Abstract
We have previously shown in infant rats that brief and repeated experiences with a stressed dam outside the homecage (maltreatment) alters methylation of DNA associated with the brain-derived neurotrophic factor (bdnf) gene within the developing and adult prefrontal cortex. BDNF is a key mediator of activity-dependent processes that have a profound influence on neural development and plasticity. Here we examined whether maltreatment also alters bdnf DNA methylation in two additional regions known to be prominently affected by diverse forms of early-life adversity in humans, the hippocampus and amygdala. We found significant bdnf DNA methylation modifications present within the adult hippocampus (dorsal and ventral) and amygdala (central/basolateral complex). We observed that the nature of change differed between sexes, gene locus (bdnf I vs. IV), and brain region. Furthermore, a manipulation that did not produce any obvious behavior difference in infants (brief and repeated experiences with a nurturing foster dam) also had long-term effects on methylation. These data provide further empirical support of DNA methylation modifications as biological consequences of caregiving environments.
Keywords: epigenetic, DNA methylation, bdnf, maltreatment, foster care, amygdala, hippocampus
Influential studies done as early as the 1950s began to delineate the profound relationship between parental care and developmental trajectories. In parallel, researchers began to pioneer psychobiological approaches to understand biological responses to disruptions in the early caregiving environment. These approaches have helped us realize that there is a sensitive period during early postnatal development in which the developing brain has an incredible level of plasticity, and that caregiving experiences during this period shape neural circuits that will influence lifelong structural and functional aspects of brain and behavior (Gunnar & Quevedo, 2007; Korosi & Baram, 2009; Landers & Sullivan, 2012; Sanchez, 2006). Though it has been appreciated for some time that these long-term influences represent so-called epigenetic programming, it has only been within the past decade in which empirical evidence that directly supports this notion has been presented. With its stable and even heritable nature regarding gene regulation, DNA methylation has been the most extensively studied epigenetic modification with regards to experience-induced changes within the postnatal environment. Utilizing candidate gene and whole-genome approaches, studies with various rodent models have consistently indicated a causal relationship between the quality of the caregiving environment and DNA methylation modifications within the developing and adult brain (Jensen & Champagne, 2012; Roth, 2012; Szyf & Bick, 2013; Szyf, McGowan, & Meaney, 2008).
Postmortem and epidemiological studies have begun to reveal aberrant epigenetic marking of the human genome in individuals reporting significant early-life adversity (Borghol et al., 2012; Labonté et al., 2012a; Labonté et al., 2012b; McGowan et al., 2009; McGowan et al., 2008; Mehta et al., 2013; Smith et al., 2011). As childhood maltreatment is known to induce long-term brain alterations and has been associated with varied forms of psychopathology, epigenetic mechanisms have emerged as a leading candidate to mechanistically link these experiences and outcomes. In human epigenetic studies however it is difficult to distinguish methylation/demethylation that may have developed as a response to the caregiving environment from those that occurred as a consequence of additional experiences and environmental stimuli encountered later in life. Peripheral measures of DNA methylation (obtained from blood samples or cheek swabs) likely do not reflect the complexity of DNA methylation modifications that occur within the brain (i.e. gene- and cell-type-specific). Research with an animal model in which the timing and duration of maltreatment can be controlled and subsequent neural assessments made is thus of value in increasing our understanding of the relationship between caregiver maltreatment and epigenetic outcomes.
We have therefore investigated whether there are DNA methylation modifications in an experimental model, in which infant rats during the first postnatal week are repeatedly exposed to brief bouts of caregiver maltreatment outside the homecage. Our model is a variation on that introduced by the Baram Laboratory (Gilles, Schultz, & Baram, 1996; Ivy, Brunson, Sandman, & Baram, 2008) and used by the Sullivan Laboratory (Raineki, Moriceau, & Sullivan, 2010; Roth & Sullivan, 2005), which capitalizes on the ability of resource deprivation (nesting material) to produce aberrant caregiving behaviors that evoke later changes in brain function and behavior of the offspring. We previously showed that brief, but repeated, exposure to caregiver maltreatment (i.e. being stepped on, dropped, dragged, actively avoided, and roughly handled) produces DNA methylation modifications that are present in both the developing and adult whole (Roth, Lubin, Funk, & Sweatt, 2009) and medial (Blaze, Scheuing, & Roth, 2013) prefrontal cortex. Here we extend these results by experimentally assessing whether caregiver maltreatment (as well as repeated exposure to a nurturing foster dam, an additional experimental manipulation we always employ) alters DNA methylation within two additional behaviorally- relevant brain regions, the hippocampus and amygdala. Similar to our other studies we utilized a candidate gene approach and high-resolution mapping of cytosine sites to examine important regulatory regions of the brain-derived neurotrophic factor (bdnf) gene. We focused on the bdnf gene for the following reasons. The bdnf gene codes for a protein that plays an essential role in the development and survival of neurons, as well as neural plasticity underlying life-long cognitive processes (Bath, Akins, & Lee, 2012; Greenberg, Xu, Lu, & Hempstead, 2009; Park & Poo, 2013). There are ample data indicating that there are changes in bdnf DNA methylation in response to a variety of environmental factors, especially stress (Kundakovic, Lim, Gudsnuk, & Champagne, 2013; Lubin, Roth, & Sweatt, 2008; Roth, Zoladz, Sweatt, & Diamond, 2011; Unternaehrer et al., 2012). Furthermore, several psychiatric disorders that are often associated with early-life adversity have been linked to aberrant methylation of bdnf DNA (Fuchikami et al., 2011; Keller et al., 2010; Perroud et al., 2013; Smith, et al., 2011).
