Abstract
Background
Although pregnancy can be associated with adaptive changes in weight and eating behavior for women with eating disorders, less is known about whether these changes are maintained in the postpartum period. We used a longitudinal design to examine gestational and postpartum weight trajectories in mothers with and without eating disorders in the Norwegian Mother and Child Cohort Study (MoBa) conducted by the Norwegian Institute of Public Health.
Methods
Fifty-six women reported anorexia nervosa (AN), 636 bulimia nervosa (BN), 3,327 binge eating disorder (BED), and 69 EDNOS purging type (EDNOS-P). The referent group included 61,233 mothers with no eating disorder. We used a mixed effects model to predict weight change over time by eating disorder subtype.
Results
Mothers with AN, BN, BED and EDNOS had greater increases in BMI during pregnancy and greater decreases in BMI over the first six months postpartum. Women with AN shifted from the underweight BMI range before pregnancy to the normal weight range at 36 months postpartum
Conclusions
Patterns of maternal weight gain and retention during the perinatal period vary across eating disorder subtype and warrant clinical attention.
Keywords: eating disorders, MoBa, The Norwegian Mother and Child Cohort Study, pregnancy, postpartum, weight, anorexia nervosa, bulimia nervosa, binge eating disorder
INTRODUCTION
Women with eating disorders face unique challenges during pregnancy and the postpartum period (Bulik et al., 2007; Bulik et al., 2009; Easter et al., 2013; Micali, Simonoff, & Treasure, 2007; Micali & Treasure, 2009). Chief among these is concern about the gestational weight gain necessary for the development of a healthy fetus and the likelihood of weight retention during the postpartum period. Although pregnancy can lead to adaptive changes in weight, less is known whether these changes are maintained in the postpartum period (Bulik et al., 2007; Knoph et al., 2013; Micali et al., 2007; Stein & Fairburn, 1996).
Using data from a large population-based sample, the Norwegian Mother and Child Cohort Study (MoBa) conducted by the Norwegian Institute of Public Health, we have reported that women with eating disorders [anorexia nervosa (AN), bulimia nervosa (BN), binge eating disorder (BED), and eating disorder not-otherwise-specified, purging type (EDNOS-P)] immediately before and during pregnancy, gained significantly more weight during pregnancy (1.9–2.9 kg) on average than women without eating disorders (Bulik et al., 2009). In addition, research from another large population-based cohort in the Netherlands (Generation R) found that women with AN gained weight significantly faster during pregnancy in comparison to women without eating disorders (at a relative .07% higher increase/week). In contrast, women with BN gained weight significantly slower rate than women without eating disorders (at a rate of .05% lower relative increase/week; (Micali et al., 2012). However, to our knowledge, no studies have compared longitudinal patterns of maternal weight from pregnancy through postpartum period in women with and without eating disorders prospectively.
The postpartum period
In the postpartum period, even in women without eating disorders, dissatisfaction with body weight and shape are normative. In the first month after delivery, 75% are concerned with weight retention and by 4 months postpartum, 70% of women are attempting to lose weight (Carter, Duncan, & Wordsworth, 2000; Hiser, 1987). During this time, women report increased weight and shape concerns and an increase in food restraint such as food avoidance and/or adherence to specific food rules (Stein & Fairburn, 1996). Concern with residual gestational weight, changes to body shape and food restraint may be especially heightened for women with eating disorders and result in altered weight trajectories above and beyond the weight changes seen in women without eating disorders. Thus, the postpartum period represents a potentially precarious time for women with eating disorders.
Pregnancy can be transformational for women with eating disorders because carrying a fetus offers new motivations for behavior changes such as weight restoration and purging cessation. However, during the postpartum period, without the continued worries about the potential for harm to the fetus, women with eating disorders may find it difficult to extend their recovery behaviors in service of their own health (Astrachan-Fletcher, Veldhuis, Lively, Fowler, & Marcks, 2008). Describing change and speed at which such weight changes occur in women with and without eating disorders could index potential problems in eating and compensatory behaviors. Weight loss trajectories that differ from the expected weight change trajectory during this critical period could provide a signal to gynecological and primary care providers to assess eating disorder behavior further. Although our research group found that women with eating disorders gain significantly more weight during pregnancy and we have examined the patterns of course and predictors of maternal eating disorders during the postpartum period, we have not yet examined the rate at which women with AN, BN, EDNOS-P or BED gain gestational weight or retain or lose weight across the postpartum period (Bulik et al., 2009; Knoph et al., 2013).
Thus, the purpose of the present study was to examine gestational and postpartum weight trajectories in mothers with and without eating disorders in the MoBa sample. We compared the weight change of mothers with and without eating disorders at seven time points: pre-pregnancy, 17 weeks and 29 weeks gestation, birth, 6, 18, and 36 months postpartum.
Hypotheses
We hypothesized that mothers with eating disorders would have weight trajectories divergent from mothers without eating disorders across the observation period and that their weight trajectories would differ by eating disorder type. In particular, we anticipated that women with AN, BN and EDNOS-P would gain gestational weight more quickly during pregnancy but lose weight more quickly during the postpartum period. In contrast, we expected that women with BED would gain more quickly during pregnancy but lose gestational weight more slowly and thus, retain more gestational weight during the postpartum period.
