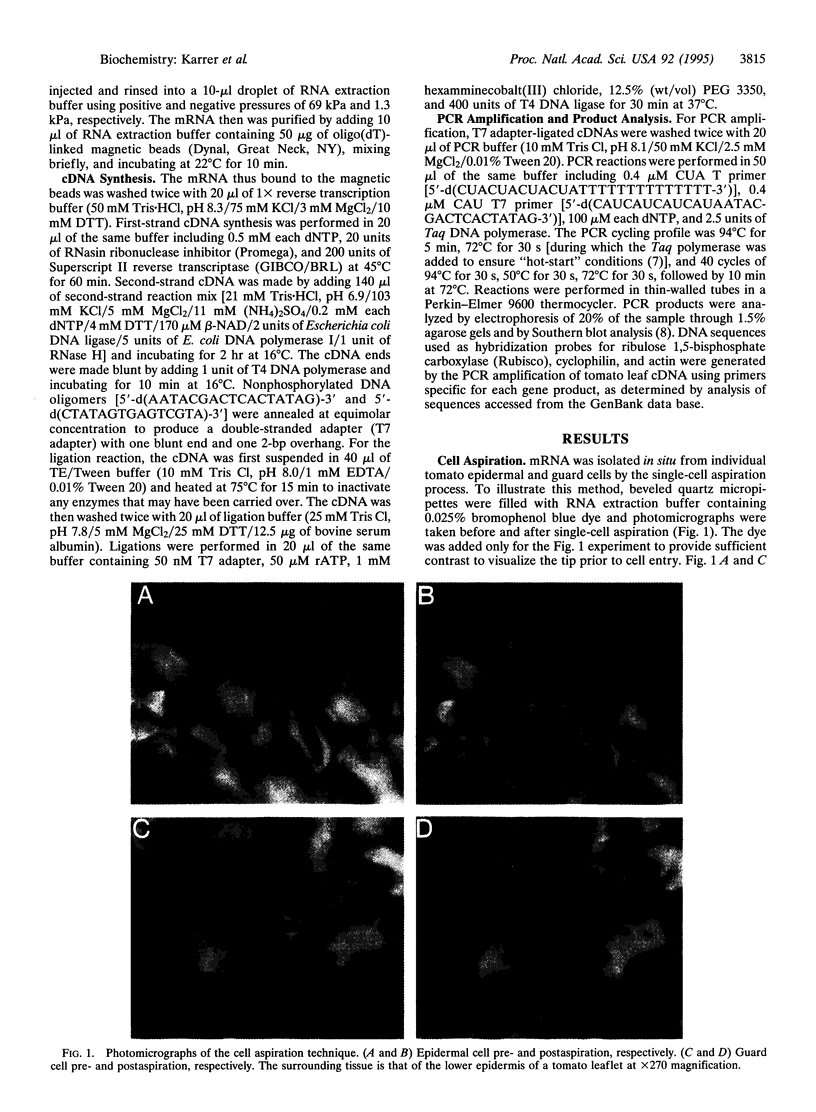
Abstract
A method for isolating and cloning mRNA populations from individual cells in living, intact plant tissues is described. The contents of individual cells were aspirated into micropipette tips filled with RNA extraction buffer. The mRNA from these cells was purified by binding to oligo(dT)-linked magnetic beads and amplified on the beads using reverse transcription and PCR. The cell-specific nature of the isolated mRNA was verified by creating cDNA libraries from individual tomato leaf epidermal and guard cell mRNA preparations. In testing the reproducibility of the method, we discovered an inherent limitation of PCR amplification from small amounts of any complex template. This phenomenon, which we have termed the "Monte Carlo" effect, is created by small and random differences in amplification efficiency between individual templates in an amplifying cDNA population. The Monte Carlo effect is dependent upon template concentration: the lower the abundance of any template, the less likely its true abundance will be reflected in the amplified library. Quantitative assessment of the Monte Carlo effect revealed that only rare mRNAs (< or = 0.04% of polyadenylylated mRNA) exhibited significant variation in amplification at the single-cell level. The cDNA cloning approach we describe should be useful for a broad range of cell-specific biological applications.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chou Q., Russell M., Birch D. E., Raymond J., Bloch W. Prevention of pre-PCR mis-priming and primer dimerization improves low-copy-number amplifications. Nucleic Acids Res. 1992 Apr 11;20(7):1717–1723. doi: 10.1093/nar/20.7.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich R. A., Delaney T. P., Uknes S. J., Ward E. R., Ryals J. A., Dangl J. L. Arabidopsis mutants simulating disease resistance response. Cell. 1994 May 20;77(4):565–577. doi: 10.1016/0092-8674(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Dresselhaus T., Lörz H., Kranz E. Representative cDNA libraries from few plant cells. Plant J. 1994 Apr;5(4):605–610. doi: 10.1046/j.1365-313x.1994.5040605.x. [DOI] [PubMed] [Google Scholar]
- Eberwine J., Yeh H., Miyashiro K., Cao Y., Nair S., Finnell R., Zettel M., Coleman P. Analysis of gene expression in single live neurons. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3010–3014. doi: 10.1073/pnas.89.7.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing N. N., Wimmers L. E., Meyer D. J., Chetelat R. T., Bennett A. B. Molecular Cloning of Tomato Plasma Membrane H-ATPase. Plant Physiol. 1990 Dec;94(4):1874–1881. doi: 10.1104/pp.94.4.