The objectives of the current study were: 1) to experimentally determine whether there were bdnf DNA methylation modifications present within the hippocampus and amygdala of adults exposed to aversive or nurturing caregiving during the first postnatal week of life; 2) to determine whether adult bdnf DNA methylation modifications linked to caregiver experiences were already present in infancy; and, 3) to determine whether DNA methylation modifications differed between males and females.
Methods
For complete details on methodologies, please see Supplement 1.
Caregiving manipulations
With a method previously used (Blaze, et al., 2013; Roth, et al., 2009) and adapted from earlier studies (Gilles, et al., 1996; Ivy, et al., 2008; Raineki, et al., 2010; Roth & Sullivan, 2005), infant rats were divided into three equal groups on postnatal day (PN) 1 using a within litter design. For 30 minutes daily beginning on PN1 and ending on PN7, up to two males and two females from the same litter were exposed to either a stressed dam in a novel environment with limited nesting material (maltreatment condition) or a dam in a familiar environment with adequate nesting material (foster care condition). Additional littermates served as controls by remaining in the home cage (normal care condition).
Methylation assays on adult brain tissue
The hippocampus (dorsal vs. ventral tissue) and amygdala (basolateral, lateral, and central nuclei were homogenized together to yield sufficient DNA) were dissected on dry ice using stereotaxic coordinates, and nucleic acid was extracted. Methylation status was estimated via direct bisulfite sequencing (BSP) on bisulfite-modified DNA as previously described (Parrish, Day, & Lubin, 2012; Roth, et al., 2009; Roth, et al., 2011). Bisulfite-treated samples were amplified by primer sets (listed in Supplement 2) targeting DNA associated with bdnf exons I and IV, which encompass transcription factor binding and transcription start sites.
Methylation assays on infant brain tissue
Since no site-specific changes were responsible for the methylation changes present in adults, we used methyl-specific real-time PCR (MSP) to simply screen the same bdnf regions in infant tissue. Bisulfite-treated samples were amplified by primers (listed in Supplement 2) designed to distinguish unmethylated and methylated DNA associated with bdnf I and IV (same sequences as targeted by BSP for adult tissue).
Statistical Analyses
Caregiver behaviors and infant vocalizations were analyzed by one-way ANOVAs and Bonferroni's post-hoc tests. BSP data were analyzed by three-way ANOVAs (levels: infant condition, sex, CG site) and Tukey-Kramer post-hoc tests. MSP data were analyzed by two-way ANOVAs (levels: infant condition, sex), one-sample t-tests (for comparisons to normal care controls), and two-tailed unpaired t-tests (for comparisons between groups and sexes). For all analyses, differences were considered to be statistically significant for p≤0.05. Non-significant trends at p<0.1 are also reported.
Results
Caregiving behaviors and infant responses
As seen in our prior studies with this rodent model (Blaze, et al., 2013; Roth, et al., 2009; Roth & Sullivan, 2005), infants assigned to our maltreatment condition were subjected to a greater proportion of aversive caregiving behaviors (i.e. being stepped on, dropped, dragged, actively avoided, and roughly handled) than infants assigned to either the foster or normal care condition (one-way ANOVAs and Bonferroni post-hoc tests are reported in Table 1). Both audible and ultrasonic vocalizations also differed significantly across conditions. While there were no differences in audible or ultrasonic vocalizations emitted between infants in the foster and normal care conditions (p's>0.05), infants in the maltreatment condition emitted significantly more audible and ultrasonic vocalizations (Table 1).
Table 1.
