Abstract
The brain responds to injury and infection by activating innate defense and tissue repair mechanisms. Working upon the hypothesis that the brain defense response involves common genes and pathways across diverse pathologies, we analysed global gene expression in brain from mouse models representing three major central nervous system disorders, cerebral stroke, multiple sclerosis and Alzheimer’s disease compared to normal brain using DNA microarray expression profiling. A comparison of dysregulated genes across disease models revealed common genes and pathways including key components of estrogen and TGF-β signaling pathways that have been associated with neuroprotection as well as a neurodegeneration mediator, TRPM7. Further, for each disease model, we discovered collections of differentially expressed genes that provide novel insight into the individual pathology and its associated mechanisms. Our data provide a resource for exploring the complex molecular mechanisms that underlie brain neurodegeneration and a new approach for identifying generic and disease-specific targets for therapy.
Keywords: cDNA microarrays, Brain inflammation, Cerebral stroke, Alzheimer’s disease, Systems biology, Gene expression profiling
Introduction
Common pathologies of the brain fall into three general categories, neurodegenerative diseases, acute injury and chronic immune-mediated diseases. The initiating triggers for these pathologies can be highly diverse and the disease processes typically complex. Nevertheless, the death of neuronal and glial cells and the activation of innate defense mechanisms whose main functions are to counteract infection and limit tissue damage are common features. In this study we used whole-genome expression profiling to analyse and compare the molecular changes in the brain during three diverse mouse models of human neurodegenerative conditions, namely Alzheimer’s disease (AD), cerebral stroke (CS) and multiple sclerosis (MS). The hypothesis underlying this study is that there are pathways associated with brain defense and tissue repair that are activated in all three disorders independently of the pathological process. Further, we believe that by subtracting these common responses, we can better identify the disease model-specific genes and obtain a novel insight into pathogenic mechanisms operating in the brain. These different gene categories may provide a matrix for selecting and evaluating therapeutic targets that are aimed at limiting neuronal damage and maintaining structural and functional integrity of the brain.
Neurodegenerative diseases are often associated with abnormalities in specific proteins, particularly those that tend to oligomerize and aggregate. AD is characterized by the loss of synapses and neurons leading to cognitive impairment and memory loss. It is estimated that there are currently 26 million people worldwide with AD and this figure is projected to increase around 4-fold by 2050. Pathognomic features are the intracellular and extracellular accumulation of aggregated beta-amyloid (Aβ) in cortex and hippocampus and intracellular neurofibrillary tangles containing hyperphosphorylated tau proteins together with activation of local inflammatory responses. Mutations or duplications in three genes that are important for regulating Aβ production, amyloid precursor protein (APP), presenelin (PSEN)1 and PSEN2, lead to early-onset familial AD while polymorphisms in the gene encoding apolipoprotein E (APOE) are a genetic risk factor for late-onset AD [1]. Under physiological conditions APP promotes trans-cellular adhesion [2] and neurite outgrowth [3]. However, the dysregulated production of soluble Aβ polymers is strongly implicated in AD aetiopathogenesis. In addition to aggregating into fibrillary amyloid deposits, Aβ can disrupt glutamatergic synaptic structure and function [4]. In this study we used APP23 transgenic mice that over-express human APP751 cDNA with the Swedish mutation specifically in neurons, 7-fold higher than endogenous murine APP, and represent a characterized mouse model for AD [5]. Mice develop progressive Aβ deposits in the neocortex and hippocampus associated with reactive astrocytes and microglia and neurodegenerative changes that include neurite distortion and hippocampal neuron loss. They also show cognitive and behavioural alterations [6] and altered brain vasculature and blood flow [7] that are typical of AD.
Stroke is the third leading cause of death and the main cause of adult disability in the USA and Europe. Approximately 600,000 strokes occur annually in the USA with around 25% fatality. Interruption of the brain blood supply results in the depletion of oxygen and glucose that initiates a cascade of events leading to ischemic injury which ultimately causes damage and death of neuronal, glial and vascular cells and leads to impaired brain function [8]. Detailed knowledge of the pathophysiological events induced by brain ischemia has come mainly from the study of animal stroke models. Excitotoxicity, peri-infarct depolarization, inflammation, necrosis, autophagy and apoptosis seem to be the major processes that lead to neuronal damage and their mediators are promising targets for the design of neuroprotective strategies. Recently, tissue injury compatible with hypoxia has also been described in other neurodegenerative diseases including acute and chronic MS [9] and AD [10]. Consequently, we felt that a model of stroke provided an important comparator for the neurodegenerative disease models. As a CS model we chose permanent occlusion of the middle cerebral artery (pMCAO) which induces ischemic injury, without reperfusion injury, mainly in a region of ipsilateral cortex [11] consistent with that in naturally occurring stroke.
MS is a chronic inflammatory disease with autoimmune features that is manifested in the central nervous system (CNS) and is characterized by demyelination and early axonal damage [12]. MS affects mostly young individuals, with an age of onset typically between 25 and 40 years of age. It is more prevalent in women than in men and has an overall incidence frequency of 80–100/100,000 worldwide. The Tg6074 TNF transgenic mouse line models neuropathological [13] and some biochemical [14] features of MS in the brain. Tg6074 mice over-express a murine TNF-α-globin transgene in premyelinating oligodendrocytes and develop a chronic progressive inflammatory demyelinating disease starting with glial cell activation and progressing to demyelination, axonal damage and the development of confluent plaques typical of chronic MS. As in MS, extracellular fibrin deposition in the brain is also a neuropathological manifestation in this model and its pharmacological depletion delays the onset of disease [15].
While no mouse model perfectly mirrors all aspects of a human disease, we believe that these models of three medically important human disorders position us uniquely to understand the response of the mammalian brain to serious insult. By looking globally for genes with altered expression profiles, we can begin to define molecular signatures of disease and use these to further understand mechanisms of neurological injury and disease.
Materials and methods
Mouse models
Transgenic mouse models for AD and chronic progressive MS were used, the APP23 line expressing APP751 cDNA with the Swedish mutation in neurons under the control of the Thy-1 promoter [5] and the Tg6074 line expressing murine TNF under its own promoter [13], respectively. Both male and female transgenic mice were used at the ages indicated below. The stroke model used was permanent focal occlusion of the left middle cerebral artery (pMCAO) in 3–4 month-old male mice as previously described [11]. Animals were sacrificed at various time points post-occlusion. Age and sex-matched wild-type control littermate (WT) animals were sacrificed at matching time points for each disease. The detailed number, sex and age of sacrifice of all the animals used in this study are shown in Supplementary Table 1. Mice were maintained in the animal facility of Hellenic Pasteur Institute under specific pathogen free conditions, in standard cages, with free access to food and water and maintained on a 12 hour light–12 hour dark cycle. All the experimental procedures conformed to the national and European guidelines for animal experimentation.
APP23 mice show deposits of amyloid β peptide (Aβ) in neocortex and hippocampus from around 6 months of age which increase in frequency with age [5]. Confluent plaque formation and the accumulation of phosphorylated tau protein are detected by 15 months of age. The plaques are surrounded by dystrophic neurites and neurite loss is detected in plaque vicinity. For our study, brain samples were grouped into early (up to 6 months, TgAPP23EARLY) and late (10–18 months, TgAPP23LATE) time points (Supplementary Table 2).
For pMCAO, the time points post-surgery chosen for analysis were divided into the acute early phase (<24 h, pMCAOEARLY) where the local response to ischemia can be studied in the absence of major inflammatory events, and the progressive chronic phase (24 h–14 days, pMCAOLATE), where lesion expansion occurs due to delayed neuronal death and repair mechanisms operate to limit neuronal damage [8] (Supplementary Table 2).
Brain samples from Tg6074 TNF transgenic mice were also grouped into early and late stages of disease. Tg6074 mice develop a chronic progressive inflammatory disease in the brain and neuropathological changes from 3 weeks of age. Early changes include oligodendrocyte apoptosis and the activation of microglia and astrocytes (up to 3 weeks, Tg6074EARLY) and late changes include immune cell infiltration accompanied by blood–brain barrier breakdown, myelin vacuolation, primary demyelination and secondary axonal damage (3–9 weeks, Tg6074LATE) (Supplementary Table 2).
RNA isolation
Total RNA was isolated from half brain dissected along the mid sagittal plane in all mice and representing the ispilateral cortex in pMCAO mice. Tissues were snap frozen in liquid nitrogen and then homogenized in Trizol reagent (Invitrogen) using an Ultra Turrax T25 (Janke and Kunkel) tissue homogenizer and briefly sonicated using an Ultrasonic homogenizer sonicator (Cole and Parmer). RNA was extracted according to the manufacturer’s instructions. Total RNA purity and concentration was determined prior to labeling and hybridization using the microfluidics-based platform 2100 Bioanalyzer (Agilent Technologies) and the RNA 6000 Nano LabChip kit.
