Abstract
BACKGROUND:
Diethylstilbestrol (DES) and other pharmaceutical estrogens have been used at ≥μM concentrations to treat advanced prostate tumors, with successes primarily attributed to indirect hypothalamic-pituitary-testicular axis control mechanisms. However, estrogens also directly affect tumor cells, though the mechanisms involved are not well understood.
METHODS:
LAPC-4 (androgen-dependent) and PC-3 (androgen-independent) cell viability was measured after estradiol (E2) or DES treatment across wide concentration ranges. We then examined multiple rapid signaling mechanisms at 0.1 nM E2 and 1μM DES optima including levels of: activation (phosphorylation) for mitogen-activated protein kinases, cell-cycle proteins, and caspase 3, necroptosis, and reactive oxygen species (ROS).
RESULTS:
LAPC-4 cells were more responsive than PC-3 cells. Robust and sustained extracellular-regulated kinase activation with E2, but not DES, correlated with ROS generation and cell death. c-Jun N-terminal kinase was only activated in E2-treated PC-3 cells and was not correlated with caspase 3-mediated apoptosis; necroptosis was not involved. The cell-cycle inhibitor protein p16INK4A was phosphorylated in both cell lines by both E2 and DES, but to differing extents. In both cell types, both estrogens activated p38 kinase, which subsequently phosphorylated cyclin D1, tagging it for degradation, except in DES-treated PC-3 cells.
CONCLUSIONS:
Cyclin D1 status correlated most closely with disrupted cell cycling as a cause of reduced cell numbers, though other mechanisms also contributed. As low as 0.1 nM E2 effectively elicited these mechanisms, and its use could dramatically improve outcomes for both early- and late-stage prostate cancer patients, while avoiding the side effects of high-dose DES treatment.
Keywords: cell cycle, apoptosis, MAP kinases, non-genomic, reactive oxygen species, estrogen receptors
Introduction
Prostate tumors are usually androgen-dependent in the beginning, so front-line therapies are initially aimed at reducing the amount of free androgens that sustain these tumors. Traditional therapies to treat androgen-dependent tumors have included radiation and surgical removal of tumors, followed by androgen receptor antagonists, or pharmaceutical castration with luteinizing-hormone-releasing hormone (LHRH) analogs that block androgen production (1). However, these treatments become ineffective against tumors that have previously regressed with treatment, and have then escaped these controls to grow again. These recurrent or advanced prostate tumors often develop androgen-independence, and as an alternative, synthetic estrogens like diethylstilbestrol (DES) have been used as treatments (2). Estrogen treatment is thought to indirectly decrease androgen production by negative feedback control on the hypothalamic-pituitary-testicular axis (3), though with the proven androgen-independence of these tumors, this may not provide much additional therapeutic benefit. Current clinical practice employs high doses of DES (≥μM), which can cause many unwanted side-effects in patients (2) including erectile dysfunction, decrease in sex drive, weight gain, gynecomastia, and cardiovascular problems.
Estrogens (or their mimics) could control cellular proliferation or death via a variety of mechanisms which we examined in these studies. They bind to both intracellular and membrane receptors that transduce extracellular signals to downstream effectors (4-7). Downstream mitogen-activated protein kinases (MAPKs) are responsive to a large number of external stimuli and are nodes of signaling integration for estrogenic signals, as well as those from other classes of ligands, via their receptors (8,9). There are three primary MAPK subclasses: extracellular signal-regulated kinases (ERKs); c-Jun N-terminal kinases (JNKs); and p38. ERKs 1 and 2 are well known for being involved in controlling cell proliferation (9) by integrating upstream signals to regulate cell cycle proteins by post-translational modifications (10). ERKs also propagate the signaling cascade to other important cellular response molecules such as c-Myc (11), Elk-1 (12), eIF4E (13) and cyclin D1 (14). On the other hand JNK is better known for triggering cellular apoptotic responses, for instance after UV radiation or other types of DNA damage (15-17). The MAPK called p38 has some functions similar to JNK, but is more typically reported to be involved in cytokine, cellular stress, and escalating inflammatory responses. The p38 MAPK can also control cell number by cyclin D1 phosphorylation at Thr-286 which ubiquitin-tags it for rapid degradation through proteasomes (18), cyclin-dependent kinase (CDK) regulation at major checkpoints (14,19), or inducing apoptosis through cellular stress mechanisms (20). Therefore, there are multiple mechanistic pathways via which MAPKs are important regulators of cell numbers.
A novel role of ERK phosphorylation is the generation of high levels of ROS, which in turn further sustain ERK activation through the inactivation of dual-specificity phosphatases. This prolonged activation of ERK will then produce even higher ROS levels, creating a positive feed-forward mechanism for ramping up this destructive response quickly. Generation of ROS can lead to cell death via the initiation of apoptosis, cell senescence mechanisms, or autophagy (21).
As cell cycle proteins drive cell division, they directly cause increased cell numbers; therefore inhibition of these mechanisms can cause decreases in cell numbers as cells die and are not replaced. The cell cycle is tightly regulated, being controlled at each major checkpoint by cyclins and CDKs, as well as inhibitory proteins acting on them. Cyclin D1 controls the G1/S transition with its partners CDK4/CDK6 (22). Cyclin D1 protein levels can be changed via p38 phosphorylation (resulting in degradation), depending on the stage of the cell cycle. CDK4/CDK6 can be regulated by the cell cycle inhibitor p16INK4A (23), which when phosphorylated binds to CDK4/CDK6, preventing the formation of the cyclin D1-CDK4/6 holoenzyme required for successful cell cycle progression. Therefore, signals that activate or inhibit this cascade have profound effects on cell numbers.
Programmed cell killing can also play a major role in controlling cell numbers. Several effector caspases feed into executioner caspases, represented in our studies by caspase 3 (24), that dismantle DNA. Necroptosis is an alternative, relatively newly described method of programmed cell death. Cells undergoing this process have morphologies similar to necrotic cells (plasma membrane integrity loss, increases in cell and organelle volume), but do not require the activation of caspases (25). Though necroptotic cells are best known to initiate the death process after Fas ligand (FasL) binding, tumor necrosis factor α (TNFα), or TNF-related apoptosis-inducing ligand (TRAIL) stimulation (26), a growing number of other receptors and stressors are being reported to be involved in this mechanism (27). Receptor-interacting protein kinase 1 (RIPK1) serves as a scaffold for this process (28), and the selective inhibitor necrostatin-1 keeps it in an inactive conformation, thus preventing necroptosis (29).
