Abstract
Recently, the century-old idea of targeting cancer with viruses (‘oncolytic viruses’) has come of age, with promise documented in early-stage and several late-stage clinical trials in a variety of cancers. While originally prized for their direct tumor cytotoxicity (‘oncolytic virotherapy’), recently, the pro-inflammatory and immunogenic effects of viral tumor infection (‘oncolytic immunotherapy’) have come into focus. Indeed, a capacity for eliciting broad, sustained anti-neoplastic effects stemming from combined direct viral cytotoxicity, innate antiviral activation, stromal pro-inflammatory stimulation and recruitment of adaptive immune effector responses is the greatest asset of oncolytic viruses. However, it also is the source for enormous mechanistic complexity that must be considered for successful clinical translation. Due to fundamentally different relationships with their hosts (malignant or not), diverse replication strategies and distinct modes of tumor cytotoxicity/ killing, oncolytic viruses should not be referred to collectively. These agents must be evaluated based on their individual merits. In this review, we highlight key mechanistic principles of cancer treatment with the polio:rhinovirus chimera PVSRIPO, and their implications for oncolytic immunotherapy in the clinic.
Keywords: poliovirus, oncolytic virus, IRES, Necl5, CD155, translation, innate antiviral response, interferon, eIF4G
Introduction
Viruses are difficult vehicles for cancer therapy because they exert very complex effects on infected tumors that -for the most part- cannot be manipulated. The generic basis for proposed oncolytic virus (OV) platforms, cytotoxicity in select laboratory cell lines and anti-neoplastic effects in corresponding xenograft models, is insufficient to predict clinical efficacy. This is because the multifaceted, aggregate biological response to virus infection of cancers rarely lends itself to therapeutic application. The past 30 years’ leap in molecular virology yielded opportunities for genetic manipulation of certain traits, most notably pathogenicity. This made dangerous, lethal pathogens amenable for clinical use in patients. However, other key aspects of the host:virus relationship, in particular those involving the immune system, are difficult to engineer.
Despite this, OVs capable of properly targeting malignancy promise anti-tumor effects of an extent and range difficult to match with other therapeutic modalities. Viruses can elicit immunogenic forms of tumor cell death, present powerful pathogen- and danger associated molecular patterns (P/D-AMP), induce innate antiviral type 1 interferons (IFNα, β) responses within the tumor, evoke pro-inflammatory signals in tumor stroma and/or recruit adaptive immune responses directed against the infected tumor. The demands on a successful OV platform, however, are enormous. Such viruses must (i) be categorically non-pathogenic; (ii) be genetically stable upon intratumoral replication; (iii) target/infect notoriously heterogeneous tumors; (iv) elicit efficient killing of infected tumor cells; (v) act in the presence of neutralizing antibodies and innate antiviral immune activation; (vi) reverse the immune-repressive tumor/stromal micro-environment; (vii) be capable of recruiting immune effector arms against the tumor target. In addition, there are many unique practical and regulatory aspects to consider, such as (i) particle stability (including within the clinically intended delivery apparatus); (ii) reliable and efficient manufacture; (iii) public health implications, e.g. the availability of protective vaccines/anti-virals. Combining these features in a single agent is a tremendous challenge. Establishing a proper mechanistic rationale for clinical use of any OV platform requires pre-clinical research efforts that typically far exceed those for simple, mono-mechanistic therapeutics.
Descriptions of OV strategies often refer to such agents collectively with common mechanistic implications. Yet, the proposed OVs have very little (or nothing) in common with regard to tumor tropism, mechanism of tumor cell killing, replication strategy, response to innate antiviral immune activation, etc. This makes it difficult to summarize the OV experience in cogent texts or to extrapolate the clinical experience with one agent to its peers. Therefore, while highlighting general aspects of oncolytic virotherapy, we limit this discussion to our experience with poliovirus and its effects on glioblastoma (GBM). The particular oncolytic agent we are discussing is PVSRIPO, the type 1 (Sabin) live-attenuated poliovirus vaccine replicating under control of a heterologous internal ribosomal entry site of human rhinovirus type 2 (Fig. 1).1,2 PVSRIPO is showing early promise in currently accruing Phase-I clinical trials with intratumoral, convection-enhanced delivery (CED) in patients with recurrent GBM.3
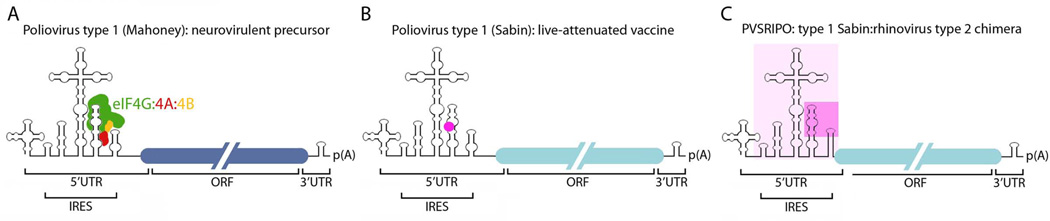
Figure 1.
Genetic structure of PVSRIPO. Poliovirus has a single-strand, +sense RNA genome of ~7,400 nucleotide length; overall genome organization is outlined (ORF; open reading frame). (A) Poliovirus type 1 (Mahoney), isolated 1941 from stool of healthy children in Cleveland, is the prototype neurovirulent strain. The approximate binding region for the eIF4G:4A:4B translation initiation helicase complex in the internal ribosomal entry site (IRES) is indicated.38 (B) Serial passage in primate tissue culture/primates yielded the type 1 (Sabin) live-attenuated vaccine.30 The position of a key attenuating point mutation in the IRES (G480A) is shown (•). (C) PVSRIPO is the type 1 (Sabin) vaccine containing a human rhinovirus type 2 IRES (pink box; a region of major sequence divergence is shaded darker).
Tumor Targeting and Tropism
Utilizing animal viruses as oncolytic agents in essence requires diverting their natural, ingrained pathogenicity towards a therapeutic effect. Therefore, the outcome of deliberate tumor infection is determined by factors that naturally define the virus:host relationship. The single most important such factor is target cell tropism. We define tropism [gr. τρέπειν (trepein), to turn, to direct, to alter, to change] as a virus’ ability to recognize cell surface features that mediate viral genome entry into the host cell. The term ‘cell-internal tropism’, referring to factors determining virus susceptibility after host cell entry, is confusing in the context of OVs. Virus entry that does not lead to productive infection can have important biological consequences, e.g. provoke a host response. A tight definition is therefore needed to strictly distinguish instances with transfer of viral genetic material into target tumor cells from those without.
