Abstract
High-throughput screens (HTS) of compound toxicity against cancer cells can identify thousands of potential new drug-leads. But only limited numbers of these compounds can progress to expensive and labor intensive efficacy studies in mice, creating a ‘bottle-neck’ in the drug development pipeline. Approaches that triage drug-leads for further study are greatly needed. Here, we provide an intermediary platform between HTS and mice by adapting mouse models of pediatric brain tumors to grow as orthotopic xenografts in the brains of zebrafish. Freshly isolated mouse ependymoma, glioma and choroid plexus carcinoma cells expressing red fluorescence protein (RFP) were conditioned to grow at 34°C. Conditioned tumor cells were then transplanted orthotopically into the brains of zebrafish acclimatized to ambient temperatures of 34°C. Live in vivo fluorescence imaging identified robust, quantifiable and reproducible brain tumor growth as well as spinal metastasis in zebrafish. All tumor xenografts in zebrafish retained the histological characteristics of the corresponding parent mouse tumor and efficiently recruited fish endothelial cells to form a tumor vasculature. Finally, by treating zebrafish harboring ERBB2-driven gliomas with an appropriate cytotoxic chemotherapy (5-fluorouracil) or tyrosine kinase inhibitor (Erlotinib), we show that these models can effectively assess drug efficacy. Our data demonstrate, for the first time, that mouse brain tumors can grow orthtopically in fish and serve as a platform to study drug efficacy. Since large cohorts of brain tumor bearing zebrafish can be generated rapidly and inexpensively, these models may serve as a powerful tool to triage drug-leads from HTS for formal efficacy testing in mice.
Introduction
Brain tumors include the most lethal forms of childhood cancer, but few new treatments of these diseases have been developed during the last 30 years1. This impasse has resulted, in part, from a lack of disease models that can be used in preclinical HTS and drug efficacy testing. Recently, our group and others have developed accurate mouse models of pediatric medulloblastoma2–5, glioma6, ependymoma7 and choroid plexus carcinoma. Together these models represent >60% of childhood brain tumors and therefore have great potential to discover new therapies for all patients. The development of the Smoothened inhibitor Vismodegib for the treatment of SHH-subtype medulloblastoma using the Ptch+/−; Tp53−/− mouse model provides proof-of-principle of this approach8. We have also shown that mouse brain tumor models can be used in HTS campaigns; identifying bolus 5-flourouracil as a new treatment of ependymoma that has now entered clinical trial9.
The integration of cell culture and robotic technologies has made HTS a powerful drug discovery tool, identifying hundreds of lead compounds with potent anticancer cell activity10, 11. But further triaging of these compounds for resource-intensive, in vivo testing in mice can be difficult. We reasoned that zebrafish might provide a relatively inexpensive and high-throughput system to triage compounds between HTS and definitive mouse model studies. Zebrafish have proved an excellent tool for studying the biology and treatment of cancer, including human tumor xenografts, but they have not been studied as a host for brain tumors or mouse cancer xenografts12, 13. Here we report an efficient system that allows mouse brain tumors to be grown in the brains of zebrafish. These tumors recapitulate the histology of the parent mouse tumor and can be used to test drug efficacy. Since large cohorts of zebrafish bearing brain tumors can be generated rapidly and inexpensively, these models may serve as a powerful new tool in the drug development pipeline between HTS and formal efficacy testing in mice.
Results and Discussion
Establishment of orthotopic mouse brain tumor xenografts in zebrafish
Red fluorescence protein (RFP) expressing tumor cells were isolated from three mouse brain tumor models generated exactly as described previously7, including a mouse model of glioblastoma generated by transducing Ink4a/Arf−/−, mouse embryonic neural stem cells (NSC) with an ERBB2-RFP retrovirus (GBMERBB2-RFP); and two separate ependymoma models generated from NSC transduced with RTBDN-RFP (EPRTBDN-RFP) or EPHB2-RFP retroviruses (EPEPHB2-RFP). We also isolated cells from a new mouse model of choroid plexus carcinoma that we recently developed by transducing the choroid plexus of embryonic Tp53flx/flx;Rbflx/flx;PTENflx/flx mice with Cre-recombinase-RFP (CPCRFP, unpublished).
As a first step to test if mouse brain tumors can grow orthotopically in zebrafish, we implanted 10 zebrafish with 2×105 of each tumor cell type. Cells were injected via the intranasal route into the cerebrum of anesthetized, 30 day old, dexamethasone-immunosuppressed, zebrafish. Ten control fish were similarly implanted with RFP-only transduced NSC. Similar amounts of RFP+ cells were readily detected by direct intravital fluorescence microscopy (IFM) in all zebrafish brains 24 hours following implantation; but no tumor cells were detectable in recipient zebrafish after three days and no fish developed tumors (data not shown).
