Abstract
Background
Observational and experimental data support a potential breast cancer chemopreventive effect of green tea.
Methods
We conducted an ancillary study using archived blood/urine from a phase IB randomised, placebo-controlled dose escalation trial of an oral green tea extract, Polyphenon E (Poly E), in breast cancer patients. Using an adaptive trial design, women with stage I–III breast cancer who completed adjuvant treatment were randomised to Poly E 400 mg (n = 16), 600 mg (n = 11) and 800 mg (n = 3) twice daily or matching placebo (n = 10) for 6 months. Blood and urine collection occurred at baseline, and at 2, 4 and 6 months. Biological endpoints included growth factor [serum hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF)], lipid (serum cholesterol, triglycerides), oxidative damage and inflammatory biomarkers.
Results
From July 2007-August 2009, 40 women were enrolled and 34 (26 Poly E, eight placebo) were evaluable for biomarker endpoints. At 2 months, the Poly E group (all dose levels combined) compared to placebo had a significant decrease in mean serum HGF levels (−12.7% versus +6.3%, P = 0.04). This trend persisted at 4 and 6 months but was no longer statistically significant. For the Poly E group, serum VEGF decreased by 11.5% at 2 months (P = 0.02) and 13.9% at 4 months (P = 0.05) but did not differ compared to placebo. At 2 months, there was a trend toward a decrease in serum cholesterol with Poly E (P = 0.08). No significant differences were observed for other biomarkers.
Conclusions
Our findings suggest potential mechanistic actions of tea polyphenols in growth factor signalling, angiogenesis and lipid metabolism.
Keywords: breast cancer, chemoprevention, tea polyphenols
Introduction
Observational studies have correlated green tea intake with reduced risk of breast cancer incidence and recurrence (Seely et al., 2005; Sun et al., 2006; Ogunleye et al., 2010). The most abundant and possibly most potent polyphenol in green tea is epigallocatechin-3-gallate (EGCG; Yang et al., 2009). In preclinical studies, EGCG inhibits growth factors, such as hepatocyte growth factor (HGF), which influences tumour cell growth, migration and invasion (Bigelow & Cardelli, 2006), and vascular endothelial growth factor (VEGF), which is involved in angiogenesis (Masuda et al., 2002; Sartippour et al., 2002). In addition, tea polyphenols have potent antioxidant and anti-inflammatory effects in in vitro and animal models (Senthil Kumaran et al., 2008), as well as human studies (Hakim et al., 2003; Hsu et al., 2007; Nguyen et al., 2012). EGCG has also been proposed to promote weight loss and have a favourable effect on metabolic markers related to obesity (Moon et al., 2007). These same pathways have been implicated in breast cancer development.
Secondary cancer prevention trials targeting breast cancer survivors are useful clinical models for studying the effects of potential chemopreventive agents on biomarker endpoints (Stearns et al., 2004; Crew et al., 2012). These women have a risk of developing contralateral breast primaries of 0.5–1% per year (Malone et al., 2010). One study demonstrated a high concordance of 70% among women diagnosed with a hormone receptor (HR)-negative primary breast cancer having a HR-negative contralateral breast cancer (Swain et al., 2004). Therefore, these women who are not candidates for adjuvant hormonal therapy represent a highly motivated patient population for testing chemopreventive agents targeting HR-negative breast cancer.
We conducted a phase IB dose escalation trial in 40 women with a history of stage I–III HR-negative breast cancer given an oral green tea extract, Polyphenon E (Poly E), at three different dose levels or matching placebo for 6 months (Crew et al., 2012). The primary outcome of the parent trial was to determine the maximum tolerated dose (MTD) of Poly E, defined as the dose that causes a dose-limiting toxicity (DLT; grade 2 or higher) in approximately 25% of participants. We present the results of secondary exploratory biomarker analyses using archived blood and urine samples collected from this trial to evaluate the effects of Poly E on biomarkers associated with breast cancer risk.
Materials and methods
Study population
From July 2007 to August 2009, 40 women were enrolled at four sites, as described previously (Crew et al., 2012). Institutional review board approval was obtained at each participating site (Columbia University, MD Anderson Cancer Center, Memorial Sloan-Kettering Cancer Center and Baylor College of Medicine) and all participants provided their written informed consent in English or Spanish (NCT00516243).
Eligible participants, as described previously (Crew et al., 2012), included women aged 21–65 years with a history of histologically-confirmed resected stage I–III oestrogen receptor (ER)-negative and progesterone receptor (PR)-negative breast carcinoma without evidence of disease and a minimum of 6 months since completing adjuvant treatment. Participants had to abstain from all tea consumption, including supplements that contain tea compounds, for 30 days prior to baseline and during the 6-month intervention.
Of the 40 participants, 34 completed their 6-month study evaluations (26 Poly E and eight placebo) and were included in the secondary biomarker analyses. In total, 28 participants completed the drug intervention (20 Poly E and eight placebo), four were lost to follow-up (two Poly E and two placebo), five developed a DLT and had to come off study drug, as per protocol (five Poly E, including four who completed their study assessments), and three were non-adherent to the study drug (three Poly E, including two who completed their study assessments; Crew et al., 2012).