We and others have previously shown that rapidly initiated (nongenomic) steroid-induced signaling events are involved in the modulation of tissue size/cell number changes (involving both cell proliferation and cell killing) in tumor cell types that contain membrane estrogen receptors (7,30,31). We now predict that these mechanisms could also be active in prostate cancer cells, where the expected direct response to estrogens would be to mediate rapid cellular signaling leading to cell killing, or the slowing of cell proliferation; direct mechanisms of action of estrogens on prostate tumor cells are still relatively understudied. Better understanding of such a direct effect could result in significant improvements in treatment strategies to suppress tumor growth while reducing harmful side effects due to current high dose DES treatments.
Materials and Methods
Cell Lines and Reagents
We chose cell lines representing the two main types of human prostate cancers -- androgen-dependent vs. androgen-independent. LAPC-4 androgen-dependent prostate cancer cells (32) were maintained to sub-confluence in phenol red-free Iscove’s Modified Dulbecco’s Medium (IMDM, MediaTech - Manassas, VA) with 10% fetal bovine serum (FBS) (Atlanta Biologicals – Lawrenceville, GA), 4 mM L-glutamine (Sigma-Aldrich – St. Louis, MO), and 10 nM dihydrotestosterone (Sigma-Aldrich). PC-3 androgen-independent prostate cancer cells (33) were maintained by growth in phenol red-free RPMI 1640 (Sigma-Aldrich) with 10% FBS and 2 mM L-glutamine. Both cell lines were propagated at 37°C and 5% CO2. E2 and DES (Sigma-Aldrich) were dissolved in ethanol to a stock concentration of 10 mM (final concentration of EtOH 0.0001%) before serial dilution into IMDM or RPMI 1640 at concentrations ranging from 10−14 M to 10−6 M for our studies.
MTT Cell Viability Assay
Cells were plated at 5,000 cells/well in poly-D-lysine-coated 96-well assay plates (BD Biosciences – Bedford, MA; 96-well assay plates: Corning – Tewksbury, MA), and then allowed to attach overnight. The next day, 100 μl of medium containing 1% four times charcoal-stripped FBS, plus either vehicle, E2, or DES was added at indicated concentrations. After three days, treatment solutions were removed and 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich) was added for 1 hr. Cells were then lysed and the signal read at 590 nm in a Wallac 1420 plate reader (Perkin Elmer – Waltham, MA) .
Plate Immunoassays
Phosphorylated proteins were assayed by recognition with antibodies (Abs) specific for these post-translationally modified epitopes: pERK1/2 (Thr202/Tyr204), pJNK (Thr183/Tyr185), phospho-p38 (Thr180/Tyr182), phospho-cyclin D1 (Thr286) (all from Cell Signaling – Danvers, MA), or phospho-p16INK4A (Ser152) (Thermo Scientific – Rockford, IL). Changes in cyclin D1 levels were measured by using an Ab to the total cyclin D1 (recognizing both modified and unmodified protein; Cell Signaling Cat. No. 2922). A plate immunoassay developed previously by our lab (34), was adapted for use with the BIOMEK FXP workstation (Beckman Coulter – Brea, CA) to automate the majority of the liquid handling, thereby reducing experimental variability and increasing experimental output.
Prostate cancer cells were plated at 10,000 cells/well in 96-well assay plates, allowed to attach overnight, and weaned from steroids and other small molecules in the growth media by treatment with 100 μl of media with 1% four times charcoal-stripped FBS for 48 hr. Cells were then treated with E2 or DES for up to 60 min on the workstation, followed by simultaneous fixation and permeabilization (2% paraformaldehyde, 1% glutaraldehyde, 0.5% Nonidet P-40, 0.15 M Sucrose). Primary Ab to the phosphorylated epitopes was then added and incubated with the cells overnight. The next day, biotinylated anti-mouse/anti-rabbit IgG secondary Ab (Vector Labs – Burlingame, CA) was added for 1 hr. Next, cells were incubated with avidin-biotinylated conjugated alkaline phosphatase (ABC-AP, Vector Labs) for 1 hr, then for 30 min with para-nitrophenylphosphate substrate (Sigma Aldrich), allowing the yellow color of the para-nitrophenol product to accumulate. Plates were read at 405 nm in a Wallac 1420 plate reader. Readings were then normalized to cell number, estimated by the crystal violet dye (Sigma-Aldrich) assay, as described previously (35).
ER Antagonist Assays
To investigate the involvement of different ERs and their potential role in altering ROS formation or cyclin D1 phosphorylation, the following ER antagonists were used at their most selective concentrations: for ERα, 10−7 M 1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride (MPP, Tocris Bioscience – Minneapolis, MN); for ERβ, 10−6 M 4-[2-phenyl-5,7-bis(trifluoromethyl) pyrazolo[1,5-a]pyrimidin-3-yl] phenol PHTPP (Tocris); and for GPR30, 10−6 M G15 (Tocris). Cells were pre-incubated with antagonists for 30 min prior to estrogen treatments.
Caspase 3 Assays
To determine estrogen-induced activations of caspase 3, cells were seeded into black optical 96-well plates (Corning) at a density of 5,000 cells/well and allowed to attach overnight. Estrogen treatments were started the next day in media with 1% four times charcoal-stripped FBS, for times ranging from 2-24 hr (only the 8 hr optimum time point data are shown in Fig. 5). After treatment, plates were centrifuged at 300 g for 5 min, and treatment-containing media were suctioned off. Cells were then lysed with 50 μL of lysis buffer (10 mM HEPES; 2 mM EDTA; 0.1% CHAPS; 1 mM DTT; pH 7.4) and stored at −20°C until assay. Assay buffer (50 μL of 50 mM HEPES; 100 mM NaCl; 0.1% CHAPS; 1 mM EDTA; 10% glycerol; 10 mM DTT; pH 7.4) containing a 50 μM final concentration of Ac-DEVD-AFC caspase-3 assay substrate (Enzo Life Sciences – Farmingdale, NY) was added. The cellular enzyme-catalyzed release of 7-Amino-4-trifluoromethylcoumarin was monitored using a FlexStation 3 microplate reader (Molecular Devices – Sunnyvale, CA) at an excitation wavelength of 400 nm and an emission wavelength of 505 nm. Staurosporine at 1μM was used as a positive control for inducing caspase activity.
Figure 5. Caspase 3 activity levels after E2 or DES treatments.
LAPC-4 and PC-3 cells were treated with 0.1 nM E2 or 1 μM DES and caspase 3 activity measured after 8 hr (which is the response optimum; time course not shown). White bars denote LAPC-4 cells, and black bars PC-3 cells. * denotes significance from vehicle (V) at p<0.05. The shaded gray horizontal bars represent the response to vehicle ± SEM. Staurosporine (Stauro) at 1μM was the positive control for caspase 3 activation.
