Abstract
Background & Aims
We investigated whether antimicrobial prophylaxis alters the incidence of bloodstream infection in patients with acute liver failure (ALF), and whether bloodstream infections affect overall mortality within 21 days after development of ALF.
Methods
We performed a retrospective cohort analysis of 1551 patients with ALF enrolled by the US Acute Liver Failure Study Group from January 1998 through November 2009. We analyzed data on infections in the first 7 days after admission and the effects of prophylaxis with antimicrobial drugs on development of bloodstream infections and 21-day mortality.
Results
In our study population, 600 patients (39%) received antimicrobial prophylaxis and 226 (14.6%) developed at least 1 bloodstream infection. Exposure to antimicrobial drugs did not affect the proportion of patients who developed bloodstream infections (12.8% in patients with prophylaxis vs 15.7% non-prophylaxed; P=.12) but a greater percentage who received prophylaxis received liver transplants (28% vs 22%; P=.01). After adjusting for confounding factors, overall mortality within 21 days was independently associated with age (odds ratio [OR]=1.014), model for end-stage liver disease score at admission (OR=1.078), and vasopressor administration at admission (OR=2.499). Low grade of coma (OR=0.47) and liver transplantation (OR=0.101) reduced mortality. Although bloodstream infection was significantly associated with 21-day mortality (P=.004), an interaction between bloodstream infection and etiology was detected: blood stream infection affected mortality to a greater extent in non-acetaminophen ALF patients (OR=2.03) than in acetaminophen ALF patients (OR=1.14).
Conclusions
Based on a large, observational study, antimicrobial prophylaxis does not reduce incidence of bloodstream infection or mortality within 21 days of ALF. However, bloodstream infections were associated with increased 21-day mortality in patients with ALF—to a greater extent in patients without than with acetaminophen-associated ALF. Our findings do not support routine use of antimicrobial prophylaxis in patients with ALF.
Keywords: liver damage, APAP, antibiotic, compensatory anti-inflammatory response syndrome, systemic inflammatory response syndrome
Introduction
Patients with acute liver failure (ALF) are susceptible to infection due to multiple immunological deficits. Despite this, it is difficult to diagnose significant infections in ALF given the similarities in hemodynamic profile compared with septic shock 1. The relationship between infection and development of the Systemic Inflammatory Response Syndrome (SIRS) and multiorgan dysfunction (MOD) in ALF have been debated 2. The physiologic profile of SIRS is characterized by increases in both pro-inflammatory 3, 4 and anti-inflammatory cytokines 5, 6, culminating in a blunted immune response to microbial infection. Tolerance of circulating monocytes to bacterial endotoxins further impedes host immunity 7. Reduced production of HLA-DR and pro-inflammatory cytokines, along with augmented levels of the anti-inflammatory IL-10 (compensatory anti-inflammatory response, CARS), are associated with increased incidence of infection and inferior outcomes8.
Despite its physiological plausibility, the impact of infection in ALF patients remains to be defined. While several studies suggest poorer outcomes in ALF subjects with SIRS criteria, bloodstream infection (BSI) has not been linked to impaired survival although it is frequently assumed to be so 9–11. Similarly, while antimicrobial prophylaxis or empirical therapy may prevent or treat early infection, no study to date has documented a clear mortality benefit.
The Acute Liver Failure Study Group (ALFSG) has collected detailed clinical and laboratory information on more than 2,000 patients with ALF over the past 13 years. We performed a retrospective, cohort study utilizing the ALFSG database to answer the following questions:
What is the impact of antimicrobial prophylaxis on rates of infection (particularly BSI) and 21-day survival in ALF patients?
What is the association between BSI and severity of illness and 21-day survival in ALF patients?
Methods
The reporting of this study followed the STROBE guideline 12.
Study Design and Setting
We performed a retrospective cohort study of 1551 patients prospectively enrolled by the U.S. ALFSG between January 1998 and November 2009. All participating centers are tertiary academic liver transplant (LT) referral centers. The Institutional Review Board at each participating center approved all protocols.
Operational Definitions
For the purposes of this study, ALF is defined as INR ≥ 1.5 and hepatic encephalopathy within the first 26 weeks of liver disease in a patient with an acute hepatic insult 13. Hepatic encephalopathy (HE) grade is defined by the West Haven Criteria (summarized); grade 1 ~ any alteration in mentation, grade 2 being somnolent or obtunded but easily rousable or presence of asterixis, grade 3 being rousable with difficulty and, grade 4: unresponsive to deep pain14.
Participants and variables
All patients met criteria for ALF as defined above13. Standardized case report forms gathering clinical, biochemical and microbiologic data were utilized and placed in a comprehensive database. Detailed data was collected for 7 days post admission to study (unless patient discharged, received LT or died before 7-days). Information regarding LT and 21-day survival were also available.
The exposure of interest was culture-positive infection (particularly BSI) during the first seven days in the study and the primary outcome assessed was 21-day overall survival. Confounding factors assessed included age, gender, severity of liver disease (MELD), severity of illness (requirement for organ support ~ vasopressors, mechanical ventilation (MV), renal replacement therapy (RRT), HE grade (West Haven Criteria14), requirement for LT and use of antimicrobial prophylaxis. In a secondary analysis, we examined the association between anti-microbial prophylaxis (exposure) on the subsequent development of BSI and 21-day mortality.