Average percent occurrences of infant-directed behaviors and vocalizations across the 7 exposure periods.
| Normal | Foster | Maltreatment | |
|---|---|---|---|
| Adverse caregiving behaviors | |||
| Step on F(2,64)=28.98, p<0.001 | 6.4%*** | 6.9%*** | 18.5% |
| Drop F(2,64)=15.43, p<0.001 | 1.3%*** | 0.9%*** | 5.4% |
| Drag F(2,64)=2.45, p=0.09 | 4.1% | 4.1% | 5.6% |
| Avoid F(2,64)=26.5, p<0.001 | 0.5%*** | 0.6%*** | 12.3% |
| Rough handle F(2,64)=40.36, p<0.001 | 3.9%*** | 3.0%*** | 13.6% |
| Nurturing caregiving behaviors | |||
| Lick/groom F(2,64)=12.77, p<0.001 | 33.4%** | 34.7%*** | 17.4% |
| Hover/nurse F(2,64)=18.44, p<0.001 | 50.4%*** | 49.8%*** | 27.2% |
| Pup vocalizations | |||
| Audible F(2,56)=9.49, p<0.001 | 35.0%* | 27.4%*** | 50.3% |
| Ultrasonic F(2,36)=62.43, p<0.001 | 50.4%*** | 47.6%*** | 85.8% |
p<0.05,
p<0.01,
p<0.001 vs. maltreatment.
Note: Normal vs. foster comparisons were not significant.
Methylation patterns in the adult hippocampus
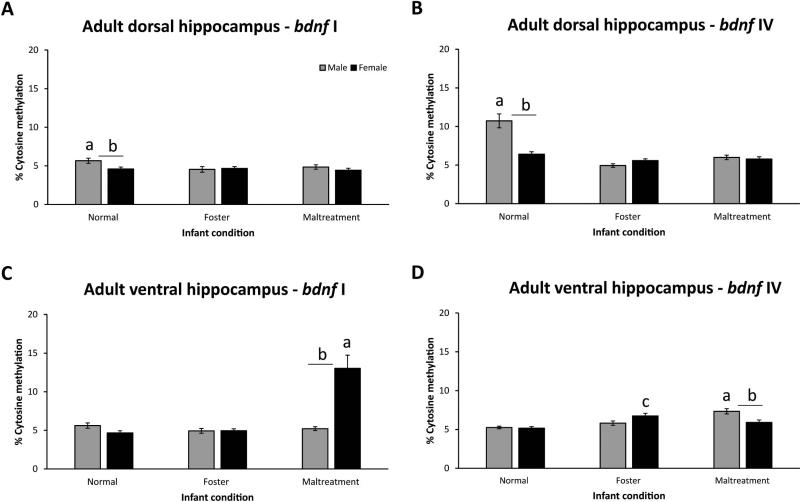
To assess the effects of our caregiving environments on adult methylation patterns, methylation of bdnf DNA was measured approximately 3 months following the last exposure session (in animals at PN90). BSP was used to estimate methylation for each cytosine within the targeted regions of bdnf I (10 sites) and IV (12 sites). Since methylation patterns did not vary substantially across estrus stages in females, data were collapsed. A three-way ANOVA for bdnf I methylation within the dorsal hippocampus (Figures 1a and S1) revealed main effects of infant condition [F(2,568)=4.53, p=0.01], sex [F(1,568=7.56, p<0.01], and CG site [F(9,568)=70.29, p<0.001]. There was also a significant interaction between the factors of infant condition x sex [F(2,568)=4.49, p=0.01]. Follow-up Tukey-Kramer tests showed higher average methylation (i.e. across the region) in normal males than both foster care (p<0.01) and maltreated-males (p<0.05) as well as normal females (p=0.001). Likewise, a three-way ANOVA for bdnf IV methylation within the dorsal hippocampus (Figures 1b and S2) revealed similar effects and patterns: main effects of infant condition [F(2,696)=33.02, p<0.001], sex [F(1,696=13.02, p<0.001], and CG site [F(11,696)=12.25, p<0.001], as well as an infant condition x sex interaction [F(2,696)=18.67, p<0.001]. Tukey-Kramer tests showed higher levels of methylation in normal males than foster care and maltreated-males and normal females (all p's<0.001).
Figure 1.
Average percent methylation of bdnf I and IV DNA in the adult dorsal (panels A-B) and ventral (panels C-D) hippocampus. Data presented are average estimated methylation levels across the 10 (bdnf I, panels A-C) and 12 (bdnf IV, panels B-D) CG sites. Statistics are provided within the text. Panels A and B- ap-values significant vs. foster and maltreatment males; bp-value significant vs. normal females. Panels C- ap-values significant vs. normal and foster care females; bp-value significant vs. maltreated-males. Panel D- ap-value significant vs. normal and foster care males; bp-value significant vs. maltreated-females; cp-value signficiant vs. normal care females. Males, n=7-14/group; females, n=11-12/group; error bars=SEM.