Necroptosis Assays
This mechanism was defined by the use of a selective necroptosis-inhibitor in the MTT assay (described above). Cells were treated for three days with EtOH (0.0001%) vehicle or 10−10 M E2 or 10−6 M DES; TNFα (10 ng/ml, Millipore) plus cyclohexamide (10 μg/ml, Sigma-Aldrich) were used together to provide a positive control for necroptosis. Necrostatin-1 (20 μM, Millipore – Billerica, MA) was used to specifically define necroptosis by the inhibition of RIP1 kinase (29).
ROS Assays
Cells were plated at 10,000 cells/well in a 96-well plate, allowed to attach overnight, and then treated with 100 μl of media containing 1% four times charcoal-stripped FBS for 48 hr. Cells were loaded with 15μM 2’,7’-Dichlorodihydrofluorescein diacetate (DCDHF) (Enzo Life Sciences) for 1 hr. Then the production of ROS was measured in cells after E2 or DES treatment for 15 min. Hydrogen peroxide (1μM, Fisher Scientific – Pittsburg, PA) and ethanol (0.0001%) were used as positive and negative controls, respectively. For both E2 and DES treatments, the concentrations spanned 10−14 to 10−6 M. Dichlorofluorescein production, formed as a result of ROS/DCDHF interaction, was measured at an excitation of 485 nm, and an emission of 538 nm in a SpectraMax M3 Multi-Mode Microplate Reader (Molecular Devices). For studies with MEK inhibitor U0126 (Promega – Madison, WI), cells were co-incubated with 10−7M inhibitor during the last 30 min of the DCDHF incubation.
Statistics
One-way analysis of variance was conducted for each experiment. A Holm-Sidak post hoc test was used to measure the significance of each treatment vs. the vehicle control. Significance was set at p<0.05.
Results
Cell Viability
E2 treatment (represented by triangles in this and all subsequent line graphs) for three days effectively decreased the number of viable cells by 20-30% below the vehicle treatment level at 10−14 to 10−6 M concentrations in LAPC-4 androgen-dependent (early-stage) prostate cancer cells (represented by white symbols and bars in all figures) (Fig. 1A) and at 10−10 to 10−8 M concentrations in PC-3 androgen-independent (late-stage) prostate cancer cells (represented by black symbols and bars in all figures). DES treatment (represented by circles in all line graphs) decreased cell viability by as much as 20% in LAPC-4 cells at 10−14 to 10−12 M and 10−6 M, while the cell viability of PC-3 cells was not significantly decreased by DES at any concentration (Fig. 1B). For comparison (positive control), when these cells were grown in complete serum-containing media, LAPC-4 cell numbers increased by 27%, while PC-3 increased by 19% (not shown).
Figure 1. Cell viability after 3 days of E2 or DES treatment.
LAPC-4 and PC-3 prostate cancer cells were treated with log incremental concentrations of each estrogen, and cell viability was measured by the MTT assay. In all figures throughout the manuscript white symbols denote LAPC-4 cells and black symbols PC-3 cells; triangles represent E2 treatments, and circles represent DES treatments. *denotes significance from vehicle (V) controls (squares) at p<0.05, and shaded horizontal gray bars represent the response to vehicle ± SEM.
Phospho-ERK Driven ROS Accumulation and Phospho-JNK Driven Caspase Activation
In both LAPC-4 and PC-3 cells, most concentrations of E2 and DES elicited similar rapid ERK and JNK activations and deactivations in a typical oscillating time pattern (36-38) (Figs. 2 & 4). However, there were some instances where values for ERK activations at some concentrations did differ (for E2 at 1 and 10 min, and for DES at 1 and 15min); we chose the nonvariable 5 min time point for subsequent measurements at single time points. To better visualize the composite temporal pattern, the insets in these figures show the averages of these changes for all concentrations. Because of these largely similar responses (most not significantly different from one another due to concentration), we chose conditions for all further assays to mimic physiologically (10−10 M E2)- and clinically (10−6 M DES)-relevant concentrations that were also active in reducing cell viability (see Fig. 1). The response for those chosen concentrations is shown in bold lines for each in Figs. 2 and 4. At these selected concentrations E2-treated LAPC-4 cells showed a rapid and significant increase in phospho-ERK levels (16%) after 5 min (Fig. 2A), and a maximal response (26%) after 15 min of treatment. After falling to control levels, activation was seen again at 60 min, as we have often observed in other cell lines (34,37,38). A similar but less robust response was seen in PC-3 cells [ERK activated by 8% after 10 min of E2 treatment, with another rise to 18% at 60 min (Fig. 2B)]. In both cell lines, DES at most concentrations moderately, though significantly, decreased phospho-ERK levels rapidly (Fig. 2C & D). In LAPC-4 cells, the ≤20% decrease in phospho-ERK levels was mostly maintained throughout the time course. The activations of ERK in our studies were not consistent with the traditional role for ERK in causing cell proliferation, as our cells numbers instead declined.
Figure 2. Phospho-ERK (pERK) levels in LAPC-4 and PC-3 cells after E2 or DES treatments.
Cells were treated with E2 or DES at different concentrations (different color of symbols and lines). pERK was measured by plate immunoassay for up to 60 min. Insets show the average of all [E2] (A & B) or all [DES] (C & D) treatments. * denotes significance from vehicle response (shown as 0 time) at p<0.05. The shaded gray horizontal bars represent the response to vehicle ± SEM.
Figure 4. Phospho-JNK (pJNK) levels in LAPC-4 and PC-3 after E2 or DES treatments.
Cells were treated with different concentrations of E2 or DES (each line) and then pJNK was measured by plate immunoassay for up to 60 min. Insets show the average response values of all [E2] (A & B) or all [DES] (C & D) treatments. * denotes significance from the vehicle response (time 0) at p<0.05. The shaded gray horizontal bars represent the response to vehicle ± SEM.
Therefore, we next investigated the role of sustained ERK in the generation of ROS. The production of ROS and associated sustained activation of ERK can lead to cell death, via a positive feed forward mechanism (21). To test this possibility we treated LAPC-4 and PC-3 cells with E2, which increased ROS across all concentrations after 15 min (the optimum in the time course, not shown) in both cell lines (Figs. 3A & C). Addition of MEK inhibitor U0126 inhibited ROS increases by ~50% in both cells lines, indicating ERK1/2 is involved in this E2-mediated pathway, but not exclusively responsible for ROS generation. However, DES did not significantly alter the levels of ROS at any concentration in either cell line (3B & D), consistent with the more robust cell-killing effect by E2.
Figure 3. ROS measured in LAPC-4 vs. PC-3 cells treated with 0.1 nM E2 or 1 μM DES.
ROS levels were measured after 15 min of each estrogen treatment (A-D). Black stars represent response to 1 μM H2O2, a positive control for ROS generation. White symbols denote LAPC-4 cells, black symbols for PC-3 cells; triangles represent E2, and circles DES treatments. * denotes significance from vehicle control at p<0.05. The shaded gray horizontal bars represent the response to vehicle ± SEM.