Data sources/measurement
As per standard intensive care unit (ICU) protocols, standard aerobic and anaerobic (2 × 10 ml of blood) paired samples were taken on admission from new central lines inserted as well as one peripheral site. Blood cultures were also taken upon clinical suspicion of BSI (presence of fever, SIRS features, hypotension, abnormalities of central line site). Urine and sputum (tracheal aspirates for intubated patients) were obtained upon admission and when clinically indicated (unexplained leukocytosis or new infiltrates on chest x-ray). Antibiotics were recorded as either prophylaxis (absence of positive cultures) or treatment on case report forms. Choice of antimicrobial regimen was individualized according to physician discretion. Culture results were documented for a maximum of 7 days after enrollment.
Positive blood, urine or sputum cultures were defined as isolation of recognized pathogenic microorganisms – both bacterial and fungal were used as the criteria for infection. The microbiological database recorded isolation of S. aureus, S. pneumonia, E. coli, Klebsiella pneumoniae, and Candida species. Additional organisms isolated via culture were classified as “other”; a manual review of respective case report forms to further characterize these infections was performed. The absence of urinalysis data and chest x-ray findings precluded fulfillment of diagnostic criteria for urinary tract infections and pneumonia. Use of antimicrobial therapy for prophylactic or therapeutic intent was specified on the case reporting form during the initial 7-days of study; however, the decision to use prophylactic antibiotics was made at each study site according to local protocol. Culture results and sensitivities were used to tailor antimicrobial therapy as indicated. Outcomes including survival and requirement of liver transplantation were recorded.
Statistical Methods
Statistical analyses were performed using SAS Version 9.2 (SAS Institute, Cary, NC). Descriptive statistics were used to report demographics, antimicrobial prophylaxis, presence of infection (blood, urine, sputum) and other variables describing illness severity, treatment, and outcomes. Differences in categorical variables between groups were tested using the chi-square test with one degree of freedom and a two-sided significance level of 0.05. Differences in continuous variables were determined using the Wilcoxon rank sum test and student t-test and presented as medians (Interquartile range) or means (standard deviations) after normality testing. Logistic regression analysis was used to study the effects of pre-specified prognostic variables on the a) probability of 21-day mortality and b) development of BSI. Survival was defined as dichotomous outcome - alive at 21-days after enrollment into the Registry (mortality ~ converse). Pre-specified variables included in the final model were age, etiology of ALF, HE grade on the day of admission to the registry, MELD score (admission), development of BSI during the seven-day data collection period, receiving antimicrobial prophylaxis, and receiving a transplant during the 21-days after entry to study. Etiology was collapsed into two groups: Acetaminophen toxicity (APAP) and all other etiologies (non-APAP). HE grade was categorized into two groups: low (grade 1 or 2) and high (grade 3 or 4). Variables that achieved a statistical significance of p=0.10 on univariable analysis (see Table 3) and were not collinear with other variables were included in the multivariable model. The inclusion of interaction terms were guided by clinical relevance and statistical significance of p <0.15. Model performance was assessed using the c-statistic and the Hosmer-Lemeshow test for goodness of fit. Multivariate associations are reported as odds ratios (OR) with 95% confidence limits.
Table 3.
Multivariable analysis of 21 day mortality for 1452 patients with Acute Liver Failure
| Univariate | Multivariate (N=1452, c=0.827) | ||||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | Included in model? |
OR | 95% CI | p-value | |
| Age | 1.021 | 1.013 – 1.028 | <0.0001 | Yes | 1.014 | 1.005 – 1.024 | 0.003 |
| APAP etiology* | 0.693 | 0.556 – 0.864 | 0.0011 | Yes | <0.0001 | ||
| Low Coma grade (admission) | 0.34 | 0.271 – 0.426 | <0.0001 | Yes | 0.470 | 0.330 – 0.670 | <0.0001 |
| MELD (admission) | 1.073 | 1.060 – 1.087 | <0.0001 | Yes | 1.078 | 1.063 – 1.094 | <0.0001 |
| Mechanical Ventilation (admission) | 2.967 | 2.366 – 3.719 | <0.0001 | Yes | 1.398 | 0.970 – 2.017 | 0.0726 |
| Vasopressors (admission) | 4.272 | 3.274 – 5.574 | <0.0001 | Yes | 2.499 | 1.773 – 3.521 | <0.0001 |
| Antimicrobial prophylaxis | 1.017 | 0.814 – 1.271 | 0.8787 | Yes | 0.892 | 0.677 – 1.175 | 0.42 |
| BSI* | 1.740 | 1.300 – 2.327 | 0.0002 | Yes | 0.004 | ||
| Transplant (during 21 days) | 0.192 | 0.134 – 0.274 | <0.0001 | Yes | 0.101 | 0.066 – 0.154 | <0.0001 |
| APAP*BSI | Yes | 0.11 | |||||
- Statistical significance at alpha=0.05 (p-value), OR ~ Odds Ratio, 95% CI ~ 95% Confidence Intervals.
- Abbreviations: APAP ~ Acetaminophen, BSI ~ bloodstream infection, Non-APAP ~ Non-acetaminophen
- Etiology was stratified as APAP vs. non-APAP etiologies
- Low HE grade = Grade One or Two (comparison to High HE grade (Three or Four) by West Haven Criteria14
- *Renal replacement therapy was not significant on univariable analysis (See Table 2).
- Etiology (APAP) and presence of BSI both significantly impacted 21-day mortality in the univariate models and there was a significant interaction in the multivariate model.
