicated to the research team. Finally, because of its scope, the study did not include an on-the-ground assessment of the procedures at each laboratory and is therefore unable to determine the cause for the observed deficiencies. However, root cause analysis and remediation has been initiated at some of the laboratories following the study.
In conclusion, this study supports the implementation of EQAS for transfusion infectious disease screening in SSA. The findings highlight deficiencies that could be remedied by improved quality assurance and validation of laboratory screening. Following communication of the results to participating laboratories, we received positive feedback with a number of requests for follow-up investigation; some laboratories have expressed an interest in participating in ongoing proficiency testing. Therefore, we encourage the adoption of ongoing EQAS in Africa, ideally facilitated by the World Health Organization (WHO) and/or the Centers for Disease Control and Prevention (CDC), both of which have expressed their support for continued activities. Implementation of quality assurance systems is feasible, even in remote settings [20].
Supplementary Material
Acknowledgments
We are grateful to Safe Blood for Africa Foundation for assistance in identifying and facilitating the participation of a number of laboratories and to the Institut National de la Transfusion Sanguine (INTS), Paris, France for serving as our central laboratory and especially Françoise Bouchardeau and Isabelle Houdoin who prepared the panels. The authors are also grateful to Drs. Daniel Kimani at the Centers for Disease Control and Prevention in Kenya, Peter Mwamba at University of Nairobi and Leslie Tobler for their invaluable contribution.
Funding: Blood Systems Research Institute, Institut National de la Transfusion Sanguine and NHLBI career development award K24-HL-75036 to Dr. Murphy
APPENDIX
The Anglophone Africa Transfusion Research Group includes:
-
Botswana
Mukendi K. Kayembe (National Blood Transfusion Service Botswana)
Anderson Chinorumba (National Blood Transfusion Service Botswana)
Gilbert G. Gonnetsweng (National Blood Transfusion Centre Gaborone, Botswana)
Joseph Mphele (Francistown Regional Blood Transfusion Center, Botswana)
-
Cape Verde
Maria Conceição Ramos Pinto (Serviço de Sangue, Cape Verde)
José Rocha (ELISA Lab, Ag. Neto Hospital, Cape Verde)
-
Ghana
Mavis Okyere (Accra Area Blood Centre, Ghana)
Elliot Dogbe (Komfo Anokye Teaching Hospital Kumasi, Ghana)
Asamoah Michael (Accra Area Blood Centre, Ghana)
Carboo Tetteh (Accra Area Blood Centre, Ghana)
Patrick Asebga (Area Transfusion Centre of Tamale Teaching Hospital, Ghana)
-
Kenya
Margaret Oduor (National Blood Transfusion Service Kenya)
Charles Rombo Oliech (National Blood Transfusion Service Kenya)
Daniel Kimani (Centers for Disease Control, Nairobi, Kenya)
Grace Kitonyi (African Society of Blood Transfusion Kenya)
Alice N Mbui (National Blood Transfusion Service, Testing Laboratory, Kenya)
Miriam L Okiya (National Blood Transfusion Centre, Kenya)
June A Akoth (Regional Blood Transfusion Centre Mombasa, Kenya)
Benard O Odindo (Regional Blood Transfusion Centre Kisumu, Kenya)
John Agata (Regional Blood Transfusion Centre Nakuru, Kenya)
Reuben Welanunu (Regional Blood Transfusion Centre Eldoret, Kenya)
Steven K Mutukaa (Regional Blood Transfusion Centre Embu, Kenya)
Jamilla A. Rajab (The Mater Hospital, Kenya)
-
Lesotho
Maleqhoa Nyopa (National Blood Transfusion Service Lesotho)
-
Mauritius
Janaki Sonoo (National Blood Transfusion Service Mauritius)
-
Nigeria
Idris A Saliu (Safe Blood for Africa, Nigeria)
Agba Janet C. (National Blood Transfusion Services Abuja, Nigeria)