For most animal viruses, host cell tropism conveyed by a specific viral receptor(s), shapes their pathogenic properties. It is therefore intuitively obvious that an effective OV must have tumor target tropism in patients. Yet, the decisive issue of tropism remains vague for many OV platforms. Early clinical investigations included viruses with documented poor cancer tropism, such as adenovirus type 5,4 which is due to absent, low or erratic expression of the coxsackie-adenovirus receptor5 in human cancers.6 In some instances, (clinical) cancer-tropism of viruses for which the host-cell receptor is unknown was predicted based on virus killing of select laboratory cancer cell lines.7 This is risky because viral receptors, many of which are involved in cell adhesion functions, may be induced by the artificial conditions of tissue culture. For example intercellular adhesion molecule-1 (ICAM-1), a receptor for many human enteroviruses, is induced upon cell adherence/ spread on tissue culture plastic-ware.8 Thus, ex vivo culture of cells from tissues that naturally resist infection by ICAM-1-tropic viruses, may make them permissive to infection in vitro. Also, clonal selection of cancer cell lines can yield non-representative frequency of receptor expression. For these reasons, there is no substitute for documenting virus tropism for patients’ tumors directly, through protein expression studies in tumor tissues.9
Obtaining solid, empirical support for viral tropism for a tumor target in the clinic does not imply that therapeutic efficacy rests on homogeneous targeting/infection of the entire tumor mass. As with any other molecular target in cancer, viral receptors are unlikely to be homogeneously expressed in all tumor cells and virus-resistant subpopulations likely exist even if receptor is abundant in the tumor as a whole. This is not of primary concern for devising OV strategies. First, it is inconceivable that all malignant cells can be reached, infected and eliminated, even with aggressive dosing and invasive administration. Second, the major thrust behind OV strategies in the clinic is their potential for recruitment of host immunogenic responses directed against the tumor. Therefore, tumor tropism is a prerequisite for establishing the conditions for an immunogenic response; it cannot mediate in toto eradication of the tumor mass and its satellites without such a response.
The concept of targeting cancer with poliovirus is largely built on its restrictive tropism for an intriguing cell surface molecule intricately linked to cancer, tumor stroma and proliferating tumor vasculature.2 Poliovirus recognizes a single host cell receptor, which is necessary and sufficient to convey virus susceptibility.10 The poliovirus receptor (PVR), a.k.a. Nectin-like molecule 5 (Necl5), or CD155, is a cell adhesion molecule of the Ig-like superfamily11 expressed during embryonic development.12 The nectin/nectin-like family of genes encode proteins involved in cell:cell adhesions, polarization, differentiation, migration, proliferation and survival.11 Necl5’s physiologic roles are poorly understood, but involve control over cell movement, e.g. through participation in leading-edge dynamics in cooperation with αvβ3 integrin and the platelet-derived growth factor receptor13 (reviewed in Takai et al.).11 Accordingly, Necl5 is a major determinant of GBM invasiveness and dissemination in the CNS.14,15 Studies in a rat liver injury model suggest that the Necl5 gene is rapidly induced upon acute tissue disruption and is down-regulated once proper cell-to-cell contacts are restored.16 The same study showed that constitutive, ectopic Necl5 expression upon malignant transformation of hepatocellular carcinoma cells is associated with a loss of Necl5 control.16 Necl5 is broadly expressed in laboratory GBM cell lines,2 in primary GBM cell lines grown in culture,9 in primary (pre-culture) explant GBM cells obtained from patients at surgery,17 in GBM biopsy-derived xenotransplants, and in surgically excised GBM tissues.9 Moreover, widespread ectopic Necl5 expression has been documented in patient tissue samples of many other types of cancers, e.g. colorectal carcinoma,18 lung adenocarcinoma,19 breast cancers,20 and melanoma.21
An intriguing, and possibly relevant factor for therapy is that Necl5 is physiologically expressed in antigen-presenting cells (APCs), i.e. macrophages and dendritic cells.22 Curiously, polio-infection of APCs, even with neurovirulent strains, is abortive/non-lethal and does not interfere with their ability to engage T cells (discussed below).23 PVSRIPO infection of patient peripheral blood mononuclear cells differentiated into macrophage/ dendritic lineage generates potent innate and adaptive responses, as reported previously for poliovirus.23 Tumor-associated macrophages (TAMs), a significant stromal component of GBM,24 broadly promote tumor progression via pro-angiogenic, -proliferative/invasive and -survival signals, including repression of cytotoxic T cell activity.25 These properties are the result of TAM polarization effects of the local tumor microenvironment, e.g. cytokine/chemokine production or extracellular matrix interactions.26 Given the key roles of TAMs in tumor support and immune repression, re-polarization towards a more pro-inflammatory, anti-neoplastic phenotype has been an attractive, if elusive, goal in cancer immunotherapy. Few events may be as effective in TAM repolarization as virus infection. This hypothesis is difficult to test in vivo, because TAM phenotypes are difficult to recapitulate in animal tumor models and patient explant TAM assays are challenging. However, the plausible in vitro evidence suggests that PVSRIPO-targeting of TAM may well contribute to its anti-neoplastic potential (Fig. 4).
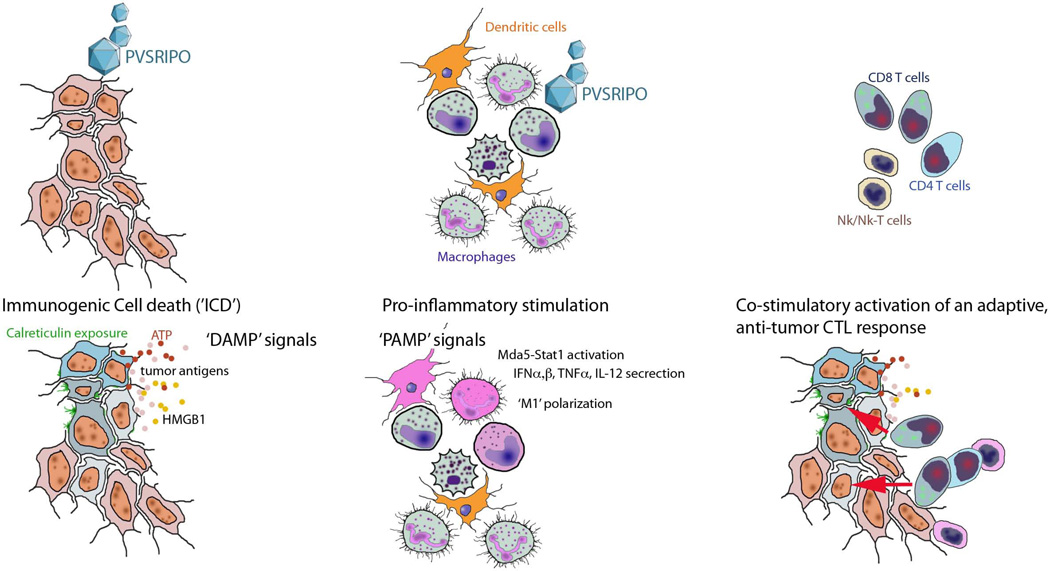
Figure 4.