- In the presence of the interaction, BSI was independently associated with increased 21-day mortality:
- Non-APAP ALF patients OR 2.034 (1.257–3.292)
- APAP ALF patients OR 1.136 (0.672–1.922)
- In a separate mortality model for APAP-ALF patients, presence of BSI was NOT independently associated with 21-day mortality (p=0.68)
- 99 of 1551 patients had missing data
Results
Review of microbiology
Baseline characteristics of 1551 enrolled ALF patients are shown in Table 1. The most common etiology was acetaminophen (n=719, 46%). A total of 531 (34%) patients experienced at least one culture-documented infection and 226 (14.6%) patients had at least one episode of bloodstream infection (BSI), 223 (14.4%) had at least one positive sputum culture/tracheal aspirate, and 258 patients (16.6%) had at least one positive urine culture. Amongst BSI ALF patients, 35.4% (n=80) were infected with a gram-positive isolate, 16.8%, (38) gram-negatives, 1% (2) were polymicrobial (gram positive/gram negative) and 9% (20) had fungemia. Eighty-six (38%) of BSI infected patients were classified as “other” and were therefore unspecified, despite manual review.
Table 1.
Baseline characteristics of 1551 ALF patients stratified for antimicrobial prophylaxis
| N | Total Cohort (N=1551) |
Prophylaxis (N=600) |
No Prophylaxis (N=951) |
p-value | |||
|---|---|---|---|---|---|---|---|
| Age | 1551 | 39.0 (29.0–50.0) | 38.0 (29.0–50.0) | 39.0 (29.0–51.0) | 0.31 | ||
| Female | 1551 | 1066 (68.7%) | 411 (68.5%) | 655 (68.9%) | 0.88 | ||
| Etiology | 1551 | 0.60 | |||||
| APAP | 719 (46.4%) | 271 (45.2%) | 448 (47.1%) | ||||
| Viral Hepatitis | 153 (9.9%) | 65 (10.8%) | 88 (9.3%) | ||||
| DILI | 174 (11.2%) | 69 (11.5%) | 105 (11.0%) | ||||
| Indeterminate | 206 (13.3%) | 86 (14.3%) | 120 (12.6%) | ||||
| Other | 299 (19.3%) | 109 (18.2%) | 190 (20.0%) | ||||
| Biochemistry* | |||||||
| APACHE II | 430 | 16.0 (10.0–21.0) | N/A | 16.0 (10.0–21.0) | |||
| MELD | 1485 | 31.7 (24.8–38.7) | 32.4 (25.5–39.8) | 31.3 (24.3–37.9) | 0.01 | ||
| INR | 1496 | 2.7 (2.0–4.2) | 2.7 (2.0–4.1) | 2.7 (2.0–4.3) | 0.49 | ||
| Bilirubin (mg/dl) | 1542 | 7.3 (3.9–20.5) | 7.9 (4.3–22.3) | 6.9 (3.7–19.6) | 0.01 | ||
| Creatinine (mg/dl) | 1548 | 1.6(0.9–3.1) | 1.8 (1.0–3.3) | 1.5 (0.8–2.9) | 0.002 | ||
| Lactate (mg/dl) | 799 | 4.4 (2.6–9.7) | 4.2 (2.6–8.7) | 4.7 (2.6–10.5) | 0.08 | ||
| ALT | 1534 | 1983 (649–4510) | 1944.0 (627–4305) | 2003 (684–4720) | 0.20 | ||
| WBC | 1542 | 10.5 (7.1–15.2) | 10.4 (6.8–15.5) | 10.6 (7.2–15.1) | 0.32 | ||
| Platelet Count | 1539 | 132 (86–195) | 129 (82–185) | 133 (91–201) | 0.03 | ||
| Organ support | |||||||
| Mechanical ventilation | |||||||
| Admission* | 1545 | 707 (45.8%) | 309 (51.6%) | 398 (42.1%) | <0.001 | ||
| 7-days** | 1551 | 944 (60.9%) | 406 (67.7%) | 538 (56.6%) | <0.001 | ||
| Vasopressors | |||||||
| Admission* | 1522 | 293 (19.3%) | 145 (24.6%) | 148 (15.9%) | <0.001 | ||
| 7-days** | 1543 | 513 (33.3%) | 240 (40.1%) | 273 (28.9%) | <0.001 | ||
| Renal Replacement Therapy | |||||||
| Admission* | 1536 | 321 (20.9%) | 175 (29.3%) | 146 (15.6%) | <0.001 | ||
| 7-days** | 1544 | 522 (33.8%) | 257 (42.9%) | 265 (28.0%) | <0.001 | ||
| Coma Grade* | 1547 | <0.001 | |||||
| 1 or 2 (Low) | 807 (52.2%) | 271 (45.2%) | 536 (56.5%) | ||||