Oreh A Adaeze (National Blood Transfusion Services Abuja, Nigeria)
Eneas J. Konobe (National Blood Transfusion Service, North West Zonal Centre, Nigeria)
Stephen F Ajala (National Blood Transfusion Service, North West Zonal Centre, Nigeria)
Dr. Uwem Oyekan (Lagos State Blood Transfusion Service, Nigeria)
Mrs F.I Oyediran (National Blood Transfusion Service, South West Zonal Centre, Nigeria)
Oluwale O Egbewumi (National Blood Transfusion Service, South West Zonal Centre, Nigeria)
Vitalis Aguguo (National Blood Transfusion Service, South East Zonal Centre, Nigeria)
Dr Deni O.C Onyetenu (National Blood Transfusion Service, South East Zonal Centre, Nigeria)
Oloyede Benson (National Blood Transfusion Service South-South Zonal Centre, Nigeria)
Omomene Okubor (National Blood Transfusion Service South-South Zonal Centre, Nigeria)
Olalekan Rufai (National Blood Transfusion Service, Nigeria)
-
South Africa
Marion Vermeulen (South African National Blood Service)
Lilian Gaggia (South African National Blood Service)
Wendy Sykes (Donation Testing Department, South African National Blood Service)
Allan Naidoo (Donation Testing Department, South African National Blood Service)
Arthur Bird (Western Province Blood Transfusion Service)
Russell T. Cable (Western Province Blood Transfusion Service)
-
Tanzania
Dunstan Haule (National Blood Transfusion Service Tanzania)
Efesper Nkya (National Blood Transfusion Service Tanzania)
Ndeonasia A Towo (Eastern Zone Blood Transfusion Centre, Tanzania)
Aloyce Ole Sulul (Northern Zone Blood Transfusion Centre, Tanzania)
Abdul Mahamoud (Lake Zone Blood Transfusion Centre, Tanzania)
Senyael Marco Urassa (Lake Zone Blood Transfusion Centre, Tanzania)
Jackson Ndaskoy (Southern Highland Zone Blood Transfusion Centre, Tanzania)
Omar Juma Kidua (Zanzibar Blood Transfusion Centre, Tanzania)
Fatma I Ahmeid (Zanzibar Blood Transfusion Centre, Tanzania)
Daniel H Kuhanda (Western Zone Blood Transfusion Center, Tanzania)
Johnl C Mtimba (Western Zone Blood Transfusion Center, Tanzania)
Charles Masanja (Southern Zone Blood Transfusion Center, Tanzania)
Vincent R Mtweve (Southern Zone Blood Transfusion Center, Tanzania)
-
Uganda
Dorothy Kyeyune-Byabazaire (Uganda Blood Transfusion Service)
Julius O. Onencan (Arua Regional Blood Bank Laboratory, Uganda Blood Transfusion Service)
Wabuyi Patrick (Mbarara Regional Blood Bank, Uganda)
Ezra Musisi (Nakasero Blood Bank, Uganda)
Hannington Dheyongera Hans (Kitovu Sub-Regional Blood Bank, Uganda)
Paul Akankwasa (Kitovu Sub-Regional Blood Bank, Uganda)
Enoch Osana (Mbale Regional Blood Bank, Uganda)
Komakech C Ojok (Gulu Regional Blood Bank, Uganda)
Grace Otekat (Fort Portal Regional Blood Bank)
Sarah M Katusiime (Fort Portal Regional Blood Bank)
-
Zambia
David Chama (Zambia National Blood Transfusion Service)
Mundia Hendrix (Lusaka Provincial Blood Centre, Zambia)
-
Zimbabwe
Lucy M Marowa (National Blood Service Zimbabwe)
Sisodwa Z Nkomo (National Blood Service Zimbabwe)
References
- 1.Tagny CT, Mbanya D, Tapko JB, et al. Blood safety in Sub-Saharan Africa: a multi-factorial problem. Transfusion. 2008;48:1256–1261. doi: 10.1111/j.1537-2995.2008.01697.x. [DOI] [PubMed] [Google Scholar]
- 2.Dini L, Frean J. Quality assessment of malaria laboratory diagnosis in South Africa. Trans R Soc Trop Med Hyg. 2003;97:675–677. doi: 10.1016/s0035-9203(03)80101-3. [DOI] [PubMed] [Google Scholar]
- 3.WHO. World Health Organization: Policy and Procedures of the WHO/NICD Microbiology External Quality Assessment Programme in Africa. 2007 [Google Scholar]