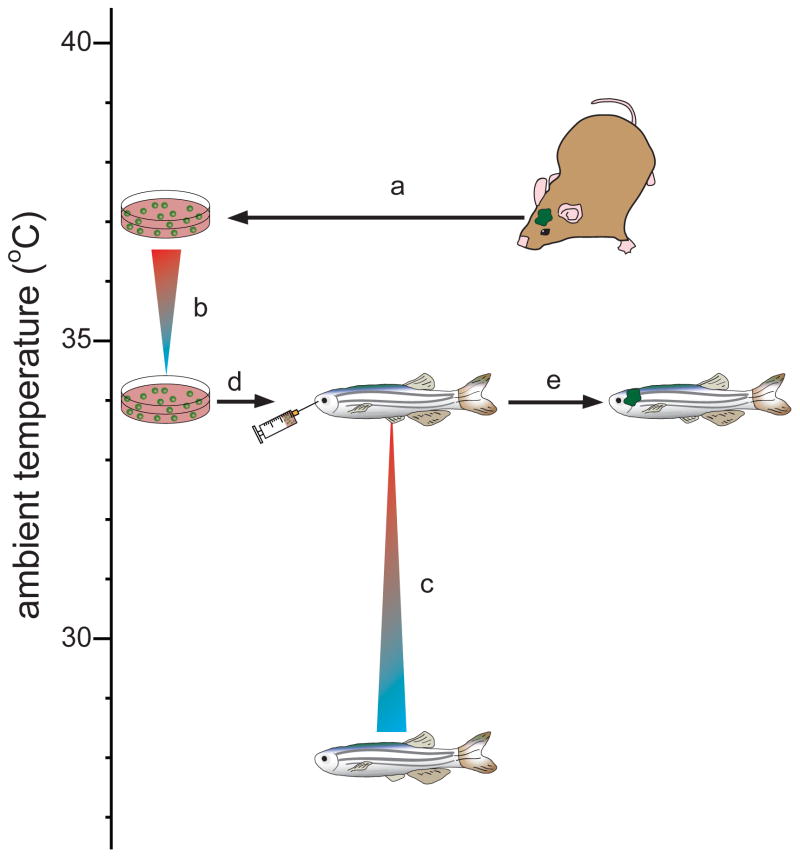
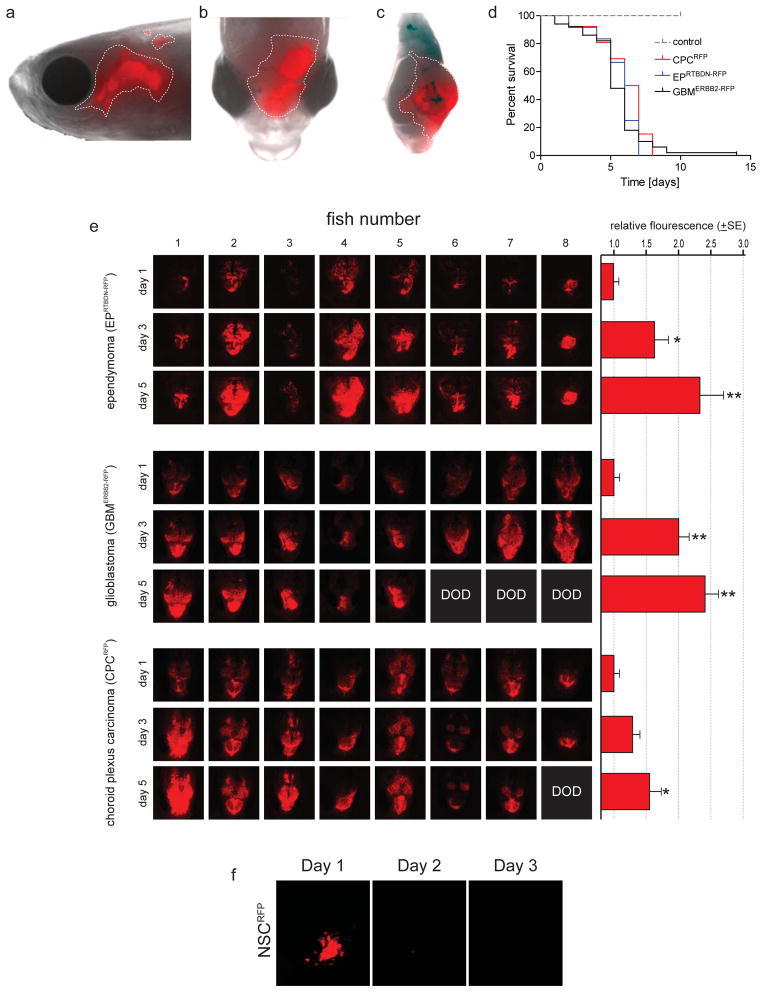
We reasoned that the marked difference in body temperature between mice (37°C) and zebrafish (~28°C) might account for the failed engraftment of tumors. Therefore, we conditioned freshly isolated cultures of mouse tumors (or control NSCRFP) to grow at 34°C by reducing the temperature of cultured cells by 0.75°C per week for 4 weeks (Figure 1a,b). Similarly, we acclimatized zebrafish to an ambient temperature of 34°C by increasing their water temperature by 1°C per day for six days (Figure 1c). Remarkably, under these conditions, all four tumor cell types demonstrated robust and rapid engraftment in zebrafish brains, resulting in the death of recipient fish from brain tumors within a median of five to seven days (Figures 1d,e and 2). Serial IFM of zebrafish readily detected reproducible and significant increases in brain fluorescence, indicative of tumor growth (EPRTBDN-RFP, p=0.001; EPEPHB2-RFP, p=0.008; GBMERBB2-RFP, p=0.002, CPCRFP, p=0.02; Mann-Whitney Figure 2e). In contrast, no fish harboring NSCRFP cells displayed increased fluorescence or tumor development (Figure 2d, f).