| 3 or 4 (High) | 740 (47.8%) | 328 (54.8%) | 412 (43.5%) | ||||
| Infection** | |||||||
| Bloodstream | 1551 | 226 (14.6%) | 77 (12.8%) | 149 (15.7%) | 0.12 | ||
| Sputum | 1551 | 223 (14.4%) | 82 (13.7%) | 141 (14.8%) | 0.53 | ||
| Urine | 1551 | 258 (16.6%) | 92 (15.3%) | 166 (17.5%) | 0.27 | ||
| LTx Listing | Listed for LT | 1547 | 611 (39.5%) | 263 (43.8%) | 348 (36.6%) | 0.0048 | |
| Listed for LT (APAP) | 719 | 180 (25.0%) | 84/271(31.0%) | 96/448 (21.4%) | 0.0041 | ||
| Listed for LT (non-APAP) | 828 | 431 (52.1%) | 179/328 (54.4%) | 252/500 (50.1%) | 0.48 | ||
| Outcomes*** | |||||||
| Liver transplant | 1551 | 373 (24.1%) | 167 (27.8%) | 206 (21.7%) | 0.006 | ||
| Alive at 21 days | 1551 | 1084 (69.9%) | 418 (69.7%) | 666 (70.0%) | 0.88 | ||
- Normally distributed data presented as mean (Standard deviation)
- Non-normally distributed data presented as Median (IQR)
- Categorical data presented as percentage (chi-square test)
- 600 ALF patients received antimicrobial prophylaxis
- *~On admission, **~During 7-day inpatient phase ***~21-day post-enrollment
Outcomes: Impact of antimicrobial prophylaxis
Six hundred (39%) ALF patients had received antimicrobial prophylaxis at some point in their course (Table 1). More than 95% of participating sites (27/28) employed antimicrobial prophylaxis. Of the 600 ALF patients receiving prophylaxis 47% (n=283) received extended spectrum beta-lactam (e.g. piperacillin-tazobactam, ticarcillin-clavulinate), 39% (235) vancomycin, 27% (160) fluoroquinolones, and 20% (121) 3rd or 4th generation cephalosporins. 19 % (116) of patients were prophylactically on fluconazole (Supplementary File 1). On admission to study, ALF patients receiving antimicrobial prophylaxis had higher MELD score (32.4 vs. 31.3, p=0.01), bilirubin (7.9 vs. 6.9 mg/dl, p=0.01) and creatinine levels (1.8 vs. 1.5 mg/dl, p=0.02). These ‘prophylaxed’ patients were more likely to have higher coma grades (Grade III/IV 55% vs. 43%, p< 0.001) and were more likely to require organ support (MV ~ 52% vs. 42%, vasopressors 25% vs. 16%, and RRT ~ 29% vs. 16%, p<0.001 for all comparisons) on admission. There was no significant difference in the probability of having a BSI based on receiving prophylaxis (12.8%) or not (15.7% p=0.12). Likewise, there were no differences in rates of positive sputum cultures/tracheal aspirates (13.7% vs. 14.8%; p=0.53), or bacteruria (15.3% vs. 17.5%; p =0.27) respectively.
In the APAP subgroup (n=719), more patients on antimicrobial prophylaxis were listed for LT (31% vs. 21.4%, p=0.004). Overall, patients receiving antimicrobial prophylaxis were more likely to go on to LT (27.8% vs. 21.7%, p=0.006) but there was no difference in unadjusted overall 21-day survival (70% vs. 70%, p=0.88).
Association between bloodstream infection (BSI) and 21-day survival
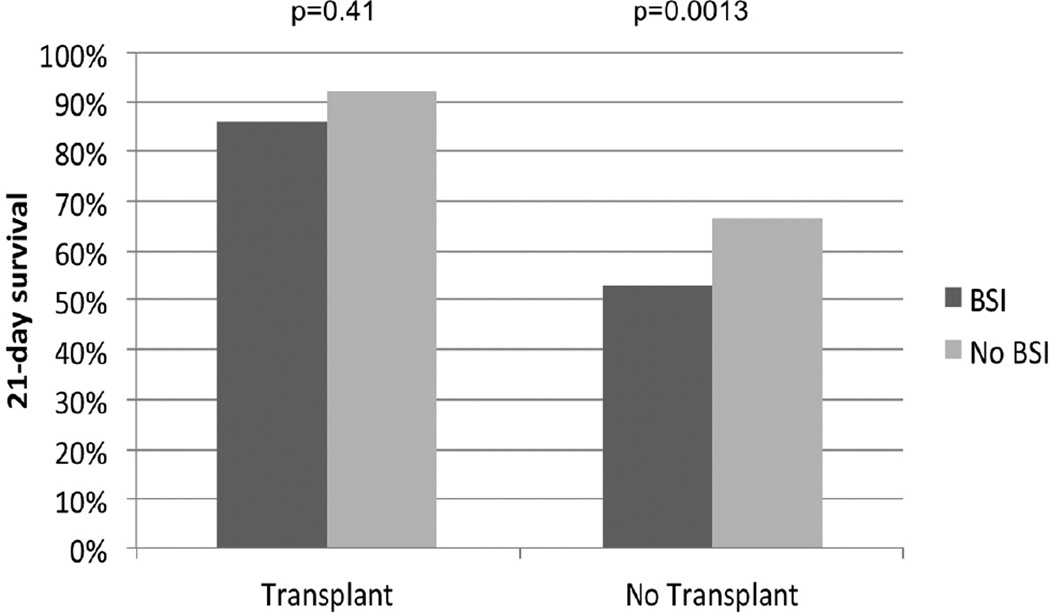
Data on 226 ALF patients who developed BSI (14.6%) are shown in Table 2. ALF patients developing BSI had decreased 21-day survival (59% vs. 72%; p=0.0002) compared to those without BSI. This effect on 21-day survival was seen particularly amongst non-LT patients (BSI 53% vs. No BSI 65%, p=0.0013) but not in LT patients (86% vs. 91%, p=0.41, Figure 1). The difference in frequency of LT between ALF patients with and without BSI was not significant (19% vs. 25%; p=0.06).
Table 2.
