Introduction
Enteral (EN) and parenteral nutrition (PN) support is provided to intensive care unit (ICU) patients under the assumption they will slow the loss of lean body mass (LBM) loss and improve outcomes 1–3. Despite the extensive use of these feedings in critically ill populations for over four decades, evidence to support their efficaciousness is extremely limited. A 2007 meta-analysis of prospective randomized clinical trials (PRCTs) that compared EN to standard care (SC) or PN in a number of disease conditions, including critical care 4 found almost no evidence of their benefit. Only three trials compared EN to SC in critically ill populations 5–7 and all were conducted in well nourished, surgical ICU patients. No differences in mortality 7, duration of time on the ventilator 5, 6, or length of stay in the ICU 5 or hospital 7 were observed in these trials, suggesting that EN did not improve outcomes in normal nourished, surgical intensive care unit patients.
We postulated three major design flaws impeded the ability of these trials to discern the influence of nutrition support on clinically relevant outcomes. First, the “conventional wisdom” that feeding is always beneficial and withholding invasive nutritional support from malnourished patients is unethical resulted in their exclusion from most of these trials. The failure to demonstrate a positive benefit of EN on mortality or morbidity may, in part, be due to the restriction of only well-nourished participants in these trials. These individuals have little ability to show quantifiable benefit from EN. Second the outcomes selected to assess efficacy in these previous trials take many days of adequate nutritional intake to alter their course of development, yet the nutritional interventions were generally of short duration (7–10 days). Finally, participants were provided with an aggressive, invasive EN and/or PN during the catabolic phase of their hospitalization; a time when repletion was not possible. Although the nutrition support was discontinued when the critical illness resolved, it was followed by inadequate oral nutrient intake with little attention to adequacy; at time when repletion was possible. This “nutritional feast” during the phase of illness when repletion is not possible, followed by “famine” during the interval when recuperation is possible resulted in all participants receiving poor intake [i.e., receiving standard care (SC)] during their hospitalization. These previous designs likely attenuated the abilities to detect differences in any nutritional-related post-ICU outcomes examined, such as length of stay or total infections while hospitalized.
Because of these limitations in previous trials, we designed and conducted a PRCT to determine if a comprehensive nutrition program from Acute Lung Injury (ALI) diagnosis to hospital discharge could improve morbidity and influence mortality in normal and malnourished ICU patients. Patients with ALI were selected because they typically they have functional GI tracts and a high prevalence of malnutrition. We hypothesized patients randomized to receive the intensive medical nutrition intervention (IMNT) would have fewer infections, shorter hospital and ICU lengths of stay (LOS) and lower mortality than those randomized to standard care (SC).
Overview, study design and patient recruitment
This single center PRCT was originally designed to detect the influence of the provision of IMNT from ALI diagnosis to hospital discharge compared to SC on nosocomial infections in ICU patients. Secondary endpoints included days to weaning from mechanical ventilation, ICU and hospital length of stay and death. Adult (≥ 18 yrs) patients were recruited from the medical or surgical ICUs of an urban tertiary care hospital with a diagnosis of ALI, as defined by the American-European Consensus Conference (AECC)8. To improve internal validity and control disease acuity, patients were excluded if they were previously admitted to the ICU during the same hospitalization, had medical orders not to resuscitate, severely immunosuppressed (e.g., post-stem cell transplant, receiving chemotherapy), immobility or severe neurologic disease prior to admission. Within 24 hours of ALI diagnosis patients meeting eligibility criteria were approached for participation. A written informed consent was obtained from the participant or a designated surrogate prior to enrollment, an identification number was assigned, and randomization to either SC or IMNT occurred. Group assignment was determined using a computer generated list of random numbers that had been placed in sequentially numbered, sealed opaque envelopes. A non-hospital based research member, blind to all aspects of the participant’s profile, opened the enveloped and assigned the feeding group when notified of a new enrollment. Ethical approval for this study was granted by the Medical Center’s and University’s Institutional Review Boards and the study was conducted between 2009–2013.
Standard Care
Participants randomized to SC received the nutritional care provided by their physicians and registered dietitians (RDs). The protocol for SC includes nutrition assessments completed by an RD and feeding recommendations conveyed in daily multidisciplinary patient care rounds and via the electronic medical record, specifying formula, type of feeding (eg, bolus, intermittent, or continuous), initiation rate, and goal infusion. Enteral feeding devices were placed by the medical service and EN was administered at the discretion of the attending service. Per the ICU EN feeding protocol, nursing staff members held EN if gastric residual volumes exceeded 250 ccs during a 4-hour infusion period, vomiting occurred, or aspiration was suspected. As deemed appropriate by the attending service, PN was initiated when patients could not be fed via the enteral route within 72–96 hours of intubation. All efforts to transition patients from PN to EN were completed following the institutional protocols. Following extubation, oral diets were initiated and prescribed by the managing medical services. Snacks, multivitamin supplements and dietary counseling were only provided when ordered.