E2 treatment evoked different JNK responses from androgen-dependent compared to androgen-independent cell lines (Fig. 4). In LAPC-4 cells, JNK was slightly deactivated after 1 min, and was intermittently deactivated thereafter (Fig. 4A). However, in PC-3 cells, JNK was activated (20%) at 1-10 min of E2 treatment, (Fig. 4B) followed by a return to control levels and a 10% decrease at 30 min, rising again to baseline at 1 hr. In contrast, DES decreased JNK activation in both cell lines by 5-10% as early as 5 min, an effect that mostly persisted through the remainder of the time course (Fig. 4C & D). This is mostly inconsistent with the typical role of JNK in decreasing cell viability compared to the cell line-specific viability responses we saw (Fig. 1). Therefore, we next examined caspase activation to determine if apoptosis contributed to any of the cell number declines observed in our viability assays (using staurosporine as a positive control) (39). A time-course study at 2-24 hrs showed that the highest levels of caspase 3 activity were seen after eight hrs in both cell lines (entire time course not shown); this time was therefore chosen for the comparative studies. Caspase 3 was significantly activated in LAPC-4 cells by both E2 and DES, but not in PC-3 cells (Fig. 5). Though not entirely consistent with the cell-killing effects we saw in our viability assays, nor with the traditional role of JNK in activating caspases, these data could contribute some mechanistic explanations for cell-killing to the balance of multiple mechanisms affecting cell number.
Necroptosis
We next examined the possibility that this alternative mechanism of cell death contributed to these reductions in cell viability caused by estrogens. This form of programmed cell death was not observed in either cell line after either estrogen treatment (Fig. 6). The combination of TNFα and cyclohexamide served as a positive control for necroptosis. Addition of the necroptosis inhibitor necrostatin-1 blocked the cell-killing effects of the positive control, but did not alter the cell number decreases caused by the estrogens. Therefore, necroptosis did not appear to be involved in the killing of these cells by estrogens.
Figure 6. Necroptosis after E2 or DES treatments.
LAPC-4 and PC-3 cells were treated with 0.1 nM E2 or 1 μM DES and cell viability was measured via MTT assays after 3 days. White bars denote LAPC-4 cells; black bars are PC-3 cells. * denotes significance from vehicle (V) at p<0.05. The shaded gray horizontal bars represent the response to vehicle ± SEM.
TNFα plus cyclohexamide (TNF/Cyclo) were positive controls for inducing necroptosis in these cells which is defined by necrostatin (NecS) reversal of the response. The cell viability in complete growth medium is shown for comparison at this 3 day time point (Complete).
Phospho-p16INK4A
Next we turned to modulation of the cell cycle through phosphorylation of the CDK inhibitor, p16INK4A. We hypothesized that activation of p16INK4A would interfere with CDK‘s partnering with cyclin D1, and thereby hinder progression through the cell cycle, leading to reduced cell numbers. In LAPC-4 cells, the levels of phospho-p16INK4A rapidly increased ~45% in1-5 min after both E2 and DES treatment (Fig. 7), and then fell to 20-25% increase for the duration of the time course (Figs.7A & B). In PC-3 cells, E2 initially activated p16 less robustly, but increasing to about 37% by 15 min and sustaining this level throughout the time course (Fig. 7C). However, DES activation of p16 in PC-3 cells though significant, was severely blunted (Fig. 7D). Therefore, these data could help explain in part the data of Fig. 1 where DES-treated PC-3 cell numbers did not decline.
Figure 7. Phospho-p16 (p-p16) levels after E2 or DES treatments.
LAPC-4 and PC-3 cells were treated with 0.1 nM E2 or 1 μM DES and p-p16 was measured for up to 60 min by plate immunoassays. White symbols denote LAPC-4 cells, and black symbols PC-3 cells. Triangles represent E2, and circles DES. * denotes significance from vehicle (at time 0) at p<0.05. The shaded gray horizontal bars represent the response to vehicle ± SEM.
Phospho-p38
Cyclin D1 can be directly phosphorylated by p38 at a site that directs its degradation (14), so we next examined whether p38 was activated by estrogens in our model systems. In both LAPC-4 cells and PC-3 cells, both E2 and DES phospho-activated p38 rapidly (within 1 min) with similar patterns (Fig. 8). The most robust response was In LAPC-4 cells, where DES activated p38 by almost 100% after 5 min of treatment (Fig. 8B). All other increases were in the ~50% ranges (Figs. 8A, C, and D). Interestingly, these activations were sustained for at least 60 min under all conditions, and could therefore be available to direct phospho-activation of cyclin D1.
Figure 8. Activated p38 (p-p38) levels after treatment with 0.1 nM E2 and 1 μM DES.
LAPC-4 and PC-3 cells were treated with E2 or DES and p-p38 was measured for up to 60 min via plate immunoassays. White symbols denote LAPC-4 cells, black PC-3 cells. Triangles represent E2, and circles DES. * denotes significance from vehicle (time 0) at p<0.05. The shaded gray horizontal bars represent the response to vehicle ± SEM.
Phospho-cyclin D1 and Total Cyclin D1
As p38-mediated phosphorylation of cyclin D1 leads to its degradation, this could in turn slow or halt progress through the cell cycle (14,18), causing cell numbers to decline. Both estrogen treatments of LAPC-4 cells caused a rapid rise in cyclin D1 phosphorylation (Fig. 9A and B), as did E2 treatment of PC-3 cells (Fig. 9C), though the latter was not as well sustained. However, DES treatment of PC-3 cells instead caused a dephosphorylation of cyclin D1 (Fig. 9D). Although this decrease in phosphorylation fluctuated, it lasted at least 30 min. If these cyclin phosphorylations affect the levels of cyclin proteins as expected, they could explain our estrogen-driven changes in cell numbers. Therefore, we next examined total cyclin D1 levels to determine if phosphorylation did indeed correlate with degradation shortly thereafter. To assess this, we treated LAPC-4 and PC-3 cells with E2 or DES over a time course of 5-950 min (16 hrs.). Decreases in total cyclin D1 were observed in each scenario where cyclin D1 was phosphorylated (Fig. 9E, 9F, 9G). E2 caused a significant decrease in total cyclin D1 within an hr in both LAPC-4 and PC-3 cells, and DES acted similarly in LAPC-4 cells. Maximum reductions at 16 hrs in these three cases were ~25%. However, there was no significant decrease in total cyclin D1 levels in PC-3 cells after DES treatment (Fig. 9H), which correlated with its lack of phosphorylation and its inability to reduce the numbers of viable cells in this case. The ratio of p-cyclin D1 to total cyclin D1 shows a very rapid change in cyclin D1 kinase “activity” which in this case is a marking of the protein for degradation, except in DES-treated PC-3 cells (Fig. 9I-9L). This last view of the data best correlates with the pattern of estrogen-induced decrease in cell number.