Comparison of 226 patients with bloodstream infections with 1325 patients without BSI
| BSI (N=226) | No BSI (N=1325) | p-value | |||
|---|---|---|---|---|---|
| Age | 40.5 (30.0–51.0) | 39.0 (29.0–50.0) | 0.49 | ||
| Female | 143 (63.3%) | 923 (69.7%) | 0.06 | ||
| Etiology | 0.30 | ||||
| APAP | 100 (44.3%) | 619 (46.7%) | |||
| Viral Hepatitis | 15 (6.6%) | 138 (10.4%) | |||
| DILI | 27 (12.0%) | 147 (11.1%) | |||
| Indeterminate | 34 (15.0%) | 172 (13.0%) | |||
| Other | 50 (22.1%) | 249 (18.8%) | |||
| Biochemistry* | |||||
| APACHE II | 17.0 (14.0–22.0) | 15.0 (10.0–21.0) | 0.02 | ||
| MELD | 31.7 (26.0–38.1) | 31.7 (24.5–38.8) | 0.96 | ||
| INR | 2.6 (1.9–4.1) | 2.7 (2.0–4.2) | 0.14 | ||
| Bilirubin (mg/dl) | 7.6 (4.1–20.2) | 7.3 (3.7–20.5) | 0.50 | ||
| Creatinine (mg/dl) | 1.8 (1.0–3.1) | 1.6 (0.9–3.1) | 0.14 | ||
| Lactate (mg/dl) | 4.9 (2.8–10.9) | 4.4 (2.5–9.5) | 0.10 | ||
| ALT (U/L) | 1461.0 (492.5–3794.5) | 2076.5 (674.0–4645.0) | 0.02 | ||
| WBC | 10.3 (6.8–16.3) | 10.5 (7.2–15.1) | 0.86 | ||
| Platelet Count | 120.0 (81.0–187.0) | 133.0 (87.0–197.0) | 0.07 | ||
| Organ Support | |||||
| Mechanical Ventilation | |||||
| Admission* | 128 (56.9%) | 579 (43.9%) | <0.001 | ||
| 7-days** | 164 (72.6%) | 780 (58.9%) | 0.0001 | ||
| Vasopressors | |||||
| Admission* | 51 (23.0%) | 242 (18.6%) | 0.13 | ||
| 7-days** | 95 (42.4%) | 418 (31.7%) | 0.002 | ||
| Renal Replacement Therapy | |||||
| Admission* | 50 (22.3%) | 271 (20.7%) | 0.57 | ||
| 7-days** | 79 (35.1%) | 443 (33.6%) | 0.65 | ||
| Antimicrobial Prophylaxis** | 77 (34.1%) | 523 (39.5%) | 0.12 | ||
| Coma Grade* | 0.002 | ||||
| 1 or 2 (Low) | 96 (42.5%) | 711 (53.8%) | |||
| 3 or 4 (High) | 130 (57.5%) | 610 (46.2%) | |||
- Normally distributed data presented as mean (Standard deviation)
- Non-normally distributed data presented as Median (IQR)
- Categorical data presented as percentage (chi-square test)
- *~On admission
- **~During 7-day inpatient phase
- ***~21-day post-enrollment
Figure 1.
Comparison of 21-day overall survival (post-transplant and transplant free) between ALF patients who did and did not acquire a BSI.
• Development of BSI conferred worse 21-day survival in non-transplanted ALF patients (53% vs. 65%, p=0.0013) but not in transplanted ALF patients (86% vs. 91%, p=0.41).
In comparing ALF patients with BSI and without BSI (Table 2), ALF patients who went on to develop BSI had higher HE (Grade III/IV 57.5% vs. 46.2%, p=0.002) on admission and were more likely to require MV (57% vs. 44%, p<0.001).
Neither positive sputum cultures/tracheal aspirates (69% vs. 70%; p=0.70) nor urine cultures (71% vs. 70%; p=0.60) significantly impacted survival and were not analyzed further.
Multivariable analysis: Predictors of 21-day mortality
Multivariable logistic regression analysis was performed on the entire cohort of 1551 subjects to determine if the probability of mortality at three weeks was affected by pre-specified prognostic variables (Table 3). The final model (n=1452, 99 had missing data) included the following variables: age, etiology (APAP vs. Non-APAP), HE grade (low vs. high), MELD (admission), MV (admission), vasopressors (admission), antimicrobial prophylaxis, BSI and LT. After controlling for confounding, antimicrobial prophylaxis did not confer a significant effect on 21-day mortality. Furthermore, there was no significant interaction between prophylaxis and BSI (i.e. prophylaxis was not associated with decreased rates of infection). Variables which had a significant association with 21-day mortality included Age (Odds Ratio ~ 1.014 per year (95% CI 1.005– 1.024), MELD (admission) (OR 1.078 per increment (1.063–1.094), and requirement for vasopressors on admission (OR 2.499 (1.773–3.521). Low HE grade on admission (OR 0.47 (0.33–0.67) and receipt of LT (OR 0.101 (0.066–0.154)) were protective. The model c-statistic was 0.83 indicating good predictive accuracy. When site was added as a fixed effect to the multivariable model, performance did not significantly improve (c-statistic 0.84, data not shown).
While BSI did significantly impact 21-mortality (P=0.004) in the overall model, there was a statistically significant quantitative interaction between etiology (APAP/non-APAP) and presence of BSI (P=0.11). The magnitude of the association between BSI and increased 21-day mortality was greater in non-APAP ALF patients (OR 2.034(1.257–3.292)) than in APAP ALF patients (OR 1.136 (0.672–1.922)). Further exploration of the APAP population in a separate mortality model showed that BSI was not significant for predicting death (p=0.68).
Multivariate analysis: Predictors of bloodstream infection
The relationship between covariates and the development of BSI was explored with multivariable logistic regression using similar variables as included in the previous model. The model had poor fit and did not show any statistically significant relationships (data not shown).