Figure 1. Generation of orthotopic mouse brain tumor xenografts in zebrafish.
(a) Mouse brain tumor cells were harvested from mice and cultured under conditions that promote neural stem cell growth (ref. 7). (b) The temperature of brain tumor cell cultures was reduced by 0.75°C per week for 4 weeks. (c) Three month old wild-type (AB) or Fli1:eGFP transgenic zebrafish were acclimatized to an ambient temperature of 34°C by increasing tank water temperature by 1°C per day for six days. Zebrafish were immunosuppressed by addition of dexamethasone (15mg/ml) to tank water 2 days to prior to implantation. (d) Immunosuppressed zebrafish were anesthetized using 0.04% Tricaine, placed in a 30mm petri dish under an intravital microscope. 2×105 of tumor (or control) cells were injected into the cerebral hemisphere via the intranasal route using a 30 gauge 1μl Hamilton syringe. (e) Zebrafish were subject to intravital fluorescence microscopy to monitor tumor growth. Conditioning methodology used for successful engraftment of mouse cells in adult zebrafish.
Figure 2. Orthotopic mouse brain tumor growth in zebrafish.
(a) RFP+ mouse brain tumors were imaged in live, anesthetized zebrafish using an Olympus SZX10® stereomicroscope fitted with a 594nm filter. Representative intra-vital lateral (a) and dorsal (b) images of ependymoma EPRTBDN-RFP. (c) This same zebrafish was sacrificed, the brain macrodisected and imaged, confirming the RFP+ tumor is present within the cerebral hemisphere. (d) Survival curve of zebrafish harboring the indicated tumor types or control NSCs. The survival of zebrafish harboring tumors was significantly reduced vs. control NSCRFP (P<0.001; Log-Rank). Zebrafish were implanted with (e) the indicated tumor cells or (f) control NSCRFP and imaged exactly as described in (a). The mean intensity of tumor fluorescence was measured using NIS Elements (v 3.2) software. Serial day 1, 3 and 5 IFM images of eight zebrafish each harboring EPRTBDN-RFP, GBMERBB2-RFP or CPCRFP tumors are shown. Average +SE RFP-fluorescence relative to day 1 is shown in the graphs left (Mann-Whitney U test; *=p<0.05; **=p<0.005; DOD=dead of disease). No NSCRFP were detectable in zebrafish brains one day following implantation (f).
Zebrafish brain tumors recapitulate the histology of the parent tumor and metastasize through the central nervous system
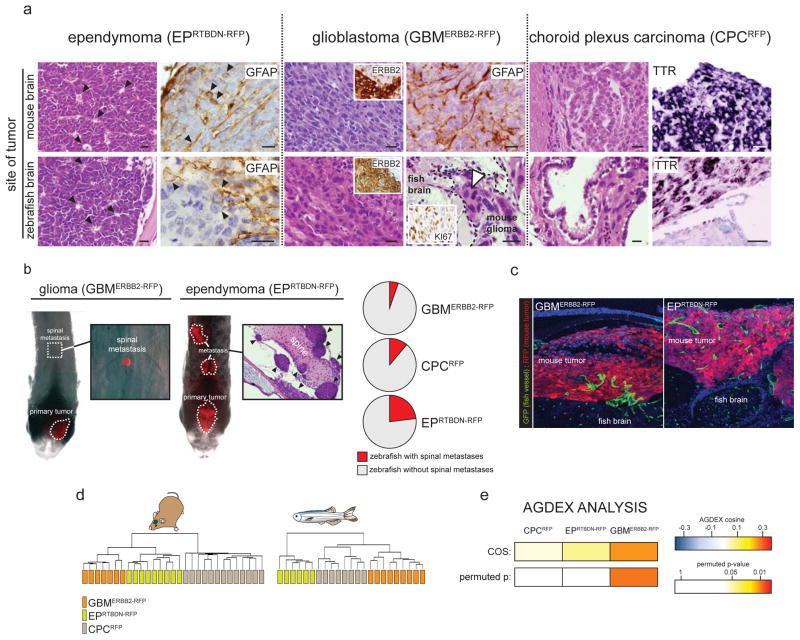
As an initial test of the fidelity of our brain tumor xenografts, we reviewed the histology and immunophenotype of all tumors (Figure 3a). Each mouse brain tumor retained its hallmark morphological features when xenografted into zebrafish brains. EPRTBDN-RFP and EPEPHB2-RFP tumors in both mouse and zebrafish contained pseudorosettes and were glial fibrillary acidic protein (GFAP) immunoreactive. GBMERBB2-RFP tumors in both species displayed cellular pleomorphism, had high mitotic rates (indicated by robust Ki67 nuclear immunoreactivity), and were intensely ERBB2 and GFAP immunoreactive. Finally, CPCRFP tumors displayed a typical epithelioid morphology and expressed transthyretin receptor (Ttr) that is highly restricted to normal and malignant choroid plexus14 (Figure 3a).