A three-way ANOVA for bdnf I methylation within the ventral hippocampus (Figures 1c and S3) revealed main effects of infant condition [F(2,590)=22.14, p<0.001], sex [F(1,590=14.29, p<0.001], and CG site [F(9,590)=9.06, p<0.001]. There was also a significant interaction between the factors of infant condition and sex [F(2,590)=23.57, p<0.001]. Tukey-Kramer tests showed that maltreated-females had higher methylation than both foster and normal care females as well as maltreated-males (all p's<0.001). Finally, a three-way ANOVA for bdnf IV methylation within the ventral hippocampus (Figures 1d and S4) revealed main effects of infant condition [F(2,695)=15.08, p<0.001] and CG site [F(11,695)=21.41, p<0.001], and a significant infant condition x sex interaction [F(2,695)=8.96, p<0.001). Post-hoc tests revealed that maltreated-males had significantly greater methylation than normal (p<0.001) and foster care males (p<0.01) and maltreated-females (p<0.001). Additionally, foster care females had higher methylation than normal (p<0.001) but not maltreated-females (p=0.22).
Methylation patterns in adult amygdala
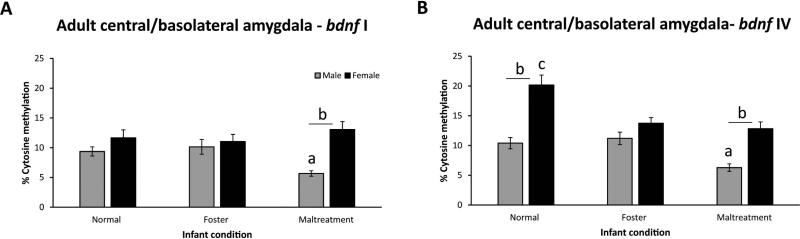
A three-way ANOVA for bdnf I methylation within the amygdala (Figures 2a and S5) revealed main effects of sex [F(1,709=16.08, p<0.001] and CG site [F(9,709)=9.17, p<0.001]. There was also a significant interaction between the factors of infant condition and sex [F(2,709)=5.02, p<0.01] and sex and CG site [F(9,709)=2.34, p=0.01]. Tukey-Kramer tests showed that maltreated-males had less methylation than foster care males (p<0.05) and maltreated-females (p<0.001). A three-way ANOVA for bdnf IV methylation within the amygdala (Figures 2b and S6) showed main effects of infant condition [F(2,786)=14.14, p<0.001), sex [F(1,786)=51.9, p<0.001], and CG site [F(11,786)=3.06, p<0.001]. There was also an interaction between infant condition and sex [F(2,786)=6.05, p<0.01]. Maltreated-males showed less methylation than foster (p=0.01) and normal care males (p=0.08) and maltreated-females (p<0.001). Normal females showed higher methylation than foster care and maltreated-females and normal males (all p's <0.001).
Figure 2.
Average percent methylation of bdnf I (panel A) and IV (panel B) DNA in the adult amygdala. Data presented are average estimated methylation levels across the 10 (bdnf I) and 12 (bdnf IV) CG sites. ap-value significant vs. foster and normal males; bp-values significant vs. other sex within same group; cp-values significant vs. foster and maltreatment females. Males, n=12-13/group; females, n=11-14/group; error bars=SEM.
Methylation patterns in the infant hippocampus and amygdala
To assess the effects of our caregiving environments on infant methylation patterns, DNA methylation was measured in rats 24 hrs after the last exposure session (at PN8). For the dorsal hippocampus, two-way ANOVAs for either bdnf I (Figure S7a) or IV (Figure S7b) did not reveal any main effects of infant or sex, nor an interaction between the factors (all p's>0.1). One-sample t-tests however showed that foster males had less methylation of bdnf I than normal care controls [t(9)=2.27, p<0.05] within the dorsal hippocampus. For the ventral hippocampus, a two-way ANOVA for bdnf I (Figure S7c) revealed no main effects of infant condition, sex, or an interaction (all p's>0.1). One-sample t-tests showed that both foster care males [t(9)=2.52, p<0.05] and females [t(9)=2.47, p<0.05] had higher methylation of bdnf I in the ventral hippocampus than normal care controls. A two-way ANOVA for bdnf IV (Figure S7d) revealed no main effect of infant condition (p>0.1), a main effect of sex [F(1,32)=4.09, p=0.05], and no factor interaction (p>0.1). Maltreated-females did however have higher methylation than normal care controls [t(8)=3.17, p=0.01] and maltreated-males [t(16)=2.11, p=0.05].