DISCUSSION
Key findings
In this study, ALF patients who received antimicrobial prophylaxis displayed increased severity of illness on admission (MELD, HE grade and requirement for organ support). Use of antimicrobial prophylaxis did not appear either to alter rates of infection or to improve overall survival on crude or adjusted (multivariable) analysis. Nonetheless, patients who went on to LT were more likely to have received antimicrobial prophylaxis. The presence of BSI was associated with increased severity of illness (HE grade, requirement for organ support) and 21-day mortality on univariable analysis, an effect that was more pronounced in non-acetaminophen etiologies of ALF after adjusting for confounding factors. In all ALF patients, age, MELD, and the requirement for vasopressors on admission were also independently associated with increased 21-day mortality.
Comparison with previous studies
Prior reports of the incidence of ALF-related infections have varied considerably, with bacteremia reported between 22% and 80% 15–17. Early studies emphasized gram-positive infections likely related to pulmonary sepsis and concerns of tracheal suctioning exacerbating intracranial hypertension 18. Studies by Rolando et al. noted the negative impact of bacteremia on survival in ALF, with attributable mortality ranging from 10 to 52% 19–21. In contrast, two recent studies failed to demonstrate any significant impact due to infection nonetheless with a shift towards more gram-negative pathogens 11, 22. Our study demonstrates that the development of BSI is associated with increased 21-day mortality in ALF patients, particularly in non-APAP etiologies of ALF after controlling for confounding. Non-APAP patients often follow a subacute pattern (encephalopathy >8 weeks after development of synthetic dysfunction) and are at lower risk of developing complications (cerebral edema, acute kidney injury) compared with APAP (hyperacute liver failure) patients 13. Although not conclusively shown, the prolonged course of illness and the development of the Compensatory anti-inflammatory response (CARS); a relative state of immunosuppression may explain the increased risk of BSI in this group 2. In contrast, for APAP patients with hyperacute liver failure (hepatic encephalopathy within seven days of hepatic dysfunction) that demonstrate a high risk of cerebral edema but also a high rate of spontaneous recovery, the severity of the ALF syndrome, and not infectious complications, may be driving mortality. Thus, BSI appears to play an important role only in slower evolving or sub-acute ALF etiologies 23.
Previous studies suggest a relationship between infection, HE grade, and outcomes in ALF. Higher rates of infection have been shown in subjects with higher HE grade and escalating number of SIRS components 9. Vaquero et al. demonstrated a link between culture-positive infection and progression to advanced coma grade 11. In this cohort we found an association between advanced HE grade on admission, the subsequent development of BSI and decreased 21-day survival (low coma grade was protective after adjusting for confounding factors). Coma grade would appear to be a surrogate for increased susceptibility, but this cannot be concluded from our data.
The utility of antimicrobial prophylaxis in ALF remains controversial. Previous studies have shown the use of antimicrobial prophylaxis decreases the incidence of infection in ALF patients without an effect on mortality 20, 24. Similarly, while our results suggest that patients with an increasing burden of illness/MOD on admission are more likely to be placed on antimicrobial prophylaxis (Table 1), its use did not affect rates of BSI or 21-day mortality in ALF patients after controlling for severity of illness (organ support, MELD). While data is limited, the United States Acute Liver Failure Study Group has previously recommended antimicrobial prophylaxis in patients with advanced HE, refractory hypotension, the presence of SIRS components and for patients listed for LT 25. However the most recent guidelines from the American Association for the Study of Liver Disease do not advocate the routine use of prophylaxis 26.
Study limitations and strengths
This study has limitations that warrant consideration. First, while we included data from 23 liver transplant centers across the United States, it is a retrospective analysis of prospectively collected data and thus is observational in nature, potentially predisposing to bias and residual confounding. Only association and not causation can be inferred. Data on infection was only collected during the first 7-days of study and due to the retrospective format, we cannot conclusively state that all infections included in this analysis were clinically significant (colonization vs. infection). We were also unable to determine presence or duration of antibiotics prior to referral if not present on day 1 (admission) of data collection or subsequent to this day in the registry. Finally, despite a complete review of electronic and original paper charting we were unable to obtain complete microbiological information on all pathogens and as such cannot comment on changes in trends of bacterial flora (i.e., gram positive vs. gram negative). However to date, the impact of clinically significant infection on outcomes such as transplant and mortality have not been evaluated in a prospective and/or clinical trial setting. Hence there is value in replicating observational studies of this nature to assess for consistency and generalizability across studies. To our knowledge this is the largest retrospective study to date assessing the role of infection in ALF. Of interest, our study validates previous studies by Rolando, which did not show a significant benefit with antimicrobial prophylaxis 27.
Conclusions
ALF patients who received antimicrobial prophylaxis had evidence of significant organ dysfunction on admission. While antimicrobial prophylaxis did not appear to either alter rates of infection or to improve overall survival on unadjusted or adjusted (multivariable) analysis, patients who went on to LT were more likely to have received antimicrobial prophylaxis. The presence of BSI was associated with increased 21-day mortality with a greater impact in patients with non-APAP ALF patients after controlling for confounding. Age, MELD, and the requirement for vasopressors on admission were independently associated with increased 21-day mortality in all ALF patients. These findings do not support the routine use of antimicrobial prophylaxis in ALF patients.