Figure 3. Mouse brain tumors retain key biologic characteristics when xenorafted into zebrafish brains.
(a) Zebrafish harboring tumors were euthanized using 0.04% Tricaine and fixed in 4% paraformaldehyde overnight. Zebrafish were then decalcified using 0.5M EDTA (AMRESCO®) for 5 days, rinsed in phosphate buffered saline, dehydrated and paraffin wax embedded. 5μm sections were stained using hematoxylin and eosin (H&E) or subjected to standard immunohistochemistry using the indicated primary antibodies (GFAP 1:500, Dako rabbit polyclonal Z0334; ERBB2 1:40, Vector mouse monoclonal VP-C380; Ki67, 1:1000, Vector rabbit polyclonal vp-K451). TTR expression was visualized by in situ hybridization using a full length TTR cRNA template (BC032069, generous gift of Dr. Edwin Monuki). Paraffin sections were treated with RNAzip (Ambion, Austin, TX) and de-waxed to water. Probe hybridization was performed at 60°C overnight in standard hybridization buffer. Sections of the same tumors growing in mouse brains were analyzed in parallel for comparison. Arrows in EPRTBDN-RFP H&E and GFAP mark pseudorosettes and GFAP+ tumor cells respectively. Dotted line in bottom right GBMERBB2-RFP H&E demarcates tumor invading normal brain. Arrow indicates mitotic tumor cells (scale bars=15μm). (b) Zebrafish were implanted with the indicated tumors and the entire dorsal brain and spine imaged daily exactly as described in Figure 1 and 2. Arrows in H&E of EPRTBDN-RFP tumors mark individual metastatic deposits. Pie charts report the proportion of zebrafish in which the indicated tumors metastasized. (c) Fli1:eGFP zebrafish harboring tumors were euthanized fixed and decalcified as described above. Brains containing tumor were then cryo-protected in 30% sucrose for 2 days and frozen in tissue freezing media (TBS®). 12μm sections were counter stained using DAPI containing hard set mounting media and imaged by confocal microscopy with 488nm and 594nm filters. (d) Total RNA was extracted from the indicated brain tumors grown in mouse and zebrafish brains. Microarray gene expression profiles were generated using Affymetrix 430v2 arrays and subject to unsupervised hierarchical clustering exactly as described7. Transcriptomes of each tumor type grown in mouse and human brains were directly compared using the Agreement of Differential Expression (AGDEX) algorithm exactly as described was used7.
Brain tumors have a propensity to disseminate through the central nervous system (CNS). Therefore, to test if zebrafish xenografts also metastasize, we generated cohorts of zebrafish implanted with EPRTBDN-RFP, GBMERBB2-RFP, or CPCRFP cells and monitored the spines of each animal using daily IFM (Figure 3b). 5.3% (n=5/94), 11.1% (n=3/27), and 23.1% (n=3/13) of zebrafish bearing GBMERBB2-RFP, CPCRFP, and EPRTBDN-RFP tumors, respectively, developed RFP+ masses along the spinal axis that were distinct from the main implanted tumor. In each case, spinal masses were undetectable immediately following cell implantation but developed one to two days post implantation. Histologic analyses of the complete CNS of fish confirmed that these masses were independent, distant deposits of tumor rather than direct extensions of the main tumor mass (Figure 3b). Thus, implanted mouse brain tumors disseminate through the CNS of zebrafish and IFM of these tumors may serve as a useful tool for studying brain tumor metastasis in vivo.
Xenografted mouse brain tumors recruit zebrafish CNS vasculature
The recruitment of normal blood vessels by cancer cells represents a critical step in tumorigenesis and metastasis15. Fli1:enhanced Green Fluorescence Protein (eGFP) transgenic zebrafish that express eGFP specifically in endothelial cells16 have been used effectively to study angiogenesis in peripheral solid tumor xenografts17. Therefore, to test if mouse brain tumor cells are competent to induce angiogenesis in zebrafish, we generated EPRTBDN-RFP and GBMERBB2-RFP tumors in Fli1:eGFP fish. Dual immunofluorescence of tumor sections confirmed that all RFP+ mouse brain tumors effectively recruited an eGFP+ zebrafish vasculature (Figure 3c). In contrast to the uniform, regularly spaced and non-tortuous vessels in normal brain, eGFP+ blood vessels in mouse xenografts were frequently branched, highly variable in size and tortuous (Figure 3c). Thus, mouse brain tumors implanted in zebrafish recruit host vasculature. Since fluorescent tumor cells and blood vessels can be imaged by IFM in live animals, this system holds great promise to study brain tumor angiogenesis in vivo.