For bdnf I methylation in the amygdala (Figure S8a), there were no main effects of infant condition or sex (p's>0.1) but there was an interaction between infant condition and sex [F(1,28)=4.87, p<0.05]. Maltreated-females had less methylation than normal [t(7)=2.7, p<0.05] and foster care females [t(14)=2.12, p=0.05]. Similarly, for exon IV (Figure S8b) there were no main effects of infant condition or sex (p's>0.1), but there was an interaction between the factors of infant condition and sex [F(1,25)=4.38, p<0.05]. Maltreated-males had marginally higher methylation than foster care males [t(13)=1.99, p=0.07].
Discussion
Results from the present study support the hypothesis that exposure to maltreatment early in life produces CNS DNA methylation alterations. This was experimentally assessed in male and female rats using a model where infants (from the same litter) could be assigned to different, recurring treatments. Our observations of caregiver behaviors during the exposure sessions indicate that within the maltreatment condition infants were subjected to frequent occurrences of aversive caregiving, including bouts of being stepped on, actively avoided, and roughly handled. Infants within the foster care condition instead were subjected to more nurturing and positive actions composed predominately of crouching, nursing, anogenital licking, and grooming. Behaviors displayed by the foster care dams and the biological mothers (normal condition) did not differ from one another, demonstrating that infants with these two conditions experienced similar (quality and quantity) care during the 30 minute sessions. Our measures of audible and ultrasonic vocalizations indicate that maltreated-infants differentially responded to their caregiving environment. Specifically, these infants emitted significantly more vocalizations in comparison to infants from the foster and normal care groups (with no difference between the latter two). Together, behavioral observations here replicate those initially observed with this model (Blaze, et al., 2013; Roth, et al., 2009) and are in-line with similar observations when nesting material is restricted within the homecage (Ivy, et al., 2008; Raineki, et al., 2010).
In our biochemical findings for both the hippocampus and amygdala, we found that there was no one specific cytosine site showing a change in methylation status with treatment (though some sites showed higher methylation than others, regardless of infant condition), and instead experience-induced DNA methylation modifications occurred across cytosines within the amplified regions. Changes in methylation status were markedly affected by infant condition and were found to vary between sexes, brain regions, and bdnf locus. Changes common to our foster care and maltreatment groups were lower levels of methylation for both bdnf I and IV in the dorsal hippocampus of males and lower levels of bdnf IV methylation in the amygdala of females. This suggests methylation outcomes in these cases were influenced by a common factor between the conditions, such as exposure to a novel environment and/or an unfamiliar caregiver or removal from the homecage. Changes evoked by our foster care condition are interesting in their own right, as these are long-term changes from a manipulation that did not result in an apparent behavioral response from the infants (i.e. no change in vocalizations).
Changes unique to our maltreatment group were greater methylation of bdnf I in the ventral hippocampus of females, greater methylation of bdnf IV in the ventral hippocampus of males, and lower methylation of bdnf I and IV in the amygdala of males. In one of our more recent studies, we likewise found methylation changes at bdnf I and IV within the medial prefrontal cortex specific to our maltreatment group, with different outcomes between males and females (Blaze, et al., 2013). Together, our results provide empirical support of the ability of caregiver maltreatment to produce DNA methylation alterations across behaviorally-relevant brain networks known to be affected by childhood maltreatment- the prefrontal cortex, hippocampus, and amygdala. They also provide insight of the ability of maltreatment to differentially affect brain regions at the molecular level. Our observations of differential epigenetic marking of the bdnf gene are in agreement with other reports (albeit in adult animals) where environmental stimuli and conditions have been shown to produce a complex pattern of DNA methylation modifications that vary across bdnf gene loci and brain region (Fuchikami, et al., 2011; Kundakovic, et al., 2013; Lubin, et al., 2008; Roth, et al., 2011; Tian, Marini, & Lipsky, 2010). Our observations of epigenetic alterations that differ between males and females parallel a similar phenomenon reported by others (Kosten, Huang, & Nielsen, 2014; Kundakovic, et al., 2013; Mueller & Bale, 2008; Schwarz, Nugent, & McCarthy, 2010).
As studies have shown the ability of postnatal experiences to cause changes in DNA methylation that persist into adulthood, we examined methylation patterns in infant animals to determine whether adult patterns were likewise present in infants. Since no site-specific changes were responsible for the methylation changes present in adults, we used MSP to simply screen the same bdnf regions in infant tissue. The methylation patterns for bdnf I and IV that we observed in adults were not detected in infants. We have observed a similar occurrence of differing patterns between developing and adult rats in the medial prefrontal cortex for bdnf I and IV methylation (Blaze, et al., 2013) and the whole prefrontal cortex for exon IV (Roth, et al., 2009). Later-emerging group differences in DNA methylation have too been reported for another gene locus in the hypothalamus of male mice subjected to early-life stress (Murgatroyd et al., 2009). Interestingly, we have observed persisting maltreatment-induced methylation changes (i.e. hypermethylation in infants, adolescents, and adults) at exon IX of the bdnf gene in tissue from the prefrontal cortex (Roth, et al., 2009). These data demonstrate the ability of early-life adversity to produce both persisting and later emerging DNA methylation alterations.