IMNT Intervention
Participants randomized to IMNT received all of the SC practices described above; however, several factors did specifically differ. First, EN tubes were placed more rapidly and EN was initiated within 6 hours of hemodynamic stability. Second, to improve overall energy provision the following changes were made: 1) EN infusions received were closely monitored and rates were increased to achieve estimated daily needs when feeding interruptions occurred; and 2) feedings were prescribed during a 24-hour period (eliminating bolus or intermittent feeding prescriptions). To avoid the complications of prolonged overfeeding, EN volumes were reviewed on a daily basis and altered as needed to match estimated energy needs. Following extubation, oral dietary intake was initiated as soon as swallowing allowed. Increased dietary intake was facilitated by eliminating unnecessary therapeutic dietary prescriptions, assisting with feeding, and by providing increased snacking options and meal tickets for cafeteria purchases. Additionally, the study staff visited participant during meal times to address and resolve specific feeding issues and to discuss the importance of eating during recovery.
Energy and protein estimations and intake
The use of indirect calorimetry is considered the ’gold standard’ for energy needs assessment; however, due to prohibitory percentages of fraction of inspired oxygen (ie, >50%) and the frequency of weaning trials for extubation, this was impractical in this patient population. Therefore, energy prescriptions were calculated using 30 kcal/kg admission weight or obesity-adjusted ideal body weight.9 Protein prescriptions were estimated using 1.5 g protein/kg ideal body weight for patients without obesity and using adjusted ideal body weight for patients with obesity 9–11. Measurement of energy and protein intake was assessed from physician orders and nursing graphics for daily EN and/or PN received and multivitamin/mineral supplements consumed. EN data included the formula name, composition and daily volume ordered and received. The daily PN prescription (% carbohydrate, protein and fat) ordered and received was recorded. Because propofol was used for used for sedation, the caloric contributions of this lipid-based medication were also included in the total energy intake, as appropriate. All energy provided via dextrose containing IV fluids was also included in the total energy intake. As previously described, study personnel obtained calorie counts using a multiple-pass 24-hour recall approach12. Briefly, menus were saved for each meal and reviewed with the patient and/or family members each day. Patients were asked to recall everything they had consumed at each of the meals. Clarification was sought for of any food mentioned but not on the menu, or any food that was not listed but appeared on the menu. Foods consumed between meals were also recorded. Upon collection of daily oral intake the energy and protein content was calculated using the University of Minnesota Nutrient Data System for Research.
Demographics and clinical variables
Demographic and medical information including gender, race/ethnicity, age, diagnosis, admission height and weight were collected from the electronic medical record. Body mass index (BMI) was calculated based on admission weight (kg)/height (m)2. The ICU admission Acute Physiology and Chronic Health Evaluation (APACHE) II score were used to assess severity of illness 13. These scores range from 0–71, with higher scores indicating more severe illness. The Sequential Organ Failure Assessment (SOFA) scores14, 15 assessed daily from enrollment to ICU discharge were used to characterize participants organ function while critically ill. These scores (range 0–24) are based on an individual’s respiratory, cardiovascular, hepatic, coagulation, renal and neurologic systems; higher scores indicated more severe organ compromise. Nutritional status was assessed at baseline using subjective global assessment (SGA) 16. Use of steroids, vasopressor, inotropic agents and number of transfusions for packed red blood cells and platelets were also recorded.
Venous bloods draws occurred daily between 6:00–8:00 am. Participants white blood cell count per unit volume (nl range 4.50–11.0 × 109//L), high and low serum glucose (mg/dL) levels and baseline C-reactive protein (mg/dL) were recorded. The hyper- and hypoglycemic events were defined as blood glucose levels above 180 mg/dL or below 60 mg/dL respectively, based on the results from the 2009 NICE Sugar Study17. The presence of new infections (blood, sputum, lungs, urinary tract or wounds) were identified in the laboratory reports and affirmed from the physician progress notes and discharge summaries.
Power analysis and sample size calculations were performed using Power Analysis and Sample Size (NCSS Statistical and Power Analysis Software, PASS, 2005) for the log-rank test18 based on findings reported by Rubinson et al19. On the basis of these results and a significance level of 0.05% a sample size of 200 (100/group) provided 87% power to detect a hazard ratio of 2.5 or more and was the recruitment goal for this trial.
Statistics
Standardized descriptive statistics including measures of means, median, standard deviations, ranges and standard errors for continuous variables were calculated to describe the groups. For dichotomous variables (e.g. hypo-, hyperglycemia, infections, death), chi-square tests were used to obtain unadjusted comparisons between the two groups and Student’s t-tests and Wilcoxon rank-sum tests were used for unadjusted comparisons of continuous variables (e.g. number of infections, energy and protein intake, days on mechanical ventilation) that were normally and non-normally distributed, respectively. Time until death was estimated within groups using survival analysis methods, treating discharge alive from the ICU as right-censoring. Cumulative incidence of death was estimated within groups using the Kaplan-Meier method; unadjusted comparisons between the groups were made using the log-rank test; Cox proportional hazards multiple regression was used to adjust for additional factors including BMI, age, gender, SOFA, APACHE II, ethnicity, total and daily dose of insulin, and number of hyper-and hypoglycemic days, PN and/or parenteral lipids. All analyses were done using the statistical program SAS (version 9.2, 2009, SAS Institute Inc).