Mouse gliomas retain the transcriptome of their parent tumor in zebrafish
To test more comprehensively if our xenografts recapitulate the biology of the corresponding parent mouse brain tumor, we used Affymetrix M430 microarrays to compare the transcriptomes of GBMERBB2-RFP, EPRTBDN-RFP, and CPCRFP tumors growing in both species. Unsupervised, hierarchical clustering of tumor transcriptomes confirmed that, regardless of host species, samples of the same tumor type are related to each other, but are distinct from other tumors (Figure 3d). To test if the transcriptome of any given tumor type grown in mice correlates with the transcriptome of the same tumor type grown in zebrafish, we used our recently developed AGDEX algorithm that estimates the degree of similarity between transcriptomes derived from different tissues7, 18. A positive AGDEX score indicates a positive correlation in transcriptome between compared tissues; the significance of this correlation is assigned a permuted p-value that controls for false discovery. All three tumor types displayed positive AGDEX scores, indicating that the mouse and zebrafish brain tumor transcriptomes are positively related (Figure 3e). GBMERBB2-RFP correlated particularly closely with the parent mouse tumor suggesting that zebrafish xenografts of this tumor are especially faithful reproductions of the parent tumor (AGDEX score 0.24, permuted p=0.02; Figure 3e).
Brain tumor xenografts in zebrafish can be used as a preclinical drug development tool
Since GBMERBB2-RFP xenografts most closely recapitulated the histology and transcriptome of their parent mouse tumors, we used this model to test our system as a preclinical drug development tool. Current cancer chemotherapies can be broadly divided into non-specific cytotoxic drugs and small molecule inhibitors of specific tumorigenic proteins. We selected one drug each from these two classes that have known activity against human GBM: the cytotoxic antimetabolite 5-fluorouracil (5-FU)19 and tyrosine kinase inhibitor, Erlotinib20 that targets ERBB2 that is mutated in human GBM21 and drives GBMERBB2-RFP.
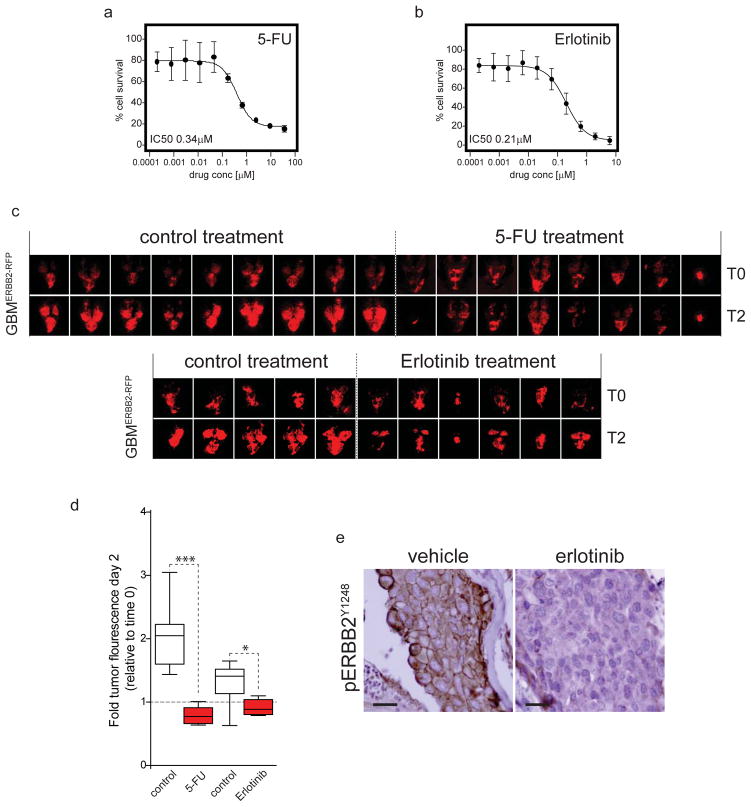
First, we confirmed that GBMERBB2-RFP cells are sensitive to 5-FU and Erlotinib with an effective concentration to inhibit growth by 50% (EC50) of 0.34μM and 0.21μM, respectively (Figure 4a,b). Next, 28 adult zebrafish were implanted with GBMERBB2-RFP cells exactly as described above and imaged using IFM to confirm the presence of RFP+ tumor cells. Zebrafish were then randomly assigned to one of four treatment groups: vehicle control (n=9, 5-FU; n=5, Erlotinib); 5-FU (n=8) or erlotinib (n=6). Erlotinib is water insoluble, and was therefore delivered by oral gavage as described previously22. Briefly, 10μl of a 5% DMSO solution containing an equivalent of 200mg/kg erlotinib was delivered via a p10 pipette tip inserted into the oral cavity of anaesthetized fish so that the tip was positioned adjacent to the gills. We showed previously that this dose blocks ERBB2 signaling in brain tumors in mice23. The ‘erlotinib group’ was dosed once per day on two consecutive days. Control fish were treated by oral gavage of vehicle only (5% DMSO). 5-FU was administered to zebrafish by adding the drug directly to tank water. We first determined the maximum tolerated dose of 5-FU by adding increasing concentrations of drug to the tank water of non-tumor bearing fish (data not shown). The ‘5-FU group’ were then treated at this maximum tolerated dose (4.7mM) on two consecutive days. Five control zebrafish received DMSO only. Tumor development was measured in all zebrafish by IFM. GBMERBB2-RFP tumors in zebrafish were highly sensitive to 5-FU therapy, displaying a ~50% reduction in tumor size relative to baseline, while tumors in vehicle treated fish doubled in size over the same time period (Figure 4c,d; p<0.0001). Erlotinib also inhibited brain tumor growth relative to control treatment, although this effect was less significant than that of 5-FU (Figure 4c, d; P<0.01).