There were some limitations in our current study. First, the design of our exposure chambers did not allow us to distinguish caregiving behaviors directed at males verses those at females. As it is known that males are licked more by the dam within the nest (Hao, Huang, Nielsen, & Kosten, 2011; Moore & Morelli, 1979) it is possible that differences in care toward males and females during our manipulations and within the homecage (before and after treatment) contributed to methylation differences. Second, while keeping animals in strictly controlled rearing and housing conditions has obvious value for experimentally assessing the link between early-life experiences and later outcomes, this experimental design excludes the reality of complex social environments and the summing effects of total life experiences that are responsible for behavioral outcomes in humans. Third, the techniques we used in this study do not allow us to draw any conclusions regarding cytosine methylation verses hydroxymethylation, subregion specificity (for example CA1 vs. dentate), or cell-type specificity (i.e. glia vs. neurons), though increasing evidence suggests these are important considerations to keep in mind when interpreting methylation data (Brown, Weaver, Meaney, & Szyf, 2008; Huang et al., 2010; Jin, Kadam, & Pfeifer, 2010; Lister et al., 2013; Roth, et al., 2011; Shulha, Cheung, Guo, Akbarian, & Weng, 2013). We also acknowledge here with the large number of BSP measurements and tests on these data, the expected number of false rejections is increased above 5%. It will thus be important in future work to confirm BSP observations for these brain regions with additional techniques and testing, though we point out that in previous related work we have successfully verified BSP results with MSP and methylated DNA immunoprecipitation (e.g. Lubin, et al., 2008; Roth, et al., 2009).
Finally, we did not assess corresponding mRNA transcripts or protein levels, which may be useful in helping us appreciate the variation in magnitude of alterations (i.e. a very strong effect in the ventral hippocampus of females) and their functional relevance. While gene expression data can certainly be helpful in interpreting methylation data, the complexity between the relation between DNA methylation and gene transcription is beginning to be realized. While most studies have been conducted under the framework that environmentally-driven increases in DNA methylation will correlate with sustained decreases in basal levels of gene expression, a handful of studies have now indicated that DNA methylation can also be associated with transcriptional activation (Chahrour et al., 2008; Uchida et al., 2011). The complexity between the relationship between DNA methylation and gene transcription is further realized when one considers that DNA methylation changes do not always result in basal changes in gene expression, but can instead prime transcriptional responses to subsequent stimuli and neural activation (Baker-Andresen, Ratnu, & Bredy, 2013).
In conclusion, the gene locus, brain region, and sex-specific effects we identified highlight the complexity of the effects of early-life experiences that extend far beyond the period of early development. Our data suggest that methylation/demethylation of the bdnf gene, a gene intimately associated with brain development and function, could subserve developmental trajectories and gender differences associated with exposure to caregiver maltreatment. Future work will be necessary to experimentally link the observed methylation changes with behavior outcomes. As bdnf epigenetic marks are susceptible to change through therapeutic approaches (D'Addario et al., 2012; Koppel & Timmusk, 2013; Perroud, et al., 2013; Roth, et al., 2009; Sales et al., 2011; Tian, et al., 2010), it may be possible to target interventions to reverse maltreated-induced epigenetic alterations.
Supplementary Material
Acknowledgments
This work was supported by a Young Investigator Award from the Brain & Behavior Research Foundation, and grants from the University of Delaware Research Foundation and The National Institute of General Medical Sciences (1P20GM103653). We thank Thomas DiChiara, Emily Ellis, Hannah Evans, Samantha Jones, Hillary Porter, Brittany Rider, Lisa Scheuing, and Megan Warren for their help in generating animals, behavior coding, biochemistry, and gel electrophoresis.