Results
A total of 1028 patients that required MV between July 10, 2009 and May 1, 2013 were evaluated for potential study enrollment. The reasons for exclusion and randomization into IMNT or SC are depicted in Figure 1. The demographic and baseline clinical parameters of the 78 participants that were enrolled (40 IMNT, 38 SC) are provided in Table. 1. Overall Participants had a mean (SD) age of 55.4 yrs (17), were predominantly obese and approximately 37% were classified as malnourished (moderate or severe) by SGA. No differences in age, gender, race/ethnicity, BMI, nutritional status, baseline SOFA score, PaO2:FiO2 ratio, proportion of participants with PaO2:FiO2 < 200, baseline CRP, WBC or glucose levels occurred between the groups. The APACHE II score at ICU admission was significantly lower in IMNT than SC (23.4 vs. 27.7 respectively, p = 0.03).
Figure 1.
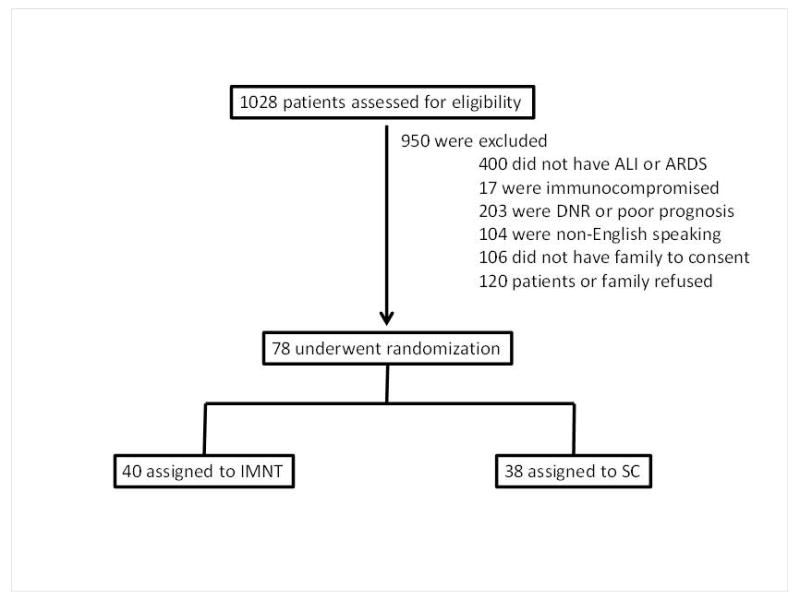
Table 1.
Demographic Profile and Baseline Clinical Parameters of Participants (N = 78).a
| Variable | Intervention (n = 40) | Control (n = 38) | P Value |
|---|---|---|---|
| Age, y | 52.5 (17.1) | 58.6 (16.2) | .11 |
| Female, n (%) | 21 (51.2) | 17 (44.7) | .56 |
| Race, n (%) | |||
| African American | 10 (25) | 11 (29) | |
| Caucasian | 22 (55) | 18 (47) | |
| Hispanic | 8 (20) | 9 (24) | |
| Height, cm | 170.1 (10.5) | 170.7 (10.1) | .81 |
| Weight, kg | 86.3 (27.6) | 88.6 (27.2) | .71 |
| Body mass index, kg/m2 | 29.8 (9.3) | 30.1 (8.9) | .89 |
| BMI ≥30, n(%) | 18 (45) | 18 (47) | .83 |
| SGA category, n (%) | .15 | ||
| Normal | 27 (65.8) | 23 (60.5) | |
| Moderate | 11 (26.8) | 15 (39.5) | |
| Severe | 3 (7.3) | 0 | |
| Baseline SOFA | 9.3 (3.8) | 9.4 (3.4) | .97 |
| APACHE II | 23.4 (9.3) | 27.7 (7.9) | .03 |
| PaO2:FiO2, ratio | 195 (105) | 183 (122) | .69 |
| PaO2:FiO2, ratio ≤200, n (%) | 23 (58) | 21 (55) | .84 |
| CRP, mg/dL | 102.2 (92.2) | 131.2 (92) | .20 |
| White blood cell count, cells × 109/L | 13.3 (12.2) | 11.1 (6.2) | .34 |
| Glucose, mg/dL | 154 (48.9) | 150.9 (55.1) | .78 |
APACHE II, Acute Physiology and Chronic Health Evaluation; BMI, body mass index; CRP, C-reactive protein; SGA, Subjective Global Assessment; SOFA, Sequential Organ Failure Assessment:
Values are means (standard deviations) unless otherwise indicated.