Study design and intervention
The study design was a randomised, double-blinded, placebo-controlled, dose escalation trial of Poly E 400, 600 or 800 mg of EGCG (2–4 capsules) twice daily or matching placebo (3 : 1 randomisation) for 6 months (Crew et al., 2012). Participants and study investigators were blinded to drug assignment (Poly E versus placebo) but not to dose level. Assignment to dose level was based upon the time-to-event continual reassessment method (TITE-CRM; Cheung & Chappell, 2000), which assesses long-term toxicity with staggered enrollment and, on average, allocates more participants to the MTD. Of the 30 participants randomised to Poly E, 16 were assigned to 400 mg, 11–600 mg and 3–800 mg of Poly E twice daily. There were no intra-participant dose modifications.
Poly E is a well-defined pharmaceutical-grade decaffeinated oral green tea extract, containing 65% EGCG and lesser amounts of epicatechin, epigallocatechin and epicatechin gallate (Chang et al., 2003). Each capsule contains 200 mg of EGCG, equivalent to two to three cups of brewed green tea. Therefore, the doses tested in this trial were equivalent to the EGCG content of eight to 24 cups of brewed green tea daily. Five DLTs occurred during this phase I trial (Crew et al., 2012), including indigestion, transaminitis, insomnia, rectal bleeding (all grade 3) and weight gain (grade 2). Based upon these findings, Poly E 600 mg twice daily was defined as the MTD with a DLT rate of 27% (three of 11).
Measurement of biomarkers
Blood samples and spot urine samples were collected at baseline, and at 2, 4 and 6 months during the 6-month drug intervention. Biological endpoints included growth factor (serum HGF, VEGF), lipid (total serum cholesterol, triglycerides), oxidative damage [urine 8-oxo-7,8-dihydrodeoxyguanosine (8-oxodG) and 15-F2-isoprostanes (15-F2t-IsoP), plasma protein carbonyls] and inflammatory [serum high-sensitivity C-reactive protein (hsCRP), urine prostaglandin E2 metabolite (PGE-M)] biomarkers, as well as total urinary tea polyphenols. An enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN, USA) was used to detect serum HGF and VEGF levels. Duplicate aliquots of each sample were run and averaged, and concentrations were determined based on standard curves. Pretreatment and post-treatment samples from the same patients were assayed in the same batch for the ELISA. The intra-assay coefficient of variation for HGF and VEGF was <7.0% and the interassay coefficient of variation for HGF and VEGF was <9.0%. Standard serum lipid panels (total cholesterol, triglycerides) were measured at the clinical core laboratory of each participating site. Urine 8-oxodG was measured by a competitive ELISA (Rossner et al., 2006) and 15-F2t-IsoP by immunoassay kits (Oxford Biomedical Research, Oxford, MI, USA). Both biomarkers were expressed as nmol mmol−1 L−1 urinary creatinine. Each sample was analysed in duplicate; the interassay coefficients of variation for 8-oxodG and 15-F2t-IsoP were 25% and 8.5%, respectively. Plasma protein carbonyls were measured by a noncompetitive ELISA (Winterbourn & Buss, 1999) and serum hsCRP was measured by ELISA (United Biotech, Mountain View, CA, USA). Urinary PGE-M levels were measured using a liquid chromatography/tandem mass spectrometric method (Murphey et al., 2004). The lower limit of detection of PGE-M was 40 pg and the coefficient of variation was 7.2%. Five urinary metabolites of tea catechins [epicatechin, epigallocatechin, methylepigallocatechin (4′-MeEGC), 5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone (M4) and 5-(3′,4′-dihydroxyphenyl) -γ-valerolactone (M6)] were assayed by high-performance liquid chromatography, which allows the determination of free and conjugated forms of tea catechins, as described previously (Inoue-Choi et al., 2010). These urinary metabolites were summed as a total tea polyphenols index and the concentration was expressed in units of urinary creatinine by weight (mmol g−1 Cr) to account for varying urine volumes. All samples were run in the same batch at the same facility and analysed in a blinded fashion.
Statistical analysis
Descriptive statistics were conducted on each of the biomarker endpoints for the Poly E and placebo groups. Because of the small numbers and the fact that urinary tea polphenols did not differ significantly by dose level (data not shown), all three dose levels of Poly E were combined for the biomarker analyses. We calculated the mean absolute change and percentage change from baseline for each biomarker. Paired t-tests and two-sample t-tests were used to compare within-group and between-group differences for the Poly E and placebo groups, respectively. Linear regression was used to evaluate absolute change in each biomarker with treatment and baseline values as covariates. Repeated measure analysis of variance was used with a time interaction term using treatment and dose levels as main effects. There was no correction for multiple comparisons as a result of the exploratory nature of these secondary biomarker endpoints. All analyses were two-sided and conducted using sas, version 9.2 (SAS Institute, Cary, NC, USA).
Results
The baseline characteristics of the 34 participants included in the secondary biomarker analyses are summarised in Table 1. The Poly E and placebo groups were well balanced in terms of baseline characteristics. Median age was 55 years (range 39–65 years), 79% were post-menopausal and the median body mass index was 29 kg m−2 (range 21–41 kg m−2). Over half had stage II–III breast cancer, 88% had prior chemotherapy, and the median time since diagnosis was 34 months (range 10–170 months). Because the participants were at least 6 months post-adjuvant therapy prior to enrollment, we anticipated minimal residual effects of these treatments on our biomarker end-points.