METHODS
Participants
Data were collected as part of the Norwegian Mother and Child Cohort Study (MoBa) conducted by the Norwegian Institute of Public Health (Magnus et al., 2006). The study was approved by the appropriate regional committees for ethics in medical research and the Norwegian National Data Inspectorate. Informed consent was obtained from each MoBa participant upon recruitment.
A complete description of recruitment and data collection has been published elsewhere (Bulik et al., 2009; Magnus et al., 2006). In short, MoBa is a longitudinal prospective pregnancy cohort study. Pregnant women were recruited through invitation by mail after registering for a routine prenatal ultrasound at approximately 17–18 weeks gestation (www.fhi.no/morogbarn). Participants were recruited from all over Norway from 1999–2008, and 38.5% of invited women consented to participate. The cohort now includes 109,000 children, 91,000 mothers and 71,700 fathers. Follow-up is conducted by questionnaires at regular intervals and by linkage to national health registries including the Medical Birth Registry of Norway (MBRN) to capture pregnancy outcome variables (Irgens, Bergsjø, & Lie, 2000).
The current study is based on version 5 of the quality-assured data files released for research in 2010. The analysis population included MoBa participants who had: (a) within range self-reported age, weight, and height values, (b) a live, singleton birth (c) returned Questionnaire 1 before delivery, and (d) did not complete an early pilot version of Questionnaire 1 (n=2,605). If a woman enrolled in MoBa more than once (due to additional pregnancies), only the first pregnancy was included. Of the initial 103,474 mother–child records reported in version 5 of the quality assured data files, 81,322 (81%) met these criteria.
Further inclusion criteria were also applied for the current report. We included participants who had: (a) complete eating disorder classification data; (b) at least three non-missing observations across the seven time points; (c) biologically viable times for live birth (gestational age of the baby exceeded 140 days but was less than 309 days); (d) a recorded gestational age of baby per ultrasound; (e) an age greater than 18 years and (e) complete maternal height data. Of the 81,322 mother-child records, 65,532 met these additional criteria.
Data Collection Timeline
MoBa participants completed six self-report questionnaires during their pregnancy and the postpartum period. Five of the six questionnaires were used in these analyses and were completed at approximately 17 and 29 weeks gestation and 6, 18, and 36 months postpartum. Questionnaires are published on the MoBa website (www.fhi.no/moba-en).
Measures
Medical Birth Registry of Norway (MBRN)
Information on pregnancy outcomes is reported to MBRN for all deliveries after 16 weeks gestation through mandatory notification by midwives and doctors (Irgens et al., 2000).
Eating Disorders
Eating disorder items were previously used for studies of eating disorders in the Norwegian Institute of Public Health Twin Panel and were designed in accordance with DSM-IV criteria (American Psychiatric Association, 2000; Harris, Magnus, & Tambs, 2002; Reichborn-Kjennerud, Bulik, Tambs, & Harris, 2004; Reichborn-Kjennerud et al., 2003; Reichborn-Kjennerud, Bulik, Kendler, et al., 2004; Reichborn-Kjennerud, Bulik, Sullivan, Tambs, & Harris, 2004). In the analysis population, respondents completed eating disorder items at a median of 17.0 weeks gestation [interquartile range (IQR) 15.9–18.7].
Complete diagnostic algorithms and hierarchies have been reported previously (Bulik et al., 2007; Bulik et al., 2009). Because the primary focus of the present paper was to examine maternal weight trajectories of women with eating disorders immediately before pregnancy, algorithms were constructed to define the presence of eating disorders in the six months before pregnancy and/or during pregnancy (Bulik et al., 2007; Bulik et al., 2009). Our final diagnostic categories were broadly defined and included: broad AN (meeting relaxed DSM-IV criteria for AN: BMI<19.0 and the exception of amenorrhea); BN (endorsing at least weekly frequency of binge eating and either purging compensatory behaviors such as vomiting or laxative use or non-purging compensatory behaviors such as exercise, fasting); BED (at least weekly frequency of binge eating in the absence of compensatory behaviors); and EDNOS-P (purging at least weekly in the absence of binge eating). Criteria for binge eating included both eating an unusually large amount of food and the feeling of loss of control. Purging was differentiated from emesis and hyperemesis gravidarum.
As the symptom picture for many women changed before and during pregnancy, the order for our diagnostic hierarchy was: AN, BN, EDNOS-P, BED, and no-ED. All individuals who met AN criteria before pregnancy were categorized as AN. Because the diagnosis of AN depends on assessment of BMI, and an accurate measure of actual maternal BMI during pregnancy is complicated due to gestational weight gain, we did not measure new onset of AN during pregnancy. Those who met BN criteria either before or during pregnancy and who did not meet AN criteria before pregnancy were categorized as BN. If not classified as AN or BN, those who met criteria for EDNOS-P before or during pregnancy and did not endorse binge eating at either time were categorized as EDNOS-P. Similarly, individuals who endorsed BED and did not endorse purging during or before pregnancy were included in the BED group. In sum, the order for our diagnostic hierarchy was: AN, BN, EDNOSP, BED, and no-ED. Group assignment was only made when all responses were available to ensure accurate classification.