Possible host immunogenic responses to PVSRIPO oncolytic immunotherapy. (Left) PVSRIPO infects and kills GBM cells, elicits ICD, presents DAMPs and tumor antigens. (Middle) PVSRIPO-infection of APCs elicits innate antiviral type 1 IFN responses, PAMP signals for pro-inflammatory stimulation. (Right) The combination of multiplex direct viral cytotoxicity, DAMP and PAMP presentation, pro-inflammatory TAM activation, cytokine release, etc. promote an adaptive anti-tumor immune effector cell response.
Attenuation and Safety
Ectopic expression of viral receptors -such as Necl5- means that cancer cells may be susceptible to virus infection and killing. However, tropism for normal target cells (expressing receptors) remains; toxicity stemming from inherent viral pathogenicity is unacceptable in a clinical oncological setting. Modern molecular virology has had remarkable success in engineering non-pathogenic variants, even of lethal, feared pathogens. For example, genetic recombinants of the neuropathogens poliovirus and herpes simplex virus 1 with neuronal replication deficits in vitro lack neuropathogenicity in relevant non-human primates.27,28 They also did not cause encephalitis, meningitis or poliomyelitis after high-titer intratumoral inoculation in GBM patients.3,29
We devised a uniquely effective way of preventing polio virulence in the normal Central Nervous System (CNS). Poliovirus-binding to Necl5 and ensuing host cell entry lead to perforation of the viral capsid and rapid cytoplasmic release of +strand viral RNA (Fig. 2A). Establishing infection depends on immediate translation of incoming viral RNA (Fig. 2B), as ‘naked’ polio RNA cannot persist and failure to translate will abort the infectious process. Once initial viral translation has occurred, cytotoxic viral proteins re-arrange the host cell cytoplasm and intercept host gene expression to benefit viral replication. Therefore, translation initiation at incoming polio RNA constitutes a rate-limiting step. Both instances where deliberate administration of live poliovirus to human subjects has met FDA approval (the Sabin vaccines and PVSRIPO) rely on interfering with this step specifically in the CNS.
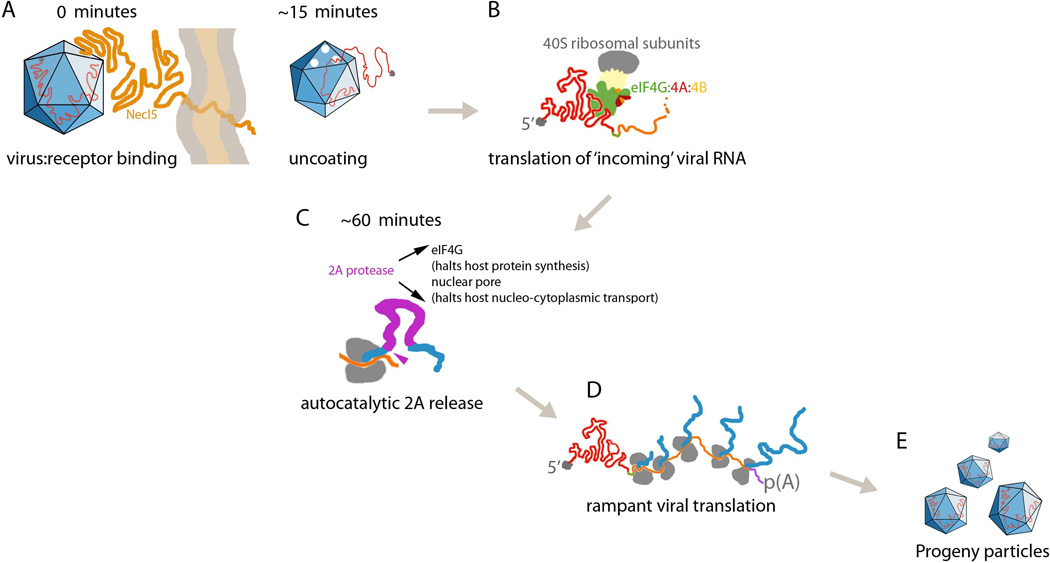
Figure 2.
Early events after PVSRIPO infection of tumor cells. (A) Approximately 15min after virus-receptor docking, empty capsids (indicating viral RNA ‘uncoating’) can be recovered from infected cells. (B) The incoming, uncapped viral RNA serves as a translation template; recruitment of the host cell translation initiation helicase complex eIF4G:4A:4B to the IRES is required for initiation of viral protein synthesis (see text). (C) The first protein released from the nascent viral polyprotein is the viral protease 2A. (D) Initial rounds of viral translation give rise to highly productive viral protein synthesis and genome replication. This is fostered by stimulatory viral polypeptides and block of competing host cell protein synthesis. (E) Viral progeny is first detected by ~4h.
The live-attenuated (Sabin) polio vaccines (types 1 and 3 resulted from serial passage of neurovirulent strains in primate cell cultures/non-human primates; type 2 essentially is a field isolate),30 feature crucial, attenuating point mutations in a cis-acting genetic element responsible for viral protein synthesis, the internal ribosomal entry site (IRES; Fig. 1B).31 Later investigations linked the Sabin attenuating IRES mutations to compromised neuronal translation initiation.32–34 PVSRIPO was engineered by exchange of the entire cognate IRES with the corresponding segment from a distant poliovirus relative, human rhinovirus type 2 (HRV2; Fig. 1C).1 Although PVSRIPO attenuation may involve a similar mechanism as the Sabin IRESes, its neuronal incompetence is more pronounced,32 has a far broader genetic footprint and -most importantly- is genetically stable upon replication in the intended target.35 The reasons for this become apparent when considering the molecular mechanism for translation initiation at enterovirus IRESes (Fig. 1A).
