Abstract
The Memory for Intentions Screening Test (MIST) is a clinical measure of prospective memory (PM). There is emerging support for the sensitivity and ecological relevance of the MIST in clinical populations. In the present study, the construct validity of the MIST was evaluated in 40 younger (18–30 years), 24 young-old (60–69 years), and 37 old-old (70+ years) healthy adults. Consistent with expectations derived from the PM and aging literature, older adults demonstrated lower scores on the MIST’s primary scale scores (particularly on the time-based scale), but slightly better performance on the semi-naturalistic 24-hour trial. Among the healthy older adults, the MIST showed evidence of both convergent (e.g., verbal fluency) and divergent (e.g., visuoperception) correlations with standard clinical tests, although the magnitude of those correlations were comparable across the time- and event-based scales. Together, these results support the discriminant and convergent validity of the MIST as a measure of PM in healthy older adults.
Keywords: Aging, Prospective memory, Construct validity, Geropsychology, Neuropsychological assessment
Prospective memory (PM) is an aspect of declarative (i.e., episodic) memory that describes the formation, maintenance, and execution of future intentions (Kliegel, Martin, McDaniel, & Einstein, 2002). A growing convergence of studies from neuroimaging and clinical populations indicates that PM is highly dependent on rostral prefrontal (e.g., Brodmann’s area 10; Burgess, Quayle, & Frith, 2001; Burgess, Scott, & Frith, 2003), medial temporal (e.g., hippocampal), and posterior parietal (Martin et al., 2007) neural systems. Activation of precuneus and parietal regions has been noted during PM task stages such as encoding, maintenance, and retrieval (see Burgess, Gonen-Yaacovi, & Volle, 2011 for review). PM is colloquially described as “remembering to remember” and involves a complex series of events, which may be conceptualized within a multi-phasic process that includes (1) forming an intention, (2) maintaining the intention over a delay during which one is engaged in other activities, (3) initiating the intended action at the appropriate time, and (4) executing the intention (Kliegel et al., 2002). As posited by McDaniel and Einstein (2000) in their multiprocess theory of PM, the process of executing an intention may be automatic or deliberate, the demands of which may vary depending on the characteristics of the PM task, target cue, and the individual. In terms of its real world relevance, PM is essential for daily activities such as remembering to take a medication at the appropriate time, remembering to pay monthly household bills, or remembering to return a telephone call. Indeed, PM plays a unique role in the successful completion of a wide array of everyday activities such as preparing a hot meal, transportation/navigation, managing finances, and doing household chores (Smits, Deeg, & Jonker, 1997; Schmitter-Edgecombe, Woo, & Greenley, 2009).
Despite its conceptual appeal and clinical relevance, PM assessments are not routinely included in even the most comprehensive of neuropsychological evaluations. A survey completed in 2005 of assessment practices of clinical neuropsychologists (Rabin, Barr, & Burton, 2005) revealed that of the top 40 assessments of memory only one test was listed that included even a brief assessment of PM, i.e. the Rivermead Behavioral Memory Test (RBMT; Wilson, Cockburn, & Baddeley, 1985). Of the 747 respondents to that survey, only 48 (6.4%) endorsed using the RBMT (Rabin et al., 2005). This low use of PM tests may reflect the scarcity of user-friendly, psychometrically sound measures of PM. Additionally, the clinical usefulness of many PM measures is restricted by factors such as time demands of administration and scoring, limited demographically-adjusted normative standards, and insufficiently standardized experimental procedures.
The Memory for Intentions Screening Test (MIST; Raskin, Buckheit, & Sherrod, 2010) was designed to efficiently measure PM, while overcoming the limitations of previous instruments. The RMBT and the Cambridge Prospective Memory Test (CAMPROMT; Wilson, Emslie, & Foley, 2004) are two measures that provide a more naturalistic assessment of PM as compared to the MIST. In contrast, the MIST was developed to resemble a traditional laboratory-based neurocognitive task. At present, there is a considerable evidence base for the construct validity of this instrument in predicting cognitive (e.g., Woods, Moran, Dawson, et al., 2008; Gupta et al., 2010; Woods, Twamley, Dawson, Narvaez, & Jeste, 2007; Raskin et al., 2011) and everyday functioning outcomes (Woods, Iudicello, et al., 2008; Woods et al., 2009; Woods et al., 2011; Doyle et al., 2012). The MIST is a standardized measure in which participants perform eight different PM tasks over approximately 30 minutes. A word-search puzzle serves as the foreground (i.e., distracter) task. There are four time-based trials (e.g. “In 2 minutes, tell me 2 things you forgot to do in the past week”, “In 15 minutes, tell me it’s time to take a break”), and four event-based trials (e.g. “When I show you a red pen, sign your name on your paper”; “When I show you a tape recorder, tell me to rewind the tape.”). The length of time between the participant being informed of the future intention and the execution of that intention is a span of either 2 minutes or 15 minutes. Participants are not allowed to write down any cues and no preparatory cues are presented prior to the cue to execute the intention. Finally, participants are instructed to call their examiner 24 hours after testing to report the length of time they slept and the quality of their sleep. Incorrect responses are coded using a detailed, comprehensive scoring system that operationalized common errors of omission (e.g., loss of time) and commission (e.g., task substitution errors). The MIST yields a summary score ranging from 0 – 48, time- and event -based scales ranging from 0 – 8, and coding for different error types, including omissions, task substitutions, loss of content, and loss of time (Raskin et al., 2010; Woods, Moran, Dawson, et al., 2008)
The construct validity of the MIST has increasingly been examined in a range of clinical populations. The current literature base provides support for the inter-rater reliability and internal consistency of the MIST (Woods, Moran, Dawson, et al., 2008). The MIST correlates with other well-validated clinical measures of memory and executive functions in studies of diverse clinical populations such as HIV infection, (Gupta et al., 2010), schizophrenia (Woods et al., 2007), and Parkinson’s disease (Raskin et al., 2011). The MIST also differentiates healthy adults from populations with HIV infection (Carey et al., 2006), substance use disorders (e.g., Iudicello et al., 2011; Weinborn, Woods, O’Toole, Kellogg, & Moyle, 2011), schizophrenia (Twamley et al., 2008; Woods, et al., 2007), traumatic brain injury (Fleming, Shum, Strong, & Lightbody, 2005; Tay, Ang, Lau, Meyyappan, & Collinson, 2010), mild cognitive impairment (e.g., Karantzoulis, Troyer, & Rich, 2009), and Parkinson’s disease (Raskin et al., 2011). In terms of its ecological validity, the MIST has been significantly associated with a variety of important everyday functioning outcomes, including declines in instrumental activities of daily living (Woods, Iudicello, et al., 2008; Woods, Weinborn, Velnoweth, Rooney, & Bucks; 2012), financial mismanagement (Pirogovsky, Woods, Filoteo, & Gilbert, 2012), medication non-adherence (Woods, Moran, Carey, et al., 2008b; Woods et al., 2009), unemployment (Woods et al., 2011), and lower health-related quality of life (Doyle et al., 2012). In fact, results from the above studies suggest that the MIST accounts for variance above and beyond other important predictors like general cognitive status, depression, disease severity, and sociodemographics (e.g., Woods et al., 2009).