Microarray study design
We chose a common reference design [16,17] in which gene expression in each sample is compared to that of a common RNA control. This design has the advantage of flexibility and allows multiple comparisons for each individual sample. As a control RNA, we chose the mouse “Universal Reference RNA” (Stratagene). For samples, we chose half brain from all mice, not specific lesioned areas. This allowed us to obtain global transcriptome signatures for each pathology and to directly compare changes in gene expression across diseases. The purpose of our study design was to allow the major brain gene changes associated with each disease to be detected and compared across the different diseases, rather than maximising the sensitivity of gene discovery for each individual disease.
cDNA Microarray fabrication, RNA labeling and hybridization
Microarrays were constructed using the National Institute of Aging (NIA) 15 K and Trans-NIH Brain Molecular Anatomy Project (BMAP) mouse cDNA clone sets, together containing a total of approximately 27,000 clones representing 19,263 unique transcripts. Polymerase chain reaction (PCR) amplicons were prepared for printing as described previously [18]. Following amplification and purification, amplicons were resuspended at 100–200 nM in 50% DMSO and printed onto UltraGAPs aminosilane coated slides (Corning, Corning, NY) using an Intelligent Automation System (IAS) arrayer (Cambridge, MA). After printing, DNA was cross-linked to the slides by UV irradiation at 120 mJ using a Stratalinker (Stratagene) and stored in a desiccated chamber until use.
Detailed cDNA target preparation and hybridization protocols are available at http://compbio.dfci.harvard.edu/protocols.html. Briefly, cDNA was synthesized at 42 °C using 10 μg of total RNA, 6 μg random hexanucleotide primers, and 200 units of SuperScript II (Invitrogen) reverse transcriptase in 1× first strand synthesis buffer containing 10 mM DTT, 25 mM dATP, 25mMdCTP, 25 mM dGTP, 15 mM dTTP, and 10 mM amino allyl-dUTP (Sigma). Following cDNA synthesis RNA was hydrolyzed for 15 min at 65 °C with 200 mM NaOH and 100 mM EDTA. Unincorporated amino allyl-dUTP was removed through chromatography on QIAquick columns (Qiagen). Columns were washed with 5 mM potassium phosphate buffer (pH 8.5). Following lyophilization, the cDNA was resuspended in 4.5 μl sodium carbonate (Na2CO3) buffer (pH 9.0), mixed with Cy3 or Cy5 NHS-ester (Amersham Biosciences) and incubated at ambient temperature for 1 h. cDNA with incorporated Cy3 or Cy5 was purified using QIAquick columns, combined and lyophilized.
Slides were prehybridized in 1% bovine serum albumin (BSA) in 5× SSC, 0.1% SDS for 40 min at 42 °C, after which slides were washed and dried. Cy3 and Cy5 labeled cDNA was resuspended in 30 μl of 50% formamide, 5× SSC, 0.1% SDS containing 0.5 μg mouse Cot-1 DNA (Gibco BRL), and 1 μg poly-dA and hybridized to the microarray at 42 °C for 16 h under glass coverslips. Following hybridization, slides were washed for 4 min at 42 °C in solution containing 2× SSC, 0.2% SDS followed by a 4 minute wash in 0.2× SSC, 0.1% SDS and a 2.5 minute wash in 0.05× SSC at ambient temperature. All samples were hybridized with dye-reversal replicates and duplicate hybridizations of randomly selected samples were performed.
Slides were dried by centrifugation and immediately scanned at 10 μm resolution using an Axon GenePix 4000B confocal laser scanner (Axon Instruments). Data were saved as 16-bit TIFF files and expression levels were extracted using Spotfinder [19]. Microarray data from individual slides was considered for further analysis when the percentage of “good” spots as identified by the SpotFinder QC parameters was greater than 75%.
Data normalization and analysis
The resulting expression data for each model were normalized using a locally weighted scatterplot smoothing regression (LOWESS) algorithm with the smoothing parameter set to 0.33, followed by standard deviation regularization across array-grids and flip-dye consistency by checking to keep data with the range of ±2.0 SD as implemented MIDAS (http://www.tm4.org) [19]. Data analysis and mining were performed using MeV (version 3.1; http://www.tm4.org) [19]. For each disease model, we compared the experimental animals to WT at equivalent time points, using a two-sample t-test with p values calculated based on the t-distribution. To perform the t-tests we pooled the expression profiles of the disease time points and compared them to the pooled expression profiles of wild-type controls at matching time points, to control for age differences in gene expression. We chose a generous cutoff of p<0.05 without correcting for multiple testing as our goal was to validate the resulting gene sets experimentally. Using these criteria, we identified 464 significant genes in our AD model, 3154 genes in stroke, and 1101 for our MS model. Samples were also divided into early and late subgroups (according to the criteria mentioned above), in order to determine the relevance of average gene expression changes to different stages of disease (Supplementary Table 3). Gene lists were loaded into an MS Access relational database and those in common between models were identified through simple database queries. Gene networks were generated with Ingenuity Pathways Analysis (Ingenuity® Systems, http://www.ingenuity.com) used to identify the canonical pathways that were most significant to the dataset. Functional gene tables were created for each disease (provided as supplementary material). Functional annotation of the genes was performed by standard Pubmed querying and by using the Bioinformatics Harvester III (http://harvester.fzk.de/harvester/). The relative expression (RE) of each gene in disease compared to the wild-type situation was based on the average expression value for all the time points. Data was compliant with the MGED society standards and criteria and is deposited to the ArrayExpress public database [20] (http://www.ebi.ac.uk/microarray-as/ae/) with experiment ID E-MTAB-1.
qRT-PCR validation
RT-PCR was used to validate the results obtained from the microarray analysis using representative samples selected from those used for the microarray hybridization (method validation) as well as to control for sampling error by using independent samples (result validation). Several genes were selected from each disease (Supplementary Table 4). Primer selection was performed using the program Primer 3, version web 0.3.0. [21], URL: http://primer3.sourceforge.net/) or ready primer sets used for the QuantiTect assay were purchased from Qiagen. A detailed list of primers is given in Supplementary Table 5. Total RNA was converted to cDNA via first strand cDNA synthesis. Briefly, 3–5 μg of total RNA was treated with RQ1 DNase enzyme (Promega) and was then reverse transcribed using MMLV reverse transcriptase (Promega). PCR conditions were checked using conventional PCR in an MJ Research PTC-200 Peltier Thermal Cycler. The cycling conditions were as follows: 95 °C for 5 min, 95 °C for 1 min, 59 °C for 1 min, and 72 °C for 2 min. qRT-PCR was performed using the Roche LightCycler (Roche), with the following conditions: 95 °C for 15 s, 59 °C for 10 s, 72 °C for 12 s and typically 45 cycles of amplification. At the end of each PCR run, melting curve analysis was also performed to verify the integrity and homogeneity of PCR products. The reactions were monitored using SYBR green I (Roche) and results were analysed using the Light Cycler software version 3.5 (Roche, Mannheim, Germany). For method validation (Supplementary Fig. 1 A–C) the same test and control samples used for microarray analysis were used for APP23 and Tg6074, and for the pMCAO model controls from sham-operated mice sacrificed at the same postoperative time points. Relative expression was calculated as follows: ΔCt target gene (actin–sample)/ ΔCt housekeeping gene (actin–sample). For experiment validation (Supplementary Fig. 1 D, E) test samples from independent Tg6074 transgenic mice and from an independent pMCAO experiment were used. Controls were non-transgenic littermates (WT) of equivalent ages for the transgenic tissues and sham-operated mice sacrificed at the same post-operative time points for the pMCAO model. Reactions were monitored using the QuantiFast™ SYBR® green RT-PCR kit (Qiagen Inc.) according to the manufacturer’s instructions. QuantiTect Primer Assays (Qiagen Inc) were used for selected genes (see Supplementary Table 5). Gene expression levels were calculated using already created standard curves for each gene. These standard curves were created by plotting threshold cycle (CT) values versus the logarithm of serial diluted RNA concentrations. Least squares method was used for the determination of A and B values in the equation . The coefficient of determination (R2) was greater than 0.99. Values were normalized using the respective β-glucuronidase (Gusb) values. For quantitative RT-PCR analyses differential expression between control and disease samples at each time point was evaluated using Mann Whitney Rank Sum Test. p values less than 0.05 were considered statistically significant.
Results
Comparison of gene expression profiles between disease models and WT mice
APP23 transgenic model for AD
Differential gene expression in APP23 mouse brain compared to WT was assessed using Student’s t-test which identified 464 cDNA elements (Student’s t-test, p<0.05) with significantly altered levels of expression (Supplementary Table 6), including several that are orthologs of human genes already implicated in the aetiopathogenesis of AD and related pathologies (Table 1). Altered expression of the genes encoding amyloid β precursor protein (APP), the related amyloid precursor-like protein 2 (APLP2) and APP-binding family A member 1/X11/Mint 1 (Apba1), an APP adaptor that binds proteins essential for synaptic vesicle exocytosis, was observed. The expression of APP and Apba1 was altered from the early stages of disease (Supplementary Table 6, APP23EARLY), prior to the development of obvious histopathological alterations.