Conventional protein synthesis occurs when the eukaryotic initiation factor (eIF) 4E binds the canonical 7-methyl-guanidine ‘cap‘ modification at the 5’ end of mRNAs.36 eIF4E recruits the large scaffold eIF4G, which assembles the eIF4G:4A:4B helicase complex (responsible for scanning and unwinding the 5’UTR until the initiation codon is found) and binds eIF3 (the bridge to the small ribosomal subunit).36 Poliovirus RNA, which is un-capped,37 cannot bind eIF4E. Therefore, it employs an unorthodox translation mechanism by recruiting the eIF4G:4A:4B helicase complex directly, independent of the 5’ end or eIF4E (Fig. 1A).38 Binding to eIF4G:4A:4B is enabled by the IRES, a ~500nt structure in the 5’UTR of the viral genome (Fig. 1A).39 Thus, the rate-limiting step of initial translation of incoming poliovirus RNA depends on attracting eIF4G:4A:4B to the IRES (Fig. 2B). By substituting the HRV2 IRES, we eliminated the ability of PVSRIPO to recruit eIF4G to the viral genome in normal CNS cells.40 The part of the HRV2 IRES responsible for this defect (the distal ~1/3 of the IRES comprising stem loop domains v and vi; Fig. 1C)41 is the genetically most divergent (from poliovirus)41 and contains the ‘footprint’ for eIF4G:4A:4B binding.33,38 The Sabin IRES mutations (Fig. 1B) and the heterologous HRV2 IRES in PVSRIPO (Fig. 1C) may interfere similarly with host cell translation factor recruitment in the CNS. However, in the former, attenuation rests on artificial (tissue culture-generated) single point mutations that spontaneously revert to the wild type sequence.42 In PVSRIPO, attenuation is due to a functionally integrated, naturally occurring, intact IRES which is inherently deficient for eIF4G:4A:4B recruitment in the CNS. We believe that this distinction explains the superior attenuation/genetic stability profile of PVSRIPO compared to its Sabin precursor.35
PVSRIPO’s neuronal deficits in recruiting host translation factors are likely due to (i) host cell (neuronal) RNA-binding proteins that interact with the HRV2 IRES and interfere with eIF4G recruitment;40,43,44 and/or (ii) absent Raf-Erk1/2 mitogen-activated protein kinase (MAPK) signals in the normal CNS, restricting ribosome recruitment to the viral IRES. This could involve effects on eIF4G:4A:4B,45,46 or signals to the MAPK interacting kinase (Mnk) that affect IRES competence.47 Importantly, despite its profound CNS-incompetence, PVSRIPO is fully translation-proficient in tumor cells (see below), indicating a truly tumor-specific viral translation/cytotoxicity/killing phenotype.2
Tumor Cytotoxicity and Cell Killing
Genetic engineering can yield non-pathogenic virus variants safe for use in cancer patients. Ideally, this does not affect the major attraction of OVs, their tumor cytocidal potential. Since host immune responses to OV tumor targeting may be key mediators of clinical efficacy,48 there is a debate about the relative contribution of direct viral cytotoxicity to therapy. Traditional views stipulate primary (direct cytotoxic) effects that may evoke, but are functionally separate from secondary (host inflammatory) responses. However, tumor cell killing and the host immunologic response may be closely integrated mechanistically and biologically. For example, an attractive prospect for therapy is the propensity of viruses to elicit ‘immunogenic cell death (ICD)’, a non-canonical form of cell demise characterized by release of the pro-inflammatory cytokine high-mobility group box 1 (HMGB1), adenosine-tri-phosphate (ATP) and pre-apoptotic exposure of calreticulin.49 ICD in essence posits the dying cancer cell as a potent immune stimulus; thus, OV efficacy may occur as a result of intertwined cytotoxicity and host immunogenic responses.
Many animal viruses exhibit replication success leading to cell killing in clonal laboratory cancer cell lines. This is unsurprising, given that common impediments to viral growth in normal living tissues, e.g. lacking tropism, intact innate antiviral defenses, an inactive cell cycle in terminally differentiated cells, silent mitogenic signaling pathways, etc., may be lifted in such hosts. However, this is not representative of exceedingly heterogeneous cancers laced with (non-malignant) stromal components in patients. To provide empirical support for clinical investigations, it is therefore of utmost importance to identify the molecular mechanisms of viral cytotoxicity in vitro for correlation with the clinical target.
PVSRIPO has a simple, short-lived and extremely toxic relationship with infected cancer cells. A scant coding capacity and primitive life cycle do not allow parasitic, long-term relations with the host. Successful infection, which depends on the rate-limiting step of initial translation of incoming viral RNA (see above), produces very rapid, irreversible and lethal alterations of host cell physiology in tumor cells.2 If initial viral protein synthesis succeeds, the first viral protein to be released is the protease 2Apro, which is autocatalytically cleaved from the nascent viral polyprotein (Fig. 2C). 2Apro intercepts host cell gene expression as early as ~60min after infection, e.g. through degradation of eIF4G50 and nuclear pore proteins.51 eIF4G cleavage selectively shuts off host cell protein synthesis; a C-terminal eIF4G fragment, capable of forming fully functional eIF4G:4A:4B helicase complexes, is sufficient for translation of poliovirus genomes.31
Contrary to the CNS, PVSRIPO drives rampant viral translation and cytotoxicity in cancer cells. This is because they offer an enormous advantage to the virus by allowing wanton viral, IRES-mediated translation.2 This is not specific to the HRV2 IRES in PVSRIPO, but relates to the initiation mechanism operational at all enterovirus IRESes.32 Repression of HRV2 IRES-mediated translation in the CNS (see above) is virtually absent in malignant cells.44 Neuron-like cells that recapitulate PVSRIPO’s CNS-incompetence become fully susceptible to viral translation upon activation of Raf-Erk1/2 MAPK signals.47 Erk1/2 MAPK networks converge on translation machinery, i.e. eIF4G and its binding partners involved in 5’UTR scanning/unwinding (eIF4B), ribosome recruitment (eIF3), RNA-binding (eIF4G:4A:4B helicase complex), the MAPK interacting kinase (Mnk) and other, less obvious factors with influence over translation control.45,46,52–54 Efforts to mechanistically unravel MAPK signaling networks are ongoing, but we have conclusive evidence that PVSRIPO’s tumor cytotoxicity rests on catalytic activity of the Erk1/2 downstream kinase Mnk.47 Thus, PVSRIPO oncolytic capacity relies on a key, central oncogenic signaling network centered on Raf-Erk1/2-Mnk signals.
Oncolytic Viruses and Innate Antiviral Immunity in Cancer
Viral infection invariably triggers anti-viral defenses stemming from tumor or stromal components. The first line of defense is activation of an innate antiviral response to non-self, pathogen-associated molecular patterns (PAMPs), such as viral genetic material.55 For example, viral RNA (structurally distinct from host mRNA) is detected by the cytoplasmic ‘RIG-I’-like receptors, RIG-I (retinoic acid-inducible gene-I) and MDA-5 (melanoma-derived antigen-5),55 which induce type 1 interferons (IFNs) that mediate autocrine/paracrine induction of 100s of IFN-stimulated genes (Fig. 3A). Most animal viruses have evolved with means for evading, blocking or counteracting antiviral defenses in their preferred host species. Thus, the pathogenic process results from an ‘arms race’ between the host’s intent of detection/elimination of infected cells, vs. the virus’ intent of deception/establishing infection. It is obvious that the virus:innate defense interplay is a major factor in oncolytic virotherapy, either in a restrictive role (preventing viral cytotoxicity, replication, spread) or, a far more exciting possibility, in a supportive role.