The cognitive aging literature was the birthplace of modern PM research and has arguably been the primary source of the most influential theoretical advances in PM over the past two decades. However, the MIST, which was developed as a clinical test, has seldom been used to measure PM in older adults. Given the prefrontal and medial temporal correlates of PM, and the overlap of these areas with those affected by age-related brain volume declines, it is no surprise that the construct of PM is frequently assessed within the aging population. A meta-analytic review of the literature on PM and aging conducted by Henry, MacLeod, Phillips and Crawford (2004) revealed that older adults tended to perform worse on tasks that rely more on self-initiated encoding, monitoring, and retrieval. Additionally, the strongest age-related differences in PM have been reported for time-based tasks, which place greater demands on self-initiated monitoring (e.g., Einstein, McDaniel, Richardson, Guynn, & Cunfer 1995; Park, Hertzog, Kidder, Morrell, & Mayhorn, 1997). In contrast, relatively automatic PM encoding, monitoring, and cue detection are spared to some extent in older adults (see McDaniel & Einstein, 2011 for review). This disruption of PM performance noted in older adults may not be due to aging per se, but may instead be related to reduced frontal lobe function seen in a subset of older adults (McFarland & Glisky 2009). Pardoxically, however, older adults may perform as well as or better than younger adults on semi-naturalistic tasks or low-demand event-based tasks (e.g. mailing postcards to the examiner and phoning the examiner daily for a period of time). This discrepancy may be explained by the higher levels of processing demands and attention monitoring required for time-based tasks relative to event-based tasks (Henry et al., 2004).
Although the MIST shows promise as a measure of PM in various other clinical populations, it has received little attention in healthy aging cohorts, which have been a major focus of the broader PM literature. We are aware of only three studies that have reported associations between age and the MIST, all of which have focused on middle-aged adults with a high prevalence of psychological and medical comorbidities. Woods, Moran, Dawson, et al. (2008) found that younger age was associated with better performance; however, the mean age of participants in that study was just over 40 years. Previous studies that used the MIST have yielded similar patterns of age effects on both event-based (Woods, Dawson, Weber, Grant, & HNRC, 2010) and time-based (Weber et al., 2011) PM, whereby “younger” adults (i.e., mean age = 31 years) perform better than “older” adults (i.e., mean age = 56 years) within the laboratory setting. However, as demonstrated by Weber et al. (2011), these same older middle-aged adults perform slightly better than younger adults on naturalistic tasks. Two other studies of note have provided evidence of the ecological validity of the MIST among genuinely older adults. Woods et al., (2012) reported that the event-based scale of the MIST was a unique predictor of self-reported instrumental activities of daily living (IADL) declines among 50 older Australians (mean age = 69.2 years), while Pirogovsky et al. (in press) showed univariate associations between the MIST and performance-based tasks of financial and medication management in 33 older adults (mean age = 71.2).
To date, however, the construct validity of the MIST has not been comprehensively evaluated in healthy older adults over the age of 70 years. Accordingly the aim of this study was to examine the construct validity of the MIST in the context of the well-documented effects of age on PM. In light of prior literature, it was hypothesized that (a) older adults would demonstrate poorer PM performance on the MIST than younger adults, (b) these effects would be driven by the lower time-based versus event-based MIST scores, (c) there would be an interaction between age group and MIST PM task such that older adults would perform worse than their younger counterparts in the laboratory setting but not on a naturalistic task. We also examined the cognitive correlates of PM performance in our older cohort and hypothesized that MIST scores would be related to executive functions, delayed memory, and verbal fluency, but not cognitive abilities such as information processing speed and visuoperception.
Method
Participants
Study participants included 61 English-speaking, community-dwelling older adults who were recruited from the Western Australian Participant Pool (RSB, director) and 40 young seronegative healthy adult cohort from the HIV Neurobehavioral Research Program (San Diego, CA). To minimize the possibility that our older participants had mild cognitive impairment, we excluded subjects if they scored ≤27 (Benson et al., 2005) on the Mini Mental State Exam (MMSE; Folstein, Folstein, & McHugh, 1975). A cutoff score of 10 on the HIV Dementia Scale (Power, Selnes, Grim, & McArthur, 1995) was used for the participants in the youngest group. We also excluded participants who reported histories of major psychiatric (e.g., mental retardation, psychosis, and recent substance dependence) or neurological (e.g., seizure disorders, closed head injuries with loss of consciousness <30 minutes, and cerebrovascular accidents) conditions that might affect cognition. Other chronic medical comorbidities (e.g., diabetes, cardiovascular disease, and cancer) that commonly accompany aging were documented. Although half of the older group reported age-related medical conditions, these were generally those that do not substantially increase brain pathology. The medical conditions present in this cohort were: arthritis (30%), diabetes (8%), cancer (9%), and cardiovascular disease (1%). Forty participants were between the ages of 18–30 (i.e., the “young” (Y) group), 24 were between 60–70 years old (i.e., the “young old” (YO) group), and 37 participants were over the age of 70 years (i.e., the “old old” (OO) group). Table 1 displays the samples’ demographic characteristics. The groups differed significantly on education (p = .01), such that the Y group had significantly more number of years of education than the YO group.
Table 1.
Demographic characteristics of the 101 study volunteers
| Variable | Young (n = 40) | Young-old (n = 24) | Old-Old (n = 37) |
|---|---|---|---|
| Age (years) mean (SD) | 25.33 (3.30) | 64.80 (2.51) | 75.10 (5.73) |
| range | (18 – 30) | 60 – 69 | 70 – 88 |
| Education (%) | |||
| Some high school | 0 | 0 | 8 |
| High school | 30 | 46 | 24 |
| Some college | 35 | 25 | 24 |
| College and higher | 35 | 29 | 44 |
| Sex (% men) | 50 | 38 | 24 |
Materials and procedure
The human research ethics office of the University of Western Australia and the institutional review board of the University of California, San Diego approved the parent studies and all participants provided written, informed consent. All participants completed the research version of the MIST (Woods, Moran, Dawson, et al., 2008), which as described above includes eight PM trials that are completed in the context of an ongoing word search puzzle. The eight tasks are balanced on the following characteristics: (1) a 2-minute or 15-minute delay; (2) a verbal (e.g., “In 2 minutes, ask me what time this session ends”) or physical (e.g., “In 15 minutes, use that paper to write down the number of medications you are currently taking”) response; and (3) a time-based (e.g., “In 15 minutes, tell me that it is time to take a break”) or event-based (e.g., “When I show you a postcard, self-address it”) cue. The cognitive load (i.e., the total number of other intentions “online” at the time each intention is supposed to be recalled) varies across the items. As an ongoing task, participants complete a series of word search puzzles to prevent overt rehearsal of the prescribed intentions. Each PM trial on the MIST is worth two possible points: one point is awarded for a correct response and one point for responding (in some manner) at the appropriate time (15% of the targets) or to the appropriate cue. For example, if a participant is 3 minutes tardy in asking what time the session ends, only one point is awarded for that trial. Similarly, one point is earned if, for example, the participant signs their name instead of self-addressing the displayed postcard (NB. this differs from the Raskin et al., 2010, instructions, which award zero points for an incorrect event-based trial).