Table 1. Baseline demographic and clinical characteristics of the subset of women with previously treated hormone receptor-negative breast cancer, participating in a Polyphenon E (Poly E) intervention study, and evaluable for biomarker analyses (n = 34).
| Characteristics | Poly E (n = 26) | Placebo (n = 8) | Total (n = 34) |
|---|---|---|---|
| Median age, years (range) | 54.5 (39–65) | 56.5 (47–61) | 55 (39–65) |
| Menopausal status, n (%) | |||
| Premenopausal | 5 (19) | 2 (25) | 7 (21) |
| Post-menopausal | 21 (81) | 6 (75) | 27 (79) |
| Race, n (%) | |||
| White | 15 (58) | 5 (63) | 20 (59) |
| Hispanic | 5 (19) | 2 (25) | 7 (20) |
| Black | 5 (19) | 1 (12) | 6 (18) |
| Asian | 1 (4) | 0 (0) | 1 (3) |
| Median body mass index (kg m−2) (range) | 28 (21–41) | 29 (23–34) | 29 (21–41) |
| Stage, n (%) | |||
| I | 13 (50) | 2 (25) | 15 (44) |
| II | 7 (27) | 4 (50) | 11 (32) |
| III | 6 (23) | 2 (25) | 8 (24) |
| Breast cancer treatments, n (%) | |||
| Chemotherapy | 23 (88) | 7 (88) | 30 (88) |
| Trastuzumab | 4 (15) | 3 (38) | 7 (21) |
| Radiation therapy | 19 (73) | 7 (88) | 26 (76) |
| Median time since diagnosis, months (range) | 31 (10–170) | 40 (17–73) | 34 (10–170) |
| Dose level of Poly E, n (%) | |||
| 400 mg bid | 14 (54) | – | |
| 600 mg bid | 11 (42) | – | |
| 800 mg bid | 1 (4) | – | |
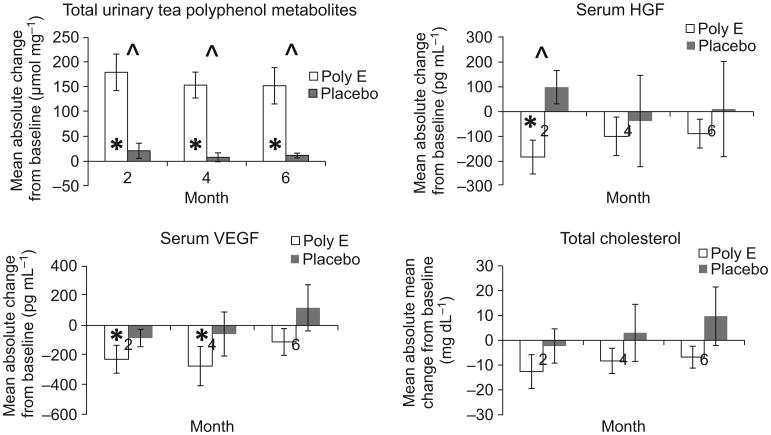
Systemic biomarker analyses are summarised in Table 2. There was an almost 10-fold increase from baseline in total urinary tea polyphenols in the Poly E-treated group. Compared to placebo, these differences were statistically significant at 2, 4 and 6 months (P < 0.001). Mean urinary tea polyphenol levels peaked at 2 months and decreased slightly at 4 and 6 months in the Poly E group (Fig. 1). At 2 months, mean serum HGF levels decreased by 12.7% for Poly E compared to a 6.3% increase for placebo (P = 0.04). This trend persisted at 4 and 6 months (Fig. 1) but was no longer statistically significant. Within the Poly E group, mean serum VEGF levels decreased by 11.5% at 2 months (P = 0.02) and 13.9% at 4 months (P = 0.05). However, these changes were not significant compared to placebo. There was a trend toward a decrease in total cholesterol with Poly E treatment, which did not reach statistical significance. No significant differences were observed in the biomarkers of oxidative damage or inflammation after treatment with Poly E compared to placebo (Table 2). No significant trends were observed with the serial blood and urine biomarkers by Poly E dose level or over time (data not shown). The results remained consistent after adjustment for baseline biomarker values.
Table 2. Biomarker results of women with previously treated hormone receptor-negative breast cancer randomised to Polyphenon E (Poly E) or placebo (n = 34).