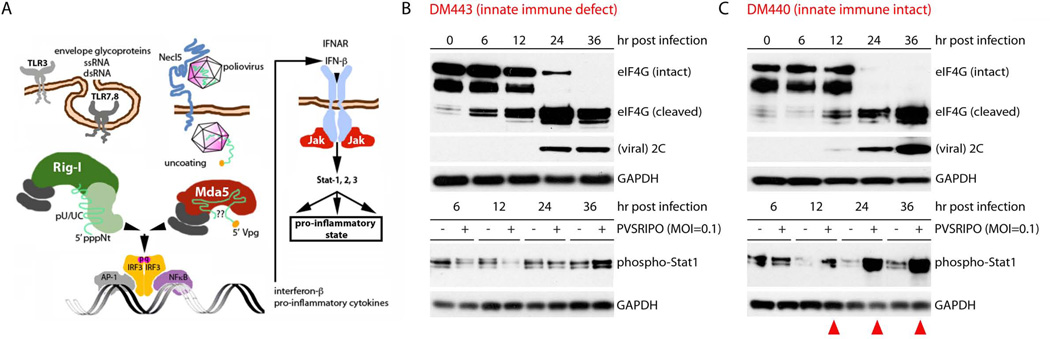
Figure 3.
The effect of innate anti-viral activation on PVSRIPO oncolysis in vitro. (A) Schematic of the RIG-I like receptors and MDA-5 activation, IFN induction by polio RNA. (B) DM443 and (C) DM440 cultures were infected with PVSRIPO at an MOI of 0.1. In DM443 cells (B), stable levels of Stat1 phosphorylation indicate failure of type 1 IFN signaling. DM440 cells responded with vigorous Stat1 phosphorylation (red arrowheads) (C). Viral cell killing (indicated by eIF4G cleavage) and expression of viral proteins (2C) were indistinguishable in both cell lines. The relatively protracted appearance of viral proteins/eIF4G cleavage is due to the low MOI.
A frequent argument in favor of tumor-specificity and cytotoxicity of OVs is the purportedly reduced state of the innate antiviral response in cancers. Viruses that are neutralized by intact innate defenses are unable to establish successful infections in normal tissues and, thus, generally lack pathogenicity. However, such viruses may replicate in and kill tumor cells with deficient innate defenses. Based on this paradigm, OV platforms were devised, whose tumor-selectivity and cytotoxicity depends on hindered innate antiviral defenses in malignant cells.56,57 Applying this concept in the clinic is problematic for several reasons. First, there is the notorious tumor heterogeneity. It is true that many laboratory tumor cell lines exhibit deficits in innate antiviral IFN responses.58 Such cells are permissive to killing by viruses that are otherwise blocked by intact antiviral defenses.57 However, the manifestation of this phenomenon in patient tumors is far from clear. Ex-vivo cultivation and passage of any type of cell, e.g. the non-malignant African Green Monkey kidney (Vero) explant cell line, can select for profound innate antiviral defense defects.59 Innate antiviral defects in laboratory cell lines may primarily reflect serial in vitro propagation/tissue culture adaptation rather than malignant transformation.
The problem is easily recognizable when screening cancer cell lines for the innate response to virus infection (Fig. 3B, C). DM440 and DM443 are two melanoma cell lines (chosen at random), derived from similar biopsy material of distinct patients with metastatic disease (courtesy of Dr. H. Seigler, Duke University Medical Center).60 DM440 (passage 43) vigorously responds to PVSRIPO infection with phosphorylation of STAT1 (indicating an IFN response; Fig. 3A) (Fig. 3C). DM443 (passage 25) does not (Fig. 3B). No information about the tumor of origin that could shed light on this issue is available, but considering the heterogeneity of advanced, metastatic and/or treatment-refractory cancers, it is safe to assume that such tumors harbor cells with a wide range of innate antiviral competency. Indeed, this has recently been shown for the status of the innate antiviral defense and RIG-I in hepatocellular carcinoma.61 OVs that were characterized in cell lines/animal tumor models with homogeneous innate antiviral deficits may exert very different biological effects in heterogeneous target tumors in patients. Second, concepts of targeting innate immune deficiency may miss out on promising modes of anti-neoplastic efficacy. Tumor cytotoxicity, e.g. the coveted ‘immunogenic’ kind,49 may result from the innate defense:virus arms race rather than unilateral toxic effects of the virus or its products alone.62 Harming/killing of defenseless cells with a blunted innate antiviral response may lack this potentially important quality. In other words, an ability to successfully overcome active defenses in tumor cells, i.e. killing in the presence of a functional innate antiviral response may be important for therapy. Third, IFN responses have significant pro-inflammatory and immune-stimulatory effects that may help to recruit immune effector cells and mediate adaptive anti-tumor immunity. For viruses whose oncolytic capacity relies on absent antiviral responses, this issue is tricky, because the (desirable) co-stimulatory effects of the innate antiviral response occur at the expense of viral cytotoxicity and spread.
Poliovirus stands out amongst its peers with regard to the innate antiviral defense: (i) the main -and perhaps only- PAMP is viral RNA; (ii) unlike other RNA viruses, poliovirus (and the picornaviridae family) are exclusively detected by MDA-5 (Fig. 3A);63 (iii) poliovirus lacks a specific strategy to block or counter host innate defenses. Rather, it is the virus’ remarkably efficient cytotoxicity that permit host cell killing in the presence of innate immune activation.64 Therefore, polioviruses uniquely exhibit almost undisturbed viral replication and cell killing in IFNα-treated cells.64 Assays in DM440 and DM443 cells confirm this scenario (Fig. 3B, C). We deliberately infected these cells with PVSRIPO at a multiplicity of infection (MOI) of 0.1. This means that initially only ~10% of cells in the culture are infected, enabling amplification of IFN signaling to both infected and uninfected cells by delaying cell death (in DM440 cells; Fig. 3C). This response could interfere with subsequent rounds of infection, cytotoxicity and cell killing of the remaining ~90% of cells in the culture. Yet, host cytotoxic effects (e.g. eIF4G cleavage) and viral translation occur at a similar rate in DM440 and DM443 cells (Fig. 3B, C). This shows that primary PVSRIPO cytotoxicity (first round of infection) and subsequent cell killing and spread are not significantly affected by intact antiviral defenses. Thus, for PVSRIPO, triggering the innate antiviral response in tumor/tumor stroma does not primarily impede oncolytic efficacy.