Figure 4. Zebrafish brain tumor models can be used for preclinical drug testing.
(a) Single cell suspensions of 750 GBMERBB2-RFP tumor cells were seeded into each well of a 96-well plate in neurobasal medium exactly as described7. 24 hours later cells were dosed with the indicated concentrations of (a) 5-FU or (b) Erlotinib, or DMSO vehicle control and incubated for a further 72 hours. Percent cell survival relative to DMSO only controls was then determined in each well using the Cell Titer Glo reagent (Promega) and Envision plate reader (Perkin-Elmer). Assays were performed in independent triplicates. (c) Zebrafish harboring GBMERBB2-RFP tumors were established exactly as described in Figure 1. After 24 hours zebrafish were treated with addition of 5-FU or vehicle (control) to the tank water (top), or vehicle (control) or Erlotinib by oral gavage on two consecutive days. All zebrafish were imaged exactly as described in Figure 2. (d) Graph reports the fold fluorescence of tumors shown in (c) at day 2 relative to fluorescence at day 0 in fish treated with vehicle, 5-FU or erolotinib. Whiskers=extreme outliers, Box=median, 25th and 75th percentiles. (e) Immunohistochemistry of active, phospho-ERBB2Y1248 receptor expression in GBMERBB2-RFP tumors taken from fish treated with erlotinib or vehicle control. Immunostaining was performed as described in Figure 3 using ERBB2Y1248 rabbit polyclonal antibody (Novus Biologicals NB 100-81960; scale bars=15μm).
Finally, since erlotinib inhibits the ERBB2 tyrosine kinase that drives GBMERBB2-RFP tumors, we used phospho-specific immunohistochemistry to test if the drug inhibited ERBB2 signaling brain tumors in zebrafish (Figure 4e). Intense, membrane and cytoplasmic pERBB2Y1248 immunoreactivity was readily detected in GBMERBB2-RFP cells in the brains of three independent, vehicle treated fish. In stark contrast, pERBB2Y1248 immunoreactivity was virtually undetectable in GBMERBB2-RFP tumors three days following the onset of erlotinib therapy (Figure 4e). Thus, erlotinib effectively inhibits ERBB2 signaling in tumor cells in the zebrafish brain.
We report the first orthotopic models of mouse brain tumors in adult zebrafish. All models recapitulate the histology of their parent tumor. In addition, our glioma model retained a similar gene expression profile to its parent tumor. We further show that metastasis can be imaged in live zebrafish. Imaging of metastasis and other cellular processes in mice is challenging, in large part because existing fluorescence, bioluminescence and radiological imaging of mice has limited resolution. The translucency, small size, and ease of husbandry of zebrafish renders them useful for studying hematopoietic and solid malignancies in peripheral tissues in vivo12, 13. Our demonstration that tumor dissemination can also be visualized in the zebrafish CNS could greatly enhance understanding of the biology and treatment of brain metastasis. This is especially important since primary brain tumors kill patients by direct or metastatic invasion of the nervous system24, and brain metastasis of peripheral solid cancers are a major cause of morbidity and mortality25.
Our zebrafish system also allows preclinical testing of potential new brain tumor therapies. Tumor-bearing zebrafish embryos are an established system for the conduct of chemical genetic and therapeutic screens of cancer12, 13, 26–28. Indeed, drug screens against melanoma in zebrafish embryos have led directly to human clinical trials of the dihydroorotate dehydrogenase inhibitor, Leflunomide. Since zebrafish embryos can be maintained and treated in multi-well plates, they are a valuable whole organism system for conducting chemical screens26. However, the bathing of embryos in drug solution does not accurately model clinical drug dosing and it is unclear if tumors growing in developing or mature tissues are equivalently responsive to drugs. Our system allows for testing of drugs against brain tumors growing in the adult brain. We further show that oral gavage (erlotinib) or direct tank water administration (5-FU) of drug can be used to treat tumors in this system.