The estimates of energy and protein needs and the percentage of estimated needs received in IMNT and SC are depicted in Table 2. Energy and protein requirements were similar between groups. Participants randomized to IMNT received significantly greater percentages of estimated energy and protein needs throughout the study and significantly greater Kcal/Kg (Table 2 and Figure 2). No significant differences in the number of participants/group that received PN (8/40 IMNT vs 5/38 SC, p= 0.42), the mean number of days of PN in participants that received any PN or the overall percentage of energy received from PN (9% intervention, 7% control, p =0.33) was found. The percentage of participants that received parenteral lipids as Propofol or via their PN prescription also did not differ between groups (32% IMNT, 16% SC, p = 0.09)
Table 2.
Nutrition Requirements and Amount of Energy/Protein Received per Day.a
| Variable | Intervention (n = 40) | Control (n = 38) | P Value |
|---|---|---|---|
| Estimated energy needs, kcal | 2158 (426) | 2219 (342) | .48 |
| Estimated protein needs, g | 109 (20) | 111 (17) | .59 |
| Energy received/d, kcal | 1798 (509) | 1221 (423) | <.0001 |
| Percentage of estimated energy needs received per day | 84.7 (22) | 55.4 (19) | <0001 |
| kcal/kg received per dayb | 25.4 (6.6) | 16.6 (5.6) | <0001 |
| Received PN, n (%) | 8 (20) | 5 (13.2) | .42 |
| Mean days of PN intake among participants who received PN | 6.4 (5.2) | 6.0 (4.1) | .89 |
| Protein received per day, g | 82 (23) | 60.4 (24) | <0001 |
| Percentage of estimated protein needs received per day | 76.1 (18) | 54.4 (21) | <0001 |
| Received parenteral lipids,c n (%) | 13 (32) | 5 (13) | .09 |
All data are means (SD).
Kcal/kg= kcal/kg admit weight or adjusted ideal body weight if body mass index ≥30 kg/m2
Includes lipids from parenteral nutrition and propofol.
Figure 2.
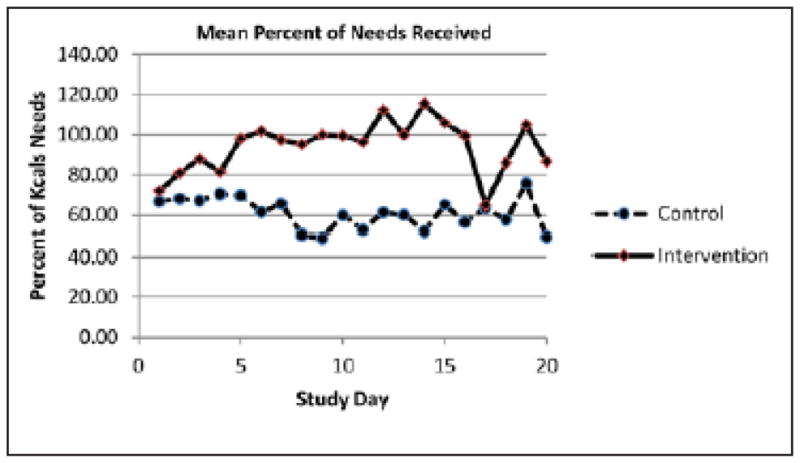
Mean percentage of energy needs received per day in the intensive medical nutrition therapy (Control) and standard nutrition support care (Intervention) groups.
Study outcomes are presented in Table 3. Overall there was no difference between group for hospital or ICU LOS, number of days on mechanical ventilation, infections or number of days between hospital admission and study enrollment day. The mean amount of insulin received was similar between groups; however when analysis was restricted to participants that received any insulin (17/40 intervention and 20/38 controls) significantly more insulin was received by the IMNT compared to the SC group (78 vs. 36 units, p=0.03). In June, 2013 review by the Data Safety Monitoring Board revealed significantly more deaths in the IMNT compared to the SC group [16/41, 39%) vs. 6/38, (16%), p =0.02]. Although no apparent differences were observed for cause of death between the two groups, the recent reports of worse outcomes in participants randomized to receive higher energy intake compared to controls in three large well designed PRCT similar to INTACT, 20–23 coupled with the highly significant differences in death rates resulted in cessation of the trial.
Table 3.
Clinical Outcomes in IMNT vs SNSC Participants (N = 78).
| Variable | IMNT (n = 40) | SNSC (n= 38) | P Value |
|---|---|---|---|
| Hospital LOS, d | 27.2 (18.2) | 22.8 (14.3) | .33 |
| ICU LOS, d | 15.5 (12.8) | 16.1 (11.5) | .83 |
| Number of days between hospital admission and enrollment | 8.8 (8.7) | 6.4 (6.6) | .17 |
| Days on ventilator (median, IQR) | 6 (4–10) | 7 (3–14) | .85 |
| Number of infections, n (%) | 5 (12) | 8 (21) | .29 |
| Any hyperglycemic event, n (%)a | 30 (73) | 26 (68) | .64 |
| Number of days with hyperglycemia | 2.2 (3.0) | 2.4 (4.0) | .85 |
| Any hypoglycemic event, n (%)a | 12 (29.3) | 11 (28.9) | .98 |
| Number of days with hypoglycemia | 0.3 (0.6) | 0.9 (0.7) | .08 |
| Insulin received per day, U | 23.6 (47.6) | 14 (23.6) | .25 |
| Insulin received per day on days insulin was received in participants who were given insulin, U | 77.7 (70.4) | 35.9 (27.9) | .03 |
| Died | 16 (40.0) | 6 (15.8) | .017 |
All values are mean (SD) unless otherwise indicated. ICU, intensive care unit; IMNT, intensive medical nutrition therapy; IQR, interquartile range; LOS, length of stay; SNSC, standard nutrition support care.