Outcomes
Maternal weight. Maternal weight was obtained through self-report questionnaires. Participants reported their current weight and pre-pregnancy weight and height at 17 weeks gestation [median(IQR) = 17.0 weeks (15.9–18.7)], their most recent weight at their prenatal check-up at 29 weeks gestation [median (IQR) = 28.6 weeks (27.5–29.7)], their weight immediately before their infants’ birth and their current weight at 6 months postpartum [median (IQR) = 6.3 months (6.2–6.5)], and their current weight at 18 months postpartum [median (IQR) = 18.3 months (18.2–18.7)] and 36 months postpartum, [median (IQR) = 36.4 months(36.2–37.0)]. Participant BMI (kg/m2) was calculated at each time point.
Covariates
Covariates included maternal pre-pregnancy BMI, maternal age at delivery, parity (defined as total number of live births), maternal education, total household income, and breastfeeding status. Weight in women covaries inversely with socioeconomic status including both education and income levels (Sobal & Stunkard, 1989). Education and income are also associated with prolonged breastfeeding, which, in turn, is associated with postpartum weight loss (Stuebe et al., 2010). Adjusting for these factors allows for the examination of the unique association between eating disorder status and maternal weight over time. Breastfeeding was categorized according to World Health Organization guidelines (WHO, 1991): ‘Predominant breastfeeding’ mothers exclusively breastfed their infants but the infant may also have received liquids (water and water-based drinks, fruit juice), ritual fluids and oral rehydration solution, drops or syrups (vitamins, minerals and medicines). ‘Partial breastfeeding’ mothers breastfed their infants but other feeding types had been used (either solid foods or formulas). ‘No breastfeeding’ mothers did not feed their infants any breast milk. All covariates were self-reported with the exception of maternal age, which was captured from the MBRN.
Statistical Analyses
To characterize the association between change in BMI over time by eating disorder subtype we used a mixed model with random effects for the intercept and continuous covariate representing time. Given the limited data points per woman (maximum n=7), the abrupt nature of weight change before and immediately after childbirth, and a preference for parsimony, the model used in this analysis is a piecewise linear regression model, also known as a bent line model, with two points of slope change: at birth and 6 months after birth (Weiss, 2005). These points of change were determined upon visual inspection of the data. Thus, time is divided into three distinct periods Period 1: Pregnancy to birth, Period 2: Birth to 6 months postpartum and Period 3: 6–36 months postpartum. Fixed effects included time, eating disorder subtype and covariates. The covariance matrix used an autocorrelation structure, dependent on the intervals of time between each BMI report. To determine the best fitting model, we evaluated the fit of a set of models that included no effects, a random intercept and both a random intercept and random slope for time with and without covariates. The model with two random effects, for intercept and slope, displayed the best fit as evaluated by log-likelihood statistics. The random effect for slope corresponds to person-level estimates. Given this mixed effects model and a maximum likelihood procedure, we assumed the data were missing at random, and included individuals who were missing data for some time points (Graham, 2009). Residuals scaled by the inverse Cholesky root of the marginal variance-covariance matrix were evaluated for proper fit.
Data values were omitted from individual time-points when: (a) the self-reported time of questionnaire completion was implausible (i.e., person reports same time for different surveys; 1,732 data values) (b) less than three time points (670 data values), or (d) the participant was pregnant with a second child after index pregnancy (17,338 data values).
All analyses were performed using SAS/STAT software, Version 9.2 of the SAS System for Windows (SAS/STAT® 9.2 User’s Guide., 2008.). Graphics were completed using R software (R Development Core Team, 2008). An alpha level of 0.05 was used to indicate statistical significance.
RESULTS
Participant Characteristics
In the sample, mean pre-pregnancy BMI (kg/m2) was 24.0 (4.2). Maternal mean age at delivery was 30.0 years (SD=4.5), 55.9% (n=36,501) of mothers were primiparous, 7.7% of mothers engaged in predominant breastfeeding and 72.8% in partial breastfeeding at five months. Maternal age, pre-pregnancy BMI, education, income level, breastfeeding status, and parity across eating disorder groups are presented in Table 1.
Table 1.