We are currently working to refine our zebrafish brain tumor models. Adult zebrafish need to be immunosuppressed to accept tumor xenografts since there are no widely available immunodeficient mutants. Current approaches to immunosuppress zebrafish include chemical or radiation immunoablation13. However, these treatments can result in unwanted morbidity and mortality, reducing the efficiency of the experimental system. Therefore, we are investigating whether recently described immunodeficient but viable mutant strains e.g., Rag1−/−29 can accept tumor xenografts without the need for exogenous immunosuppression. Additional mutant strains may also improve the utility of our model system. The relative translucency of zebrafish facilitates transcranial fluorescence imaging of brain tumors; however, the resolution of this imaging could be increased dramatically in mutants that lack melanophores or iridiophores e.g., Casper mutant30. Indeed, while reproducible growth was detected in our tumor implants, inter-tumoral variability in tumor growth was noted. This might reflect, in part, difficulties in detecting deep seated fluorescent tumor cells through a translucent cranium. Thus mutant fish may improve the resolution and sensitivity of our imaging system.
Although our models hold promise to advance the treatment of brain tumors, several questions need to be addressed before zebrafish can be formally integrated into the drug development pipeline for these diseases. In particular, the degree to which drug efficacy in zebrafish predicts activity in mice and humans remains to be determined. It will be especially important to determine differences in the pharmacokinetics of drugs among zebrafish, mice and humans. Relatively little is known about the pharmacokinetics of most drugs in zebrafish. If our fish model is to effectively triage drugs between HTS and mouse models, then it will be important to ensure that promising agents are not rejected because of unfavorable, but species specific, pharmacokinetic profiles in zebrafish. Ongoing parallel studies of zebrafish and mouse systems should answer these important questions.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01CA129541, P01CA96832 and P30CA021765, R.J.G), the Collaborative Ependymoma Research Network (CERN), and by ALSAC. We are grateful to the staff of the Hartwell Center for Bioinformatics and Biotechnology and Flow Cytometry & Cell Sorting Shared Resource at St Jude Children’s Research Hospital for technical assistance.
Footnotes
Conflict of interest: None
References
- 1.Smith MA, Seibel NL, Altekruse SF, Ries LA, Melbert DL, O’Leary M, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28(15):2625–34. doi: 10.1200/JCO.2009.27.0421. Epub 010 Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawauchi D, Robinson G, Uziel T, Gibson P, Rehg J, Gao C, et al. A mouse model of the most aggressive subgroup of human medulloblastoma. Cancer Cell. 2012;21(2):168–80. doi: 10.1016/j.ccr.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C, et al. Subtypes of medulloblastoma have distinct developmental origins. Nature. 2010;468(7327):1095–9. doi: 10.1038/nature09587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pei Y, Moore Colin E, Wang J, Tewari Alok K, Eroshkin A, Cho Y-J, et al. An Animal Model of MYC-Driven Medulloblastoma. Cancer Cell. 2012;21(2):155–67. doi: 10.1016/j.ccr.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swartling FJ, Grimmer MR, Hackett CS, Northcott PA, Fan Q-W, Goldenberg DD, et al. Pleiotropic role for MYCN in medulloblastoma. Genes & Development. 2010 May 15;24(10):1059–72. doi: 10.1101/gad.1907510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow Lionel ML, Endersby R, Zhu X, Rankin S, Qu C, Zhang J, et al. Cooperativity within and among Pten, p53, and Rb Pathways Induces High-Grade Astrocytoma in Adult Brain. Cancer Cell. 2011;19(3):305–16. doi: 10.1016/j.ccr.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson RA, Wright KD, Poppleton H, Mohankumar KM, Finkelstein D, Pounds SB, et al. Cross-species genomics matches driver mutations and cell compartments to model ependymoma. Nature. 2010;466(7306):632–6. doi: 10.1038/nature09173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romer JT, Kimura H, Magdaleno S, Sasai K, Fuller C, Baines H, et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) mice. Cancer Cell. 2004 Sep;6(3):229–40. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Atkinson Jennifer M, Shelat Anang A, Carcaboso Angel M, Kranenburg Tanya A, Arnold LA, Boulos N, et al. An Integrated In Vitro and In Vivo High-Throughput Screen Identifies Treatment Leads for Ependymoma. Cancer Cell. 2011;20(3):384–99. doi: 10.1016/j.ccr.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caponigro G, Sellers WR. Advances in the preclinical testing of cancer therapeutic hypotheses. Nat Rev Drug Discov. 2011;10(3):179–87. doi: 10.1038/nrd3385. [DOI] [PubMed] [Google Scholar]