Hyperglycemia defined as glucose >180 mg/dL; hypoglycemia defined as glucose <70 mg/dL.
The Kaplan Meier estimates of time to death and log-rank test for unadjusted comparisons between the groups are presented in Figure 3. The first death in the IMNT group occurred on study day 4 and 7 deaths had occurred by day 10. The first death in the SC group did not occur until study day 11. Estimates of the hazard ratio for death and its confidence intervals in the IMNT vs. SC assessed with Cox proportional hazards multiple regression analysis are presented in Table 4 Baseline parameters evaluated for inclusion in the model were BMI, age, gender, race/ethnicity, SGA, SOFA and APACHE II scores. Unadjusted analysis revealed participants in the IMNT groups experienced 2.65 times higher hazard of death than those randomized to SC. When adjusted for age (HR 1.04, 95% CI 1.02–1.07, p = 0.001) and baseline SOFA score (HR 1.32, 95% CI 1.14–1.54, p =0.0003) the hazard of death in the IMNT was 5.67 times (p =0.001) higher than the SC group. No other baseline variables evaluated were found to be significant additional predictors during Cox modeling.
Figure 3.
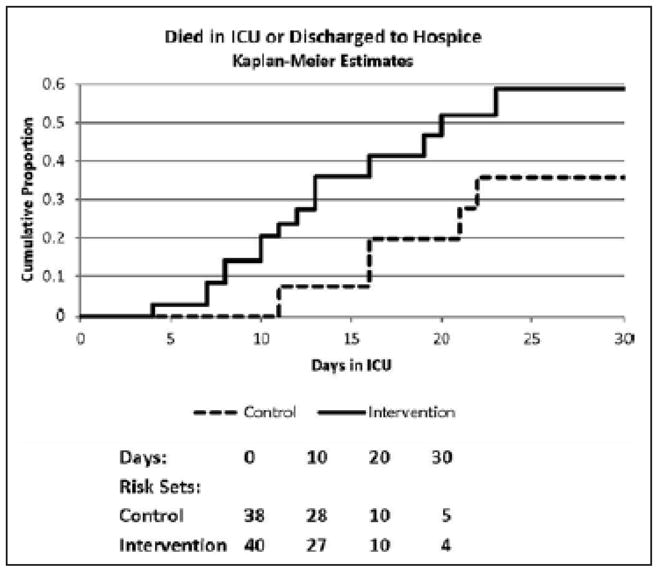
Kaplan-Meier estimates of time to death and log-rank test results for unadjusted comparisons between intensive medical nutrition therapy and standard nutrition support care.
Table 4.
Cox Regression Analysis Relating IMNT vs SNSC and Adjusted for Other Clinical Characteristics to Death.
| Independent Variable | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| Model 1 | |||
| IMNT vs SNSC | 2.65 | 1.03–6.84 | .04 |
| Model 2 | |||
| IMNT vs SNSC | 5.67 | 1.97–16.29 | .001 |
| Age | 1.04 | 1.01–1.08 | .001 |
| Baseline SOFA | 1.32 | 1.11–1.57 | .003 |
CI, confidence interval; IMNT, intensive medical nutrition therapy; SOFA, Sequential Organ Failure Assessment; SNSC, standard nutrition support care.