The Role of the Host Immune System
The arguably most intriguing, mechanistically complex and least explored aspect of OVs is their potential to elicit host immunogenic responses directed against the tumor.48 Since viruses generate responses to viral cytotoxicity in autologous tumor, they may be regarded as in situ vaccines, specific to the antigenic profile of individual tumors. A long list of events may conspire in oncolytic immunotherapy.48 For PVSRIPO, these include: (i) primary tumor cytotoxicity releasing tumor antigens; (ii) DAMPs, ICD and their pro-inflammatory stimuli; (iii) PAMP exposure/MDA-5 activation within the tumor; (iv) innate antiviral-mediated recruitment of natural killer cell responses; (v) virus-mediated alterations of intratumoral cytokine profiles; (vi) viral targeting-, PAMP exposure-, pro-inflammatory stimulation of APCs; (vii) TAM re-polarization/reversal of the immune-suppressive cancer microenvironment; (viii) recruitment of adaptive anti-tumor humoral and cytotoxic T cell responses. These possibilities are schematically depicted in Fig. 4.
Conclusion
Targeting cancer with viruses has obvious attractions that motivated efforts to quickly move prototype OV constructs towards clinical investigation. As with other areas of experimental cancer research, the empirical basis for clinical use of OVs is more complicated than anticipated. The common problem of matching pre-clinical paradigms established in experimental tumor models with the patient situation is particularly acute with OVs. This is due to the exceedingly complex, multilayered relations with their malignant host tissues, normal tumor stroma and the host organism. Recent promise of oncolytic virotherapy in the clinic, e.g. our observations with PVSRIPO, show that relentless pre-clinical, mechanistic research addressing all aspects of the virus:host relationship, can yield promising clinical results in the most difficult oncologic indications that resist all available conventional therapy.
Acknowledgments
Funding sources: This work was supported by PHS Awards R01 CA124756, R01 CA140510 (M.G.), and P50 NS20023 (D.D.B.). PVSRIPO pre-clinical development, IND-directed toxicology and IND submission were supported in part by the Rapid Access to Intervention Development (RAID) and the Experimental Therapeutics (NExT) Programs of the NCI Division of Cancer Treatment and Diagnosis, Developmental Therapeutics Program. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Financial Disclosures: Drs. Desjardins, Sampson, Henry S. Friedman, Bigner and Gromeier are co-owners of intellectual property related to the technology discussed.
References
- 1.Gromeier M, Alexander L, Wimmer E. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc Natl Acad Sci U S A. 1996;93(6):2370–2375. doi: 10.1073/pnas.93.6.2370. PMCID: PMC39803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, Wimmer E. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc Natl Acad Sci U S A. 2000;97(12):6803–6808. doi: 10.1073/pnas.97.12.6803. PMCID: PMC18745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desjardins A, Sampson JH, Peters K, Ranjan T, Vlahovic G, Threatt S, Herndon JI, Boulton S, Lally-Goss D, McSherry F, Friedman A, Friedman H, Bigner D, Gromeier M. Phase I study of an Oncolytic polio/rhinovirus recombinant (PVSRIPO) against recurrent glioblastoma. Neuro-Oncology; SNO Annual Meeting; 2013; San Francisco, CA. 2013. p. 107. [Google Scholar]
- 4.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274(5286):373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 5.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275(5304):1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 6.Mori T, Arakawa H, Tokino T, Mineura K, Nakamura Y. Significant increase of adenovirus infectivity in glioma cell lines by extracellular domain of hCAR. Oncol Res. 1999;11(11–12):513–521. [PubMed] [Google Scholar]
- 7.Poirier JT, Dobromilskaya I, Moriarty WF, Peacock CD, Hann CL, Rudin CM. Selective tropism of Seneca Valley virus for variant subtype small cell lung cancer. J Natl Cancer Inst. 2013;105(14):1059–1065. doi: 10.1093/jnci/djt130. PMCID: PMC3888137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paine R, 3rd, Christensen P, Toews GB, Simon RH. Regulation of alveolar epithelial cell ICAM-1 expression by cell shape and cell-cell interactions. Am J Physiol. 1994;266(4 Pt 1):L476–L484. doi: 10.1152/ajplung.1994.266.4.L476. [DOI] [PubMed] [Google Scholar]
- 9.Merrill MK, Bernhardt G, Sampson JH, Wikstrand CJ, Bigner DD, Gromeier M. Poliovirus receptor CD155-targeted oncolysis of glioma. Neuro Oncol. 2004;6(3):208–217. doi: 10.1215/S1152851703000577. PMCID: PMC1871993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendelsohn CL, Wimmer E, Racaniello VR. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- 11.Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol. 2008;9(8):603–615. doi: 10.1038/nrm2457. [DOI] [PubMed] [Google Scholar]
- 12.Gromeier M, Solecki D, Patel DD, Wimmer E. Expression of the human poliovirus receptor/CD155 gene during development of the central nervous system: implications for the pathogenesis of poliomyelitis. Virology. 2000;273(2):248–257. doi: 10.1006/viro.2000.0418. [DOI] [PubMed] [Google Scholar]
- 13.Amano H, Ikeda W, Kawano S, Kajita M, Tamaru Y, Inoue N, Minami Y, Yamada A, Takai Y. Interaction and localization of Necl-5 and PDGF receptor beta at the leading edges of moving NIH3T3 cells: Implications for directional cell movement. Genes Cells. 2008;13(3):269–284. doi: 10.1111/j.1365-2443.2008.01167.x. [DOI] [PubMed] [Google Scholar]
- 14.Sloan KE, Eustace BK, Stewart JK, Zehetmeier C, Torella C, Simeone M, Roy JE, Unger C, Louis DN, Ilag LL, Jay DG. CD155/PVR plays a key role in cell motility during tumor cell invasion and migration. BMC Cancer. 2004;4:73. doi: 10.1186/1471-2407-4-73. PMCID: PMC524493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sloan KE, Stewart JK, Treloar AF, Matthews RT, Jay DG. CD155/PVR enhances glioma cell dispersal by regulating adhesion signaling and focal adhesion dynamics. Cancer Res. 2005;65(23):10930–10937. doi: 10.1158/0008-5472.CAN-05-1890. [DOI] [PubMed] [Google Scholar]