Participant characteristics across eating disorder groups presented as n (%) or mean (sd)
| ANa (n=56) | BNb (n=636) | EDNOS-Pc (n=69) | BEDd (n=3,327) | No EDe (n=61,233) | |
|---|---|---|---|---|---|
| Maternal age, mean (sd) | 26.9 (4.8) | 29.3 (4.7) | 28.1 (5.1) | 30.1 (4.6) | 30.0 (4.5) |
| Combined Income, n (%) | |||||
| 0 – $36K | 23 (42.6) | 164 (27.9) | 25 (43.1) | 761 (24.7) | 11,583 (20.3) |
| $>36K | 11 (20.4) | 105 (17.9) | 13 (22.4) | 754 (24.4) | 12,171 (21.3) |
| $>89K | 11 (20.4) | 184 (31.3) | 14 (24.1) | 1,051 (34.0) | 19,605 (34.3) |
| $>125k | 9 (16.7) | 134 (22.8) | 6 (10.3) | 521 (16.9) | 13,763 (24.1) |
| Education, n (%) | |||||
| Vocational high school | 17 (33.3) | 153 (25.9) | 24 (36.4) | 855 (27.0) | 11,276 (19.4) |
| 3-year high school / Junior College | 13 (25.5) | 113 (19.1) | 11 (16.7) | 563 (17.8) | 8,668 (14.9) |
| Regional technical college / 4-year university degree | 11 (21.6) | 214 (36.2) | 23 (34.8) | 1,213 (38.3) | 24,219 (41.7) |
| University, technical college, more than 4 years | 10 (19.6) | 111 (18.8) | 8 (12.1) | 534 (16.9) | 13,858 (23.9) |
| Breastfeeding, n (%) | |||||
| No breastfeeding | 18 (39.1) | 113 (21.9) | 23 (41.1) | 669 (24.5) | 9,871 (19.2) |
| Predominant breastfeeding | 4 (8.7) | 33 (6.4) | 1 (1.8) | 193 (7.1) | 3,959 (7.7) |
| Some breastfeeding | 24 (52.2) | 371 (71.8) | 32 (57.1) | 1,874 (68.5) | 37,528 (73.1) |
| Parity (Total number of previous births), n (%) | |||||
| 0 | 37 (66.1) | 366 (57.5) | 42 (60.9) | 1,667 (50.1) | 34,389 (56.2) |
| 1 | 13 (23.2) | 176 (27.7) | 17 (24.6) | 1,071 (32.2) | 17,792 (29.1) |
| 2+ | 6 (10.7) | 94 (14.8) | 10 (14.5) | 589 (17.7) | 9,052 (14.8) |
AN= anorexia nervosa
BN= bulimia nervosa
EDNOS-P = eating disorder NOS, purging subtype
BED= binge eating disorder
No ED = no eating disorder
Eating Disorders Prevalence
The prevalence estimates for eating disorders were: 0.1% (n=56) for AN, 1% (n = 636) for BN, 0.1% (n = 69) for EDNOS-P, 5.1% (n = 3327) for BED, and 93.7% of women (n = 61,233) reported no eating disorder before pregnancy.
Not all participants participated at each time point. The number of participants across time and eating disorder diagnoses can be found in Table 2.
Table 2.
Maternal BMI and weight across eating disorder group over time presented as mean (sd).
| Time | ||||||||
|---|---|---|---|---|---|---|---|---|
| Period 1 -9 months to birth |
Period 2 Birth to 6 months postpartum |
Period 3 6–36 months postpartum |
||||||
|
|
||||||||
| Pre-pregnancy n=64,554 |
17 weeks n=62,939 |
29 weeks n=59,868 |
Birth n=52,519 |
6 months n=52,970 |
18 months n=30,684 |
36 months n=21,723 |
||
|
|
||||||||
| ANa | BMI | 18.2 (0.6) | 20.0 (1.5) | 22.3 (1.8) | 24.4 (2.2) | 19.1 (1.9) | 18.9 (1.8) | 19.5 (2.1) |
| Kg | 51.8 (4.1) | 56.8 (5.6) | 63.1 (6.2) | 69.1 (7.5) | 54.0 (5.8) | 54.0 (6.2) | 57.1 (6.6) | |
| n | 56 | 54 | 48 | 44 | 45 | 31 | 16 | |
|
| ||||||||
| BNb | BMI | 24.2 (4.4) | 25.5 (4.3) | 27.8 (4.6) | 30.1 (5.3) | 25.0 (5.1) | 25.0 (5.1) | 25.3 (5.2) |
| Kg | 68.2 (13.1) | 71.9 (12.9) | 78.6 (13.7) | 84.9 (15.4) | 70.5 (15.0) | 70.2 (14.7) | 71.3 (14.8) | |
| n | 627 | 621 | 566 | 506 | 505 | 270 | 183 | |
|
| ||||||||
| EDNOS-Pc | BMI | 23.8 (4.4) | 24.8 (4.1) | 27.5 (4.7) | 29.9 (5.0) | 24.6 (4.8) | 24.3 (4.6) | 24.6 (3.7) |
| Kg | 66.3 (13.9) | 69.1 (13.5) | 76.6 (14.4) | 83.3 (13.9) | 68.4 (13.5) | 67.9 (14.4) | 69.6 (12.4) | |
| n | 66 | 64 | 59 | 56 | 57 | 35 | 20 | |
|
| ||||||||
| BEDd | BMI | 25.9 (5.2) | 27.1 (5.0) | 29.5 (4.8) | 31.6 (5.0) | 26.6 (5.1) | 26.5 (5.4) | 26.7 (5.4) |
| Kg | 73.2 (15.4) | 76.6 (14.8) | 83.5 (14.6) | 89.4 (15.1) | 75.2 (15.2) | 74.8 (15.9) | 75.6 (15.9) | |
| n | 3,285 | 3,216 | 3,053 | 2,623 | 2,649 | 1,497 | 1,042 | |
|
| ||||||||
| No EDe | BMI | 23.9 (4.1) | 24.9 (4.1) | 27.1 (4.1) | 29.2 (4.3) | 24.3 (4.2) | 24.2 (4.3) | 24.5 (4.3) |
| Kg | 67.6 (12.4) | 70.4(12.2) | 76.8 (12.3) | 82.5 (13.0) | 68.7 (12.6) | 68.6 (12.7) | 69.4 (12.8) | |
| n | 60,520 | 58,984 | 56,142 | 49,290 | 49,714 | 24,918 | 28,851 | |
AN= anorexia nervosa
BN= bulimia nervosa
EDNOS-P = eating disorder NOS, purging subtype
BED= binge eating disorder
No ED = no eating disorder
Maternal weight across eating disorder group and time
In the sample, mean maternal BMI was 26.8 kg/m2 (SD=4.8) at 17 weeks gestation and 29.3 kg/m2 (SD=4.7) at delivery. In the postpartum period, mean maternal BMI was 28.1 kg/m2 (SD=4.3) at 6 months, 24.5 kg/m2 (SD=4.4) at 18 months, and 24.8 kg/m2 (SD=4.5) at 36 months postpartum.