Figure 9. Phosphoryated cyclin D1 (p-cyclin D1) vs. total cyclin levels and their ratios after treatment with 0.1 nM E2 and 1 μM DES.
LAPC-4 (early) and PC-3 (late) cells were treated with E2 or DES. The p-cyclin D1 levels (A-D) were measured for up to 60 min and the total cyclin D1 levels (E-H) were measured up to 16 hrs via plate immunoassays. The ratio of p-cyclin D1 to total cyclin D1 was calculated for overlapping time points at 5, 15, and 60 min (I-L). White symbols denote LAPC-4 cells and black PC-3 cells. Triangles represent E2 and circles DES. * denotes significance from vehicle (time 0) at p<0.05. The shaded gray horizontal bars represent the response to vehicle ± SEM.
ER-selective Antagonists
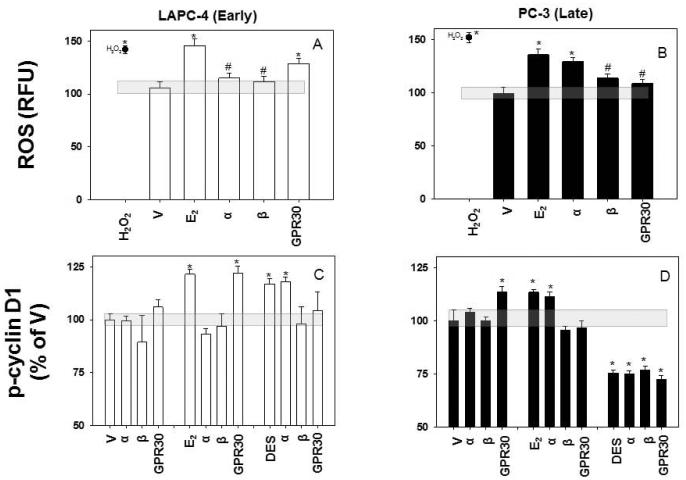
Next we examined the involvement of different estrogen receptor subtypes (Fig. 10), focusing on the mechanisms that had given the most robust of our explanations for estrogen-induced declines in cell numbers -- ROS generation and cyclin D1 phosphorylation/degradation. For this we blocked each receptor subtype with a selective antagonist at a very selective concentration, and then measured the estrogen-induced responses at time points selected as optimal for each mechanism. We found that the ROS production caused by 15 min of E2 treatment in androgen-dependent LAPC-4 cells was antagonized by both ERα- and ERβ-selective inhibitors (Fig. 10A), while the GPR30 antagonist had no effect. Androgen-independent PC-3 cells also had increased ROS levels after E2 treatment, but in this case antagonizing only ERβ and GPR30 decreased the amount of ROS produced (Fig. 10B). DES was not active in generating ROS, and so was not tested for receptor participation.
Figure 10. ER subtype-selective antagonists inhibit E2- or DES-induced ROS and p-cyclin D1 responses.
LAPC-4 and PC-3 cells were pretreated with antagonists for each of the three ER subforms: α (MPP), β (PHTPP), and GPR30 (G15) and then treated at the response time optima with 0.1 nM E2 or 1 μM DES. ROS were measured after 15 min (A & B). P-cyclin D1 levels were measured after 1 min for E2-treated PC-3 cells and 15 min for all others (C & D). White bars denote LAPC-4 cells, and black bars PC-3 cells. * denotes significance from vehicle (V) response at p<0.05. # denotes significance from the E2 response. The shaded gray horizontal bars represent the response to vehicle ± SEM.
The roles of the three known estrogen receptors in altering p-cyclin D1 levels after 15 min of E2 treatment were dictated both by the cell type in which the tests were done and the estrogen mediating the response. That is, in LAPC-4 cells, E2 again operated via ERα and ERβ to increase cyclin D1 phosphorylation (Fig. 10C), while in PC-3 cells, a 1 min E2 treatment acted via ERβ and GPR30 to increase p-cyclin D1 levels (Fig. 10D).
DES was also active in cyclin D1 activation, but only in LAPC-4 cells, where it required ERβ and GPR30. Strikingly, a 15 min DES treatment in PC-3 cells reduced cyclin D1 phosphorylation in a way that was independent of any of these three ERs (Fig. 10D), suggesting that at this high concentration this synthetic estrogen works via non-receptor-mediated mechanisms in ways that did not reduce cell number.
Discussion
Our results indicate that when several mechanisms of estrogen action are considered using cell lines that represent different stages of human prostate cancer, DES at a concentration achieved by typical clinical treatments (1 mg, three times daily leading to serum concentrations measured at ≥1 μM) (40) does not kill or otherwise reduce the numbers of cells that represent androgen-independent late-stage tumors (PC-3 cells). Ironically, these late-stage tumors are the ones usually treated with DES. While cell-killing effects have been observed at concentrations 10-100 fold higher than those used in our studies (41), such high concentrations can cause many unwanted side-effects in patients, and are unlikely to act via receptors. However, in our studies, E2 was much more potent in decreasing the numbers of both androgen-dependent and -independent prostate cancer cells in a concentration range (optimal at 0.1 nM) that may be far better tolerated by patients. The mechanisms that we examined for participation in estrogen-induced cell number decline in prostate cancer cell number are depicted in Figure 11 and their effects summarized in Table 1.
Figure 11. Direct mechanisms of E2/DES action on prostate cancer cell survival.
E2/DES initiate rapid signals at mERs and through the activation or deactivation of MAPKs. These signals can also lead to apoptosis, ROS increases, or phosphorylation of cell-cycle proteins that delay the cycle. Each of these mechanisms can contribute to the estrogenic control of cell number.
Table 1.
Summary of mechanisms contributing to estrogen-induced decline in numbers of LAPC-4 or PC-3 prostate cancer cells. E2/DES activate differing pathways (listed) in LAPC-4 vs. PC-3 cells, which can lead to cell death (apoptosis/necroptosis, ROS increases) or lack of cell proliferation (↓ pERK, phosphorylation of cell cycle proteins). Each of the mechanisms listed in red or green font were able to contribute to the estrogenic control of cell numbers in our studies. Mechanisms in the gray font indicates a pathway that was tested but does not contribute. These number of mechanisms contributing to each outcome were summed. The red text mechanisms and summary numbers indicate participation of mechanisms that will kill cells or stop their growth; the green text mechanisms and summary numbers indicate mechanisms that will cause cells to proliferate or survive. We counted changes in ERK and ROS as separate mechanisms. Only a 0.5 score is awarded to the p-p16 result for DES-treated PC-3 cells because it was a very low response compared to the others. For non-color print versions: These are the mechanisms that decrease cell number: ↑↑ERK→ROS, ↓ERK, ↑JNK, ↑ caspase 3, ↑p-p-16, ↑p-cyclin→↓total cyclin. They are summed by the top number in each panel. Mechanisms that increase cell number: ↓JNK, ↓p-cyclin→↑total cyclin -- are summed by the bottom number in each panel.