Table 1.
Genes regulated in APP23 brain that are orthologs of human genes associated with the pathogenesis of AD and related pathologies.
| GB# | Gene name | Protein name and function | AD association |
|---|---|---|---|
|
AI842005 AW550326 AW552701 |
App | Amyloid beta precursor protein. Cell adhesion at synapse with APLP1 and APLP2. Neurite outgrowth, cell proliferation. |
Neuronal intra- and extracellular accumulation. |
| AW557952 | Aplp2 | Cell adhesion at synapse with APP and APLP1. | Localized in AD plaques. |
|
AI839527 C76565 |
Apba1 | Amyloid beta precursor protein-binding family A member 1 (neuron-specific X11 protein, Mint-1). May modulate APP processing and formation of Aβ. | Participation in amyloid formation. |
| AW544505 | Soat1 | Acyl-coenzyme A. Cholesterol biosynthesis. | Gene polymorphism (SOAT1) associated with reduced risk for sporadic AD. |
| C78395 | Ap3b1 | AP-3 clathrin adaptor protein complex, beta 1 subunit. Protein cargo sorting at late Golgi and endosomes. | Reduced expression in AD. |
| AW558008 | Calu | Calumenin (Crocalbin). Calcium-binding protein of the secretory pathway. | Participation in amyloid formation. |
| C85456 | Cib1 | Calcium and integrin-binding protein 1 (Calmyrin). Calcium-binding protein that preferentially binds to and colocalizes with presenilin 2. | Reduced expression in AD. |
| AI843998 | Calna | Calcineurin A. Serine/threonine specific protein phosphatase. | May mediate disturbance in calcium homeostasis in AD. |
| C86381 | Hbp1 | High mobility group box transcription factor 1. Transcriptional regulation of cell cycle and Wnt pathway. Secreted inflammatory cytokine. Interacts with SREBPs. | Associated with senile plaques. |
| AW552451 | Crk | Proto-oncogene C-crk (P38). Regulation of cytoskeleton. Involved in axon growth and actin membrane ruffling. | Down-regulated in Down syndrome. |
| AW557606 | Grb2 | Growth factor receptor-bound protein 2. Adaptor protein involved in receptor signaling. | APP–GRB2 complexes are increased in AD. |
| AW552373 | Casp12 | Caspase 12. Cysteine protease involved in apoptosis. | Mediates ER stress-induced apoptosis in AD. |
| AU021661 | Sept3 | Neuronal-specific septin-3. GTPase enriched in presynaptic terminals with possible roles in neurite outgrowth, synaptic vesicle secretion and implicated in neurofibrillary tangle formation. | Polymorphism associated with AD. |
| AI848977 | Dbnl | Drebrin-like. F-actin binding protein which functions in maintenance and formation of dendritic spines. | Diminished in AD and aged brains. |
Genes encoding enzymes (Hmgcs1, Hmgcr, Fdft1, and Soat1) and transcription factors (Srebf1, CNBP and Hbp1) involved in cholesterol biosynthesis were all significantly up-regulated in APP23 brain in late disease stages (Supplementary Table 6, APP23LATE), indicating the existence of a causative relationship between APP and cholesterol production. Other major categories of regulated genes were those involved in vesicle trafficking (Ap2a2, Ap3b1, Smap2, Pacsin2, Tgoln1, RHOQ (Tc10), Arfgap1, Stam2, Snx2, Snx17, Tuba, Dynlrb1, and Dynlt3) including the endoplasmic reticulum (ER)-associated protein degradation (ERAD) pathway for the elimination of unassembled or misfolded proteins (SEC61G, Derl1, Gapvd1, Ecm29, and Casp12), protein modification including numerous E3 ubiquitin ligases involved in the ubiquitin–proteasome pathway for protein degradation, and cytoskeletal organization, mostly seen in the late disease stages (Table 1, APP23LATE).
A notable number of differentially regulated genes participate in mechanisms that are important for the regulation of neuronal structure and function. Further to cholesterol synthesis, vesicle trafficking and proteasome sequestration, that are all critical for axonal transport and synapse function, genes important for cell (Actg1, Cdc42ep1, Cdc42se2, cdc42bpb, Abi1, Wdr1, Fnbp4, and Crk) and synapse (Nefl, RHOQ (Tc10), Sept3, Usp14 (ax), and Dbn1) shape and plasticity were regulated, mainly at late time points (Supplementary Table 6, APP23LATE). Genes involved in neuroprotective and immunosuppressive pathways mediated by estrogen (Esr1, Grb2, Ncoa3, Phb2, Ctbp2, Gtf2e1, Polr2f, and Rbm9) and TGF-β (Grb2 and Smad4) signaling were also up-regulated.
pMCAO model for cerebral stroke
Comparison of gene expression profiles in brain samples after pMCAO with non-occluded controls identified 3154 cDNA elements with significantly altered levels of expression (Student’s t-test, p<0.05) (Supplementary Table 7). Major functional classes of genes that were regulated at early time points following occlusion were for metabolic processes such as energy production, lipid, amino acid, carbohydrate, fatty acid and heme biosynthesis, glycolysis, oxidase reduction and glutamate synthesis. Other main gene categories that were regulated from early time points (Supplementary Table 7), pMCAOEARLY) were for DNA transcription and translation, the transport of nucleic acids, ions, amino acids and proteins, the ubiquitin–proteasome pathway of protein degradation and for protein and vesicle trafficking, including genes involved in secretory, endocytosis and cell polarity pathways.
As expected, genes associated with cellular stress responses were regulated early following pMCAO notably those involved in the oxidative stress response (Map3k7, Prkcd, Prkci, Pik3c2a, Pik3cd, Map2k1, Map2k2, Mapk3, Mapk14, Nfe2l2, Crebbp, Atf4, Cdc34, Vcp, Dnaja1, Dnajb6, Dnajc11, Gstm4, Gstm6, Akr1a1, Scarb1, Sqstm1, Hmox1, Ftl1, and Sod3) (Supplementary Table 7), pMCAOEARLY: Prkcd and Scarb1), glutamate signaling (Grin2c, Gria3, Grm2, Grm3, Grid2, Gng2, and Slc38a1) and calcium mobilization (Rcn1, Cam, Camk1, Camkk1, Camk1d, Mylpf, Mrlc2, S100a16, Efhd2, Nucb1, Nucb2, Anxa6, and Clstn1) (Supplementary Table 7), pMCAOEARLY: Gria3, Gng2, Camk1d, Efhd2, Nucb2, and Clstn1), cell cycle regulation (Ccni, Ccnt2, Rif1, Cdkn1b, Ccnh, Ccng1, Rad1, Zwint, and Btg1) and DNA repair (Mcm6, Mlh1, Ddb2, Brca2, Fanca, Fancc, Ercc1, Trex1, Exo1, Cep170, Wrnip1, and Pms2) (Supplementary Table 7), pMCAOEARLY: Rif1, Cep170, and Wrnip1). Genes involved in programmed cell death (Pdcd2l, Pycard, Itm2b, Fis1, Ak2, Htra2, Slc25a4, and Casp9) and inflammasome assembly (IRAK 2, Pycard, Peli 1 and Nlrp9c), which is responsible for the activation of inflammatory processes in response to injury or infection, were also activated from early time points (Supplementary Table 7), pMCAOEARLY: Itm2b, Slc25a4, and Nlrp9c).