Individual PM trials contribute to three of the MIST’s six subscales (range = 0–8), as determined by each trial’s specific delay, cue, and response characteristics. Each subscale therefore contains four individual PM trials (see Table 3). The six subscales are then summed to create a summary score, which ranges from 0 to 48. Standardized qualitative error coding on the MIST generates the following error types: (1) no response (i.e., omission error), (2) task substitution (e.g., perseverations or intrusions), (3) loss of content (e.g., acknowledging that a response is required, but failing to recall the particulars), and (4) loss of time (i.e., performing the correct response at the wrong time). Additionally, participants complete a three-choice recognition test immediately following the completion of the MIST (range = 0–8). Finally, a 24-hour probe was administered in which participants were instructed to leave a telephone message for the examiner the following day specifying the number of hours slept the night after the assessment (scored as pass/fail based on whether or not they called at the correct time with the appropriate response). The 24-hour trial does not contribute to the MIST Summary Score. Unlike the other MIST items, participants are allowed to use any mnemonic strategy they wish for the 24-hour probe (e.g., a note in their electronic organizer or assistance from a significant other), but are not explicitly instructed to do so.
Table 3.
Descriptive data for the Memory for Intentions Screening Test (N = 101)
| MIST Variable | Young (n = 40) | Young-old (n = 24) | Old-Old (n = 37) |
|---|---|---|---|
| Summary score | 45 (42, 48) | 41 (36, 42) | 36 (33, 42) |
| Total errors (%) | 37 | 75 | 87 |
| NR errors (%) | 5 | 13 | 8 |
| TS errors (%) | 13 | 8 | 19 |
| LC errors (%) | 3 | 41 | 41 |
| LT errors (%) | 5 | 0 | 3 |
| PLO errors (%) | 0 | 0 | 0 |
| R errors (%) | 0 | 4 | 0 |
| Recognition | 8 (8, 8) | 8 (7, 8) | 8 (7, 8) |
| Distracter words | 23.5 (18, 30.75) | 13 (10.3, 16) | 12 (10.5, 15.5) |
Note: Data are presented as median values with the interquartile range in parentheses or as valid population percentages with more than one error across the various error types.
LC= loss of content; LT= loss of time; MIST= Memory for Intentions Screening Test; NR= No response; PLO= place losing omission; R=random; TS= task substitution
Participants in the older, Australian, groups were also administered a neurocognitive test battery that included the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS; Randolph, Tierney, Mohr, & Chase, 1998), the Executive Clock-Drawing Task (CLOX; Royall, Corders, & Polk, 1998), Trailmaking Test (TMT, Parts A and B; Reitan and Wolfson, 1985), the Digit Span test from the Wechsler Adult Intelligence Scale-III (WAIS-III; Psychological Corporation, 1997), Action Fluency (Piatt, Fields, Paolo, & Tröster, 1999), as well as Animal Fluency and Letter Fluency (Benton, Hamsher, & Sivan, 1994). The RBANS yielded a delayed memory index score and a visuospatial/constructional index score. A composite attention/executive functions score was derived by converting raw scores on TMT B, CLOX, and Digits backwards trial to population-based Z-scores (where higher scores correspond to better performance), which were then averaged. Similarly, a composite fluency score was generated by averaging the population-based Z-scores derived from raw scores on the Animal, Action, and Letter Fluency tests. Finally, raw scores on TMT A and RBANS Coding subtest were converted to population-based z-scores and averaged to generate a speed of information processing composite score. Descriptive data for the cognitive test scores are presented in Table 2.
Table 2.
Descriptive data for the neuropsychological battery administered to the older groups (N = 61).
| Neuropsychological Test | Young-old (n = 24) | Old-Old (n = 37) |
|---|---|---|
| RBANS | ||
| Figure Copy | 18.25 (2.0) | 15.43 (2.9) |
| (13 – 20) | (9 – 20) | |
| Line Orientation | 18.54 (1.47) | 17.19 (2.52) |
| (14 – 20) | (10 – 20) | |
| Picture Naming | 9.83 (.38) | 9.70 (.57) |
| (9 – 10) | (8 – 10) | |
| Semantic Fluency | 24.75 (6.15) | 22.62 (4.85) |
| (17 – 40) | (16 – 35) | |
| Digit Span | 12.33 (4.11) | 13.22 (3.76) |
| (8 – 29) | (7 – 22) | |
| Coding | 49.50 (8.22) | 46.05 (8.82) |
| (32 – 66) | (26 – 68) | |
| List Recall | 6.96 (2.19) | 6.14 (1.73) |
| (3 – 10) | (1 – 9) | |
| List Recognition | 19.42 (.93) | 19.27 (.90) |
| (17 – 20) | (17 – 20) | |
| Story Recall | 8.66 (2.07) | 8.73 (2.15) |
| (5 – 12) | (3 – 12) | |
| Figure Recall | 13.29 (3.97) | 10.62 (4.06) |
| (4 – 19) | (0 – 17) | |
| WAIS- III Digit Span total | 17.87 (3.80) | 16.48 (3.19) |
| (11 – 29) | (11 – 22) | |
| Trail Making Test- A | 32.17 (6.73) | 44.65 (44.92) |
| (21 – 47) | (21 – 300) | |
| Trail Making Test- B | 67.54 (16.11) | 95.48 (48.84) |
| (50 – 266) | (44 – 300) | |
| Letter Fluency | 49.42 (12.71) | 45.59 (12.15) |
| (20 – 81) | (22 – 81) | |
| Animal Fluency | 23.21 (6.21) | 19.2 4(4.09) |
| (13 – 41) | (10 – 29) | |
| Action Fluency | 18.80 (6.5) | 17.90 (5.0) |
| (8 – 34) | (7 – 30) | |
| Executive Clock Drawing Test | 12.71 (2.59) | 11.97 (2.95) |
| (4 – 15) | (5 – 15) |
Note: Data are presented as means with the standard deviations and range in the parentheses.
Data Analyses
Group differences on the MIST Summary Scale, word search, recognition trial, and error types were examined using analysis of variance (ANOVA) with education as a covariate. Next, a repeated measures ANOVA was conducted in which the between-subjects factor was age group (i.e., Y, YO, OO) and the within-subjects factor was PM cue type (i.e., time- vs. event-based), again with education as a covariate. Planned follow-up pairwise comparisons were conducted, which were complemented by Cohen’s d effect size estimates. A nominal logistic regression analysis was then conducted to determine whether education and age group predicted success on the 24-hour task. The associations between PM and measures of episodic memory, attention/executive control, fluency, speed of information processing and visuoperceptual ability were examined. Next, regression analyses were conducted using the neurocognitive measures as predictors of time- and event-based MIST scores. Although the data were non-normal, findings did not differ when parametric statistics were used and a check of the linear regression residuals nevertheless showed no serious departures from normality in their distributions. A critical alpha level of .05 was used for all analyses.
Results
Descriptive data on the MIST in the three age groups are displayed in Table 3. Of note, the medical comorbidities in the older participants were not significantly associated with PM (p’s > .1). There were no significant differences in gender across the three groups, and inclusion of gender as a covariate for the planned analyses did not alter the findings. Education corrected comparisons of MIST scores were conducted across the groups. As seen in Table 3, a significant effect of age group was noted on the MIST summary score (F(2, 97) = 10.3, p < .0001) after adjusting for education. Relative to the two older groups, the youngest participants obtained higher scores on the distracter task (p < .0001). Although the median scores were identical, non-parametric tests nevertheless showed that the youngest participants scored significantly higher on the recognition trial of the MIST, compared to the oldest group (p < .001). An analysis of group differences across error types revealed that the oldest participants made significantly more no-response and loss of content errors than the youngest participants (p’s < .05), but not other error types (p’s > .10).