Supplementary Material
Acknowledgements
This study was funded by National Institutes of Health grant (DK U-01 58369) for the United States Acute Liver Failure Study Group provided by the National Institute of Diabetes and Digestive and Kidney Diseases. Members and institutions participating in the Acute Liver Failure Study Group 1998–2011 are as follows: W.M. Lee, M.D. (Principal Investigator); Anne M. Larson, M.D., Iris Liou, M.D., University of Washington, Seattle, WA; Timothy Davern, M.D., University of California, San Francisco, CA (current address: California Pacific Medical Center, San Francisco, CA), Oren Fix, M.D., University of California, San Francisco; Michael Schilsky, M.D., Mount Sinai School of Medicine, New York, NY (current address: Yale University, New Haven, CT); Timothy McCashland, M.D., University of Nebraska, Omaha, NE; J. Eileen Hay, M.B.B.S., Mayo Clinic, Rochester, MN; Natalie Murray, M.D., Baylor University Medical Center, Dallas, TX; A. Obaid S. Shaikh, M.D., University of Pittsburgh, Pittsburgh, PA; Andres Blei, M.D., Northwestern University, Chicago, IL (deceased), Daniel Ganger, M.D., Northwestern University, Chicago, IL; Atif Zaman, M.D., University of Oregon, Portland, OR; Steven H.B. Han, M.D., University of California, Los Angeles, CA; Robert Fontana, M.D., University of Michigan, Ann Arbor, MI; Brendan McGuire, M.D., University of Alabama, Birmingham, AL; Raymond T. Chung, M.D., Massachusetts General Hospital, Boston, MA; Alastair Smith, M.B., Ch.B., Duke University Medical Center, Durham, NC; Robert Brown, M.D., Cornell/Columbia University, New York, NY; Jeffrey Crippin, M.D., Washington University, St Louis, MO; Edwin Harrison, Mayo Clinic, Scottsdale, AZ; Adrian Reuben, M.B.B.S., Medical University of South Carolina, Charleston, SC; Santiago Munoz, M.D., Albert Einstein Medical Center, Philadelphia, PA; Rajender Reddy, M.D., University of Pennsylvania, Philadelphia, PA; R. Todd Stravitz, M.D., Virginia Commonwealth University, Richmond, VA; Lorenzo Rossaro, M.D., University of California Davis, Sacramento, CA; Raj Satyanarayana, M.D., Mayo Clinic, Jacksonville, FL; and Tarek Hassanein, M.D., University of California, San Diego, CA. The University of Texas Southwestern Administrative Group included Grace Samuel, Ezmina Lalani, Carla Pezzia, and Corron Sanders, Ph.D., Nahid Attar, Linda S. Hynan, Ph.D., and the Medical University of South Carolina Data Coordination Unit included Valerie Durkalski, Ph.D., Wenle Zhao, Ph.D., Catherine Dillon, Holly Battenhouse and Tomoko Goddard
Abbreviations
- ALF
Acute Liver Failure
- APAP
Acetaminophen
- BSI
Bloodstream infection
- CARS
Compensatory anti-inflammatory response syndrome
- HE
Hepatic encephalopathy
- ICU
Intensive care unit
- LT
Liver transplantation
- MELD
Model of End-stage Liver Disease score
- MOD
Multiorgan dysfunction
- MV
Mechanical ventilation
- Non-APAP
Non-acetaminophen etiology
- RRT
Renal replacement therapy
- SIRS
Systemic inflammatory response syndrome
- US ALFSG
United States Acute Liver Failure Study Group
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: none
Format: This paper followed the STROBE guideline for reporting retrospective studies (BMJ 2007).
Author Contributions:
C.J.K. - Performed data analysis, participated in study design, drafted and revised the final manuscript.
J.C. - Participated in the design of the study, gathered data, performed data analysis, and contributed to the drafting of the manuscript.
H.B.- Participated in study design, performed statistical analysis and significantly edited the manuscript.
V.D.- Performed statistical analysis and significantly edited the manuscript.
C.S.- Was responsible for overall data retrieval, participated in the study design and editing of the manuscript.
W.M.L.- Conceived the idea of the study, participated in study design, assisted with study analysis and significantly edited the final manuscript.
All authors have reviewed and approved the final manuscript.
This work was performed jointly at the University of Texas-Southwestern (Dallas, USA) and the University of Alberta (Edmonton, Canada).
Contributor Information
Constantine J. Karvellas, Email: dean.karvellas@ualberta.ca.
Jorge Cavazos, Email: jorgecavazos1@gmail.com.
Holly Battenhouse, Email: battenho@musc.edu.
Valerie Durkalski, Email: durkalsv@musc.edu.
Jody Balko, Email: jody.balko@utsouthwestern.edu.
Corron Sanders, Email: Corron.Sanders@utsouthwestern.edu.
William M. Lee, Email: William.Lee@utsouthwestern.edu.