In counterbalance, tissue protective pathways were also activated following ischemia. Specifically, genes involved in the regulation of lipid homeostasis by the nuclear orphan receptor peroxisome proliferator-activated receptor alpha (PPAR-alpha) were up-regulated (Ins2, Sos1, Grb2, Map3k7, Chuk, Map2k1, Map2k2, Mapk3, AIP, Crebbp, Med1, Nr1h3, and Scand1). The peroxisome proliferator response operates to control the level of tissue damage after physical or chemical stress and therefore represents a protective response of the brain to ischemia [22]. A number of genes involved in the HIF signaling pathway were also regulated (Hif1a, P4hb, Birc6, Cdc34, Ube2f, Ube2g1, Ube2g2, Ube2i, Ube2j1, Ube2j2, Ube2l3, Ube2q1, Vhl, Creb1, Atf4, and Ldha), starting early after ischemia (Supplementary Table 7), pMCAOEARLY: Birc6, Ube2f, and Creb1). This pathway is important for maintaining oxygen homeostasis by sensing hypoxia and inducing genes involved in angiogenesis, erythropoiesis, glycolysis and cell survival. A large number of growth factors and receptors and components of their intracellular signaling pathways were regulated from early in disease (Esr1, Angpt2, Rbm39, Egf, Eps8l2, Megf8, Fgfr1, Fgfrl1, Ddx3x, Ghitm, Csf1r, Igfbp4, Ing1, Ing2, Ing4, Phip, Tgfb2, Tgfb3, Tgfbr2, Tgfbi, Tob1, Grb2, Sos1, Itpr1, Map2k1, Mapk3, and Stat3) (Supplementary Table 7), pMCAOEARLY: Fgfr1, Ghitm, Ing1, Grb2, and Tgfb3). As in AD, components of the estrogen receptor (Esr1, Src, Grb2, Sos1, Map2k1, Map2k2, Mapk3, Prkdc, Ctbp2, Crebbp, Carm1, Ddx5, Ccnh, Rbm9, Smarca4, Taf12, Med1, Med14, Nr3c1, Kat2b, Gtf2f1, Gtf2h1, and Gtf2h4) (Supplementary Table 7), pMCAOEARLY: Src, Grb2, Ctbp2, Med14, Nr3c1, and Kat2b) and TGF-β signaling pathways (Tgfb2, Tgfb3, Tgfbr2, Tgfbi, Nsa2, Grb2, Sos1, Smad2, Smad5, Smad6, Mapk3, Map2k1, Map2k2, Map3k7, and Crebbp) (Supplementary Table 7), pMCAOEARLY: Tgfb3 and Grb2) were activated after pMCAO.
Another category of genes regulated in the stroke model is those involved in tissue reorganization and repair of the adult CNS. These are all genes that are highly expressed in the developing nervous system. In the adult CNS they are expressed at low levels but become reactivated after injury and in several neurological diseases when the recapitulation of ontogenetic developmental programmes is thought to be important for tissue regeneration [23]. Specifically these encode semaphorin ligands and receptors (Sema3c, Sema4g, Sema6c, Nrp1, and Plxnb1) (Supplementary Table 7), pMCAOEARLY: Sema6c) and proteins implicated in axonogenesis, axon guidance, growth cone formation and orientation and neuronal tissue remodeling (Unc5a, Unc5b, Metrn, Phip, Ndrg4, and Tbc1d24) (Supplementary Table 7), pMCAOEARLY: Unc5b and Metrn).
Tg6074 model for multiple sclerosis
A total of 1101 cDNA elements were found to be significantly differentially expressed in Tg6074 compared to WT brain (Student’s t-test, p<0.05) (Supplementary Table 8). These included orthologs of many genes that have already been implicated in the pathogenesis of human MS and related pathologies, including complement components and major histocompatibility complex genes which are associated with autoimmune processes (Table 2). As expected, a large set of genes were those involved in TNF signaling pathways and inflammation, as a result of TNF transgene expression, including Adam17, Saa3, Cyba, components of the NF-κB signaling cascade (Nfkb1/p105 and Nfκbia) and apoptosis mediators (Pycard, Birc3, Faf1, and Wwox). These gene groups might correlate with cellular processes that are characteristic of this particular model, such as glial cell activation and oligodendrocyte apoptosis. Significant gene expression changes were also detected for genes that are induced by TNF but whose function in the CNS has not yet been clearly defined such as Twist1, Naf1/Tnip1, Tank, Khdc1a, Cav1, and Clec4e.
Table 2.
Genes regulated in Tg6074 brain that have been implicated in the development of human MS and related pathologies.
| GB# | Gene name | Protein name and function | MS association |
|---|---|---|---|
| AI845868 | H2-Ab1 | MHC class II H2-IA-beta chain (haplotype NOD) precursor (H2-Ab1 protein) | MHC locus is linked with MS. |
|
BE285829 AU041598 |
H2-K1 | H-2 class I histocompatibility antigen K-B alpha chain precursor (H-2K(B)) | As above. |
|
AW554748 AU040375 AI849509 |
H2-D1 | H-2 class I histocompatibility antigen D-B alpha chain precursor (H-2D(B)) | As above. |
|
BE380850 AI848245 AW541488 |
B2m | Beta-2 microglobulin, beta-chain of MHC class I molecules | MHC component. |
| AI839572 | Pdia3 | Protein disulfide isomerase associated 3. Is an integral component of the peptide-loading complex of the MHC class I pathway. Tapasin up-regulation by interferon-gamma induces sequestration of the vast majority of Pdia3 into the MHC class I peptide-loading complex. | Component of antigen processing and loading. |
| AA407026 | Tapbp | Tapasin. Involved in the association of MHC class I peptide-loading complex. | Component of antigen processing and loading. |
| AW555781 | C1qb | Complement C1q subcomponent B chain precursor. | Complement components have been identified in MS lesions [48]. |
| AW547306 | C1qc | Complement C1q subcomponent C chain precursor. C1q associates with the proenzymes C1r and C1s to yield C1, the first component of the serum complement system. | As above. |
| AW554421 | C1qa | Complement component 1, q subcomponent, alpha polypeptide. Classical complement pathway activation. | As above. |
| AU018982 | C1s | Complement component 1 s subcomponent C1s B chain is a serine protease that combines with C1q and C1s to form C1, the first component of the classical pathway of the complement system. | As above. |
| AW556041 | Cfh | Complement component factor h. Regulatory factor of the alternative complement pathway. | As above. |
| AW555904 | Cd93 | Complement component C1q receptor, CD93 antigen. Receptor for C1q, mannose-binding lectin (MBL2) and pulmonary surfactant protein A (SPA). | As above. |
|
AI838311 AW558843 |
Mmp2 | Matrix metalloproteinase-2. Cleavage of gelatin and collagen. Mmp-2 seems to be associated with the chronic progressive phase of MS. | Enhanced levels of Mmp-2 and Mmp-9 are associated with disease processes. |
| AW822201 | Mmp9 | Matrix metalloproteinase 9. Role in extracellular matrix degradation, cell proliferation and migration. Involved in blood–brain barrier (BBB) disruption in active multiple sclerosis. | Acts in active MS lesions. |
| C76038 | Cd97 | CD97 antigen. Receptor involved in both adhesion and signaling processes early after leukocyte activation. Plays an essential role in leukocyte migration. | Expressed in active MS lesions, specifically in many infiltrating T cells macrophages and microglia of active MS lesions (post-mortem brain tissue). |
| AA261175 | Tnf | Tumor necrosis factor precursor (TNF-alpha). | TNFRSF1 is an MS susceptibility locus [49]. |
| AW545573 | Il2rg | Cytokine receptor common gamma chain precursor, interleukin-2 receptor gamma chain, CD132 antigen. The gamma chain is common to the IL2, IL4, IL7, IL21 and probably also the IL13 receptors. | IL7R and IL2RA are MS susceptibility loci [50]. |
Genes encoding components of the innate immune response were regulated including molecules involved in pattern recognition (C1q, Cd93, Cd97, Cd55, and Cd68), several from early in disease (Supplementary Table 8, Tg6074EARLY: C1q and Cd93), which are required for promoting the clearance of pathogens, toxic cell debris and apoptotic cells by phagocytes. At later time points scavenger receptors (Lgals3bp and Scarb2), cytokine receptors (Il13ra1 and Ifngr2) and high mobility group box protein 1 (Hbp1) were also regulated. Genes encoding mediators important for adaptive immunity were also regulated (Ctla2a, Ctla2b, H2-Aa, H2-K, H2-D, B2m, and Cd82) some from early on (Supplementary Table 8, Tg6074EARLY: Ctla2a, H2-Aa, H2-K, and B2m) which may be consistent with the later participation of CD4+ and CD8+ T lymphocytes in the brain in this model [13].
As in the stroke model, growth factor signaling pathways were found to be highly represented from early in disease. As in the other pathologies, components of TGF-β (Tgfbr1, Tgfbr2, Tgif1, Smad1, Smad4, Smurf2, Mapk1, Mapk3, Map2k2, and Raf1) and estrogen (Esr1, Shc1, Med1, Mapk1, Mapk3, Map2k2, Gtf2h1, Ctbp2, and Ddx5) signaling pathways were regulated. The granulocyte macrophage colony-stimulating factor (GM-CSF) signaling pathway was also activated (Csf2, Csf1r, Csf2rb, Jak2, PiK3r1, Stat1, Stat3, Map2k2, Mapk1, Mapk3, and Raf1) and may be linked to disease pathogenesis, as its production is required for microglial activation associated with human MS in its animal model experimental autoimmune encephalomyelitis (EAE) [24].
Genes involved in the response to hypoxia (Hif1a, HSP90AA1, Mdm2, Nfkbia, Nfkbib, and P4hb) and NRF2-mediated oxidative stress (Kbtbd8, Dnaja1, Dnaja3, Dnajb9, Dnajc3, Gstm1, Gsto1, Nfe2l2, Pik3r1, Prkcd, and Txn1) were also regulated. Similar to the stroke model, several genes important for repair of the CNS were regulated (Sema4d, Sema6c, and Nrp1), with Sema6c being regulated at early time points in both MS (Supplementary Table 8, Tg6074EARLY) and CS models.