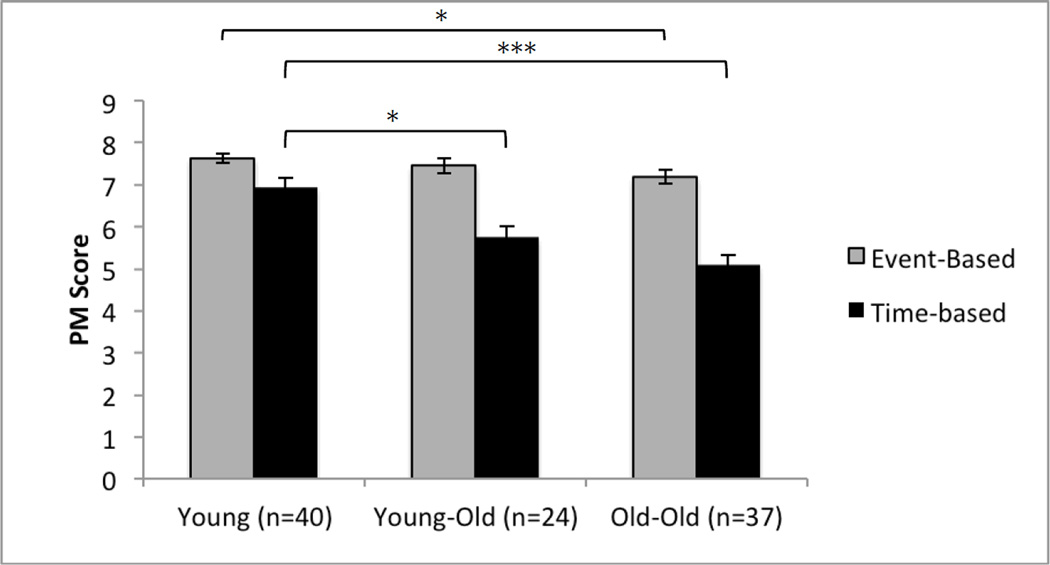
A repeated measures ANOVA with cue-type as the within subjects factor, age group as the between subjects factor, and education as a covariate revealed a significant main effect of age group, F(2, 97) = 24.5, p < .0001, as well as cue type, F(1, 97) = 14.9, p < .0001. These main effects were accompanied by a significant interaction between age group and cue type, F(2, 97) = 9.20, p < .0001. Planned follow-up pairwise comparisons (depicted in Figure 1) revealed a significant effect in that the OO group performed significantly worse than the Y group on the MIST time- (p < .0001; Cohen’s d = 1.34) and event-based tasks (p = .03; Cohen’s d = .41). The YO group performed significantly worse than the Y group on the time-based task only (p < .01; Cohen’s d = .94).
Figure 1.
Bar chart displaying the interaction between age group (i.e. Young, Young-Old, and Old-Old) and Prospective Memory (PM) cue type. Standard errors are represented in the figure by the error bars on each column.
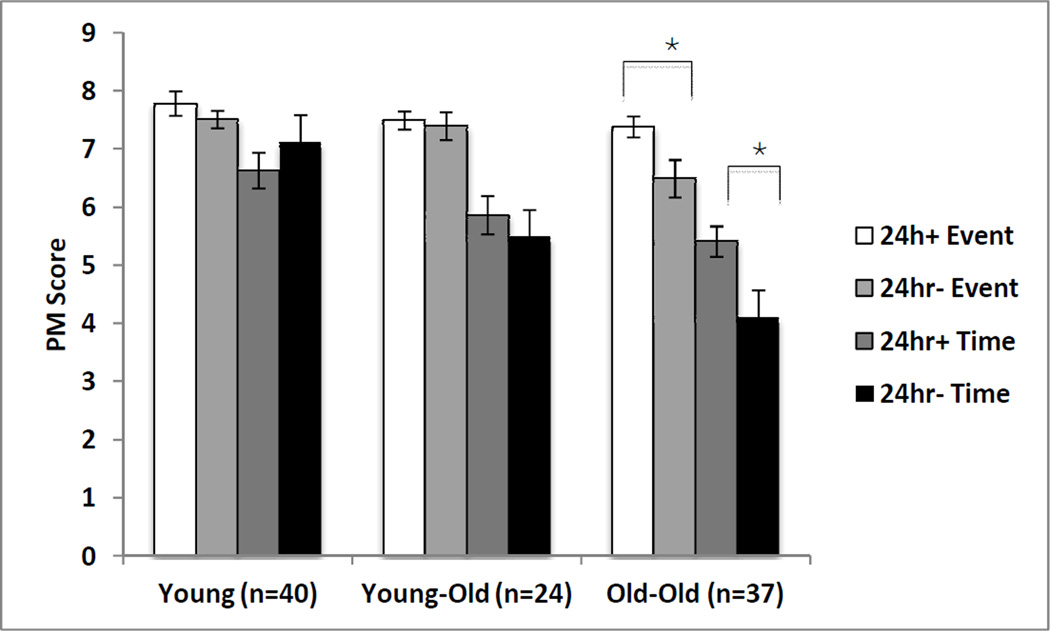
Next, a nominal logistic regression was conducted in which age group and education were entered as predictors of pass/fail status on the 24-hour MIST task. The analysis revealed that age group, but not education, was significantly associated with successful performance on the 24-hour MIST task (Wald χ2 = 13.49, p < .01; χ2 (5, N = 101) = 16.52, p = .01). Lower rates of failure on the 24-hour task were noted for the YO (33%) and OO (24%) groups compared to the Y group (64%), χ2 (2, N = 101) = 13.30, p < .01. Despite the better performance of the older groups on the semi-naturalistic task, failure on the 24-hour task was significantly associated with time- (Wilcoxon Rank Sum χ2 = 6.36, p = .02) and event-based (Wilcoxon Rank Sum χ2 = 4.33, p = .04) scores in the OO group only (see Figure 2).
Figure 2.
Time- and event-based PM scores grouped by age category (Young, Young Old, and Old Old) and naturalistic PM accuracy (i.e., successful completion of a 24-hr delay telephone task). Standard errors are represented in the figure by the error bars on each column.
Correlation analyses were conducted within the collapsed older (i.e., YO and OO) groups to examine the association between the MIST and standard neuropsychological measures. Results (displayed in Table 4) showed that the MIST summary score was moderately associated with the RBANS delayed memory, and the composite scores for attention/executive control and verbal fluency (r’s = .37 – .38). Significant correlations were also noted on the executive and verbal fluency composite scores and the time-based MIST score (p’s < .01). The magnitude of these correlations was broadly in the medium effect size range (r’s = .31 – .45). In contrast, significant associations were noted between the MIST event-based score and the RBANS delayed memory composite score (r = .29, p = .02), but not the attention/executive control and verbal fluency composite scores. Notably, education and gender were not associated with the time- or event-based MIST score among the older samples (p’s > .10). Correlation coefficients of the relationship between the three MIST scores and the composite speed of information processing score as well as the RBANS Visuospatial/Constructional composite score were also examined. Neither the time nor event-based MIST score was significantly correlated with either measure (all p’s > .10; See Table 4). We examined whether these correlations were statistically different using Steiger’s z values. Only the correlation between time-based MIST score and fluency was significantly larger than that between time-based MIST score and speed of information processing composite score (z=1.66, p=.04) and the RBANS visuospatial/constructional composite score (z=.183, p=.03). With regard to the event-based MIST score, the correlations with the neurocognitive scores did not statistically differ from each other (ps > .10). Finally, we tested whether the correlations between neurocognitive scores and MIST score differed across time- and event-based score. The only significant difference was observed for the fluency composite score (z=2.37, p=.008), such that the correlation was significantly larger for time-based MIST score compared to event-based MIST score.