| Biomarker | Poly E (n = 26) | Placebo (n = 8) | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 2 months | 4 months | 6 months | Baseline | 2 months | 4 months | 6 months | |
| Total urinary tea polyphenols (mmol g−1 Cr) | ||||||||
| Mean (SD) | 18.3 (31.2) | 191.0 (189.6) | 165.5 (131.0) | 163.8 (176.8) | 8.1 (7.0) | 29.3 (44.4) | 16.2 (30.8) | 19.9 (18.6) |
| Mean absolute change from baseline (SD) | +179.1 (186.9) | +153.5 (132.6) | +152.1 (186.4) | +21.2 (43.1) | +8.1 (25.2) | +11.8 (13.7) | ||
| Percentage change from baseline | +979 | +838 | +831 | +262 | +100 | +146 | ||
| P-value (within-group comparison)* | <0.001 | <0.001 | <0.001 | 0.207 | 0.392 | 0.046 | ||
| P-value (between-group comparison)** | <0.001 | <0.001 | <0.001 | |||||
| P-value (baseline-adjusted)*** | 0.032 | 0.003 | 0.017 | |||||
| Serum HGF (pg mL−1) | ||||||||
| Mean (SD) | 1441.2 (530.2) | 1296.4 (524.4) | 1387.9 (466.1) | 1352.4 (466.0) | 1568.5 (931.4) | 1667.1 (1088.2) | 1530.6 (925.4) | 1579.0 (1030.4) |
| Mean absolute change from baseline (SD) | −183.0 (347.0) | −99.4 (394.4) | −88.7 (296.4) | +98.5 (189.8) | -37.9 (521.1) | +10.4 (542.6) | ||
| Percentage change from baseline | −12.7 | −6.9 | −6.2 | +6.3 | −2.4 | +0.7 | ||
| P-value (within-group comparison)* | 0.013 | 0.211 | 0.140 | 0.185 | 0.843 | 0.958 | ||
| P-value (between-group comparison)** | 0.037 | 0.723 | 0.634 | |||||
| P-value (baseline-adjusted)*** | 0.037 | 0.602 | 0.405 | |||||
| Serum VEGF (pg mL−1) | ||||||||
| Mean (SD) | 1973.1 (1439.9) | 1786.8 (1330.5) | 1747.5 (1537.1) | 1861.8 (1438.5) | 921.1 (498.3) | 836.4 (460.1) | 863.0 (559.9) | 1038.9 (657.2) |
| Mean absolute change from baseline (SD) | −227.5 (478.2) | −273.5 (670.8) | −111.3 (458.0) | −84.7 (165.6) | −58.1 (418.3) | +117.8 (438.6) | ||
| Percentage change from baseline | −11.5 | −13.9 | −5.6 | −9.2 | −6.3 | +12.8 | ||
| P-value (within-group comparison)* | 0.023 | 0.048 | 0.227 | 0.191 | 0.706 | 0.472 | ||
| P-value (between-group comparison)** | 0.206 | 0.400 | 0.221 | |||||
| P-value (baseline-adjusted)*** | 0.942 | 0.508 | 0.375 | |||||
| Serum cholesterol (mg dL−1) | ||||||||
| Mean (SD) | 209.5 (43.4) | 194.6 (41.6) | 195.5 (41.4) | 200.7 (41.3) | 183.1 (27.6) | 182.1 (24.9) | 183.3 (15.8) | 191.4 (28.8) |
| Mean absolute change from baseline (SD) | −12.6 (31.7) | −8.3 (23.4) | −6.8 (20.3) | −2.3 (18.3) | +3.0 (30.4) | +9.7 (31.1) | ||
| Percentage change from baseline | −6.0 | −4.0 | −3.2 | −1.3 | +1.6 | +5.3 | ||
| P-value (within-group comparison)* | 0.076 | 0.112 | 0.139 | 0.767 | 0.803 | 0.441 | ||
| P-value (between-group comparison)** | 0.457 | 0.310 | 0.115 | |||||
| P-value (baseline-adjusted)*** | 0.760 | 0.713 | 0.255 | |||||
| Serum triglycerides (mg dL−1) | ||||||||
| Mean (SD) | 123.8 (70.8) | 125.9 (75.3) | 124.1 (71.8) | 119.2 (67.3) | 124.6 (42.1) | 132.8 (67.3) | 123.4 (78.0) | 128.3 (81.9) |
| Mean absolute change from baseline (SD) | +9.2 (72.1) | +8.2 (65.5) | −0.9 (52.6) | +15.9 (52.0) | +7.6 (59.4) | +17.5 (66.7) | ||
| Percentage change from baseline | +7.4 | +6.6 | −0.7 | +12.8 | +6.1 | +14.0 | ||
| P-value (within-group comparison)* | 0.566 | 0.554 | 0.936 | 0.450 | 0.747 | 0.549 | ||
| P-value (between-group comparison)** | 0.824 | 0.982 | 0.478 | |||||
| P-value (baseline-adjusted)*** | 0.749 | 0.991 | 0.501 | |||||
| Urine 8-oxodG (nmol mmol−1 Cr) | ||||||||
| Mean (SD) | 6.8 (8.0) | 5.9 (6.0) | 6.0 (4.8) | 5.6 (4.2) | 4.9 (2.3) | 11.8 (21.5) | 9.5 (15.4) | 11.2 (18.3) |
| Mean absolute change from baseline (SD) | −1.1 (9.0) | −0.9 (6.2) | −1.3 (8.3) | +6.9 (20.3) | +4.5 (14.1) | +6.2 (17.0) | ||
| Percentage change from baseline | −16.2 | −13.2 | −19.1 | +141 | +91.8 | +127 | ||
| P-value (within-group comparison)* | 0.525 | 0.472 | 0.440 | 0.371 | 0.392 | 0.334 | ||
| P-value (between-group comparison)** | 0.311 | 0.322 | 0.261 | |||||
| P-value (baseline-adjusted)*** | 0.178 | 0.175 | 0.130 | |||||
| Urine 15-F2t-lsoP (nmol mmol−1 Cr) | ||||||||
| Mean (SD) | 0.773 (0.386) | 0.697 (0.306) | 0.750 (0.414) | 0.757 (0.372) | 0.655 (0.345) | 0.841 (0.831) | 1.042 (0.913) | 0.748 (0.420) |