|
MAPKs are important signal integrators of external stimuli leading to cell proliferation or death. The most commonly cited mechanisms explain cell number decreases by ERK inactivation (halting proliferation) or JNK activation (inducing apoptosis via caspases). Instead, we demonstrated that E2 rapidly increased pERK levels in both early- and late-stage prostate cancer cell lines. The activation of JNK did not in any case correlate with the expected effect on caspase activity, so it did not explain any of our cell viability results. Therefore, we had to entertain other explanations and pathways for the therapeutic (tumor cell-killing) actions of these estrogens via MAPKs.
Sustained ERK activation (often throughout 60 min) associated with ROS generation-mediated cell killing (21) demonstrated in our studies did correlate with E2-induced declines in cell numbers. In this type of response ROS activates even more ERK, likely participating in a positive feed-forward loop (21). The increased ROS production we observe may not be due solely to sustained pERK, as use of MEK inhibitor U0126 only abrogated the response by about 50%. Other possible sources of E2-generated ROS include E2-induced DNA damage or intercalation of E2 into the plasma membrane, where it can undergo redox cycling (42,43). Activated JNK levels did not correlate with nor explain decreased cell numbers via apoptosis. Apoptosis, apparently not controlled by JNK, played a role only in the cell model of early-stage prostate cancer (LAPC-4) where this type of killing was caused by both estrogens. Late-stage cancer cells represented by the PC-3 cell line were unaffected by either of these mechanisms. Neither cell type underwent estrogen-induced necroptosis. Again, such a lack of response to estrogens in cells representing late-stage prostate tumors was surprising, given that this is the type of patient who is most often treated with the estrogen DES (44).
We showed that blocking the actions of proteins that drive the cell cycle was also involved in these estrogen-induced declines in cell numbers. The major cell-cycle protein CDK inhibitor p16INK4A and the cyclin D1-phosphorylating kinase p38 were both induced by estrogens in all cases, regardless of whether these treatments actually reduced cell numbers; so while these proteins may participate (be permissive), they do not alone or primarily control a critical step in determining the cell viability outcome. However cyclin D1 phosphorylation mediated by p38 (19), leading to its rapid degradation, was directly correlated with estrogen-induced cell number declines in all cases. Cyclin D1 availability and activity are major deciding factors for cell-cycle progression, and a diminished ability to move through this checkpoint readily decreases cell numbers. E2 performed much better in this pathway endpoint, providing a treatment advantage for both early- and late-stage cancer cells. It is unknown why activated p38 cannot mediate DES-induced phosphorylation of cyclin D1 in PC-3 cells, and this perhaps points to the involvement of another unknown factor.
Changes in cell viability and caspase activation were measured over a course of several hours to days, and therefore the involvement of both genomic and nongenomic signals is likely. However, for our signal transduction assays, the mechanisms we examined were activated rapidly (within minutes) after estrogen treatments, and likely were mediated by a membrane form of these estrogen receptors, as we have shown in other cancer cell types (GH3 pituitary, MCF-7 breast, PC-12 pheochromocytoma) (34,37,45). However, we have also previously seen estrogen-mediated rapid phosphorylation/activation of Elk-1 and ATF-2 transcription factors as early as 10-15 min (46). This illustrates how signaling initiated early via nongenomic mechanisms can progress to downstream genomic mechanisms. Our previous studies demonstrated rapid activation via mERs for the three MAPK activations demonstrated here. In addition, our previous work on glucocorticoid-induced killing of T lymphoma cells showed dependence on the presence of a membrane form of the glucocorticoid receptor (47). Our demonstration here of novel non-genomic actions of estrogens on prostate cancer cells should open new avenues of thinking about therapeutic approaches using estrogens of many types. Estrogen receptors play a major role in normal physiological regulation and development of many types of cells including the prostate, but also in cell survival and cancer development (48). The primary ER of the prostate gland is ERβ, with lower levels of ERα and GPR30. However, each is a possible target for cancer therapy (49). Our results using ER subtype-selective antagonists suggest that estrogens mediate specific mechanisms (increased phosphorylation of cyclin D1 resulting in its degradation, ROS generation via ERK activation) via some but not all ERs -- and not always the same ones -- to affect cell viability. E2 required ERβ to operate the most important contributory mechanisms related to cell number declines (increasing ROS and p-cyclin in both cell types), but ERα and GPR30 participated variably, especially depending upon early- vs. late-stage tumor cell type. However, the high concentration DES effects in late-stage PC-3 cells required no receptor involvement at all, were in the opposite direction from those mechanisms associated with the decline in cell numbers, and were therefore in agreement with the inability of DES to reduce cell numbers. It has previously been observed that at such high concentrations DES can bypass estrogen receptor-mediated mechanisms, and instead alter such chemical properties as fluidity, lipophilicity, and polarity of the lipid bilayer (50,51), so perhaps DES is operating on these cells via these other less productive mechanisms.
Conclusions
The degree to which these treatments are more effective because they engage more of the mechanisms that can reduce tumor cell numbers are summarized in Table 1; we listed the active mechanisms in color, and then added up how many mechanisms were active in each case toward increasing (green) or decreasing (red) cell number. Only by examining all of these responses together were we able to comprehensively consider which pathways contributed to the therapeutic cell-killing effects of estrogens on prostate cancer cells of different stages. These studies are an important example of how systematic examination of multiple mechanisms can elucidate the extent to which a therapeutic agent will be effective on tumor cell stages with distinct characteristics. We showed that estrogens have a rapid and direct effect on prostate tumor cells, and that multiple, but not all cell-killing mechanisms contribute to the therapeutic response. E2 was much more potent and efficacious than DES, suggesting that E2 could be a better form of treatment for men with all stages of prostate cancer. This represents a particularly important opportunity for treatment of advanced prostate cancers where treatment options are limited. However, these results also suggest that very early-stage developing tumors in high-risk cancer-susceptible men could benefit from low-dose E2 treatment, the natural levels of which may decline in men during aging (52,53).
Acknowledgements
We thank Dr. David Konkel for critically editing the manuscript.
This work was supported by the National Institute of Environmental Health Sciences T-32 training program in Environmental Toxicology (T-32 07254).