References
- Baker-Andresen D, Ratnu VS, Bredy TW. Dynamic DNA methylation: A prime candidate for genomic metaplasticity and behavioral adaptation. Trends in Neurosciences. 2013;36(1):3–13. doi: 10.1016/j.tins.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Bath KG, Akins MR, Lee FS. Bdnf control of adult svz neurogenesis. Developmental Psychobiology. 2012;54(6):578–589. doi: 10.1002/dev.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaze J, Scheuing L, Roth TL. Differential methylation of genes in the medial prefrontal cortex of developing and adult rats following exposure to maltreatment or nurturing care during infancy. Developmental Neuroscience. 2013;35(4):306–316. doi: 10.1159/000350716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghol N, Suderman M, McArdle W, Racine A, Hallett M, Pembrey M, et al. Associations with early-life socio-economic position in adult DNA methylation. International Journal of Epidemiology. 2012;41(1):62–74. doi: 10.1093/ije/dyr147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SE, Weaver ICG, Meaney MJ, Szyf M. Regional-specific global cytosine methylation and DNA methyltransferase expression in the adult rat hippocampus. Neuroscience Letters. 2008;440(1):49–53. doi: 10.1016/j.neulet.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong STC, Qin J, et al. Mecp2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320(5880):1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Addario C, Dell'Osso B, Palazzo MC, Benatti B, Lietti L, Cattaneo E, et al. Selective DNA methylation of bdnf promoter in bipolar disorder: Differences among patients with bdi and bdii. Neuropsychopharmacology. 2012;37(7):1647–1655. doi: 10.1038/npp.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchikami M, Morinobu S, Segawa M, Okamoto Y, Yamawaki S, Ozaki N, et al. DNA methylation profiles of the brain-derived neurotrophic factor (bdnf) gene as a potent diagnostic biomarker in major depression. PLoS ONE. 2011;6(8):e23881. doi: 10.1371/journal.pone.0023881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles EE, Schultz L, Baram TZ. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatric Neurology. 1996;15(2):114–119. doi: 10.1016/0887-8994(96)00153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of bdnf synthesis and release: Implications in cns function. The Journal of Neuroscience. 2009;29(41):12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58(1):145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Hao Y, Huang W, Nielsen DA, Kosten TA. Litter gender composition and sex affect maternal behavior and DNA methylation levels of the oprm1 gene in rat offspring. Frontiers in Psychiatry. 2011:2. doi: 10.3389/fpsyt.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Pastor WA, Shen Y, Tahiliani M, Liu DR, Rao A. The behaviour of 5-hydroxymethylcytosine in bisulfite sequencing. PLoS ONE. 2010;5(1):e8888. doi: 10.1371/journal.pone.0008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: A clinically relevant model for early-life stress. Neuroscience. 2008;154(3):1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen C, Champagne F. Epigenetic and neurodevelopmental perspectives on variation in parenting behavior. Parenting, Science and Practice. 2012;12(2-3):202–211. doi: 10.1080/15295192.2012.683358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S-G, Kadam S, Pfeifer GP. Examination of the specificity of DNA methylation profiling techniques towards 5-methylcytosine and 5-hydroxymethylcytosine. Nucleic Acids Research. 2010;38(11):e125. doi: 10.1093/nar/gkq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S, Sarchiapone M, Zarrilli F, Videtic A, Ferraro A, Carli V, et al. Increased bdnf promoter methylation in the wernicke area of suicide subjects. Archives of General Psychiatry. 2010;67(3):258–267. doi: 10.1001/archgenpsychiatry.2010.9. [DOI] [PubMed] [Google Scholar]
- Koppel I, Timmusk T. Differential regulation of bdnf expression in cortical neurons by class-selective histone deacetylase inhibitors. Neuropharmacology. 2013;75:106–115. doi: 10.1016/j.neuropharm.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Korosi A, Baram TZ. The pathways from mother's love to baby's future. Frontiers in Behavioral Neuroscience. 2009:3. doi: 10.3389/neuro.08.027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Huang W, Nielsen DA. Sex and litter effects on anxiety and DNA methylation levels of stress and neurotrophin genes in adolescent rats. Developmental Psychobiology. 2014;56(3):392–406. doi: 10.1002/dev.21106. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Lim S, Gudsnuk K, Champagne FA. Sex-specific and strain-dependent effects of early life adversity on behavioral and epigenetic outcomes. Frontiers in Psychiatry. 2013:4. doi: 10.3389/fpsyt.2013.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonté B, Suderman M, Maussion G, Navaro L, Yerko V, Mahar I, et al. Genome-wide epigenetic regulation by early-life trauma. Archives of General Psychiatry. 2012a;69(7):722–731. doi: 10.1001/archgenpsychiatry.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonté B, Yerko V, Gross J, Mechawar N, Meaney MJ, Szyf M, et al. Differential glucocorticoid receptor exon 1b, 1c, and 1h expression and methylation in suicide completers with a history of childhood abuse. Biological Psychiatry. 2012b;72(1):41–48. doi: 10.1016/j.biopsych.2012.01.034. [DOI] [PubMed] [Google Scholar]