| Mean absolute change from baseline (SD) | −0.033 (0.387) | −0.028 (0.384) | −0.047 (0.341) | +0.146 (0.616) | +0.347 (0.704) | +0.053 (0.469) | ||
| Percentage change from baseline | −4.3 | −3.6 | −6.1 | +22.3 | +53.0 | +8.1 | ||
| P-value (within-group comparison)* | 0.669 | 0.709 | 0.500 | 0.524 | 0.206 | 0.757 | ||
| P-value (between-group comparison)** | 0.330 | 0.185 | 0.515 | |||||
| P-value (baseline-adjusted)*** | 0.356 | 0.069 | 0.721 | |||||
| Plasma protein carbonyls (nmol mg−1) | ||||||||
| Mean (SD) | 0.251 (0.061) | 0.256 (0.035) | 0.262 (0.029) | 0.260 (0.038) | 0.260 (0.045) | 0.250 (0.034) | 0.254 (0.024) | 0.242 (0.035) |
| Mean absolute change from baseline (SD) | +0.003 (0.062) | +0.012 (0.059) | +0.011 (0.060) | −0.010 (0.015) | −0.006 (0.028) | −0.017 (0.021) | ||
| Percentage change from baseline | +1.2 | +4.8 | +4.4 | −3.8 | −2.3 | −6.5 | ||
| P-value (within-group comparison)* | 0.808 | 0.304 | 0.356 | 0.109 | 0.589 | 0.057 | ||
| P-value (between-group comparison)** | 0.350 | 0.254 | 0.052 | |||||
| P-value (baseline-adjusted)*** | 0.575 | 0.374 | 0.161 | |||||
| hsCRP (ng mL−1) | ||||||||
| Mean (SD) | 3.0 (4.0) | 4.1 (7.4) | 4.5 (11.0) | 5.9 (13.9) | 2.9 (2.1) | 3.2 (3.7) | 2.3 (2.6) | 2.0 (1.2) |
| Mean absolute change from baseline (SD) | +1.2 (8.0) | +1.4 (11.9) | +2.8 (14.8) | +0.3 (2.8) | −0.6 (1.9) | −0.9 (2.5) | ||
| Percentage change from baseline | +40.0 | +46.7 | +93.3 | +10.3 | −20.7 | −31.0 | ||
| P-value (within-group comparison)* | 0.440 | 0.542 | 0.341 | 0.764 | 0.373 | 0.329 | ||
| P-value (between-group comparison)** | 0.621 | 0.397 | 0.226 | |||||
| P-value (baseline-adjusted)*** | 0.750 | 0.586 | 0.445 | |||||
| Urine PGE-M (ng mg−1 Cr) | ||||||||
| Mean (SD) | 5.7 (5.2) | 4.6 (3.1) | 6.7 (9.3) | 5.6 (4.0) | 5.9 (3.2) | 5.2 (4.3) | 6.1 (3.1) | 4.8 (2.8) |
| Mean absolute change from baseline (SD) | −1.0 (5.0) | +1.1 (10.2) | −0.2 (4.8) | −0.4 (3.3) | +0.2 (2.7) | −1.2 (5.7) | ||
| Percentage change from baseline | −17.5 | +19.3 | −3.5 | −6.8 | +3.4 | −20.3 | ||
| P-value (within-group comparison)* | 0.305 | 0.581 | 0.860 | 0.770 | 0.861 | 0.582 | ||
| P-value (between-group comparison)** | 0.750 | 0.675 | 0.623 | |||||
| P-value (baseline-adjusted)*** | 0.649 | 0.848 | 0.566 | |||||
Cr, creatinine; HGF, hepatocyte growth factor; hsCRP, high-sensitivity C-reactive protein; PGE-M, prostaglandin E2 metabolite; VEGF, vascular endothelial growth factor; 8-oxodG, 8-oxo-7,8-di-hydrodeoxyguanosine; 15-F2t-lsoP, 15-F2-isoprostanes.
Comparing the absolute change from baseline within the Poly E and placebo groups using a paired t-test.
Comparing the mean absolute change from baseline at 2, 4 and 6 months in the Poly E versus placebo groups using a two-sample t-test.
Comparing the mean absolute change from baseline at 2, 4 and 6 months in the Poly E versus placebo groups using linear regression adjusting for baseline biomarker values.
Figure 1.
Changes in systemic biomarkers among women with previously treated hormone receptor-negative breast cancer given Polyphenon E (Poly E) versus placebo (n = 34) HGF, hepatocyte growth factor; VEGF, vascular endothelial growth factor. *P-value < 0.05, paired t-test comparing mean absolute change from baseline within Poly E or placebo groups. ˆ P-value <0.05, two-sample t-test comparing mean absolute change from baseline in Poly E versus placebo groups.
Discussion
We demonstrated a significant reduction in serum HGF and a favourable trend in serum VEGF and total cholesterol during a 6-month intervention of Poly E compared to placebo in women with a history of hormone receptor-negative breast cancer. Given the small sample size and a lack of adjustment for multiple comparisons, these results need to be interpreted with caution and confirmed in a larger study. Nevertheless, the findings are consistent with published results of Poly E or green tea compounds in other study populations (McLarty et al., 2009; Stendell-Hollis et al., 2010; Wu et al., 2012).