References
- 1.Cannata DH, Kirschenbaum A, Levine AC. Androgen Deprivation Therapy as Primary Treatment for Prostate Cancer. Journal of Clinical Endocrinology & Metabolism. 2012;97:360–365. doi: 10.1210/jc.2011-2353. [DOI] [PubMed] [Google Scholar]
- 2.Malkowicz SB. The role of diethylstilbestrol in the treatment of prostate cancer. Urology. 2001;58(2):108–113. doi: 10.1016/s0090-4295(01)01252-3. Supplement 1. [DOI] [PubMed] [Google Scholar]
- 3.Denmeade SR, Isaacs JT. Androgen Deprivation Strategies in the Treatment of Advanced Prostate Cancer. In: Bast RC, Kufe DW, Pollock RE, Weichselbaum RR, Holland JF, Frei E, editors. Holland-Frei Cancer Medicine. Vol. 5. BC Decker; Hamilton, ON: 2000. [Google Scholar]
- 4.Cargnello M, Roux PP. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiology and Molecular Biology Reviews. 2011;75(1):50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zivadinovic D, Gametchu B, Watson CS. Membrane estrogen receptor-alpha levels in MCF-7 breast cancer cells predict cAMP and proliferation responses. Breast Cancer Research. 2005;7(1):R101–R112. doi: 10.1186/bcr958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levin ER. Plasma membrane estrogen receptors. Trends in Endocrinology & Metabolism. 2009;20(10):477–482. doi: 10.1016/j.tem.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pietras RJ, Márquez-Garbán DC. Membrane-Associated Estrogen Receptor Signaling Pathways in Human Cancers. Clinical Cancer Research. 2007;13(16):4672–4676. doi: 10.1158/1078-0432.CCR-07-1373. [DOI] [PubMed] [Google Scholar]
- 8.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nature Reviews Cancer. 2009;9:13. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 9.Roskoski R., Jr ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacological Research. 2012;66(2):105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Chang F, Steelman LS, Shelton JG, Lee JT, Navolanic PM, Blalock WL, Franklin R, McCubrey JA. Regulation of cell cycle progression and apoptosis by Ras/Raf/MEK/ERK pathway. International Journal of Oncology. 2003;22:469–480. [PubMed] [Google Scholar]
- 11.Pintus G, Tadolini B, Posadino AM, Sanna B, Debidda M, Bennardini F, Sava G, Ventura C. Inhibition of the MEK/ERK signaling pathway by the novel antimetastatic agent NAMI-A down regulates c-myc gene expression and endothelial cell proliferation. European Journal of Biochemistry. 2002;269(23):5861–5870. doi: 10.1046/j.1432-1033.2002.03307.x. [DOI] [PubMed] [Google Scholar]
- 12.Gille H, Kortanjann M, Thomae O, Moomaw C, Slaughter C, Cobb MH, Shaw PE. ERK phosphorylation potentiates Elk-1 mediated ternary complex formation and transactivation. EMBO Journal. 1995;14(5):951–962. doi: 10.1002/j.1460-2075.1995.tb07076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meloche S, Pouyss qur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26(22):3227–3239. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- 14.Terada Y, Inoshita S, Nakashima O, Kuwahara M, Sasaki S, Marumo F. Regulation of cyclin D1 expression and cell cycle progression by mitogen-activated protein kinase cascade. Kidney Int. 1999;56(4):1258–1261. doi: 10.1046/j.1523-1755.1999.00704.x. [DOI] [PubMed] [Google Scholar]
- 15.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27(48):6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen HM, Liu ZG. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med. 2006;40(6):928–939. doi: 10.1016/j.freeradbiomed.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y-R, Wang X, Templeton D, Davis RJ, Tan T-H. The Role of c-Jun N-terminal Kinase (JNK) in Apoptosis Induced by Ultraviolet C and γ Radiation: DURATION OF JNK ACTIVATION MAY DETERMINE CELL DEATH AND PROLIFERATION. Journal of Biological Chemistry. 1996;271(50):31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 18.Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Molecular Cancer. 2007;6(24):1–16. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thornton TM, Rincon M. Non-Classical P38 Map Kinase Functions: Cell Cycle Checkpoints and Survival. International Journal of Biological Sciences. 2009;5(1):44–51. doi: 10.7150/ijbs.5.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee M-W, Park SC, Yang YG, Yim SO, Chae HS, Bach J-H, Lee HJ, Kim KY, Lee WB, Kim SS. The involvement of reactive oxygen species (ROS) and p38 mitogen-activated protein (MAP) kinase in TRAIL/Apo2L-induced apoptosis. FEBS Letters. 2002;512(1–3):313–318. doi: 10.1016/s0014-5793(02)02225-1. [DOI] [PubMed] [Google Scholar]
- 21.Cagnol S, Chambard JC. ERK and cell death: Mechanisms of ERK-induced cell death - apoptosis, autophagy and senescence. The FEBS Journal. 2010;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 22.Pillai MS, Sapna S, Shivakumar K. p38 MAPK regulates G1-S transition in hypoxic cardiac fibroblasts. The International Journal of Biochemistry & Cell Biology. 2011;43(6):919–927. doi: 10.1016/j.biocel.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Huschtscha LI, Reddel RR. p16INK4a and the control of cellular proliferative life span. Carcinogenesis. 1999;20(6):921–926. doi: 10.1093/carcin/20.6.921. [DOI] [PubMed] [Google Scholar]
- 24.Cullen SP, Martin SJ. Caspase activation pathways: some recent progress. Cell Death Differ. 2009;16(7):935–938. doi: 10.1038/cdd.2009.59. [DOI] [PubMed] [Google Scholar]
- 25.Dunai Z, Bauer P, Mihalik R. Necroptosis: Biochemical, Physiological and Pathological Aspects. Pathology & Oncology Research. 2011;17(4):791–800. doi: 10.1007/s12253-011-9433-4. [DOI] [PubMed] [Google Scholar]
- 26.Kaczmarek A, Vandenabeele P, Krysko Dmitri V. Necroptosis: The Release of Damage-Associated Molecular Patterns and Its Physiological Relevance. Immunity. 2013;38(2):209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Fulda S. The mechanism of necroptosis in normal and cancer cells. Cancer Biology & Therapy. 2013;14(11):999–1004. doi: 10.4161/cbt.26428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jouan-Lanhouet S, Arshad MI, Piquet-Pellorce C, Martin-Chouly C, Le Moigne-Muller G, Van Herreweghe F, Takahashi N, Sergent O, Lagadic-Gossmann D, Vandenabeele P, Samson M, Dimanche-Boitrel MT. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 2012;19(12):2003–2014. doi: 10.1038/cdd.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1(2):112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 30.Watson CS, Jeng Y-J, Guptarak J. Endocrine disruption via estrogen receptors that participate in nongenomic signaling pathways. The Journal of Steroid Biochemistry and Molecular Biology. 2011;127(1–2):44–50. doi: 10.1016/j.jsbmb.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly MJ, Levin ER. Rapid actions of plasma membrane estrogen receptors. Trends in Endocrinology & Metabolism. 2001;12(4):152–156. doi: 10.1016/s1043-2760(01)00377-0. [DOI] [PubMed] [Google Scholar]