- 16.Erickson BM, Thompson NL, Hixson DC. Tightly regulated induction of the adhesion molecule necl-5/CD155 during rat liver regeneration and acute liver injury. Hepatology. 2006;43(2):325–334. doi: 10.1002/hep.21021. [DOI] [PubMed] [Google Scholar]
- 17.Castriconi R, Daga A, Dondero A, Zona G, Poliani PL, Melotti A, Griffero F, Marubbi D, Spaziante R, Bellora F, Moretta L, Moretta A, Corte G, Bottino C. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J Immunol. 2009;182(6):3530–3539. doi: 10.4049/jimmunol.0802845. [DOI] [PubMed] [Google Scholar]
- 18.Masson D, Jarry A, Baury B, Blanchardie P, Laboisse C, Lustenberger P, Denis MG. Overexpression of the CD155 gene in human colorectal carcinoma. Gut. 2001;49(2):236–240. doi: 10.1136/gut.49.2.236. PMCID: PMC1728395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakai R, Maniwa Y, Tanaka Y, Nishio W, Yoshimura M, Okita Y, Ohbayashi C, Satoh N, Ogita H, Takai Y, Hayashi Y. Overexpression of Necl-5 correlates with unfavorable prognosis in patients with lung adenocarcinoma. Cancer Sci. 2010;101(5):1326–1330. doi: 10.1111/j.1349-7006.2010.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochiai H, Moore SA, Archer GE, Okamura T, Chewning TA, Marks JR, Sampson JH, Gromeier M. Treatment of intracerebral neoplasia and neoplastic meningitis with regional delivery of oncolytic recombinant poliovirus. Clin Cancer Res. 2004;10(14):4831–4838. doi: 10.1158/1078-0432.CCR-03-0694. [DOI] [PubMed] [Google Scholar]
- 21.Bevelacqua V, Bevelacqua Y, Candido S, Skarmoutsou E, Amoroso A, Guarneri C, Strazzanti A, Gangemi P, Mazzarino MC, D'Amico F, McCubrey JA, Libra M, Malaponte G. Nectin like-5 overexpression correlates with the malignant phenotype in cutaneous melanoma. Oncotarget. 2012;3(8):882–892. doi: 10.18632/oncotarget.594. PMCID: PMC3478464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freistadt MS, Fleit HB, Wimmer E. Poliovirus receptor on human blood cells: a possible extraneural site of poliovirus replication. Virology. 1993;195(2):798–803. doi: 10.1006/viro.1993.1433. [DOI] [PubMed] [Google Scholar]
- 23.Wahid R, Cannon MJ, Chow M. Dendritic cells and macrophages are productively infected by poliovirus. J Virol. 2005;79(1):401–409. doi: 10.1128/JVI.79.1.401-409.2005. PMCID: PMC538697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morantz RA, Wood GW, Foster M, Clark M, Gollahon K. Macrophages in experimental and human brain tumors. Part 1: Studies of the macrophage content of experimental rat brain tumors of varying immunogenicity. J Neurosurg. 1979;50(3):298–304. doi: 10.3171/jns.1979.50.3.0298. [DOI] [PubMed] [Google Scholar]
- 25.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33(3):119–126. doi: 10.1016/j.it.2011.12.001. PMCID: PMC3294003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobrikova EY, Goetz C, Walters RW, Lawson SK, Peggins JO, Muszynski K, Ruppel S, Poole K, Giardina SL, Vela EM, Estep JE, Gromeier M. Attenuation of neurovirulence, biodistribution, and shedding of a poliovirus:rhinovirus chimera after intrathalamic inoculation in Macaca fascicularis. J Virol. 2012;86(5):2750–2759. doi: 10.1128/JVI.06427-11. PMCID: PMC3302279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunter WD, Martuza RL, Feigenbaum F, Todo T, Mineta T, Yazaki T, Toda M, Newsome JT, Platenberg RC, Manz HJ, Rabkin SD. Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation of intracerebral injection in nonhuman primates. J Virol. 1999;73(8):6319–6326. doi: 10.1128/jvi.73.8.6319-6326.1999. PMCID: PMC112710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, Palmer CA, Feigenbaum F, Tornatore C, Tufaro F, Martuza RL. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7(10):867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 30.Sabin AB, Boulger LR. History of Sabin attenuated poliovirus oral live vaccine strains. Journal of Biological Standardization. 1973;1:115–118. [Google Scholar]
- 31.Evans DM, Dunn G, Minor PD, Schild GC, Cann AJ, Stanway G, Almond JW, Currey K, Maizel JV., Jr Increased neurovirulence associated with a single nucleotide change in a noncoding region of the Sabin type 3 poliovaccine genome. Nature. 1985;314(6011):548–550. doi: 10.1038/314548a0. [DOI] [PubMed] [Google Scholar]
- 32.Campbell SA, Lin J, Dobrikova EY, Gromeier M. Genetic determinants of cell type-specific poliovirus propagation in HEK 293 cells. J Virol. 2005;79(10):6281–6290. doi: 10.1128/JVI.79.10.6281-6290.2005. PMCID: PMC1091735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Breyne S, Yu Y, Unbehaun A, Pestova TV, Hellen CU. Direct functional interaction of initiation factor eIF4G with type 1 internal ribosomal entry sites. Proc Natl Acad Sci U S A. 2009;106(23):9197–9202. doi: 10.1073/pnas.0900153106. PMCID: PMC2695064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ochs K, Zeller A, Saleh L, Bassili G, Song Y, Sonntag A, Niepmann M. Impaired binding of standard initiation factors mediates poliovirus translation attenuation. J Virol. 2003;77(1):115–122. doi: 10.1128/JVI.77.1.115-122.2003. PMCID: PMC140626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobrikova EY, Broadt T, Poiley-Nelson J, Yang X, Soman G, Giardina S, Harris R, Gromeier M. Recombinant oncolytic poliovirus eliminates glioma in vivo without genetic adaptation to a pathogenic phenotype. Mol Ther. 2008;16(11):1865–1872. doi: 10.1038/mt.2008.184. PMCID: PMC2856473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- 37.Nomoto A, Lee YF, Wimmer E. The 5' end of poliovirus mRNA is not capped with m7G(5')ppp(5')Np. Proc Natl Acad Sci U S A. 1976;73(2):375–380. doi: 10.1073/pnas.73.2.375. PMCID: PMC335911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sweeney TR, Abaeva IS, Pestova TV, Hellen CU. The mechanism of translation initiation on Type 1 picornavirus IRESs. EMBO J. 2014;33(1):76–92. doi: 10.1002/embj.201386124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pelletier J, Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988;334(6180):320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- 40.Merrill MK, Dobrikova EY, Gromeier M. Cell-type-specific repression of internal ribosome entry site activity by double-stranded RNA-binding protein 76. J Virol. 2006;80(7):3147–3156. doi: 10.1128/JVI.80.7.3147-3156.2006. PMCID: PMC1440377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gromeier M, Bossert B, Arita M, Nomoto A, Wimmer E. Dual stem loops within the poliovirus internal ribosomal entry site control neurovirulence. J Virol. 1999;73(2):958–964. doi: 10.1128/jvi.73.2.958-964.1999. PMCID: PMC103915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horie H, Miyazawa M, Ota Y, Wakabayashi K, Yoshida H, Doi Y, Hashizume S. Analysis of the accumulation of mutants in Sabin attenuated polio vaccine viruses passaged in Vero cells. Vaccine. 2001;19(11–12):1456–1459. doi: 10.1016/s0264-410x(00)00350-9. [DOI] [PubMed] [Google Scholar]