Hepatocyte growth factor binds to a receptor tyrosine kinase called c-MET and plays a key role in epithelial– mesenchymal transition and tumour invasion (Birchmeier et al., 2003). The c-MET receptor is often overexpressed in breast cancer and is associated with a poor prognosis (Raghav et al., 2012). In an ER-negative breast cancer cell line, EGCG blocked HGF-induced phosphorylation of c-MET and AKT, and also decreased cell invasion (Bigelow & Cardelli, 2006), which supports our findings in a population of women with hormone receptor-negative breast cancer. In terms of angiogenesis, EGCG has been shown to decrease VEGF mRNA and protein levels (Masuda et al., 2002; Sartippour et al., 2002) and disrupt VEGF-receptor 2 dimerisation (Rodriguez et al., 2006). In animal models, EGCG and green tea compounds suppressed angiogenesis in a lung tumourigenesis model (Liao et al., 2004) and tumour microvessel density in a murine gastric cancer model (Zhu et al., 2007). In human studies, short-term administration of Poly E 800 mg daily for 2–6 weeks in patients with early stage prostate or breast cancer was associated with reductions in serum HGF and VEGF (McLarty et al., 2009; Campbell et al., 2010). These results support potential mechanisms of action of green tea extract in HGF signalling and angiogenesis.
Tea polyphenols are considered potent antioxidants. In in vitro and animal models, EGCG caused reduced oxidative damage and decreased levels of protein carbonylation and lipid peroxidation (Senthil Kumaran et al., 2008). In human studies, patients on hemodialysis given green tea catechins for 3 months had reduced CRP and other pro-inflammatory markers compared to controls (Hsu et al., 2007). In a randomised controlled trial of heavy smokers, four cups of decaffeinated green tea daily for 4 months reduced urinary 8-oxodG by 31% (Hakim et al., 2003). In addition, a randomised controlled trial of Poly E 800 mg daily for 3–6 weeks in 103 men with localised prostate cancer observed a favourable trend in oxidative DNA damage in blood leukocytes (Nguyen et al., 2012). We did not observe significant changes in oxidative damage or inflammatory biomarkers in this trial, possibly as a result of the small sample size. Another explanation for these inconsistent findings may be that the antioxidative effects of EGCG are only apparent in the presence of excessive oxidative stress (Yang et al., 2009).
Epigallocatechin-3-gallate has been proposed to promote weight loss and have a favourable effect on metabolic markers of obesity (Moon et al., 2007). In a randomised controlled trial of Poly E 400 or 800 mg daily for 2 months in 103 healthy post-menopausal women, low-density lipoprotein cholesterol, glucose and insulin decreased significantly with Poly E compared to placebo (Wu et al., 2012). To determine the effects of green tea on metabolic parameters, weight and body composition, 54 overweight breast cancer survivors were randomised to 6 months of daily decaffeinated green tea versus placebo tea (Stendell-Hollis et al., 2010). Green tea intake was associated with a significant increase in high-density lipoprotein cholesterol and nonsignificant trends in decreased body weight and improved glucose homeostasis. We also observed a trend toward decreased total serum cholesterol with 6 months of Poly E compared to placebo, although this did not reach statistical significance. No significant change in body weight was observed in the Poly E group compared to placebo (data not shown). Therefore, a potential cancer preventive mechanism of tea catechins may be through targeting insulin resistance and the metabolic syndrome.
The strengths of the present study include the randomised controlled design, the relatively long-term drug intervention and the placebo group, which provided reference levels for all biomarkers. A weakness is the relatively small sample size, particularly the number of placebo controls. Although 34 (85%) of the participants completed the study evaluations, only 28 (70%) completed the 6-month drug intervention, partly as a result of the five DLTs that warranted stopping the study drug. Mean urinary tea polyphenols peaked at 2 months on Poly E but decreased slightly over time during the 6-month intervention, most likely as a result of drop-outs and drug compliance. As a result, the greatest biomarker changes occurred at 2 months and decreased over time. Because effective chemopreventive drugs will likely require prolonged chronic use, safety, tolerability and long-term compliance with these agents all need to be addressed to maintain their efficacy.
There are currently limited data available regarding the effects of tea catechins for breast cancer prevention from human intervention trials. An ongoing randomised double-blind placebo-controlled trial is examining the effects of a 1-year intervention of an oral green tea extract in 800 post-menopausal women on mammographic density and other circulating biomarkers of breast cancer risk (NCT00917735). The results of our trial may inform the choice of systemic biomarkers for this larger primary prevention study.
In summary, our results suggest potential mechanisms of action of tea polyphenols in HGF signalling, angiogenesis and lipid metabolism. The optimal dose, duration and formulation of green tea and target population for breast cancer prevention have yet to be determined. In general, the public perception is that dietary supplements are safe and therefore they may gain wider acceptance in the prevention setting compared to pharmacological drugs. However, these agents need to be rigorously tested in future studies before translating to clinical practice.
Acknowledgments
The study was supported by the National Cancer Institute (grant N01-CN-35159), the National Institute of Environmental Health Sciences (grant ES009089) and the American Cancer Society (grant ACS MRSG-08-021-01-CNE).
Footnotes
Conflict of interests: The authors declare that they have no conflicts of interest.
source of funding and authorship: No funding declared.