- 4.Cham F, M M, Masango M, et al. The World Health Organization African region external quality assessment scheme for anti-HIV serology. Afr J Lab Med. 2012;1(1) doi: 10.4102/ajlm.v1i1.39. Art. #39:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birx D, de Souza M, Nkengasong JN. Laboratory challenges in the scaling up of HIV, TB, and malaria programs: The interaction of health and laboratory systems, clinical research, and service delivery. Am J Clin Pathol. 2009;131:849–851. doi: 10.1309/AJCPGH89QDSWFONS. [DOI] [PubMed] [Google Scholar]
- 6.Petti CA, Polage CR, Quinn TC, et al. Laboratory medicine in Africa: a barrier to effective health care. Clin Infect Dis. 2006;42:377–382. doi: 10.1086/499363. [DOI] [PubMed] [Google Scholar]
- 7.Tapko J, Mainuka P, Diarra-Nama AJ. Status of Blood Safety in the WHO African Region: Report of the 2006 Survey. WHO Regional Office for Africa; Brazzaville, Republic of Congo: 2006. http://www.afro.who.int/en/divisions-a-programmes/dsd/health-technologies-a-laboratories.html. [Google Scholar]
- 8.Bloch EM, Vermeulen M, Murphy E. Blood transfusion safety in Africa: a literature review of infectious disease and organizational challenges. Transfus Med Rev. 2012;26:164–180. doi: 10.1016/j.tmrv.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laperche S. Multinational assessment of blood-borne virus testing and transfusion safety on the African continent. Transfusion. 2013;53:816–826. doi: 10.1111/j.1537-2995.2012.03797.x. [DOI] [PubMed] [Google Scholar]
- 10.Laperche S, Boukatou G, Kouegnigan L, et al. Transfusion safety on the African continent: an international quality control of virus testing in blood banks. Transfusion. 2009;49:1600–1608. doi: 10.1111/j.1537-2995.2009.02239.x. [DOI] [PubMed] [Google Scholar]
- 11.Learmonth KM, Chiu CY, Galang H, et al. Assessment of the heat stability of seven rapid HIV assays. Trans R Soc Trop Med Hyg. 2011;105:388–395. doi: 10.1016/j.trstmh.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Chappel RJ, Wilson KM, Dax EM. Immunoassays for the diagnosis of HIV: meeting future needs by enhancing the quality of testing. Future Microbiol. 2009;4:963–982. doi: 10.2217/fmb.09.77. [DOI] [PubMed] [Google Scholar]
- 13.UNAIDS. Voluntary Counselling and Testing: UNAIDS Technical Update. 2000:12. [Google Scholar]
- 14.McKenna SL, Muyinda GK, Roth D, et al. Rapid HIV testing and counseling for voluntary testing centers in Africa. AIDS. 1997;11(Suppl 1):S103–110. [PubMed] [Google Scholar]
- 15.Pronyk PM, Kim JC, Makhubele MB, et al. Introduction of voluntary counselling and rapid testing for HIV in rural South Africa: from theory to practice. AIDS Care. 2002;14:859–865. doi: 10.1080/0954012021000031921. [DOI] [PubMed] [Google Scholar]
- 16.Pai NP, Tulsky JP, Cohan D, et al. Rapid point-of-care HIV testing in pregnant women: a systematic review and meta-analysis. Trop Med Int Health. 2007;12:162–173. doi: 10.1111/j.1365-3156.2006.01812.x. [DOI] [PubMed] [Google Scholar]
- 17.WHO. Annex 8 - HIV and AIDS statistics, by WHO and UNICEF regions. 2010 [Google Scholar]
- 18.Bloch EM, Vermeulen M, Murphy E. Blood transfusion safety in Africa: a literature review of infectious disease and organizational challenges. Transfus Med Rev. 2011;26:164–180. doi: 10.1016/j.tmrv.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayaraman S, Chalabi Z, Perel P, et al. The risk of transfusion-transmitted infections in sub-Saharan Africa. Transfusion. 2010;50:433–442. doi: 10.1111/j.1537-2995.2009.002402.x. [DOI] [PubMed] [Google Scholar]
- 20.Amukele TK, Michael K, Hanes M, et al. External quality assurance performance of clinical research laboratories in sub-saharan Africa. Am J Clin Pathol. 2012;138:720–723. doi: 10.1309/AJCP8PCM4JVLEEQR. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