- Landers MS, Sullivan RM. The development and neurobiology of infant attachment and fear. Developmental Neuroscience. 2012;34(2-3):101–114. doi: 10.1159/000336732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, Mukamel EA, Nery JR, Urich M, Puddifoot CA, Johnson ND, et al. Global epigenomic reconfiguration during mammalian brain development. Science. 2013;341(6146) doi: 10.1126/science.1237905. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of bdnf gene transcription in the consolidation of fear memory. Journal of Neuroscience. 2008;28(42):10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, Huang TCT, Unterberger A, Suderman M, Ernst C, et al. Promoter-wide hypermethylation of the ribosomal rna gene promoter in the suicide brain. PLoS ONE. 2008;3(5):e2085. doi: 10.1371/journal.pone.0002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Klengel T, Conneely KN, Smith AK, Altmann A, Pace TW, et al. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proceedings of the National Academy of Sciences. 2013;110(20):8302–8307. doi: 10.1073/pnas.1217750110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C, Morelli G. Mother rats interact differently with male amd female offspring. Journal of Comparative and Physiological Psychology. 1979;93(4):677–684. doi: 10.1037/h0077599. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. The Journal of Neuroscience. 2008;28(36):9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nature Neuroscience. 2009;12(12):1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Park H, Poo M. Neurotrophin regulation of neural circuit development and function. [10.1038/nrn3379]. Nature Reviews Neuroscience. 2013;14(1):7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Parrish RR, Day JJ, Lubin FD. Direct bisulfite sequencing for examination of DNA methylation with gene and nucleotide resolution from brain tissues. Current Protocols in Neuroscience. 2012;60:7.24.21–27.24.12. doi: 10.1002/0471142301.ns0724s60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroud N, Salzmann A, Prada P, Nicastro R, Hoeppli ME, Furrer S, et al. Response to psychotherapy in borderline personality disorder and methylation status of the bdnf gene. Translational Psychiatry. 2013;3:e207. doi: 10.1038/tp.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Moriceau S, Sullivan RM. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biological Psychiatry. 2010;67(12):1137–1145. doi: 10.1016/j.biopsych.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL. Epigenetics of neurobiology and behavior during development and adulthood. Developmental Psychobiology. 2012;54(6):590–597. doi: 10.1002/dev.20550. [DOI] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the bdnf gene. Biological Psychiatry. 2009;65(9):760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: Neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biological Psychiatry. 2005;57(8):823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Roth TL, Zoladz PR, Sweatt JD, Diamond DM. Epigenetic modification of hippocampal bdnf DNA in adult rats in an animal model of post-traumatic stress disorder. Journal of Psychiatric Research. 2011;45(7):919–926. doi: 10.1016/j.jpsychires.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales AJ, Biojone C, Terceti MS, Guimarães FS, Gomes MVM, Joca SRL. Antidepressant-like effect induced by systemic and intra-hippocampal administration of DNA methylation inhibitors. British Journal of Pharmacology. 2011;164(6):1711–1721. doi: 10.1111/j.1476-5381.2011.01489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM. The impact of early adverse care on hpa axis development: Nonhuman primate models. Hormones and Behavior. 2006;50(4):623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Nugent BM, McCarthy MM. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology. 2010;151(10):4871–4881. doi: 10.1210/en.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulha HP, Cheung I, Guo Y, Akbarian S, Weng Z. Coordinated cell type–specific epigenetic remodeling in prefrontal cortex begins before birth and continues into early adulthood. PLoS Genet. 2013;9(4):e1003433. doi: 10.1371/journal.pgen.1003433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B, et al. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2011;156(6):700–708. doi: 10.1002/ajmg.b.31212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M, Bick J. DNA methylation: A mechanism for embedding early life experiences in the genome. Child Development. 2013;84(1):49–57. doi: 10.1111/j.1467-8624.2012.01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M, McGowan P, Meaney MJ. The social environment and the epigenome. Environmental and Molecular Mutagenesis. 2008;49(1):46–60. doi: 10.1002/em.20357. [DOI] [PubMed] [Google Scholar]
- Tian F, Marini AM, Lipsky RH. Effects of histone deacetylase inhibitor trichostatin a on epigenetic changes and transcriptional activation of bdnf promoter 1 by rat hippocampal neurons. Annals of the New York Academy of Sciences. 2010;1199(1):186–193. doi: 10.1111/j.1749-6632.2009.05175.x. [DOI] [PubMed] [Google Scholar]
- Uchida S, Hara K, Kobayashi A, Otsuki K, Yamagata H, Hobara T, et al. Epigenetic status of gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron. 2011;69(2):359–372. doi: 10.1016/j.neuron.2010.12.023. [DOI] [PubMed] [Google Scholar]
- Unternaehrer E, Luers P, Mill J, Dempster E, Meyer AH, Staehli S, et al. Dynamic changes in DNA methylation of stress-associated genes (oxtr, bdnf) after acute psychosocial stress. Translational Psychiatry. 2012;2:e150. doi: 10.1038/tp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.