Table 4.
Associations between prospective memory and neurocognitive measures within the older samples (N = 61).
| Memory for Intentions Screening Test | |||
|---|---|---|---|
| Neurocognitive measure | Summary | Time-based | Event-based |
| Attention/Executive Control | 0.37** | 0.31* | 0.20 |
| Fluency | 0.38** | 0.45*** | 0.08 |
| Speed of information processing | 0.16 | 0.19 | 0.04 |
| RBANS Delayed Memory | 0.32* | 0.24 | 0.29* |
| RBANS Visuospatial/Construction | 0.21 | 0.15 | 0.19 |
p < .05,
p < .01,
p < .001
Finally, multiple linear regression analyses were conducted within the collapsed older sample (n = 61) and only those neuropsychological domains with significant correlations with the MIST time- and event-based scores were entered as predictors. Multivariable regression with age and the composite scores for verbal fluency and attention/executive control entered as predictors of time-based MIST scores showed that only the verbal fluency composite score (β = .35, p < .01) was significantly associated with performance on this task. The full model accounted for a significant amount of variance in the criterion, adjusted R2 = .23, p < .001. Similarly, age and RBANS delayed memory composite score were included as predictors of the event-based score. The full model was significant (adjusted R2 = .11, p = .01), and revealed that the RBANS delayed memory composite score (β = .25, p = .04) was the only significant predictor of performance on the MIST event-based scale.
Discussion
Although literature on the construct validity of the MIST as a measure of PM in clinical populations has greatly increased in recent years, there remains a dearth of studies examining this instrument in healthy aging cohorts, particularly older adults over the age of 70 years. In the present study, the construct validity of the MIST was examined by using three different age groups (i.e., young, young-old, and old-old). Consistent with our primary hypothesis, lower MIST summary scores were noted for the oldest group compared to the youngest group, suggesting that in laboratory settings, oldest adults demonstrate worse PM performance than young adults (Henry et al., 2004). Analysis of specific error types revealed that the oldest participants made more no-response (i.e., omission) and loss of content errors relative to the youngest subjects. The no-response errors, which suggest dysfunction in monitoring abilities (e.g., Doyle et al., 2013), should be interpreted with caution as they may be impacted not only by underlying cognitive abilities of interest, but also unrelated patient attributes (e.g., reluctance to make errors). While our data do not allow us to examine the personality factors linked to no response errors, a post-hoc examination of the cognitive architecture of omission errors in the older group demonstrated that these errors were significantly associated with the verbal fluency score, but not that of other executive functions. This pattern of findings, which is consistent with that noted for cognitively impaired HIV+ persons (Doyle et al., 2013), may reflect the sensitivity of monitoring abilities to the executive aspects (i.e., task switching abilities) of verbal fluency tasks. The abilities measured by our executive and attention tasks (i.e., speeded divided attention, auditory working memory, and visual planning) may be relatively less involved in intact monitoring, and consequently were not associated with omission errors (see Doyle et al., 2013). Compared to the youngest group, the two older groups demonstrated worse performance on the recognition and distracter tasks; a finding that is interpreted with caution, given the comparable median scores. At the global level, these data suggest that older adults show overall worse PM performance on the MIST, which includes deficits in encoding (recognition), monitoring and cue detection (omission errors), and the retrospective memory (loss of content errors) aspects of executing future intentions. Such findings are broadly consistent with the PM literature on aging (Henry et al., 2004) and provide further evidence for the discriminant validity of the MIST as a measure of PM ability.
Also consistent with our a priori predictions, we observed an interaction between age and time- versus event-based PM cues. A comparison of the young and young-old groups indicated a differential effect of MIST cue-type, such that the time-based task alone discriminated between the youngest and young-old groups, with the latter group demonstrating worse performance on this task only. Furthermore, our findings suggest that in the oldest group (i.e., individuals over the age of 70), performance on time- as well as event-based tasks is impaired. Notably, the oldest adults performed worse on the MIST time-based task relative to the event-based task. These findings further support the utility of using the MIST as a measure of PM in older adults and extend the current literature on PM by examining this construct in individuals over the age of 70 years. The possibility that the differential age-related deficits are linked to task difficulty rather than unique task characteristics is mitigated by the divergent cognitive and neural mechanisms involved in focal (e.g., event-based) and non-focal (e.g., time-based) PM described below. Nonetheless, the possible impact of the difficulty confound on the present study results cannot be discounted. As recommended by Chapman and colleagues (e.g., Miller, Chapman, Chapman, & Collins, 1995), future psychometric investigations may consider comparing tasks to ascertain the magnitude of impact of task difficulty on PM performance in older adults, as well as in relevant clinical populations.
The current body of literature examining MIST performance in older adults (thus far consisting of individuals younger than 70 years of age) attributes the poor performance of older adults on time-based tasks to deficits in self-initiated retrieval (Henry et al., 2004). Neuroimaging studies have found that sustained anterior prefrontal cortex activation during strategically-demanding time-based PM tasks is related to better PM performance (Burgess, Scott, & Frith, 2003; Burgess, Quayle, &Frith, 2001; Simons, Schölvinck, Gilbert, Frith, & Burgess, 2006; Reynolds, West, & Braver, 2009). However, the ability to sustain activation in this region declines with age (Braver et al., 2001; Jimura & Braver, 2010), and is thought to underlie the strategically demanding PM deficits noted for older adults compared to their younger counterparts (Henry et al., 2004; Kliegel, Mackinlay, & Jäger, 2008; McDaniel & Einstein, 2007). Future studies may wish to explicitly examine the role of time monitoring (e.g., clock checks) in the expression of time-based PM deficits on the MIST in older adults.
Event-based PM tasks on the other hand, are generally expected to have lower strategic processing and monitoring demands compared to time-based tasks, based on the notion that – all other things being equal – event-based cues are more salient, particularly if they are focal to ongoing processing (Kliegel et al., 2008). Imaging studies have shown that medial-temporal processes underlie performance on focal event-based PM tasks (Martin et al., 2007). These reflexive-associative retrieval processes are relatively spared in older adults (Scullin, Bugg, McDaniel, & Einstein, 2011). The planning and executive demands are considerably lower for event-based PM tasks and age-associated decline in frontal processes is not as problematic for the successful completion of event-based tasks. Consequently, the performance of older adults on event-based tasks may be comparable to that of younger adults (see McDaniel & Einstein, 2011 for review). However, in the current study, MIST event-based scores were significantly lower in the oldest-old group compared to the youngest group. Although, consistent with the expected differential attentional requirements for time- and event-based tasks, the oldest adults obtained worse scores on the time-based MIST tasks. Taken together, these results suggest that in adults over the age of 70 years, age-related declines impact the mechanisms involved not just in the planning and executive demands of the more strategically demanding time-based tasks, but also those underlying the completion of event-based tasks. The observed age-related event-based PM differences are commensurate with evidence demonstrating age-associated declines in episodic memory (e.g., Kausler, 1994; Singer et al., 2003), which may involve failures to adequately link target items with other items or their respective contexts (reviewed in Old & Naveh-Benjamin, 2008). This specific failure type may contribute to impaired event-based PM as well.