Mean maternal weight and BMI across eating disorder groups are presented in Table 2. The mean BMI for mothers with AN fell within the underweight range (BMI<18.5) at the pre-pregnancy time-point but within the normal weight range (18.5≤BMI<25.0) at 6, 18, and 36 months postpartum. The mean BMI for mothers with BN fell within the normal weight range at the pre-pregnancy time-point but within overweight range (25.0≤BMI<30.0) at 6, 18, and 36 months postpartum on average. The mean BMI for mothers with BED fell within the overweight range at all pre and postpartum time points. The mean BMI for mothers with EDNOS-P and mothers with no EDs fell within the normal weight range at all pre- and postpartum time points.
At their child’s birth, women with AN had 4.6 units lower mean BMI than the referent: women with BN, EDNOS-P, or BED had a 1.0, 0.5, 2.2 units higher mean BMI than the referent, respectively. All differences were significant with the exception of those with EDNOS-P.
Regression parameter estimates for weight across eating disorder group and time
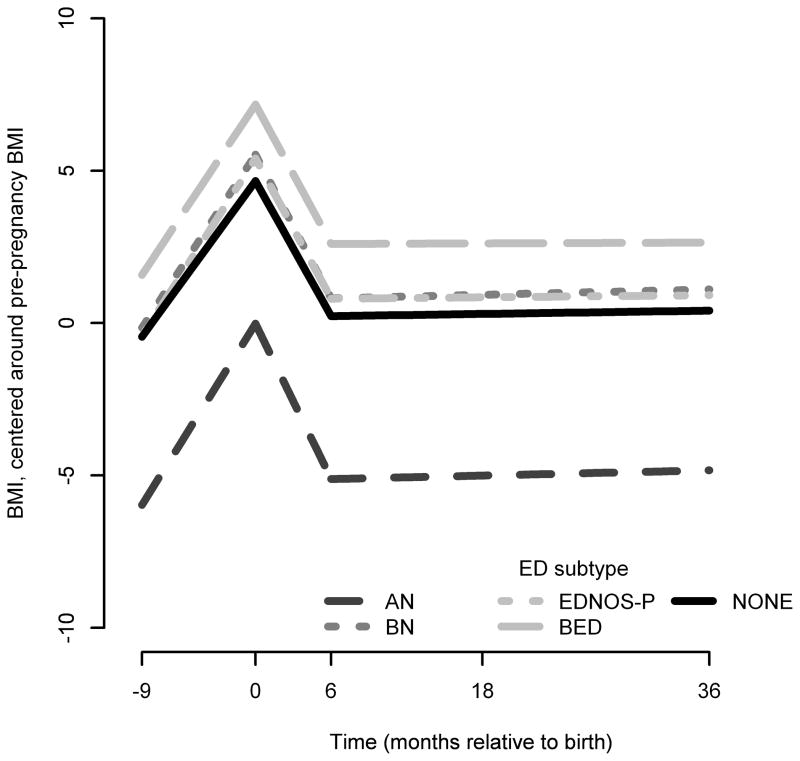
The fitted line for BMI across eating disorder group and time is presented in Figure 1.
FIGURE 1.
Fitted lines For BMI vs. time by ED Subtype
In period 1 (pregnancy to birth) in the referent group, mean BMI increased 0.57 units per month (Table 3). BMI change over time for all eating disorder groups was significantly greater relative to the referent group with no eating disorder. These estimates did not significantly change upon adjustment for covariates.
Table 3.