REFERENCES
- 1.Trewby PN, Williams R. Pathophysiology of hypotension in patients with fulminant hepatic failure. Gut. 1977;18:1021–1026. doi: 10.1136/gut.18.12.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoniades CG, Berry PA, Wendon JA, et al. The importance of immune dysfunction in determining outcome in acute liver failure. J Hepatol. 2008;49:845–861. doi: 10.1016/j.jhep.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Calandra T, Gerain J, Heumann D, et al. High circulating levels of interleukin-6 in patients with septic shock: evolution during sepsis, prognostic value, and interplay with other cytokines. The Swiss-Dutch J5 Immunoglobulin Study Group. Am J Med. 1991;91:23–29. doi: 10.1016/0002-9343(91)90069-a. [DOI] [PubMed] [Google Scholar]
- 4.Martin C, Boisson C, Haccoun M, et al. Patterns of cytokine evolution (tumor necrosis factor-alpha and interleukin-6) after septic shock, hemorrhagic shock, and severe trauma. Crit Care Med. 1997;25:1813–1819. doi: 10.1097/00003246-199711000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Friedman G, Jankowski S, Marchant A, et al. Blood interleukin 10 levels parallel the severity of septic shock. J Crit Care. 1997;12:183–187. doi: 10.1016/s0883-9441(97)90030-7. [DOI] [PubMed] [Google Scholar]
- 6.Lehmann AK, Halstensen A, Sornes S, et al. High levels of interleukin 10 in serum are associated with fatality in meningococcal disease. Infect Immun. 1995;63:2109–2112. doi: 10.1128/iai.63.6.2109-2112.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolk K, Docke WD, von Baehr V, et al. Impaired antigen presentation by human monocytes during endotoxin tolerance. Blood. 2000;96:218–223. [PubMed] [Google Scholar]
- 8.Sfeir T, Saha DC, Astiz M, et al. Role of interleukin-10 in monocyte hyporesponsiveness associated with septic shock. Crit Care Med. 2001;29:129–133. doi: 10.1097/00003246-200101000-00026. [DOI] [PubMed] [Google Scholar]
- 9.Rolando N, Wade J, Davalos M, et al. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734–739. doi: 10.1053/jhep.2000.17687. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt LE, Larsen FS. Prognostic implications of hyperlactatemia, multiple organ failure, and systemic inflammatory response syndrome in patients with acetaminophen-induced acute liver failure. Crit Care Med. 2006;34:337–343. doi: 10.1097/01.ccm.0000194724.70031.b6. [DOI] [PubMed] [Google Scholar]
- 11.Vaquero J, Polson J, Chung C, et al. Infection and the progression of hepatic encephalopathy in acute liver failure. Gastroenterology. 2003;125:755–764. doi: 10.1016/s0016-5085(03)01051-5. [DOI] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342:273–275. doi: 10.1016/0140-6736(93)91818-7. [DOI] [PubMed] [Google Scholar]
- 14.Atterbury CE, Maddrey WC, Conn HO. Neomycin-sorbitol and lactulose in the treatment of acute portal-systemic encephalopathy. A controlled, double-blind clinical trial. Am J Dig Dis. 1978;23:398–406. doi: 10.1007/BF01072921. [DOI] [PubMed] [Google Scholar]
- 15.Rolando N, Philpott-Howard J, Williams R. Bacterial and fungal infection in acute liver failure. Semin Liver Dis. 1996;16:389–402. doi: 10.1055/s-2007-1007252. [DOI] [PubMed] [Google Scholar]
- 16.Wyke RJ, Canalese JC, Gimson AE, et al. Bacteraemia in patients with fulminant hepatic failure. Liver. 1982;2:45–52. doi: 10.1111/j.1600-0676.1982.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 17.Rolando N, Harvey F, Brahm J, et al. Fungal infection: a common, unrecognised complication of acute liver failure. J Hepatol. 1991;12:1–9. doi: 10.1016/0168-8278(91)90900-v. [DOI] [PubMed] [Google Scholar]
- 18.Wade J, Rolando N, Philpott-Howard J, et al. Timing and aetiology of bacterial infections in a liver intensive care unit. J Hosp Infect. 2003;53:144–146. doi: 10.1053/jhin.2002.1363. [DOI] [PubMed] [Google Scholar]
- 19.Rolando N, Wade JJ, Fagan E, et al. An open, comparative trial of aztreonam with vancomycin and gentamicin with piperacillin in patients with fulminant hepatic failure. J Antimicrob Chemother. 1992;30:215–220. doi: 10.1093/jac/30.2.215. [DOI] [PubMed] [Google Scholar]
- 20.Rolando N, Gimson A, Wade J, et al. Prospective controlled trial of selective parenteral and enteral antimicrobial regimen in fulminant liver failure. Hepatology. 1993;17:196–201. [PubMed] [Google Scholar]
- 21.Rolando N, Wade JJ, Stangou A, et al. Prospective study comparing the efficacy of prophylactic parenteral antimicrobials, with or without enteral decontamination, in patients with acute liver failure. Liver Transpl Surg. 1996;2:8–13. doi: 10.1002/lt.500020103. [DOI] [PubMed] [Google Scholar]
- 22.Karvellas CJ, Pink F, McPhail M, et al. Predictors of bacteraemia and mortality in patients with acute liver failure. Intensive Care Med. 2009;35:1390–1396. doi: 10.1007/s00134-009-1472-x. [DOI] [PubMed] [Google Scholar]
- 23.Stravitz RT. Critical management decisions in patients with acute liver failure. Chest. 2008;134:1092–1102. doi: 10.1378/chest.08-1071. [DOI] [PubMed] [Google Scholar]
- 24.Salmeron JM, Tito L, Rimola A, et al. Selective intestinal decontamination in the prevention of bacterial infection in patients with acute liver failure. J Hepatol. 1992;14:280–285. doi: 10.1016/0168-8278(92)90171-k. [DOI] [PubMed] [Google Scholar]
- 25.Stravitz RT, Kramer AH, Davern T, et al. Intensive care of patients with acute liver failure: recommendations of the U.S. Acute Liver Failure Study Group. Crit Care Med. 2007;35:2498–2508. doi: 10.1097/01.CCM.0000287592.94554.5F. [DOI] [PubMed] [Google Scholar]
- 26.Polson J, Lee WM. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179–1197. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 27.Rolando N, Harvey F, Brahm J, et al. Prospective study of bacterial infection in acute liver failure: an analysis of fifty patients. Hepatology. 1990;11:49–53. doi: 10.1002/hep.1840110110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.