- 11.Macarron R, Banks MN, Bojanic D, Burns DJ, Cirovic DA, Garyantes T, et al. Impact of high-throughput screening in biomedical research. Nat Rev Drug Discov. 2011;10(3):188–95. doi: 10.1038/nrd3368. [DOI] [PubMed] [Google Scholar]
- 12.White R, Rose K, Zon L. Zebrafish cancer: the state of the art and the path forward. Nat Rev Cancer. 2013;13(9):624–36. doi: 10.1038/nrc3589. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoletov K, Klemke R. Catch of the day: zebrafish as a human cancer model. Oncogene. 2008;27(33):4509–20. doi: 10.1038/onc.2008.95. [DOI] [PubMed] [Google Scholar]
- 14.Herbert J, Cavallaro T, Dwork AJ. A marker for primary choroid plexus neoplasms. Am J Pathol. 1990;136(6):1317–25. [PMC free article] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg Robert A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Lawson ND, Weinstein BM. In Vivo Imaging of Embryonic Vascular Development Using Transgenic Zebrafish. Developmental Biology. 2002;248(2):307–18. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- 17.Stoletov K, Montel V, Lester RD, Gonias SL, Klemke R. High-resolution imaging of the dynamic tumor cell–vascular interface in transparent zebrafish. Proceedings of the National Academy of Sciences. 2007 Oct 30;104(44):17406–11. doi: 10.1073/pnas.0703446104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pounds S, Gao CL, Johnson RA, Wright KD, Poppleton H, Finkelstein D, et al. A Procedure to Statistically Evaluate Agreement of Differential Expression for Cross-Species Genomics. Bioinformatics. 2011;27(15):2098–103. doi: 10.1093/bioinformatics/btr362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walbert T, Gilbert M, Groves M, Puduvalli V, Alfred Yung WK, Conrad C, et al. Combination of 6-thioguanine, capecitabine, and celecoxib with temozolomide or lomustine for recurrent high-grade glioma. Journal of Neuro-Oncology 2011. 2011 Apr 01;102(2):273–80. doi: 10.1007/s11060-010-0313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.rados MD, Lamborn KR, Chang S, Burton E, Butowski N, Malec M, et al. Phase 1 study of erlotinib HCl alone and combined with temozolomide in patients with stable or recurrent malignant glioma. Neuro-Oncology. 2006 Jan 1;8(1):67–78. doi: 10.1215/S1522851705000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.TCGA. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collymore C, Rasmussen S, Tolwani RJ. Gavaging Adult Zebrafish 2013. 2013 Aug 11;(78):e50691. doi: 10.3791/50691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernan R, Fasheh R, Calabrese C, Frank AJ, Maclean KH, Allard D, et al. ERBB2 Up-Regulates S100A4 and Several other Prometastatic Genes in Medulloblastoma. Cancer Res. 2003;63(1):140–8. [PubMed] [Google Scholar]
- 24.Lacy J, Saadati H, Yu JB. Complications of Brain Tumors and Their Treatment. Hematology/Oncology Clinics of North America. 2012;26(4):779–96. doi: 10.1016/j.hoc.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Lin NU, Wefel JS, Lee EQ, Schiff D, van den Bent MJ, Soffietti R, et al. Challenges relating to solid tumour brain metastases in clinical trials, part 2: neurocognitive, neurological, and quality-of-life outcomes. A report from the RANO group. The Lancet Oncology. 2013;14(10):e407–e16. doi: 10.1016/S1470-2045(13)70308-5. [DOI] [PubMed] [Google Scholar]
- 26.White RM, Cech J, Ratanasirintrawoot S, Lin CY, Rahl PB, Burke CJ, et al. DHODH modulates transcriptional elongation in the neural crest and melanoma. Nature. 2011;471(7339):518–22. doi: 10.1038/nature09882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong S-K, Tsang M, Dawid IB. The Mych Gene Is Required for Neural Crest Survival during Zebrafish Development. PLoS One. 2008;3(4):e2029. doi: 10.1371/journal.pone.0002029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ridges S, Heaton WL, Joshi D, Choi H, Eiring A, Batchelor L, et al. Zebrafish screen identifies novel compound with selective toxicity against leukemia. Blood. 2012 Jun 14;119(24):5621–31. doi: 10.1182/blood-2011-12-398818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wienholds E, Schulte-Merker S, Walderich B, Plasterk RHA. Target-Selected Inactivation of the Zebrafish rag1 Gene. Science. 2002 Jul 5;297(5578):99–102. doi: 10.1126/science.1071762. [DOI] [PubMed] [Google Scholar]
- 30.White RM, Sessa A, Burke C, Bowman T, LeBlanc J, Ceol C, et al. Transparent Adult Zebrafish as a Tool for In Vivo Transplantation Analysis. Cell Stem Cell. 2008;2(2):183–9. doi: 10.1016/j.stem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]