Regarding the possible cognitive mechanisms underlying the age-associated PM deficit, our findings support the influential role of verbal fluency, attention/executive control, and retrospective memory with evidence for the separability of the time- and event-based PM abilities. For instance, time-based, but not event-based, PM was uniquely associated with attention/executive control, as well as verbal fluency suggesting that time-based PM is more heavily dependent on self-initiated retrieval compared to event-based PM as measured by the MIST. On the other hand, delayed memory alone was a significant predictor of performance on the event-based tasks. Of course, caution is warranted in interpreting the numerical discrepancies between these correlations, as very few survived the more rigorous test of direct statistical comparison of their relative magnitudes. In this regard, TB PM was significantly more strongly associated with verbal fluency than was EB PM. A similar pattern of stronger association of time-based PM with fluency and executive control measures compared to event-based performance paired with the relationship between event-based scores and delayed memory has previously been reported in the MIST literature, specifically among persons with Parkinson’s disease (e.g., Raskin et al., 2011) and HIV infection (Zogg et al., 2011). Our findings are also commensurate with the multi-process theory, which posits that time-based PM requires higher-level cognitive components such as self-initiated monitoring (e.g., clock checking) and retrieval (e.g., time perception) processes (McDaniel & Einstein, 2000). Among older adults, related abilities such as self-initiated, strategic switching and executive control underlie performance on word generation and “switching” tasks (Moscovitch, 1994; Piatt et al., 1999; Troyer, Moscovitch, & Winocur, 1997). Thus, it is likely that the association noted between time-based PM and verbal fluency performance reflects the multifaceted nature of this PM ability. The associations noted between event- and time-based PM scores with neurocognitive tasks suggests that there was sufficient variability in the PM scores, and consequently ceiling effects are unlikely to play a role in the age by PM task interaction observed.
This study also provides preliminary evidence of the divergent validity of the MIST in an older cohort. For example, PM performance was dissociable from visuoperceptual ability. Similarly, as expected given the minimal processing speed demands of the MIST, divergence was observed for performance on this instrument and speed of information processing abilities.
In spite of the evidence for moderate age-associated PM impairments in the laboratory, older individuals performed better than their younger counterparts on the seminaturalistic 24-hour MIST task. This phenomenon is known as the age-PM paradox (Rendell & Thomson, 1999). Older adults are more likely to establish and use external cues to act as reminders to help them complete these non-laboratory PM tasks (e.g., Maylor, 1990). It is also posited that young and old adults may differ in their motivation to complete naturalistic PM tasks (Patton & Meit, 1993; Rendell & Craik, 2000). Our results suggest that in the youth group, failure on the 24-hour MIST task is not associated with performance on the time- or event-based tasks administered in the laboratory. However, the pathways to failure on the 24-hour task in older adults (particularly the oldest group) appear to be associated with both time- and event-based PM performance, as has previously been shown in clinical samples, including HIV infection (Zogg et al., 2010) and methamphetamine users (Iudicello et al., 2011). The disruption in PM performance on lab-based tasks appears to be related to failures by the older adults on the semi-naturalistic task. This is consistent with the demonstrated association between PM impairment and self-reported as well as performance-based functional impairment in healthy older adults (Woods et al., 2012; Pirogovsky et al., in press).
This study is not without its limitations. The youth (Y) group was recruited in San Diego, CA whereas the remaining two groups were composed of Australians. Concerns about cross-cultural differences impacting our findings are mitigated by important cohort characteristics: (1) all participants were native English speakers, and (2) these two countries have comparable educational systems and resources. Another limitation is that our naturalistic measure of PM consisted of a single trial with a large window of time for a correct response (±15% of the 24 hour target). Multiple naturalistic trials would have been ideal, as they might have increased the task difficulty and might also have generated greater variability in performance. The limited demands of the naturalistic task and possible floor effects may also increase our risk of Type II error. To better detect meaningful relationships between cognitive and PM variables in an older cohort, conservative statistical methods were not utilized. To minimize Type I error, the number of correlation analyses were limited to the primary PM scales, summary neuropsychological domains were mostly utilized, and a select number of non-PM domains were included to cover the relevant constructs to demonstrate convergent and divergent validity. Nevertheless, based on our findings, future studies may conduct a more comprehensive examination of PM in elderly subjects. In our analyses, we used education as a covariate to minimize the loss of statistical power. Given that the three age groups differed on this variable, the suitability of this method is impacted (see Miller and Chapman, 2001). However, in a smaller subset that was matched on education, we noted that the pattern of findings was unchanged. Finally, although our primary interest was to examine the cognitive correlates of PM performance in participants over the age of 70 years, the study may have benefitted from parallel neurocognitive data for the youngest participants.
To summarize, the present study supports the construct validity of the MIST in individuals over the age of 70 years. We also found that older adults with impaired PM performance on lab-based tasks were more vulnerable to semi-naturalistic task failures. In our cohort of older adults, we noted that verbal fluency, executive dysfunction, episodic memory, and general cognitive function were associated with PM deficits. These findings add to the nascent body of psychometric literature on the MIST. Continued use of this instrument in future investigations will serve to bolster its construct validity. For example, it would be useful to examine the relationship between MIST scores and biomarkers (e.g., telomere length and tau) that have been shown to be associated with age-related changes in memory. Furthermore, imaging studies examining the neural correlates of performance on the MIST in older adults would serve to better describe the neuroanatomical substrates of PM in this cohort. Also, given the relevance of PM in the successful execution of everyday functioning tasks, the association of MIST scores to a wider range of activities of daily living (e.g., automobile driving, shopping, medication adherence) in older cohorts warrants examination. Future studies may also benefit from examining longitudinal changes in PM in relation to healthy aging, as well as the predictive value of MIST performance for the development of dementia in older adults. Based on findings in healthy older adults (Woods et al., 2012), patients with Parkinson’s disease (Pirogovsky et al., 2012) and individuals with mild cognitive impairment (Karantzoulis, Troyer, & Rich, 2009) it has been posited that the formal assessment of PM is clinically relevant. The present findings bolster this argument and support the potential clinical utility of using the MIST to aid in the evaluation of older individuals’ cognitive status and ability to execute instrumental activities of daily living.
Acknowledgements
Data were collected as part of the Doctor of Psychology thesis project of Aimee Velnoweth. The current study was also supported in part by National Institute of Mental Health grants R01-MH073419 to Dr. Woods. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government. The authors thank the study volunteers for their participation, Rebecca Lachovitzki and Brenton Maxwell for their assistance with data collection and coding, and Dr. Sarah Raskin for providing us with the MIST.