AW, BA, CH, HG, HLM, JC, MR, NR, PB, SML and TLC participated in the design and coordination of the study and helped to draft the manuscript. DLH conceived the study, participated in its design and coordination and drafted the manuscript. J Cardelli conducted the HGF and VEGF biomarker assays and helped to draft the manuscript. KAH performed the statistical analysis and drafted the manuscript. KDC conceived the study, participated in its design and coordination and drafted the manuscript. RMS conducted the oxidative damage biomarker assays and helped to draft the manuscript. All authors read and approved the final manuscript.
References
- Bigelow RL, Cardelli JA. The green tea catechins, (–)-epigallocatechin-3-gallate (EGCG) and (–)-epicatechin-3-gallate (ECG), inhibit HGF/Met signaling in immortalized and tumorigenic breast epithelial cells. Oncogene. 2006;25:1922–1930. doi: 10.1038/sj.onc.1209227. [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- Campbell JS, Cardelli JA, McLarty J, Hershman DL, Taback B, Refice SF, Wang S, Bigelow R, Crew KD. Effects of Presurgical Administration of An Oral Green Tea Extract in Women with Operable Breast Cancer. Philadelphia, PA: American Association for Cancer Research, Frontiers in Cancer Prevention; 2010. [Google Scholar]
- Chang PY, Mirsalis J, Riccio ES, Bakke JP, Lee PS, Shimon J, Phillips S, Fairchild D, Hara Y, Crowell JA. Genotoxicity and toxicity of the potential cancer-preventive agent Polyphenon E. Environ Mol Mutagen. 2003;41:43–54. doi: 10.1002/em.10129. [DOI] [PubMed] [Google Scholar]
- Cheung YK, Chappell R. Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics. 2000;56:1177–1182. doi: 10.1111/j.0006-341x.2000.01177.x. [DOI] [PubMed] [Google Scholar]
- Crew KD, Brown P, Greenlee H, Bevers TB, Arun B, Hudis C, McArthur HL, Chang J, Rimawi M, Vornik L, Cornelison TL, Wang A, Hibshoosh H, Ahmed A, Terry MB, Santella RM, Lippman SM, Hershman DL. Phase IB randomized, double-blinded, placebo-controlled, dose escalation study of Polyphenon E in women with hormone receptor-negative breast cancer. Cancer Prev Res (Phila) 2012;5:1144–1154. doi: 10.1158/1940-6207.CAPR-12-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim IA, Harris RB, Brown S, Chow HH, Wiseman S, Agarwal S, Talbot W. Effect of increased tea consumption on oxidative DNA damage among smokers: a randomized controlled study. J Nutr. 2003;133:3303S–3309S. doi: 10.1093/jn/133.10.3303S. [DOI] [PubMed] [Google Scholar]
- Hsu SP, Wu MS, Yang CC, Huang KC, Liou SY, Hsu SM, Chien CT. Chronic green tea extract supplementation reduces hemodialysis-enhanced production of hydrogen peroxide and hypochlorous acid, atherosclerotic factors, and proinflammatory cytokines. Am J Clin Nutr. 2007;86:1539–1547. doi: 10.1093/ajcn/86.5.1539. [DOI] [PubMed] [Google Scholar]
- Inoue-Choi M, Yuan JM, Yang CS, Van den Berg DJ, Lee MJ, Gao YT, Yu MC. Genetic association between the COMT genotype and urinary levels of tea polyphenols and their metabolites among daily green tea drinkers. Int J Mol Epidemiol Genet. 2010;1:114–123. [PMC free article] [PubMed] [Google Scholar]
- Liao J, Yang GY, Park ES, Meng X, Sun Y, Jia D, Seril DN, Yang CS. Inhibition of lung carcinogenesis and effects on angiogenesis and apoptosis in A/J mice by oral administration of green tea. Nutr Cancer. 2004;48:44–53. doi: 10.1207/s15327914nc4801_7. [DOI] [PubMed] [Google Scholar]
- Malone KE, Begg CB, Haile RW, Borg A, Concannon P, Tellhed L, Xue S, Teraoka S, Bernstein L, Capanu M, Reiner AS, Riedel ER, Thomas DC, Mellemkjaer L, Lynch CF, Boice JD, Jr, Anton-Culver H, Bernstein JL. Population-based study of the risk of second primary contralateral breast cancer associated with carrying a mutation in BRCA1 or BRCA2. J Clin Oncol. 2010;28:2404–2410. doi: 10.1200/JCO.2009.24.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, Weinstein IB. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J Exp Ther Oncol. 2002;2:350–359. doi: 10.1046/j.1359-4117.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- McLarty J, Bigelow RL, Smith M, Elmajian D, Ankem M, Cardelli JA. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev Res (Phila) 2009;2:673–682. doi: 10.1158/1940-6207.CAPR-08-0167. [DOI] [PubMed] [Google Scholar]