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser C. S., Gunning D. A., Budelier K. A., Brown S. M. Structure and expression of cytosolic cyclophilin/peptidyl-prolyl cis-trans isomerase of higher plants and production of active tomato cyclophilin in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9519–9523. doi: 10.1073/pnas.87.24.9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross P., Julius C., Schmelzer E., Hahlbrock K. Translocation of cytoplasm and nucleus to fungal penetration sites is associated with depolymerization of microtubules and defence gene activation in infected, cultured parsley cells. EMBO J. 1993 May;12(5):1735–1744. doi: 10.1002/j.1460-2075.1993.tb05821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Koning A. J., Tanimoto E. Y., Kiehne K., Rost T., Comai L. Cell-specific expression of plant histone H2A genes. Plant Cell. 1991 Jul;3(7):657–665. doi: 10.1105/tpc.3.7.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C. J. Plant disease resistance genes in signal perception and transduction. Cell. 1994 Feb 11;76(3):419–422. doi: 10.1016/0092-8674(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Lambert K. N., Williamson V. M. cDNA library construction from small amounts of RNA using paramagnetic beads and PCR. Nucleic Acids Res. 1993 Feb 11;21(3):775–776. doi: 10.1093/nar/21.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melaragno J. E., Mehrotra B., Coleman A. W. Relationship between Endopolyploidy and Cell Size in Epidermal Tissue of Arabidopsis. Plant Cell. 1993 Nov;5(11):1661–1668. doi: 10.1105/tpc.5.11.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisson P. E., Rashtchian A., Watkins P. C. Rapid and efficient cloning of Alu-PCR products using uracil DNA glycosylase. PCR Methods Appl. 1991 Nov;1(2):120–123. doi: 10.1101/gr.1.2.120. [DOI] [PubMed] [Google Scholar]
- Nuovo G. J., Forde A., MacConnell P., Fahrenwald R. In situ detection of PCR-amplified HIV-1 nucleic acids and tumor necrosis factor cDNA in cervical tissues. Am J Pathol. 1993 Jul;143(1):40–48. [PMC free article] [PubMed] [Google Scholar]
- Pichersky E., Bernatzky R., Tanksley S. D., Cashmore A. R. Evidence for selection as a mechanism in the concerted evolution of Lycopersicon esculentum (tomato) genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3880–3884. doi: 10.1073/pnas.83.11.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R. Structure, function and regulation of plasma membrane H(+)-ATPase. FEBS Lett. 1993 Jun 28;325(1-2):108–111. doi: 10.1016/0014-5793(93)81424-x. [DOI] [PubMed] [Google Scholar]
- Yang W. C., Katinakis P., Hendriks P., Smolders A., de Vries F., Spee J., van Kammen A., Bisseling T., Franssen H. Characterization of GmENOD40, a gene showing novel patterns of cell-specific expression during soybean nodule development. Plant J. 1993 Apr;3(4):573–585. doi: 10.1046/j.1365-313x.1993.03040573.x. [DOI] [PubMed] [Google Scholar]