Inter-disease transcriptome comparisons reveal commonly regulated genes and pathways
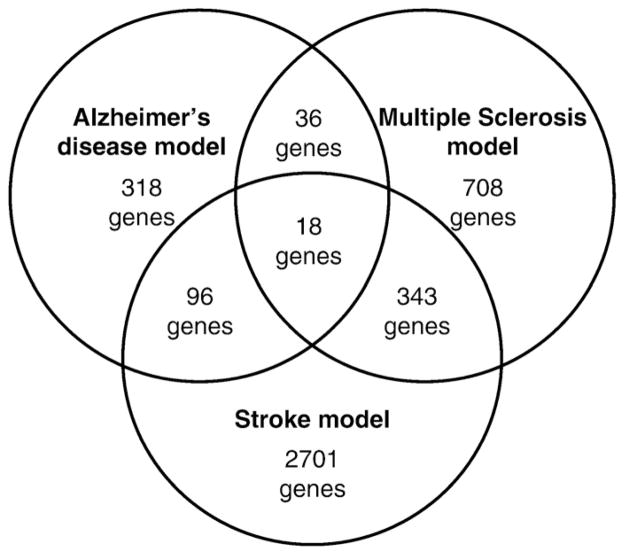
Comparison between disease-induced gene expression changes showed that APP23 brain had the smallest number of differentially regulated genes compared to WT, and the smallest number of overlapping genes with other models (Fig. 1). The stroke model showed the largest number of gene expression changes compared to WT, and the greatest overlap with other diseases (Fig. 1). The finding that less genes were significantly regulated in the AD model compared to wild-type brain (p<0.05) suggests that the aetiopathogenic mechanism in this model induces fewer changes in brain gene expression than those in the MS and stroke models. Indeed, amyloid plaques form slowly in the absence of acute inflammatory responses in this model [5]. On the other hand, the proinflammatory action of TNF in the MS model, and oxygen and nutrient deprivation in the ischemia model, induce more acute stress of CNS tissues that are associated with greater numbers of regulated genes.
Fig. 1.
Venn diagram representing the common and disease-specific genes that resulted from inter-disease comparisons of the significantly (p<0.05) differentially regulated genes from three pathologies.
A comparison across the three pathologies revealed 18 common genes (Table 3). Several could be functionally classified as genes involved in metabolism, notably cholesterol biosynthesis (Fdft1, Npepps, and Soat1), protein modification (Tpp2 and Usp33), DNA transcription and chromatin remodelling (Ctbp2, Ep400, Hbp1, Mef2b, Nono, and Stat3) and immune response (Ctsz and Cd9). One gene encodes an RNA binding protein of unknown function (Larp4b). The other genes regulated across the diseases were as follows: Esr1, which encodes the estrogen receptor alpha (ER1), a master regulatory protein in cellular signaling of nervous system cells and a potent neuroprotective molecule (reviewed in [25]); Sparc, which encodes Secreted Protein, Acidic and Rich in Cysteine) (SPARC, osteonectin), a matricellular glycoprotein that is expressed by many different types of cells, including glial and neuronal cells of the CNS, and is associated with development, remodeling, cell turnover, and tissue repair including neuronal outgrowth in lesions [26]; Rnh1 encoding ribonuclease/angiogenin inhibitor 1, whose expression in human cerebral endothelial cells is regulated by serum from MS patients with active disease [27]; and lastly, Trpm7, which encodes member 7 of the transient receptor potential ion channel family (transient receptor potential melastatin 7, TRPM7) which resides in the membrane of synaptic vesicles complexed with synaptic vesicle proteins and is essential to neurotransmitter release in sympathetic neurons [28]. Trpm7 is an essential mediator of neuronal death that is triggered by hypoxia in vitro [29] and in vivo [30] and underlies ion channel dysfunction in cells expressing AD-associated presenelin mutations [31]. Its dysregulation in all three disease models studied here suggests it may be involved in different types of neurodegneration and represent a novel therapeutic target for inhibition in a diverse collection of CNS disorders.
Table 3.
Common genes found to be regulated in the AD, MS and CS mouse models.
| Gene symbol | Protein name | Function |
|---|---|---|
| Ctbp2 | C-terminal binding protein 2 | Transcriptional corepressor enriched in stem cells and important for their maintenance and regulation of their differentiation. |
| Ctsz | Cathepsin Z | Lysosomal cysteine protease involved in protein trafficking and implicated in cancer pathogenesis. |
| Cd9 | CD9 antigen | Tetraspanin involved in cell adhesion, cell motility and tumor metastasis. Interacts with beta 1 integrins. Mediates lateral association of MHC class II molecules on the dendritic cell surface. Expected to enhance T cell receptor stimulation by DCs. |
| Ep400 | E1A binding protein P400 | A SWI2/SNF2 family ATPase which is a core subunit of NuA4/Tip60 histone acetyltransferase complex involved in chromatin-remodelling. Plays a critical role in development. |
| Esr1 | Estrogen receptor 1 (alpha) | Neuroprotective activity. ERalpha-expressing cells exhibit decreased calpain enzymatic activity and increased survival when exposed to the Ca2+ ionophore, ionomycin. |
| Fdft1 | Farnesyl diphosphate farnesyl transferase 1 | Cholesterol biosynthesis. |
| Hbp1 | High mobility group box transcription factor 1 | Transcriptional repressor. Also functions as a secreted proinflammatory cytokine involved in the innate immune response to infection and injury. Associated with senile plaques. |
| Larp4b | La-related protein 5 | Nucleotide binding protein of unknown function. |
| Mef2b | Myocyte enhancer factor 2B | Transcription factor which binds specifically to the mef2 element present in the regulatory regions of many muscle-specific genes. May be involved in muscle-specific and/or growth factor-related transcription. |
| Nono | Non-pou-domain-containing, octamer binding protein | DNA and RNA binding protein, involved in several nuclear processes. Involved in DNA damage response and RNA processing. |
| Npepps | Aminopeptidase puromycin sensitive | Involved in proteolytic events essential for cell growth and viability. May act as regulator of neuropeptide activity. Highest expression in brain. |
| Rnh1 | Ribonuclease/angiogenin inhibitor 1 | Inhibitor of pancreatic RNase and angiogenin. May also function in the modulation of cellular activities. Putative ATP-dependent DEAD-box RNA-helicase. |
| Soat1 | Sterol O-Acyltransferase 1 | Acyl-coenzyme A:cholesterol acyltransferase. Catalyzes the formation of fatty acid–cholesterol esters. Plays a role in lipoprotein assembly and dietary cholesterol absorption. |
| Sparc | Secreted acidic cysteine rich glycoprotein/osteonectin | Matricellular glycoprotein that mediates interactions between cells and their microenvironment. Roles in tissue remodelling, cell adhesion and tumor progression. |
| Stat3 | Signal transducer and activator of transcription 3 | Signal transducer and activator of transcription 3. Transcription factor that binds to the interleukin-6 (IL-6)-responsive elements identified in the promoters of various acute-phase protein genes. STAT3B interacts with the N-terminal part of JUN to activate such promoters in a cooperative way. |
| Tpp2 | Tripeptidyl peptidase II | Tripeptidyl peptidase II (TPP-II). Component of the proteolytic cascade acting downstream of the 26 S proteasome in the ubiquitin–proteasome pathway. Release of an N-terminal tripeptide from a polypeptide. |
| Trpm7 | Transient receptor potential cation channel, subfamily M, member 7 | Transient receptor potential cation channel subfamily M member 7. Essential ion channel and serine/threonine–protein kinase. Divalent cation channel permeable to calcium and magnesium. Has a central role in magnesium ion homeostasis and in the regulation of anoxic neuronal cell death. Phosphorylates annexin A1. |
| Usp33 | Ubiquitin-specific peptidase 33 | Deubiquitinating enzyme, originally identified as a VHL tumor suppressor protein-interacting enzyme. Roles in axon guidance and Slit signaling. |
Comparison of data from the mouse models with transcriptional profiles for the equivalent human disorders
To evaluate our data we compared the results from our transcriptome analysis with equivalent data obtained from human patients with AD, CS and MS (Supplementary Table 9). We selected the most relevant published studies to the mouse models used here. For AD we used the animal Resourcerer annotation tool [32], to find the common cDNA elements (orthologous genes) between the array platforms used in the mouse and human studies, while for CS and MS this was performed manually. Commonly differentially regulated genes from each mouse model and the equivalent human data were extracted using Microsoft Access database queries.