Regression parameter estimates (se) for model of fitted line for BMI over time with knots at birth and 6 months postpartum by eating disorder subtype. All significant differences between the slope of the eating disorder group and the slope of the referent group with no eating disorder are in bold.
| Model | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No adjustment | Adjusted 1b | Adjusted 2c | ||||||||||||||
| Timea | Intercept (value at birth) |
Period 1 slope |
Period 2 slope |
Period 3 slope |
Intercept (value at birth) |
Period 1 slope |
Period 2 slope |
Period 3 slope |
Intercept (value at birth) |
Period 1 slope |
Period 2 slope |
Period 3 slope |
||||
| EDd | ||||||||||||||||
| ANe | −0.02 (0.56) | 0.66** (0.03) | −0.85** (0.04) | 0.01 (0.01) | −1.25 (0.58) | 0.67*** (0.03) | −0.86** (0.04) | 0.01 (0.01) | −1.39 (0.63) | 0.69*** (0.03) | -.87** (.04) | 0.01 (0.01) | ||||
| BNf | 5.53 (0.17) | 0.63*** (0.01) | −0.79*** (0.01) | 0.01 (<0.005) | 4.19 (0.22) | 0.63*** (0.01) | −0.79*** (0.01) | 0.01 (<0.005) | 4.20 (0.24) | 0.64*** (0.01) | −0.79*** (0.01) | 0.01 (<0.005) | ||||
| EDNOSg | 5.40 (0.51) | 0.65** (0.02) | −0.78 (0.04) | <0.005 (0.01) | 3.99 (0.56) | 0.67*** (0.03) | −0.81 (0.04) | 0.01 (0.01) | 3.71 (0.61) | 0.69*** (0.03) | −0.84* (0.04) | 0.01 (<0.005) | ||||
| BEDh | 7.18 (0.07) | 0.62*** (<0.005) | −0.76*** (0.01) | <0.005* (<0.005) | 5.69 (0.16) | 0.62*** (<0.005) | −0.77*** (0.01) | 0.005* (<0.005) | 5.48 (0.17) | 0.63*** (<0.005) | −0.77*** (0.01) | <0.005* (<0.005) | ||||
| No EDi | 4.67 (0.02) | 0.57 (<0.005) | −0.74 (<0.005) | 0.01 (<0.005) | 3.31 (0.14) | 0.57 (<0.005) | −0.74 (<0.005) | 0.01 (0.00) | 3.25 (0.15) | 0.58 (<0.005) | −0.75 (<0.005) | 0.01 (<0.005) | ||||
Period 1 = -9 months to birth; Period 2 = birth to 6 months postpartum; Period 3 = 6 months to 36 months postpartum
With covariates adjusting for age of mother, breastfeeding status, parity, education and income.
With covariates adjusting for age of mother, parity, education and income.
ED= eating disorder
AN= anorexia nervosa
BN= bulimia nervosa
EDNOS-P = eating disorder NOS, purging
BED= binge eating disorder subtype
No ED = no eating disorder
<.01
<.05
<.001
Note: All p-values were adjusted using the Benjamini-Hochberg False Discovery Rate procedure
In period 2 (birth to the 6 months postpartum) in the referent group, mean BMI declined 0.74 units per month (Table 3). Women in the AN, BN and BED groups had significantly different BMI changes than the referent group with no eating disorder. Women with AN, BN and BED had BMIs that declined more quickly than the referent population. These relationships did not change significantly after adjusting for covariates. There was no significant difference between women with EDNOS and the referent group in the unadjusted model. However, after adjusting for all covariates, women with EDNOS also had a significantly faster decline in BMI during this period.
In period 3, (6 months to 36 months postpartum) in the referent population, BMI increased a negligible amount (0.01 BMI units for one unit change in month; Table 3). During this period, women with AN, BN and EDNOS-P did not differ significantly different from the referent. In contrast, women with BED had a significant change in BMI over time when compared with the referent. These findings indicate that women with BED also had increasing BMI at this time but this increase was attenuated in comparison to women in the referent group.
In post hoc analyses, we estimated the difference between mean BMI at 36-month postpartum from mean pre-pregnancy BMI from the unadjusted regression parameter estimates as mentioned above. In all groups, the calculated difference was statistically significant; an increase greater than zero indicated an increase in BMI over time. On average, women in the referent group had a BMI increase of 0.86 (CI:.0.84 – 0.88), the BN group had a 1.13 unit increase (CI: 0.92 – 1.34), the EDNOS-P group had a 1.35 unit increase (CI:0.74 – 1.96), the BED group had a 1.06 (CI: 0.97 – 1.15) unit increase, and the AN group had 1.13 unit increase (CI: 0.46 – 1.81).
CONCLUSIONS
In this large, population-based sample of Norwegian women, mothers with AN, BN, EDNOS and BED gained weight more quickly during pregnancy, and lost weight more quickly over the first 6 months postpartum than mothers without eating disorders.