Discussion
Current Canadian, European and US guidelines for nutrition support of ICU patients recommend early EN initiation (within 24–48 hours)1–3. The major findings from this PRCT demonstrate that providing IMNT from diagnosis to hospital discharge, which included early EN, to ICU patients with ALI resulted in significantly higher mortality rates. These findings are similar to three recent large, well-designed PRCTs that found superior outcomes (i.e. lower infection rates, shorter hospital and ICU stays and better survival) in those randomized to lower vs. higher nutrient intakes in the first ~ 7-days of ICU stay20, 21, 23. Casear et al21 compared ICU patients (medical and surgical) randomized to full feeding via EN/PN (n=2312) in the first days of ICU admission to minimal EN feedings (n=2328). They found fewer ICU infections and greater likelihood of discharge alive from the ICU in patients receiving minimal feedings. Post-hoc analysis indicated the lowest dose of macronutrients received was associated with the fastest recovery, regardless of route (i.e. PN or EN) and protein rather than glucose appeared to explain the results 22. Further, a sub-set of patients (n=517) that were randomized to late PN and could not receive EN (i.e. these patients received essentially 0 nutrition for the first week) had lower infection rates (29.9% vs 40%, p = 0.01) and a 20% greater likelihood ICU survival (hazard ratio 1.2, 95% CI 1.00–1.44, p = 0.05) than those receiving early EN/PN. Arabi et al 20 randomized medical/surgical ICU patients (n= 240) to EN at estimated energy requirements vs. permissive underfeeding. Significantly higher hospital mortality occurred in patients randomized to target compared to underfeeding regimens (42.5% versus 30%, RR: 0.71 95% CI 0.50–0.99, p<0.05). Singer et al23 also reported negative outcomes from greater energy exposure in their single center PRCT in patients (N=130) expected to require MV for at least 72 hours duration. They found that MV (16.1 ± 14.7 versus 10.5 ± 8.3 days, p<0.05), ICU length of stay (17.2 ± 14.6 versus 11.7 ± 8.4 days, p<0.05) and total infection rates (28% versus 14%, p<0.05) were higher in the intervention vs. control group. No difference was observed in ICU length of stay, however a trend for lower hospital mortality in the intervention group occurred (p = 0.058). Collectively, these findings challenge the national and international nutrition support recommendations for early EN feeding in ICU patients.
Our trial was stopped prior to meeting enrollment goals due to greater mortality in the IMNT group. The only significant clinical difference between the groups was the higher energy and protein received in those randomized to IMNT. Specifically, these participants received 84.7% of their estimated needs which averaged 25.4 (6.6) Kcal/Kg compared to 55.4% and 16.6 (5.6) Kcal/Kg in SC (p < 0.0001). The differences in intake between groups began at enrollment and continued throughout the trial. All of the PRCTs described in the preceding paragraph used different predictive equations or dosing weights to estimate requirements and only one investigation 20 reported the average kcals/kg received in their participants, limiting the ability to make comparisons between the studies. Two other recent studies26,24 found no difference in outcomes between study groups; however, calorie delivery among subjects in their intervention groups were was actually closer to the control groups of Singer,23 Casaer21 and our trial. We speculate our effectiveness in delivering goal energy intake early after ALI diagnosis and consistently in our IMNT patients contributed to their higher mortality rates compare to those in other PRCT.
The first death in our IMNT group occurred on study day 4 and 7 of the 16 deaths (44%) occurred by day 10. In the SC group the first death occurred on day 11. This suggests early exposure to higher energy intake in the IMNT group, rather than overall intake increased their mortality rates compared to SC. The infusion of nonvolitional highly refined feedings, delivered over 24 hours has been postulated to interfere with autophagy (i.e. cellular cleaning), to adversely impact gut microbiota and to impair immune response 22, 27–29. Our findings support these hypotheses and the need for trials to investigate these proposed mechanisms.
Enteral nutrition is an artificial mode of nutritional provision. It is infused in ICU populations under the assumption it will slow LBM loss and improve outcomes 1, 3, 30. Our findings and other recent PRCT suggest withholding non-volitional nutrition during the acute phase of illness may be beneficial. A true control group (i.e. no EN or PN) was not included in any of these trials, so it is unknown if eliminating them would have influenced results. No PRCT in an ICU population has assessed their influence on changes in LBM and its subsequent influence on outcomes; thus, it is unknown if this link exists. Enteral feeding is a medical intervention with inherent risks and costs31–35 and should not be equated with the oral consumption of food. It is biologically feasible that withholding nonvolitional EN/PN until the catabolism of critical illness has resolved has no negative effect and the natural physiologic response of anorexia and loss of appetite due to cytokine producing disease is indeed beneficial.
Our study has certain limitations that merit consideration. First, it was a small single center trial that was stopped early due to higher mortality rates in the IMNT group. Our final sample size was underpowered to detect differences in infection rates which was our a priori primary outcome. This limits our ability to perform sub-group analysis. Second, we used energy and protein equations that provided only estimates of nutrition needs. Nutrition is provided to support the maintenance of LBM and presumably patients classified as normally nourished would have healthy levels of lean mass. However, utilizing computed tomography, we recently reported that SGA was unable to detect low levels of lean body mass in critically ill individuals. Thus, while our nutrient estimates were conservative (30 kcals/kg) and participants in the IMNT received approximately 25 Kcal/Kg provision (a widely accepted level for estimating basal energy needs), this level may have resulted in overfeeding in the intervention group. Studies utilizing indirect calorimetry combined with accurate body composition methodologies are needed to more accurately assess and guide nutrient delivery in a research setting. Finally, while these results are generalizable to patients with ALI, they should not be extrapolated to all critically ill patients in the medical ICU or to populations in other ICUs.
In conclusion we found IMNT provided from ALI diagnosis to hospital discharge resulted in greater mortality than SC. These findings are similar to other recent trials in ICU populations and challenge the current national ICU feeding guidelines. Future studies of nonvolitional feeding during acute illness are needed to discern the optimal method, dose and timing of nutrition support, including mechanistic outcomes.