To compare our gene expression data from APP23 brain to that from AD we selected the 2852 AD-related genes described in a study by Blalock et al. [33] using hippocampal specimens from 22 patients. The 38 genes that were commonly differentially expressed in the APP23 model and human AD fell into the major functional categories that were already defined in the mouse model, specifically cytoskeleton (TUBA3C and TUBA1A), extracellular matrix (SPARC and PRELP), protein and vesicle trafficking (PACSIN2, SERINC3, RHOQ, and DYNLRB1), transport (IPO7, CIB1, and BCAP29), metabolism, particularly cholesterol biosynthesis (SREBF1, FDFT1, and LPHN3), protein modification (UBE2E3, FECH, and LXN) including synapse structure and plasticity (USP14) and signal transduction (SMAD4, PPICK1, AFIQ, ING1L, ITPKC, and RAPGEFL1) (Supplementary Table 10).
For CS we selected the 41 genes described in a study by Mitsios et al. [34], that were commonly regulated in rat pMCAO and postmortem tissue from 12 acute middle cerebral artery stoke patients, after analysis using Atlas 1.2 nylon filter microarrays. The 8 genes that were commonly differentially expressed in the mouse and rat pMCAO models and human CS were growth factor receptors (FGFR1 and CSF1R), signaling components (GRB2 and CAMK1) and transcription factors (ID2, HMGA1, EGR1 and STAT3) (Supplementary Table 11).
For MS we selected the 361 genes described in a study by [35] profiling normal-appearing white matter from 10 primary or secondary progressive MS patients. The 42 genes that were commonly differentially expressed included those involved in cytoskeleton (PTK2, ZFX, DIAPH1, and CTNNA1), transport (PTPRG, ATP2B2, and TFRC), metabolism (AIMP1 and GSTM1), transcription (SF1, MXD1, STAT3, ETV6, ZFP36L1, HIF1A, and EGR1), growth factors (PDGFRB, CSF1, and CSF1R) and signal transduction (JAK2, GNA13, RAP1A, IL6ST, PIK3R1, AXL, MAPK1, RPS6KA3, MAPKAPK2, RALGDS, SMAD4, OSTF1, RAF1, PRKACA, PPP2CB, and NR2F6) (see Supplementary Table 12). Additionally, for MS, we performed a comparison of the differentially expressed genes in Tg6074 brain with 49 genes that were commonly regulated in all published microarray studies from human and animal samples, in brain and peripheral blood samples as presented in a study by Comabella and Martin [36]. This analysis revealed 14 common genes that included the ones involved in cytoskeleton (ARPC1B and CAPG), transcription (MYC and STAT1), signal transduction (CSF1R, NFKBIA, GNAI2, and GRN), immune function (B2M, C1QB, and IFITM3), cell cycle (BTG1) and protein modification (SERPINA3).
Discussion
In the brain, nervous tissue structure and function are preserved within an immune-privileged environment. Unlike many other organs the brain lacks specific lymphoid tissue and under normal healthy conditions is nonpermissive, or even inhibitory, for the priming of adaptive immune responses [37]. However, immune-privilege is conditional. Following injury or infection the brain up-regulates expression of many molecules that are necessary for immune responses, neuroprotection and tissue repair, and activates mechanisms of tissue defense that are only now beginning to be elucidated.
In this study, we used microarray technology to characterize and compare the mouse brain transcriptome in three diverse models of neurodegeneration induced by ischemia, inflammation and the aberrant expression of amyloid β. By comparing them with the transcript profile of normal C57BL/6 mouse brain controls we obtained a unique insight into the molecular events underlying each individual pathology. Thus, the APP23 dataset showed that dysregulated neuronal APP production is sufficient to activate genes involved in processes that are critical for correct neuronal and synaptic functioning and further supports the hypothesis that dysregulated APP expression induces cognitive deficits through disruption of synaptic signaling. After cerebral ischemia, large numbers of trophic factors, developmental genes and disease-associated genes were up-regulated that may provide novel neuroprotective targets for other, more chronic diseases. Chronically inflamed brain, triggered in our model by expression of a TNF transgene, resembled EAE brain and MS lesions, in showing the expression of numerous genes typical of adaptive immune responses and autoimmunity. The observation that a large number of genes identified in the mouse models were also dysregulated in the equivalent human pathologies supports the relevance of our findings for human disease.
The versatile design of this study also enabled us to directly compare disease-model transcriptomes and to identify the genes and pathways that are commonly dysregulated across the three brain disorders thus opening a window onto basic mechanisms of tissue defense that are activated in the brain upon injury. Further to the identification of specific genes that were commonly regulated in diseases, a comparison of functional gene categories revealed that several important mechanisms were activated across the three disease models studied in detail here, even though specific genes were not always shared. Two prominent neuroprotective pathways were regulated in the three mouse models studied, the estrogen and TGF-β signaling pathways. The gene for the ER1 estrogen receptor was up-regulated in all disease models studied. Both ER1 and its ligand estrogen are expressed in male and female brains, by various cell types including neurons and astrocytes, where they exert important non-reproductive effects such as vasoprotection, neuroprotection and synaptic plasticity [25]. Relevant for AD, estrogen also prevents neuronal tau hyperphosphorylation in cultured neurons [38] and slows plaque formation in APP23 mice [39]. TGF-β and its receptors are also expressed by neurons and glial cells in the adult brain and have multiple functions including neuroprotection [40] and the modulation of brain inflammatory responses through the suppression of microglial activation [41]. Deficient TGF-β type II receptor expression has been associated with neurodegeneration and AD pathology in humans and transgenic mouse models [41]. Our findings suggest that ER1 and TGF-β signaling pathways represent fundamental defense mechanisms of the brain that are up-regulated in response to a wide range of pathological triggers to provide beneficial neuroprotective and immune-modulatory effects.
A large number of genes found to be dysregulated in disease-specific models have unknown function in the brain or have not previously been recognised as being regulated in the adult brain under pathological conditions. Of note, four polycomb genes (Asxl2, Epc2, Bmi1/Pcgf4 and Phc2) were differentially regulated following pMCAO and some also showed regulation in MS (Bmi1). Proteins from the Polycomb group (PcG) are epigenetic chromatin modifiers involved in the maintenance of embryonic and adult stem cells. Even though members of this family such as Bmi1 have been implicated in proliferation and self renewal of neuronal progenitors during development [42] there is no previous evidence that these molecules are activated in the adult nervous system after injury or chronic inflammatory diseases and their involvement remains to be elucidated.
Another important category of regulated genes contains CNS disease-associated mutations and chromosome loci. Following pMCAO, numerous such genes were observed including Wbscr22 and NSUN5C (located in the Williams–Beuren syndrome critical region on chromosome 7; region is deleted in Williams–Beuren syndrome and its duplication is associated with epilepsy), Cln3 (mutations result in severe neurodegeneration (Batten disease)), Cln6 (mutations associated with variant late-onset infantile neuronal ceroid lipofuscinosis (vLINCL)), Aspm (autosomal recessive primary microcephaly (MCPH) and schizophrenia), Dgcr2, Dgcr8 and Dgcr14 (genes within the DiGeorge syndrome critical region, associated with schizophrenia), Als2 (recessive mutations cause juvenile amyotrophic lateral sclerosis (ALS2) and related motoneuron disorders), Stradb (localized in chromosomal region associated with ALS), Fxr1, Atrx, Cyfip2, Arhgef6 and Arhgef7 (associated with X-linked mental retardation), Smcr8 (candidate gene for Smith–Magenis syndrome involving developmental delay and mental retardation), Rcan1 (gene of the Down syndrome critical region on chromosome 21), TRAPPC10 (Epilepsy, Holoprosencephaly Candidate-1; candidate gene for EPM1 epilepsy located on chromosome 21) and Ndrg3 (associated with Hereditary Motor and Sensory Neuropathy-Lom (HMSNL)). In APP23 brain, most of the genes associated with CNS pathology have direct relevance to AD and Down syndrome (Table 1, Supplementary Table 6). Also regulated in APP23 brain were Sip1 (reduced expression of Sip1 in spinal muscular atrophy (SMA)) and Usp14 (ax (ataxia) mutation in mice causes defective synaptic transmission). In Tg6074 brain several genes that have been implicated in MS were altered, as well as genes associated with other conditions including Hexb (deficiency is associated with Sandhoff disease in which neurodegeneration is a prominent feature), Alad, Add3 and Stradb (associated with amytropic lateral sclerosis), Gapdh (polymorphisms associated with AD susceptibility), Dld (deficiency results in neurological dysfunction), Sip1 (see AD above), Eif2b2 (defects cause leukoencephalopathy with vanishing white matter), Bag5 (promotion of neurodegeneration in sporadic Parkinson’s disease), Syngr2 (mutations associated with schizophrenia) and APP and Soat1 (mutations/polymorphisms associated with AD, Table 1). The physiological functions of these genes and the relevance of their altered expression in pathologies representing Alzheimer’s disease, cerebral stroke and multiple sclerosis will be important to determine.