Reasons for weight gain and weight retention may vary across eating disorder subtype. In pregnancy, for women with AN, increased weight gain is in line with Institute of Medicine (IOM) guidelines which recommend a weight gain of 12.7 kg to 18 kg for women with a BMI of less than 18.5. Thus, a greater weight gain in women with AN could be protective for the nutritional demands of the developing fetus (Bulik et al., 2007; Bulik et al., 2009; Micali et al., 2007; Micali & Treasure, 2009). This increased rate of weight gain for pregnant women with AN is also directly comparable to the findings of the Generation R study (Micali et al., 2012). Both studies found that the rate of weight change over time was significantly greater for women with AN in comparison to women without eating disorders.
However, for women with BN, these results conflict the Generation R study’s findings that women with BN had lower relative weight increases during pregnancy compared to referent women (Micali et al., 2012). Women with BN this sample had significantly higher weight change over their pregnancy than women without eating disorders. In addition, women with EDNOS-P and BED also had significantly higher weight change over time, a finding that is new. It may be that increased gestational weight gain for BN, EDNOS-P and BED in the present sample, may be reflective of ongoing binge eating or in the case of EDNOS-P and BN better control of purging behaviors (Siega-Riz et al., 2008).
In the postpartum period, the transition to motherhood is accompanied by significant physical changes. The combination of increased body fat and abdominal muscle loss may precipitate or exacerbate dissatisfaction with body shape and composition. Mothers of newborns are also often home alone which gives ample opportunity to engage in eating disorder behaviors in secret (Stein & Fairburn, 1996). Women with eating disorders are also at higher risk for experiencing postpartum depression and anxiety, which may also adversely influence postpartum eating behavior (Mazzeo et al., 2006; Meltzer-Brody et al., 2011). Although increased concern with shape and weight in the first six months postpartum is normative, the fact that women with AN, BN, EDNOS and BED lose weight more quickly during this period may suggest that they are resorting to familiar restriction, compensatory measures, or other extreme weight-control behaviors (Stein & Fairburn, 1996). Without the fear of harming the fetus, women with eating disorders may find it difficult to continue to recover for their own health (Astrachan-Fletcher et al., 2008).
However, between 6 and 36 months postpartum, women in the referent group and women with AN, BN, and EDNOS-P begin to demonstrate a ~0.1 % increase in BMI per month. It may be that eating behavior has stabilized during this time or this slow steady increase may be what most women in this sample are experiencing as they age. Women in the BED group are also experiencing the same slow steady increase in weight at this time but their weight is not increasing as quickly as women in the referent group suggesting that they may still be mindful of their postpartum weight retention. Thus, despite the more rapid changes in weight during pregnancy and the immediate postpartum period, the weight change for women with AN, BN, EDNOS, and referent women remained relatively stable from 6 months to 3 years postpartum.
Limitations
One limitation of the present study is the generalizability of these findings to women outside of Norway. Although, a recent World Health Organization Study found similar patterns of postpartum weight change in Norway, Brazil and the US, we are unable to state conclusively whether these findings could be generalized to women in other nations (Onyango et al., 2011).
Another limitation is a somewhat low response rate. Of invited women, only 38.5% agreed to participate in MoBa. However, this rate is characteristic for large epidemiologic studies and does not necessarily imply a biased sample (Hartge, 2006). MoBa participants are also more educated, more likely to live with a partner and to take multivitamins and folic acid supplements on average (Nilsen et al., 2009). Smokers and mothers with more than two previous births and with previous stillbirths were underrepresented (Nilsen et al., 2009). Also, because some MoBa participants have not reached the 36-month postpartum time period, the number of observations at the last time point, 36 months, is less than half that of the starting point, pre-pregnancy.
In addition, eating disorder status was assessed by self-report. The expansive size and cost of the MoBa effort prohibits structured interviews and observational measures of weight status. It is possible that the self-report questionnaires simply failed to capture some cases. Prevalence estimates of eating disorders in the six months prior and during pregnancy were somewhat lower than point prevalence estimates reported in other population-based studies (Favaro, Ferrara, & Santonastaso, 2003; Hoek, 2006). However, when these same eating disorder questions were used in studies of the Norwegian Twin panel, they yielded prevalence estimates and comorbidity profiles similar to those seen in other large population-based samples (Reichborn-Kjennerud, Bulik, Tambs, et al., 2004; Reichborn-Kjennerud et al., 2003; Reichborn-Kjennerud, Bulik, Kendler, et al., 2004; Reichborn-Kjennerud, Bulik, Sullivan, et al., 2004).
One possibility is that women with eating disorders who participated in MoBa may represent the healthier end of the eating disorder severity spectrum because they had to be well enough to conceive and participate. This likely resulted in fewer cases overall and fewer cases with more severe illness. Also, on average, mothers in MoBa are more likely to be older—30 years old on average at recruitment—which is past the period of highest risk for eating disorder incidence (Favaro, Caregaro, Tenconi, Bosello, & Santonastaso, 2009; Nilsen et al., 2009).