- 43.Merrill MK, Gromeier M. The double-stranded RNA binding protein 76:NF45 heterodimer inhibits translation initiation at the rhinovirus type 2 internal ribosome entry site. J Virol. 2006;80(14):6936–6942. doi: 10.1128/JVI.00243-06. PMCID: PMC1489066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neplioueva V, Dobrikova EY, Mukherjee N, Keene JD, Gromeier M. Tissue type-specific expression of the dsRNA-binding protein 76 and genome-wide elucidation of its target mRNAs. PLoS One. 2010;5(7):e11710. doi: 10.1371/journal.pone.0011710. PMCID: PMC2909144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dobrikov M, Dobrikova E, Shveygert M, Gromeier M. Phosphorylation of eukaryotic translation initiation factor 4G1 (eIF4G1) by protein kinase C{alpha} regulates eIF4G1 binding to Mnk1. Mol Cell Biol. 2011;31(14):2947–2959. doi: 10.1128/MCB.05589-11. PMCID: PMC3133411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dobrikov MI, Dobrikova EY, Gromeier M. Dynamic Regulation of the Translation Initiation Helicase Complex by Mitogenic Signal Transduction to Eukaryotic Translation Initiation Factor 4G. Mol Cell Biol. 2013;33(5):937–946. doi: 10.1128/MCB.01441-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goetz C, Everson RG, Zhang LC, Gromeier M. MAPK signal-integrating kinase controls cap-independent translation and cell type-specific cytotoxicity of an oncolytic poliovirus. Mol Ther. 2010;18(11):1937–1946. doi: 10.1038/mt.2010.145. PMCID: PMC2990508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prestwich RJ, Errington F, Diaz RM, Pandha HS, Harrington KJ, Melcher AA, Vile RG. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum Gene Ther. 2009;20(10):1119–1132. doi: 10.1089/hum.2009.135. PMCID: PMC2829276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 50.Etchison D, Milburn SC, Edery I, Sonenberg N, Hershey JW. Inhibition of HeLa cell protein synthesis following poliovirus infection correlates with the proteolysis of a 220,000-dalton polypeptide associated with eucaryotic initiation factor 3 and a cap binding protein complex. J Biol Chem. 1982;257(24):14806–14810. [PubMed] [Google Scholar]
- 51.Gustin KE, Sarnow P. Effects of poliovirus infection on nucleo-cytoplasmic trafficking and nuclear pore complex composition. EMBO J. 2001;20(1–2):240–249. doi: 10.1093/emboj/20.1.240. PMCID: PMC140206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dobrikov MI, Shveygert M, Brown M, Gromeier M. Mitotic phosphorylation of eukaryotic initiation factor (eIF) 4G1 at Ser1232 by Cdk1:Cyclin B inhibits eIF4A helicase complex binding with RNA. Mol Cell Biol. 2013 doi: 10.1128/MCB.01046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lawson SK, Dobrikova EY, Shveygert M, Gromeier M. p38alpha mitogen-activated protein kinase depletion and repression of signal transduction to translation machinery by miR-124 and-128 in neurons. Mol Cell Biol. 2013;33(1):127–135. doi: 10.1128/MCB.00695-12. PMCID: PMC3536301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shveygert M, Kaiser C, Bradrick SS, Gromeier M. Regulation of eukaryotic initiation factor 4E (eIF4E) phosphorylation by mitogen-activated protein kinase occurs through modulation of Mnk1-eIF4G interaction. Mol Cell Biol. 2010;30(21):5160–5167. doi: 10.1128/MCB.00448-10. PMCID: PMC2953056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilkins C, Gale M., Jr Recognition of viruses by cytoplasmic sensors. Curr Opin Immunol. 2010;22(1):41–47. doi: 10.1016/j.coi.2009.12.003. PMCID: PMC3172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282(5392):1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 57.Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N, Bell JC. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6(7):821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 58.Worthington M, Aaronson SA. Interferon system in cells from human tumors and from persons predisposed to cancer. Infect Immun. 1971;3(3):424–428. doi: 10.1128/iai.3.3.424-428.1971. PMCID: PMC416169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Desmyter J, Melnick JL, Rawls WE. Defectiveness of interferon production and of rubella virus interference in a line of African green monkey kidney cells (Vero) J Virol. 1968;2(10):955–961. doi: 10.1128/jvi.2.10.955-961.1968. PMCID: PMC375423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Augustine CK, Yoo JS, Potti A, Yoshimoto Y, Zipfel PA, Friedman HS, Nevins JR, Ali-Osman F, Tyler DS. Genomic and molecular profiling predicts response to temozolomide in melanoma. Clin Cancer Res. 2009;15(2):502–510. doi: 10.1158/1078-0432.CCR-08-1916. [DOI] [PubMed] [Google Scholar]
- 61.Hou J, Zhou Y, Zheng Y, Fan J, Zhou W, Ng IO, Sun H, Qin L, Qiu S, Lee JM, Lo CM, Man K, Yang Y, Yang Y, Yang Y, Zhang Q, Zhu X, Li N, Wang Z, Ding G, Zhuang SM, Zheng L, Luo X, Xie Y, Liang A, Wang Z, Zhang M, Xia Q, Liang T, Yu Y, Cao X. Hepatic RIG-I predicts survival and interferon-alpha therapeutic response in hepatocellular carcinoma. Cancer Cell. 2014;25(1):49–63. doi: 10.1016/j.ccr.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 62.Agol VI. Cytopathic effects: virus-modulated manifestations of innate immunity? Trends Microbiol. 2012;20(12):570–576. doi: 10.1016/j.tim.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441(7089):101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 64.Morrison JM, Racaniello VR. Proteinase 2Apro is essential for enterovirus replication in type I interferon-treated cells. J Virol. 2009;83(9):4412–4422. doi: 10.1128/JVI.02177-08. PMCID: PMC2668472. [DOI] [PMC free article] [PubMed] [Google Scholar]