- Moon HS, Lee HG, Choi YJ, Kim TG, Cho CS. Proposed mechanisms of (–)-epigallocatechin-3-gallate for anti-obesity. Chem Biol Interact. 2007;167:85–98. doi: 10.1016/j.cbi.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Murphey LJ, Williams MK, Sanchez SC, Byrne LM, Csiki I, Oates JA, Johnson DH, Morrow JD. Quantification of the major urinary metabolite of PGE2 by a liquid chromatographic/mass spectrometric assay: determination of cyclooxygenase-specific PGE2 synthesis in healthy humans and those with lung cancer. Anal Biochem. 2004;334:266–275. doi: 10.1016/j.ab.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Nguyen MM, Ahmann FR, Nagle RB, Hsu CH, Tangrea JA, Parnes HL, Sokoloff MH, Gretzer MB, Chow HH. Randomized, double-blind, placebo-controlled trial of Polyphenon E in prostate cancer patients before prostatectomy: evaluation of potential chemopreventive activities. Cancer Prev Res (Phila) 2012;5:290–298. doi: 10.1158/1940-6207.CAPR-11-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunleye AA, Xue F, Michels KB. Green tea consumption and breast cancer risk or recurrence: a meta-analysis. Breast Cancer Res Treat. 2010;119:477–484. doi: 10.1007/s10549-009-0415-0. [DOI] [PubMed] [Google Scholar]
- Raghav KP, Wang W, Liu S, Chavez-MacGregor M, Meng X, Hortobagyi GN, Mills GB, Meric-Bernstam F, Blumenschein GR, Jr, Gonzalez-Angulo AM. cMET and phospho-cMET protein levels in breast cancers and survival outcomes. Clin Cancer Res. 2012;18:2269–2277. doi: 10.1158/1078-0432.CCR-11-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez SK, Guo W, Liu L, Band MA, Paulson EK, Meydani M. Green tea catechin, epigallocatechin-3-gallate, inhibits vascular endothelial growth factor angiogenic signaling by disrupting the formation of a receptor complex. Int J Cancer. 2006;118:1635–1644. doi: 10.1002/ijc.21545. [DOI] [PubMed] [Google Scholar]
- Rossner P, Jr, Gammon MD, Terry MB, Agrawal M, Zhang FF, Teitelbaum SL, Eng SM, Gaudet MM, Neugut AI, Santella RM. Relationship between urinary 15-F2t-isoprostane and 8-oxodeoxyguanosine levels and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:639–644. doi: 10.1158/1055-9965.EPI-05-0554. [DOI] [PubMed] [Google Scholar]
- Sartippour MR, Shao ZM, Heber D, Beatty P, Zhang L, Liu C, Ellis L, Liu W, Go VL, Brooks MN. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J Nutr. 2002;132:2307–2311. doi: 10.1093/jn/132.8.2307. [DOI] [PubMed] [Google Scholar]
- Seely D, Mills EJ, Wu P, Verma S, Guyatt GH. The effects of green tea consumption on incidence of breast cancer and recurrence of breast cancer: a systematic review and meta-analysis. Integr Cancer Ther. 2005;4:144–155. doi: 10.1177/1534735405276420. [DOI] [PubMed] [Google Scholar]
- Senthil Kumaran V, Arulmathi K, Srividhya R, Kalaiselvi P. Repletion of antioxidant status by EGCG and retardation of oxidative damage induced macromolecular anomalies in aged rats. Exp Gerontol. 2008;43:176–183. doi: 10.1016/j.exger.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Stearns V, Gallagher A, Kleer CG, Singh B, Freedman M, Haddad BR, Isaacs C, Warren R, Brown M, Cullen J, Trock B, Hayes DF. A pilot study to establish a clinical model to perform phase II studies of breast cancer chemopreventive agents in women at high risk with biomarkers as surrogate endpoints for activity. Clin Cancer Res. 2004;10:8332–8340. doi: 10.1158/1078-0432.CCR-04-0297. [DOI] [PubMed] [Google Scholar]
- Stendell-Hollis NR, Thomson CA, Thompson PA, Bea JW, Cussler EC, Hakim IA. Green tea improves metabolic biomarkers, not weight or body composition: a pilot study in overweight breast cancer survivors. J Hum Nutr Diet. 2010;23:590–600. doi: 10.1111/j.1365-277X.2010.01078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CL, Yuan JM, Koh WP, Yu MC. Green tea, black tea and breast cancer risk: a meta-analysis of epidemiological studies. Carcinogenesis. 2006;27:1310–1315. doi: 10.1093/carcin/bgi276. [DOI] [PubMed] [Google Scholar]
- Swain SM, Wilson JW, Mamounas EP, Bryant J, Wickerham DL, Fisher B, Paik S, Wolmark N. Estrogen receptor status of primary breast cancer is predictive of estrogen receptor status of contralateral breast cancer. J Natl Cancer Inst. 2004;96:516–523. doi: 10.1093/jnci/djh097. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC, Buss IH. Protein carbonyl measurement by enzyme-linked immunosorbent assay. Methods Enzymol. 1999;300:106–111. doi: 10.1016/s0076-6879(99)00118-4. [DOI] [PubMed] [Google Scholar]
- Wu AH, Spicer D, Stanczyk FZ, Tseng CC, Yang CS, Pike MC. Effect of 2-month controlled green tea intervention on lipoprotein cholesterol, glucose, and hormone levels in healthy postmenopausal women. Cancer Prev Res (Phila) 2012;5:393–402. doi: 10.1158/1940-6207.CAPR-11-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu BH, Zhan WH, Li ZR, Wang Z, He YL, Peng JS, Cai SR, Ma JP, Zhang CH. (–)-Epigallocatechin-3-gallate inhibits growth of gastric cancer by reducing VEGF production and angiogenesis. World J Gastroenterol. 2007;13:1162–1169. doi: 10.3748/wjg.v13.i8.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]