- 32.Klein KA, Reiter RE, Redula J, Moradi H, Zhu XL, Brothman AR, Lamb DJ, Marcelli M, Belldegrun A, Witte ON, Sawyers CL. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nat Med. 1997;3(4):402–408. doi: 10.1038/nm0497-402. [DOI] [PubMed] [Google Scholar]
- 33.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Investigative urology. 1979;17(1):16–23. [PubMed] [Google Scholar]
- 34.Bulayeva NN, Gametchu B, Watson CS. Quantitative measurement of estrogen-induced ERK 1 and 2 activation via multiple membrane-initiated signaling pathways. Steroids. 2004;69(3):181–192. doi: 10.1016/j.steroids.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lottering M-L, de Kock M, Viljoen TC, Grobler CJS, Seegers JC. 17β-Estradiol metabolites affect some regulators of the MCF-7 cell cycle. Cancer Letters. 1996;110(1–2):181–186. doi: 10.1016/s0304-3835(96)04489-8. [DOI] [PubMed] [Google Scholar]
- 36.Vinas R, Watson C. Bisphenol s disrupts estradiol-induced nongenomic signaling in a rat pituitary cell line: effects on cell functions. Environ Health Perspect. 2013;121(3):352–358. doi: 10.1289/ehp.1205826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zivadinovic D, Watson CS. Membrane estrogen receptor-alpha levels predict estrogen-induced ERK1/2 activation in MCF-7 cells. Breast Cancer Research. 2005;7(1):R130–R144. doi: 10.1186/bcr959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watson C, Jeng Y-J, Kochukov M. Nongenomic actions of estradiol compared with estrone and estriol in pituitary tumor cell signaling and proliferation. The FASEB Journal. 2008;22(9):3328–3336. doi: 10.1096/fj.08-107672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeng Y-J, Kochukov M, Watson C. Membrane estrogen receptor-alpha-mediated nongenomic actions of phytoestrogens in GH3/B6/F10 pituitary tumor cells. Journal of Molecular Signaling. 2009;4(2):1–11. doi: 10.1186/1750-2187-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kemp HA, Read GF, Riad-Fahmy D, Pike AW, Gaskell SJ, Queen K, Harper ME, Griffiths K. Measurement of Diethylstilbestrol in Plasma from Patients with Cancer of the Prostate. Cancer Research. 1981;41(11):4693–4697. Part 1. [PubMed] [Google Scholar]
- 41.Robertson CN, Roberson KM, Padilla GM, O'Brien ET, Cook JM, Kim C-S, Fine RL. Induction of Apoptosis by Diethylstilbestrol in Hormone-Insensitive Prostate Cancer Cells. Journal of the National Cancer Institute. 1996;88(13):908–917. doi: 10.1093/jnci/88.13.908. [DOI] [PubMed] [Google Scholar]
- 42.Simpkins JW, Dykens JA. Mitochondrial mechanisms of estrogen neuroprotection. Brain Res Rev. 2008;57(2):421–430. doi: 10.1016/j.brainresrev.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D. Estrogens as endogenous genotoxic agents--DNA adducts and mutations. J Natl Cancer Inst Monogr. 2000;(27):75–93. doi: 10.1093/oxfordjournals.jncimonographs.a024247. [DOI] [PubMed] [Google Scholar]
- 44.Clemons J, Glodé LM, Gao D, Flaig TW. Low-dose diethylstilbestrol for the treatment of advanced prostate cancer. Urologic Oncology: Seminars and Original Investigations. 2013;31(2):198–204. doi: 10.1016/j.urolonc.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alyea R, Laurence S, Kim S, Katzenellenbogen B, Katzenellenbogen J, Watson C. The roles of membrane estrogen receptor subtypes in modulating dopamine transporters in PC-12 cells. J Neurochem. 2008;106:1525–1533. doi: 10.1111/j.1471-4159.2008.05491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jeng Y-J, Watson C. Proliferative and anti-proliferative effects of dietary levels of phytoestrogens in rat pituitary GH3/B6/F10 cells - the involvement of rapidly activated kinases and caspases. BMC Cancer. 2009;9(334):1–17. doi: 10.1186/1471-2407-9-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gametchu B, Watson CS. Plasma membrane-associated glucocorticoid hormone receptor in human leukemic patients: Clinical implications. In: Gametchu B, editor. Glucocorticoid Receptor Structure and Leukemic Cell Responses, Molecular Biology Intelligence Unit. R.G. Landes Company; Austin: 1995. pp. 163–176. [Google Scholar]
- 48.Pearce ST, Jordan VC. The biological role of estrogen receptors α and β in cancer. Critical Reviews in Oncology/Hematology. 2004;50(1):3–22. doi: 10.1016/j.critrevonc.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Bonkhoff H, Berges R. The Evolving Role of Oestrogens and Their Receptors in the Development and Progression of Prostate Cancer. European Urology. 2009;55:533–542. doi: 10.1016/j.eururo.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 50.Golden GA, Mason RP, Tulenko TN, Zubenko GS, Rubin RT. Rapid and opposite effects of cortisol and estradiol on human erythrocyte Na+,K+-ATPase activity: relationship to steroid intercalation into the cell membrane. Life Sci. 1999;65(12):1247–1255. doi: 10.1016/s0024-3205(99)00360-4. [DOI] [PubMed] [Google Scholar]
- 51.Whiting KP, Restall CJ, Brain PF. Steroid hormone-induced effects on membrane fluidity and their potential roles in non-genomic mechanisms. Life Sci. 2000;67(7):743–757. doi: 10.1016/s0024-3205(00)00669-x. [DOI] [PubMed] [Google Scholar]
- 52.Orwoll E, Lambert LC, Marshall LM, Phipps K, Blank J, Barrett-Connor E, Cauley J, Ensrud K, Cummings S. for the Osteoporotic Fractures in Men Study G. Testosterone and Estradiol among Older Men. Journal of Clinical Endocrinology & Metabolism. 2006;91(4):1336–1344. doi: 10.1210/jc.2005-1830. [DOI] [PubMed] [Google Scholar]
- 53.Finkelstein JS, Lee H, Burnett-Bowie S-AM, Pallais JC, Yu EW, Borges LF, Jones BF, Barry CV, Wulczyn KE, Thomas BJ, Leder BZ. Gonadal Steroids and Body Composition, Strength, and Sexual Function in Men. New England Journal of Medicine. 2013;369(11):1011–1022. doi: 10.1056/NEJMoa1206168. [DOI] [PMC free article] [PubMed] [Google Scholar]