References
- Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. Iowa City: AJA Associates; 1994. [Google Scholar]
- Benson AD, Slavin MJ, Tran TT, Petrella JR, Doraiswamy PM. Screening for Early Alzheimer's Disease: Is There Still a Role for the Mini-Mental State Examination? Primary care companion to the Journal of clinical psychiatry. 2005;7(2):62. doi: 10.4088/pcc.v07n0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Kelley WM, Buckner RL, Cohen NJ, Miezin FM, Petersen SE. Direct comparison of prefrontal cortex regions engaged by working and long-term memory tasks. Neuroimage. 2001;14:48–59. doi: 10.1006/nimg.2001.0791. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Gonen-Yaacovi G, Volle E. Functional neuroimaging studies of prospective memory: what have we learnt so far? Neuropsychologia. 2011;49(8):2246–2257. doi: 10.1016/j.neuropsychologia.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Scott SK, Frith CD. The role of the rostral frontal cortex (area 10) in prospective memory: a lateral versus medial dissociation. Neuropsychologia. 2003;41:906–918. doi: 10.1016/s0028-3932(02)00327-5. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Quayle A, Frith CD. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia. 2001;39(6):545–555. doi: 10.1016/s0028-3932(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Heaton RK, Grant I The HNRC Group. Prospective memory in HIV-1 infection. Journal of Clinical and Experimental Neuropsychology. 2006;28:536–548. doi: 10.1080/13803390590949494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle K, Weber E, Atkinson JH, Grant I, Woods SP the HNRP Group. Aging, Prospective Memory, and Health-Related Quality of Life in HIV Infection. AIDS and Behavior. 2012:1–10. doi: 10.1007/s10461-011-0121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA, Richardson SL, Guynn MJ, Cunfer AR. Aging and prospective memory: examining the influences of self-initiated retrieval processes. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21:996–1007. doi: 10.1037//0278-7393.21.4.996. [DOI] [PubMed] [Google Scholar]
- Fleming JM, Shum D, Strong J, Lightbody S. Prospective memory rehabilitation for adults with traumatic brain injury: a compensatory training programme. Brain Injury. 2005;19(1):1–10. doi: 10.1080/02699050410001720059. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Golomb J, Kluger A, de Leon MJ, Ferris SH, Convit A, Mittelman MS, et al. Hippocampal formation size in normal human aging: a correlate of delayed secondary memory performance. Learning & Memory. 1994;1(1):45–54. [PubMed] [Google Scholar]
- Gupta S, Woods SP, Weber E, Dawson MS, Grant I The HNRC Group. Is prospective memory a dissociable cognitive function in HIV infection? Journal of Clinical and Experimental Neuropsychology. 2010;32(8):898–908. doi: 10.1080/13803391003596470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JD, MacLeod MS, Phillips LH, Crawford JR. A meta-analytic review of prospective memory and aging. Psychology and Aging. 2004;19(1):27–39. doi: 10.1037/0882-7974.19.1.27. [DOI] [PubMed] [Google Scholar]
- Iudicello JE, Weber E, Grant I, Weinborn M, Woods SP the HNRP group. Misremembering future intentions in methamphetamine-dependent individuals. The Clinical Neuropsychologist. 2011;25(2):269–286. doi: 10.1080/13854046.2010.546812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K, Braver TS. Age-related shifts in brain activity dynamics during task switching. Cerebral Cortex. 2010;20(6):1420–1431. doi: 10.1093/cercor/bhp206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karantzoulis S, Troyer AK, Rich JB. Prospective memory in amnestic mild cognitive impairment. Journal of the International Neuropsychological Society. 2009;15(3):407–415. doi: 10.1017/S1355617709090596. [DOI] [PubMed] [Google Scholar]
- Klauser DH. Learning and Memory in Normal Aging. New York, NY: Academic Press; 1994. [Google Scholar]
- Kliegel M, Mackinlay R, Jäger T. Complex prospective memory: development across the lifespan and the role of task interruption. Developmental Psychology. 2008;44(2):612–617. doi: 10.1037/0012-1649.44.2.612. [DOI] [PubMed] [Google Scholar]
- Kliegel M, Martin M, McDaniel MA, Einstein GO. Complex prospective memory and executive control of working memory: A process model. Psychologische Beitrage. 2002;44(2):303–318. [Google Scholar]
- Manns JR, Hopkins RO, Squire LR. Semantic memory and the human hippocampus. Neuron. 2003;38(1):127–133. doi: 10.1016/s0896-6273(03)00146-6. [DOI] [PubMed] [Google Scholar]
- Martin T, McDaniel MA, Guyunn MJ, Houck JM, Woodruff CC, Bish JP. Brain regions and their dynamics in prospective memory retrieval: a multiprocess framework. Applied Cognitive Psychology. 2007;14:127–144. [Google Scholar]
- Maylor EA. Age and prospective memory. The Quarterly Journal of Experimental Psychology. 1990;42(3):471–493. doi: 10.1080/14640749008401233. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO. The neuropsychology of prospective memory in normal aging: a componential approach. Neuropsychologia. 2011;49:2147–2155. doi: 10.1016/j.neuropsychologia.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO. Prospective memory: an overview and synthesis of an emerging field. Los Angeles, CA: Sage Publications; 2007. [Google Scholar]
- McDaniel MA, Einstein GO. Strategic and automatic processes in prospective memory retrieval: a multiprocess framework. Applied Cognitive Psychology. 2000;14:S127–S144. [Google Scholar]
- McFarland CP, Glisky EL. Frontal lobe involvement in a task of time-based prospective memory. Neuropsychologia. 2009;47:1660–1669. doi: 10.1016/j.neuropsychologia.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of abnormal psychology. 2001;110(1):40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Miller MB, Chapman JP, Chapman LJ, Collins J. Task difficulty and cognitive deficits in schizophrenia. Journal of abnormal psychology. 1995;104(2):251. doi: 10.1037//0021-843x.104.2.251. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Cognitive resources and dual-task interference effects at retrieval in normal people: The role of the frontal lobes and medial temporal cortex. Neuropsychology. 1994;8(4):524. [Google Scholar]
- Old SR, Naveh-Benjamin M. Differential Effects of Age on Item and Associative Measures of Memory: A Meta-Analysis. Psychology and Aging. 2008;23(1):104–118. doi: 10.1037/0882-7974.23.1.104. [DOI] [PubMed] [Google Scholar]
- Park DC, Hertzog C, Kidder DP, Morrell RW, Mayhorn CB. Effect of age on event-based and time-based prospective memory. Psychology and Aging. 1997;12:314–327. doi: 10.1037//0882-7974.12.2.314. [DOI] [PubMed] [Google Scholar]
- Patton GW, Meit M. Effect of aging on prospective and incidental memory. Experimental Aging Research. 1993;19(2):165–176. doi: 10.1080/03610739308253929. [DOI] [PubMed] [Google Scholar]
- Persson J, Nyberg L, Lind J, Larsson A, Nilsson LG, Ingvar M, et al. Structure-function correlates of cognitive decline in aging. Cerebral Cortex. 2006;16(7):907–915. doi: 10.1093/cercor/bhj036. [DOI] [PubMed] [Google Scholar]
- Piatt AL, Fields JA, Paolo AM, Tröster AI. Action (verb naming) fluency as an executive function measure: convergent and divergent evidence of validity. Neuropsychologia. 1999;37(13):1499–1503. doi: 10.1016/s0028-3932(99)00066-4. [DOI] [PubMed] [Google Scholar]
- Pirogovsky E, Woods SP, Filoteo JV, Gilbert PE. Prospective memory deficits are associated with poorer everyday functioning in Parkinson’s disease. Journal of the International Neuropsychological Society. i2012;18(6):986–995. doi: 10.1017/S1355617712000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C, Selnes OA, Grim JA, McArthur JC. HIV Dementia Scale: a rapid screening test. Journal of acquired immune deficiency syndromes and human retrovirology. 1995;8(3):273. doi: 10.1097/00042560-199503010-00008. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation. WAIS-III and WMS-III technical manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Rabin LA, Barr WB, Burton LA. Assessment practices of clinical neuropsychologists in the United States and Canada: a survey of INS, NAN, and APA Division 40 members. Archives of Clinical Neuropsychology. 2005;20(1):33–65. doi: 10.1016/j.acn.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The repeatable battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. Journal of Clinical and Experimental Neuropsychology. 1998;20(3):310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Raskin S. Memory for intentions screening test: psychometric properties and clinical evidence. Brain Impairment. 2009;10(1):23–33. [Google Scholar]