The validity of our microarray data was confirmed by comparison of the differentially regulated genes to those described in previously published studies. In particular, several studies describe gene expression changes following pMCAO ischemia in the rat [43–46]. Although these latter analyses were performed in another species, specific time points following pMCAO and in selected brain regions, a significant number of genes identified in our study overlapped with previously identified genes/proteins including NGFIA/Krox24/Egr1 [43,45], Tcp1, Carm1, Eif2, Eif3, 4F2hc/Slc3a2, Srpr, Coronin-like and Tagln2 [45], 14-3-3 protein, Heme oxygenase, S-100, Gadd45g, Ctsc, Syndecan and Calponin [44] and Cacna1h, Rb1, Gabr, Grid2, Ppap2b, Ppp2r5d and Rgs8 [46]. As far as we are aware, microarray analyses of brain changes in mouse pMCAO or the APP23 and Tg6074 transgenic mouse models have not yet been published.
The validity of our microarray data for the study of human disease was evaluated by the comparison of genes regulated in the mouse models to those regulated in the equivalent human pathologies [33–35]. Interestingly, major functional gene categories of regulated genes identified in the mouse models were also observed in the human disease. Specifically, genes involved in cytoskeleton organization, extracellular matrix, protein and vesicle trafficking, transport, cholesterol biosynthesis, protein modification and signal transduction showed significant overlap in the AD mouse model and human hippocampal AD datasets. Similarly genes regulated in brain from patients with middle cerebral artery occlusion [34] contained several growth factor receptor and signaling components as seen in the mouse pMCAO model. Genes commonly regulated in MS patients and the Tg6074 mouse model notably lacked signature innate or adaptive immune response components, perhaps because the primary and secondary progressive forms of MS that were used for this comparison [35] are characterized by chronic neuroaxonal degeneration rather than immune-mediated relapses and are refractory to conventional immunomodulatory therapies [47]. However, genes involved in cytoskeleton, transport, signal transduction and gene transcription including neuroprotective growth factors and a stem cell marker were well represented and may provide clues to the pathogenic and repair mechanisms that operate in such MS lesions.
Conclusions
We show here that there are a number of gene pathways, which have well-established neuroprotective and immune-regulatory effects, that are activated in the mouse brain in response to a diverse range of pathogenic stimuli and therefore may represent constitutive defense responses of the brain to injury. By subtracting these commonly regulated pathways we have also identified genes and pathways that are associated with the development of specific pathologies caused by dysregulated APP production, chronic inflammation and occlusive ischemia that might prove useful for the purpose of drug discovery or disease diagnosis. The validity of our analysis was verified at three independent levels, the microarray-based discovery by use of an alternative method of gene expression analysis (qRT-PCR), multiple testing error by the use of independent animal samples and most importantly, the identification of genes that have already been associated with the aetiopathogenesis of the selected human brain pathologies. We believe that this dataset will provide an important source of information for the validation of independent studies focusing on the identification of risk factors for disease development, for understanding the molecular cues leading to neuropathological phenotypes and for the identification and evaluation of novel therapeutic targets.
Supplementary Material
Acknowledgments
This work was supported by a bidirectional collaboration research program from the Greek Secretariat of Research and Technology, EPAN Greece–USA and by the 6th Framework Program of the European Union, NeuroproMiSe, LSHM-CT-2005-018637. We wish to thank Matthias Staufenbiel (Novartis Pharma Ltd., Basel, Switzerland) for providing the APP23 mice. We wish to extend our special thanks to Adriana Ahumada, Razvan Sultana, Tove Anderson, Brian Frank and other members of the Quackenbush laboratory and Nikos Simos, Anda Voulgari-Kokota and Christos Georgiadis from the Probert laboratory for help and discussions.
Abbreviations
- EAE
experimental autoimmune encephalomyelitis
- CNS
central nervous system
- pMCAO
permanent middle cerebral artery occlusion
- AD
Alzheimer’s disease
- MS
multiple sclerosis
- CS
cerebral stroke
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ygeno.2010.04.004.
Footnotes
Contributions
VT, JQ and LP designed research, VT and RR performed research, VT, JW, SSV, ET, JQ, LP performed analysis, EP provided the pMCAO model, and VT, JQ and LP wrote the paper.
Contributor Information
Vivian Tseveleki, Email: vtseveleki@pasteur.gr.
Renee Rubio, Email: rrubio@jimmy.harvard.edu.
Sotiris-Spyros Vamvakas, Email: vamvakas@bio.demokritos.gr.
Joseph White, Email: jwhite@jimmy.harvard.edu.
Era Taoufik, Email: etaoufik@pasteur.gr.
Edwige Petit, Email: epetit@cyceron.fr.
John Quackenbush, Email: johnq@jimmy.harvard.edu.
Lesley Probert, Email: lesley@pasteur.gr.
References
- 1.Bertram L, Tanzi RE. Thirty years of Alzheimer’s disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci. 2008;9:768–778. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- 2.Soba P, Eggert S, Wagner K, Zentgraf H, Siehl K, Kreger S, Lower A, Langer A, Merdes G, Paro R, Masters CL, Muller U, Kins S, Beyreuther K. Homo- and heterodimerization of APP family members promotes intercellular adhesion. EMBO J. 2005;24:3624–3634. doi: 10.1038/sj.emboj.7600824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young-Pearse TL, Chen AC, Chang R, Marquez C, Selkoe DJ. Secreted APP regulates the function of full-length APP in neurite outgrowth through interaction with integrin beta1. Neural Dev. 2008;3:15. doi: 10.1186/1749-8104-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Burki K, Frey P, Paganetti PA, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, Sommer B. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci U S A. 1997;94:13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Dam D, D’Hooge R, Staufenbiel M, Van Ginneken C, Van Meir F, De Deyn PP. Age-dependent cognitive decline in the APP23 model precedes amyloid deposition. Eur J Neurosci. 2003;17:388–396. doi: 10.1046/j.1460-9568.2003.02444.x. [DOI] [PubMed] [Google Scholar]
- 7.Meyer EP, Ulmann-Schuler A, Staufenbiel M, Krucker T. Altered morphology and 3D architecture of brain vasculature in a mouse model for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2008;105:3587–3592. doi: 10.1073/pnas.0709788105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 9.Aboul-Enein F, Lassmann H. Mitochondrial damage and histotoxic hypoxia: a pathway of tissue injury in inflammatory brain disease? Acta Neuropathol. 2005;109:49–55. doi: 10.1007/s00401-004-0954-8. [DOI] [PubMed] [Google Scholar]
- 10.Peers C, Pearson HA, Boyle JP. Hypoxia and Alzheimer’s disease. Essays Biochem. 2007;43:153–164. doi: 10.1042/BSE0430153. [DOI] [PubMed] [Google Scholar]
- 11.Welsh FA, Sakamoto T, McKee AE, Sims RE. Effect of lactacidosis on pyridine nucleotide stability during ischemia in mouse brain. J Neurochem. 1987;49:846–851. doi: 10.1111/j.1471-4159.1987.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 12.Dutta R, Trapp BD. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology. 2007;68:S22–S31. doi: 10.1212/01.wnl.0000275229.13012.32. [DOI] [PubMed] [Google Scholar]
- 13.Akassoglou K, Bauer J, Kassiotis G, Pasparakis M, Lassmann H, Kollias G, Probert L. Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice: models for multiple sclerosis with primary oligodendrogliopathy. Am J Pathol. 1998;153:801–813. doi: 10.1016/S0002-9440(10)65622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mastronardi FG, Wood DD, Mei J, Raijmakers R, Tseveleki V, Dosch HM, Probert L, Casaccia-Bonnefil P, Moscarello MA. Increased citrullination of histone H3 in multiple sclerosis brain and animal models of demyelination: a role for tumor necrosis factor-induced peptidylarginine deiminase 4 translocation. J Neurosci. 2006;26:11387–11396. doi: 10.1523/JNEUROSCI.3349-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akassoglou K, Adams RA, Bauer J, Mercado P, Tseveleki V, Lassmann H, Probert L, Strickland S. Fibrin depletion decreases inflammation and delays the onset of demyelination in a tumor necrosis factor transgenic mouse model for multiple sclerosis. Proc Natl Acad Sci U S A. 2004;101:6698–6703. doi: 10.1073/pnas.0303859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon RM, Dobbin K. Experimental design of DNA microarray experiments. Biotechniques. 2003;(Suppl):16–21. [PubMed] [Google Scholar]