Contributor Information
Carol A. Braunschweig, Email: braunsch@uic.edu, University of Illinois at Chicago, Department of Kinesiology and Nutrition, 1919 W. Taylor, Room 650, Chicago, IL 60612; phone: 312-996-2575; fax 312-413-0219.
Patricia M. Sheean, Email: psheean1@luc.edu, Loyola University Chicago, Health Sciences Campus, 2160 South First Avenue, Building 105, Room 2582, Maywood, IL 60153; phone 708-216-0344.
Sarah J. Peterson, Email: Sarah_J_Peterson@rush.edu, Rush University Medical Center, Department of Food and Nutrition, 1650 W. Harrison Street, Chicago, IL 60612.
Sandra Gomez Perez, Email: slgomez@uic.edu, University of Illinois at Chicago, Institute for Health Policy and Research, M/C 275, 1747 West Roosevelt Road, Chicago, IL 60608.
Sally Freels, Email: sallyf@uic.edu, Department of Epidemiology and Biostatistics, University of Illinois at Chicago, 953 SPHPI, MC 923, Chicago IL, 60612.
Omar Lateef, Email: omar_P_Lateef@rush.edu, Professor of Medicine, Department of Medicine, Rush University Medical Center, 1650 W. Harrison Street, Chicago, IL 0612.
David Gurka, Email: David_P_Gurka@rush.edu, Department of Medicine, Rush University Medical Center, 1650 W. Harrison Street, Chicago, IL 0612.
Giamila Fantuzzi, Email: Giamila@uic.edu, Department of Kinesiology and Nutrition, University of Illinois at Chicago, 1919 W. Taylor, Room 650, Chicago, IL.
References
- 1.McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) JPEN J Parenter Enteral Nutr. 2009 May-Jun;33(3):277–316. doi: 10.1177/0148607109335234. [DOI] [PubMed] [Google Scholar]
- 2.Dhaliwal R, Cahill N, Lemieux M, Heyland DK. The Canadian Critical Care Nutrition Guidelines in 2013: An Update on Current Recommendations and Implementation Strategies. Nutr Clin Pract. 2013 Dec 2; doi: 10.1177/0884533613510948. [DOI] [PubMed] [Google Scholar]
- 3.Singer P, Berger MM, Van den Berghe G, et al. ESPEN Guidelines on Parenteral Nutrition: intensive care. Clin Nutr. 2009 Aug;28(4):387–400. doi: 10.1016/j.clnu.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 4.Koretz RL, Avenell A, Lipman TO, Braunschweig CL, Milne AC. Does enteral nutrition affect clinical outcome? A systematic review of the randomized trials. Am J Gastroenterol. 2007 Feb;102(2):412–429. doi: 10.1111/j.1572-0241.2006.01024.x. quiz 468. [DOI] [PubMed] [Google Scholar]
- 5.Hasse JM, Blue LS, Liepa GU, et al. Early enteral nutrition support in patients undergoing liver transplantation. JPEN J Parenter Enteral Nutr. 1995 Nov-Dec;19(6):437–443. doi: 10.1177/0148607195019006437. [DOI] [PubMed] [Google Scholar]
- 6.Dvorak MF, Noonan VK, Belanger L, et al. Early versus late enteral feeding in patients with acute cervical spinal cord injury: a pilot study. Spine. 2004 May 1;29(9):E175–180. doi: 10.1097/00007632-200405010-00020. [DOI] [PubMed] [Google Scholar]
- 7.Moore EE, Jones TN. Benefits of immediate jejunostomy feeding after major abdominal trauma--a prospective, randomized study. J Trauma. 1986 Oct;26(10):874–881. doi: 10.1097/00005373-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994 Mar;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 9.Krenitsky J. Adjusted body weight, pro: evidence to support the use of adjusted body weight in calculating calorie requirements. Nutr Clin Pract. 2005 Aug;20(4):468–473. doi: 10.1177/0115426505020004468. [DOI] [PubMed] [Google Scholar]
- 10.Ishibashi N, Plank LD, Sando K, Hill GL. Optimal protein requirements during the first 2 weeks after the onset of critical illness. Crit Care Med. 1998 Sep;26(9):1529–1535. doi: 10.1097/00003246-199809000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Shaw JH, Wildbore M, Wolfe RR. Whole body protein kinetics in severely septic patients. The response to glucose infusion and total parenteral nutrition. Ann Surg. 1987 Mar;205(3):288–294. doi: 10.1097/00000658-198703000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peterson SJ, Tsai AA, Scala CM, Sowa DC, Sheean PM, Braunschweig CL. Adequacy of oral intake in critically ill patients 1 week after extubation. J Am Diet Assoc. 2010 Mar;110(3):427–433. doi: 10.1016/j.jada.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985 Oct;13(10):818–829. [PubMed] [Google Scholar]