- Raskin S, Buckheit C, Sherrod C. MIST Memory for Intentions Test professional manual. Lutz, FL: Psychological Assessment Resources; 2010. [Google Scholar]
- Raskin SA, Woods SP, Poquette AJ, McTaggart AB, Sethna J, Williams RC, et al. A differential deficit in time- versus event-based prospective memory in Parkinson’s disease. Neuropsychology. 2011;25(2):201–209. doi: 10.1037/a0020999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- Rendell PG, Craik FIM. Virtual week and actual week: Age-related differences in prospective memory. Applied Cognitive Psychology. 2000;14:S43–S62. [Google Scholar]
- Rendell PG, Thomson DM. Aging and prospective memory: Differences between naturalistic and laboratory tasks. Journal of Gerontology: Psychological Sciences. 1999;54B:P256–P269. doi: 10.1093/geronb/54b.4.p256. [DOI] [PubMed] [Google Scholar]
- Reynolds JR, West R, Braver T. Distinct neural circuits support transient and sustained processes in prospective memory and working memory. Cerebral Cortex. 2009;19(5):1208–1221. doi: 10.1093/cercor/bhn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royall DR, Cordes JA, Polk M. CLOX: an executive clock drawing task. Journal of Neurology, Neurosurgery & Psychiatry. 1998;64(5):588–594. doi: 10.1136/jnnp.64.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Woo E, Greeley D. Characterizing multiple memory deficits and their relation to everyday functioning in individuals with mild cognitive impairment. Neuropsychology. 2009;23:168–177. doi: 10.1037/a0014186. [DOI] [PubMed] [Google Scholar]
- Scullin MK, Bugg JM, McDaniel MA, Einstein GO. Prospective memory and aging: preserved spontaneous retrieval, but impaitred deactivation, in older adults. Memory & Cognition. 2011;39(7):1232–1240. doi: 10.3758/s13421-011-0106-z. [DOI] [PubMed] [Google Scholar]
- Simons JS, Schölvinck ML, Gilbert SJ, Frith CD, Burgess PW. Differential components of prospective memory? Evidence from fMRI. Neuropsychologia. 2006;44(8):1388–1397. doi: 10.1016/j.neuropsychologia.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Singer T, Verhaeghen P, Ghisletta P, Lindenberger U, Baltes PB. The fate of cognition in very old age: six-year longitudinal findings in the Berlin Aging Study (BASE) Psychology and Aging. 2003;18(2):318–331. doi: 10.1037/0882-7974.18.2.318. [DOI] [PubMed] [Google Scholar]
- Smits CH, Deeg DJ, Jonker C. Cognitive and emotional predictors of disablement in older adults. Journal of Aging and Health. 1997;9(2):204–221. doi: 10.1177/089826439700900204. [DOI] [PubMed] [Google Scholar]
- Tay SY, Ang BT, Lau XY, Meyyappan A, Collinson SL. Chronic impairment of prospective memory after mild traumatic brain injury. Journal of Neurotrauma. 2010;27:77–83. doi: 10.1089/neu.2009.1074. [DOI] [PubMed] [Google Scholar]
- Troyer AK, Moscovitch M, Winocur G. Clustering and switching as two components of verbal fluency: evidence from younger and older healthy adults. Neuropsychology. 1997;11:138–146. doi: 10.1037//0894-4105.11.1.138. [DOI] [PubMed] [Google Scholar]
- Troyer AK, Moscovitch M, Winocur G, Alexander MP, Stuss D. Clustering and switching on verbal fluency: The effects of focal frontal-and temporal-lobe lesions. Neuropsychologia. 1998;36(6):499–504. doi: 10.1016/s0028-3932(97)00152-8. [DOI] [PubMed] [Google Scholar]
- Twamley E, Woods S, Zurhellen C, Vertinski M, Narvaez J, Mausbach B, et al. Neuropsychological substrates ad everyday functioning implications of prospective memory impairment in schizophrenia. Schizophrenia Research. 2008;106(1):42–49. doi: 10.1016/j.schres.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E, Woods SP, Delano-Wood L, Bondi MW, Grant I HIV Neurobehavioral Research Program Group. An examiniation fo the age-prospective memeory paradox in HIV-infected adults. Journal of Clinical Experimental Neuropsychology. 2011;33(10):1108–1118. doi: 10.1080/13803395.2011.604027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinborn M, Woods SP, O’Toole S, Kellogg EJ, Moyle J. Prospective memory in substance abusers at treatment entry: associations with education, neuropsychological functioning, and everyday memory lapses. Archives of Clinical Neuropsychology. 2011;26(8):746–755. doi: 10.1093/arclin/acr071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BA, Cockburn J, Baddeley A. The Rivermead Behavioral Memory Test manual. Bury St. Edmonds, UK: Thames Valley Test Company; 1985. [Google Scholar]
- Wilson B, Emslie HC, Foley JA. A new test of prospective memory: The CAMPROMPT. Journal of the International Neuropsychological Society. 2004;10:44. [Google Scholar]
- Woods SP, Dawson MS, Weber E, Gibson S, Grant I, Atkinson JH The HIV Neurobehavioral Research Program Group. Timing is everything: antiretroviral nonadherence is associated with impairment in time-based prospective memory. Journal of the International Neuropsychological Society. 2009;15:42–52. doi: 10.1017/S1355617708090012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Dawson MS, Weber E, Grant I the HIV Neurobehavioral Research Center (HNRC) Group. The semantic relatedness of cue-intention pairings influences event-based prospective memory failures in older adults with HIV infection. Journal of Clinical Experimental Neuropsychology. 2010;32(4):398–407. doi: 10.1080/13803390903130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S, Iudicello J, Moran L, Carey C, Dawson M, Grant I, et al. HIV-associated prospective memory impairment increases risk of dependence in everyday functioning. Neuropsychology. 2008;22:110–117. doi: 10.1037/0894-4105.22.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S, Moran L, Carey C, Dawson M, Iudicello J, Gibson, et al. Prospective memory in HIV infection: Is ‘remembering to remember” a unique predictor of self-reported medication management? Archives of Clinical Neuropsychology. 2008;23:257–270. doi: 10.1016/j.acn.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moran LM, Dawson MS, Carey CL, Grant I The HNRC Group. Psychometric characteristics of the memory for intentions screening test. The Clinical Neuropsychologist. 2008;22:864–878. doi: 10.1080/13854040701595999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S, Twamley E, Dawson M, Narvaez J, Jeste D. Deficits in cue detection and intention retrieval underlie prospective memory impairment in schizophrenia. Schizophrenia Research. 2007;90:344–350. doi: 10.1016/j.schres.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Weber E, Weisz BM, Twamley EW, Grant I The HNRP Group. Prospective memory deficits are associated with unemployment in persons living with HIV infection. Rehabilitation Psychology. 2011;56(1):77–84. doi: 10.1037/a0022753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Weinborn M, Velnoweth A, Rooney A, Bucks RS. Memory for intentions is uniquely associated with instrumental activities of daily living in healthy older adults. Journal of the International Neuropsychological Society. 2012;18:143–138. doi: 10.1017/S1355617711001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zogg JB, Woods SP, Weber E, Iudicello JE, Dawson MS, Grant I, et al. HIV-associated prospective memory impairment in the laboratory predicts failures on a semi-naturalistic measure of health care compliance. Clinical Neuropsychology. 2010;24(6):945–962. doi: 10.1080/13854046.2010.501343. [DOI] [PMC free article] [PubMed] [Google Scholar]