- 17.Dobbin K, Simon R. Comparison of microarray designs for class comparison and class discovery. Bioinformatics. 2002;18:1438–1445. doi: 10.1093/bioinformatics/18.11.1438. [DOI] [PubMed] [Google Scholar]
- 18.Hegde P, Qi R, Abernathy K, Gay C, Dharap S, Gaspard R, Hughes JE, Snesrud E, Lee N, Quackenbush J. A concise guide to cDNA microarray analysis. Biotechniques. 2000;29:548-4, 556. doi: 10.2144/00293bi01. [DOI] [PubMed] [Google Scholar]
- 19.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 20.Brazma A, Parkinson H, Sarkans U, Shojatalab M, Vilo J, Abeygunawardena N, Holloway E, Kapushesky M, Kemmeren P, Lara GG, Oezcimen A, Rocca-Serra P, Sansone SA. ArrayExpress—a public repository for microarray gene expression data at the EBI. Nucleic Acids Res. 2003;31:68–71. doi: 10.1093/nar/gkg091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 22.Bordet R, Ouk T, Petrault O, Gele P, Gautier S, Laprais M, Deplanque D, Duriez P, Staels B, Fruchart JC, Bastide M. PPAR: a new pharmacological target for neuroprotection in stroke and neurodegenerative diseases. Biochem Soc Trans. 2006;34:1341–1346. doi: 10.1042/BST0341341. [DOI] [PubMed] [Google Scholar]
- 23.Cramer S, Chopp M. Recovery recapitulates ontogeny. Trends Neurosci. 2000;23:265–271. doi: 10.1016/s0166-2236(00)01562-9. [DOI] [PubMed] [Google Scholar]
- 24.Ponomarev ED, Shriver LP, Maresz K, Pedras-Vasconcelos J, Verthelyi D, Dittel BN. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:39–48. doi: 10.4049/jimmunol.178.1.39. [DOI] [PubMed] [Google Scholar]
- 25.Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Au E, Richter MW, Vincent AJ, Tetzlaff W, Aebersold R, Sage EH, Roskams AJ. SPARC from olfactory ensheathing cells stimulates Schwann cells to promote neurite outgrowth and enhances spinal cord repair. J Neurosci. 2007;27:7208–7221. doi: 10.1523/JNEUROSCI.0509-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander JS, Minagar A, Harper M, Robinson-Jackson S, Jennings M, Smith SJ. Proteomic analysis of human cerebral endothelial cells activated by multiple sclerosis serum and IFNbeta-1b. J Mol Neurosci. 2007;32:169–178. doi: 10.1007/s12031-007-0018-3. [DOI] [PubMed] [Google Scholar]
- 28.Krapivinsky G, Mochida S, Krapivinsky L, Cibulsky SM, Clapham DE. The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron. 2006;52:485–496. doi: 10.1016/j.neuron.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 29.Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 30.Sun HS, Jackson MF, Martin LJ, Jansen K, Teves L, Cui H, Kiyonaka S, Mori Y, Jones M, Forder JP, Golde TE, Orser BA, Macdonald JF, Tymianski M. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat Neurosci. 2009;12:1300–1307. doi: 10.1038/nn.2395. [DOI] [PubMed] [Google Scholar]
- 31.Landman N, Jeong SY, Shin SY, Voronov SV, Serban G, Kang MS, Park MK, Di Paolo G, Chung S, Kim TW. Presenilin mutations linked to familial Alzheimer’s disease cause an imbalance in phosphatidylinositol 4, 5-bisphosphate metabolism. Proc Natl Acad Sci U S A. 2006;103:19524–19529. doi: 10.1073/pnas.0604954103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai J, Sultana R, Lee Y, Pertea G, Karamycheva S, Antonescu V, Cho J, Parvizi B, Cheung F, Quackenbush J. RESOURCERER: a database for annotating and linking microarray resources within and across species. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-11-software0002. (SOFTWARE0002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW. Incipient Alzheimer’s disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci U S A. 2004;101:2173–2178. doi: 10.1073/pnas.0308512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitsios N, Saka M, Krupinski J, Pennucci R, Sanfeliu C, Wang Q, Rubio F, Gaffney J, Kumar P, Kumar S, Sullivan M, Slevin M. A microarray study of gene and protein regulation in human and rat brain following middle cerebral artery occlusion. BMC Neurosci. 2007;8:93. doi: 10.1186/1471-2202-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graumann U, Reynolds R, Steck AJ, Schaeren-Wiemers N. Molecular changes in normal appearing white matter in multiple sclerosis are characteristic of neuroprotective mechanisms against hypoxic insult. Brain Pathol. 2003;13:554–573. doi: 10.1111/j.1750-3639.2003.tb00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Comabella M, Martin R. Genomics in multiple sclerosis—current state and future directions. J Neuroimmunol. 2007;187:1–8. doi: 10.1016/j.jneuroim.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Wekerle H. Breaking ignorance: the case of the brain. Curr Top Microbiol Immunol. 2006;305:25–50. doi: 10.1007/3-540-29714-6_2. [DOI] [PubMed] [Google Scholar]
- 38.Alvarez-de-la-Rosa M, Silva I, Nilsen J, Perez MM, Garcia-Segura LM, Avila J, Naftolin F. Estradiol prevents neural tau hyperphosphorylation characteristic of Alzheimer’s disease. Ann N Y Acad Sci. 2005;1052:210–224. doi: 10.1196/annals.1347.016. [DOI] [PubMed] [Google Scholar]
- 39.Yue X, Lu M, Lancaster T, Cao P, Honda S, Staufenbiel M, Harada N, Zhong Z, Shen Y, Li R. Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer’s disease animal model. Proc Natl Acad Sci U S A. 2005;102:19198–19203. doi: 10.1073/pnas.0505203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhandapani KM, Brann DW. Transforming growth factor-beta: a neuroprotective factor in cerebral ischemia. Cell Biochem Biophys. 2003;39:13–22. doi: 10.1385/CBB:39:1:13. [DOI] [PubMed] [Google Scholar]
- 41.Tesseur I, Zou K, Esposito L, Bard F, Berber E, Can JV, Lin AH, Crews L, Tremblay P, Mathews P, Mucke L, Masliah E, Wyss-Coray T. Deficiency in neuronal TGF-beta signaling promotes neurodegeneration and Alzheimer’s pathology. J Clin Invest. 2006;116:3060–3069. doi: 10.1172/JCI27341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zencak D, Lingbeek M, Kostic C, Tekaya M, Tanger E, Hornfeld D, Jaquet M, Munier FL, Schorderet DF, van Lohuizen M, Arsenijevic Y. Bmi1 loss produces an increase in astroglial cells and a decrease in neural stem cell population and proliferation. J Neurosci. 2005;25:5774–5783. doi: 10.1523/JNEUROSCI.3452-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soriano MA, Tessier M, Certa U, Gill R. Parallel gene expression monitoring using oligonucleotide probe arrays of multiple transcripts with an animal model of focal ischemia. J Cereb Blood Flow Metab. 2000;20:1045–1055. doi: 10.1097/00004647-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Tang Y, Lu A, Aronow BJ, Wagner KR, Sharp FR. Genomic responses of the brain to ischemic stroke, intracerebral haemorrhage, kainate seizures, hypoglycemia, and hypoxia. Eur J Neurosci. 2002;15:1937–1952. doi: 10.1046/j.1460-9568.2002.02030.x. [DOI] [PubMed] [Google Scholar]
- 45.Roth A, Gill R, Certa U. Temporal and spatial gene expression patterns after experimental stroke in a rat model and characterization of PC4, a potential regulator of transcription. Mol Cell Neurosci. 2003;22:353–364. doi: 10.1016/s1044-7431(02)00039-8. [DOI] [PubMed] [Google Scholar]
- 46.Ford G, Xu Z, Gates A, Jiang J, Ford BD. Expression Analysis Systematic Explorer (EASE) analysis reveals differential gene expression in permanent and transient focal stroke rat models. Brain Res. 2006;1071:226–236. doi: 10.1016/j.brainres.2005.11.090. [DOI] [PubMed] [Google Scholar]
- 47.Miller DH, Leary SM. Primary-progressive multiple sclerosis. Lancet Neurol. 2007;6:903–912. doi: 10.1016/S1474-4422(07)70243-0. [DOI] [PubMed] [Google Scholar]
- 48.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 49.De Jager PL, Jia X, Wang J, de Bakker PI, Ottoboni L, Aggarwal NT, Piccio L, Raychaudhuri S, Tran D, Aubin C, Briskin R, Romano S, Baranzini SE, McCauley JL, Pericak-Vance MA, Haines JL, Gibson RA, Naeglin Y, Uitdehaag B, Matthews PM, Kappos L, Polman C, McArdle WL, Strachan DP, Evans D, Cross AH, Daly MJ, Compston A, Sawcer SJ, Weiner HL, Hauser SL, Hafler DA, Oksenberg JR International MS Genetics Consortium. Meta-analysis of genome scans and replication identify CD6, IRF8 and TNFRSF1A as new multiple sclerosis susceptibility loci. Nat Genet. 2009;41:776–782. doi: 10.1038/ng.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lundmark F, Duvefelt K, Iacobaeus E, Kockum I, Wallström E, Khademi M, Oturai A, Ryder LP, Saarela J, Harbo HF, Celius EG, Salter H, Olsson T, Hillert J. Variation in interleukin 7 receptor alpha chain (IL7R) influences risk of multiple sclerosis. Nat Genet. 2007;39:1108–1113. doi: 10.1038/ng2106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.