- 14.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996 Jul;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 15.Moreno R, Vincent JL, Matos R, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Working Group on Sepsis related Problems of the ESICM. Intensive Care Med. 1999 Jul;25(7):686–696. doi: 10.1007/s001340050931. [DOI] [PubMed] [Google Scholar]
- 16.Baker JP, Detsky AS, Whitwell J, Langer B, Jeejeebhoy KN. A comparison of the predictive value of nutritional assessment techniques. Hum Nutr Clin Nutr. 1982;36(3):233–241. [PubMed] [Google Scholar]
- 17.Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009 Mar 26;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 18.Collett D. Modelling survival data in medical research. 2. Boca Raton, Fla: Chapman & Hall/CRC; 2003. [Google Scholar]
- 19.Rubinson L, Diette GB, Song X, Brower RG, Krishnan JA. Low caloric intake is associated with nosocomial bloodstream infections in patients in the medical intensive care unit. Crit Care Med. 2004 Feb;32(2):350–357. doi: 10.1097/01.CCM.0000089641.06306.68. [DOI] [PubMed] [Google Scholar]
- 20.Arabi YM, Tamim HM, Dhar GS, et al. Permissive underfeeding and intensive insulin therapy in critically ill patients: a randomized controlled trial. Am J Clin Nutr. 2011 Mar;93(3):569–577. doi: 10.3945/ajcn.110.005074. [DOI] [PubMed] [Google Scholar]
- 21.Casaer MP, Mesotten D, Hermans G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011 Aug 11;365(6):506–517. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]
- 22.Casaer MP, Wilmer A, Hermans G, Wouters PJ, Mesotten D, Van den Berghe G. Role of disease and macronutrient dose in the randomized controlled EPaNIC trial: a post hoc analysis. Am J Respir Crit Care Med. 2013 Feb 1;187(3):247–255. doi: 10.1164/rccm.201206-0999OC. [DOI] [PubMed] [Google Scholar]
- 23.Singer P, Anbar R, Cohen J, et al. The tight calorie control study (TICACOS): a prospective, randomized, controlled pilot study of nutritional support in critically ill patients. Intensive Care Med. 2011 Apr;37(4):601–609. doi: 10.1007/s00134-011-2146-z. [DOI] [PubMed] [Google Scholar]
- 24.Doig GS, Simpson F, Sweetman EA, et al. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. JAMA. 2013 May 22;309(20):2130–2138. doi: 10.1001/jama.2013.5124. [DOI] [PubMed] [Google Scholar]
- 25.Rice TW, Mogan S, Hays MA, Bernard GR, Jensen GL, Wheeler AP. Randomized trial of initial trophic versus full-energy enteral nutrition in mechanically ventilated patients with acute respiratory failure. Crit Care Med. 2011 May;39(5):967–974. doi: 10.1097/CCM.0b013e31820a905a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. Jama. 2012 Feb 22;307(8):795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanhorebeek I, Gunst J, Derde S, et al. Insufficient activation of autophagy allows cellular damage to accumulate in critically ill patients. J Clin Endocrinol Metab. 2011 Apr;96(4):E633–645. doi: 10.1210/jc.2010-2563. [DOI] [PubMed] [Google Scholar]
- 28.Derde S, Vanhorebeek I, Guiza F, et al. Early parenteral nutrition evokes a phenotype of autophagy deficiency in liver and skeletal muscle of critically ill rabbits. Endocrinology. 2012 May;153(5):2267–2276. doi: 10.1210/en.2011-2068. [DOI] [PubMed] [Google Scholar]
- 29.Smith AR, Macfarlane S, Furrie E, et al. Microbiological and immunological effects of enteral feeding on the upper gastrointestinal tract. J Med Microbiol. 2011 Mar;60(Pt 3):359–365. doi: 10.1099/jmm.0.026401-0. [DOI] [PubMed] [Google Scholar]
- 30.Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003 Sep-Oct;27(5):355–373. doi: 10.1177/0148607103027005355. [DOI] [PubMed] [Google Scholar]
- 31.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008 Jan 12;371(9607):126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 32.Benya R, Mobarhan S. Enteral alimentation: administration and complications. J Am Coll Nutr. 1991 Jun;10(3):209–219. doi: 10.1080/07315724.1991.10718147. [DOI] [PubMed] [Google Scholar]
- 33.Cabre E, Gassull MA. Complications of enteral feeding. Nutrition. 1993 Jan-Feb;9(1):1–9. [PubMed] [Google Scholar]
- 34.Ciocon JO, Silverstone FA, Graver LM, Foley CJ. Tube feedings in elderly patients. Indications, benefits, and complications. Arch Intern Med. 1988 Feb;148(2):429–433. [PubMed] [Google Scholar]
- 35.Bohnker BK, Artman LE, Hoskins WJ. Intranasal retraction of nasogastric feeding tube: case report and suggestion for design modification. JPEN J Parenter Enteral Nutr. 1985 Jan-Feb;9(1):53–54. doi: 10.1177/014860718500900153. [DOI] [PubMed] [Google Scholar]