Finally, self-report of weight may be biased across eating disorder subtype (Meyer, McPartlan, Sines, & Waller, 2008). In general, other research has found an extremely high correlation between participants’ self-reported and measured height and weight (Ciarapica, Mauro, Zaccaria, Cannella, & Polito, 2010; McCabe, Knight, Teter, & Wechsler, 2005; Meyer et al., 2008). However, participants with AN report their weight to be ~0.5–2 lbs higher than the measured weight (Ciarapica et al., 2010; McCabe et al., 2005; Meyer et al., 2008). Participants with BN report their weight to be ~0.5–2 lbs lower than the measured weight (Ciarapica et al., 2010; McCabe et al., 2005; Meyer et al., 2008). And participants with BED report their weight to be ~2 lbs lower than the measured weight (White, Masheb, & Grilo, 2010). We hypothesize that these trends would be stable and apply to all time points of measurement. Thus, our estimates of weight trajectory slope (weight change over time) would not differ. However, we hypothesize that our intercept estimates may be biased slightly higher in the case of AN or lower in the case of BN, BED, or in the referent group.
Clinical implications
Clinically, researchers and clinicians have often noted that pregnancy and the transition to motherhood may be an opportune window for recovery. Engaging pregnant women with eating disorders in treatment could have obvious long-term health benefits for the developing fetus but it may also lead to permanent behavior change for mothers (Crow, Keel, Thuras, & Mitchell, 2004). Indeed, on average, women with AN shifted from the underweight BMI range before pregnancy to the normal weight range at 36 months postpartum. It remains to be seen, though, whether the increase in BMI for women with AN over this time period is a function of their experience with motherhood or would whether it could also be expected in a sample of AN women matched by age and length and severity of illness.
Acknowledgments
We are grateful to all the participating families in Norway who take part in this on-going cohort study. This study was supported by R01HD047186 (Bulik: PI). The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and the Ministry of Education and Research, NIH/NIEHS (grant no N01-ES-85433), NIH/NINDS (grant no.1 UO1 NS 047537-01), and the Norwegian Research Council/FUGE (grant no. 151918/S10). Drs. Skinner and Zerwas were supported by an NIH Building Interdisciplinary Careers in Women’s Health award (K12-HD01441). Dr. Zerwas is supported by a NIMH training grant (K01MH100435). Dr. Reba-Harrelson was supported by the Ruth L. Kirschstein National Research Service Award. Dr. Perrin was supported by an NIH Career Development Award (K23-HD051817). Dr. Bulik is a consultant for Shire Pharmaceuticals.
Abbreviations
- AN
anorexia nervosa
- BN
bulimia nervosa
- BED
binge eating disorder
- EDNOS-P
eating disorder not-otherwise-specified, purging type
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 2000. Text Revision. [Google Scholar]
- Astrachan-Fletcher E, Veldhuis C, Lively N, Fowler C, Marcks B. The reciprocal effects of eating disorders and the postpartum period: a review of the literature and recommendations for clinical care. J Womens Health (Larchmt) 2008;17(2):227–239. doi: 10.1089/jwh.2007.0550. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Von Holle A, Hamer R, Knoph Berg C, Torgersen L, Magnus P, Reichborn-Kjennerud T. Patterns of remission, continuation and incidence of broadly defined eating disorders during early pregnancy in the Norwegian Mother and Child Cohort Study (MoBa) Psychol Med. 2007;37(8):1109–1118. doi: 10.1017/S0033291707000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik CM, Von Holle A, Siega-Riz AM, Torgersen L, Lie KK, Hamer RM, Reichborn-Kjennerud T. Birth outcomes in women with eating disorders in the Norwegian Mother and Child cohort study (MoBa) Int J Eat Disord. 2009;42(1):9–18. doi: 10.1002/eat.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter N, Duncan E, Wordsworth P. Bone mineral density in adults with Marfan syndrome. Rheumatology (Oxford) 2000;39(3):307–309. doi: 10.1093/rheumatology/39.3.307. [DOI] [PubMed] [Google Scholar]
- Ciarapica D, Mauro B, Zaccaria M, Cannella C, Polito A. Validity of self-reported body weight and height among women including patients with eating disorders. Eat Weight Disord. 2010;15(1–2):e74–80. doi: 10.1007/BF03325282. [DOI] [PubMed] [Google Scholar]
- Crow SJ, Keel PK, Thuras P, Mitchell JE. Bulimia symptoms and other risk behaviors during pregnancy in women with bulimia nervosa. Int J Eat Disord. 2004;36(2):220–223. doi: 10.1002/eat.20031. [DOI] [PubMed] [Google Scholar]
- Easter A, Bye A, Taborelli E, Corfield F, Schmidt U, Treasure J, Micali N. Recognising the symptoms: how common are eating disorders in pregnancy? Eur Eat Disord Rev. 2013;21(4):340–344. doi: 10.1002/erv.2229. [DOI] [PubMed] [Google Scholar]
- Favaro A, Caregaro L, Tenconi E, Bosello R, Santonastaso P. Time trends in age at onset of anorexia nervosa and bulimia nervosa. J Clin Psychiatry. 2009;70(12):1715–1721. doi: 10.4088/JCP.09m05176blu. [DOI] [PubMed] [Google Scholar]
- Favaro A, Ferrara S, Santonastaso P. The spectrum of eating disorders in young women: a prevalence study in a general population sample. Psychosom Med. 2003;65(4):701–708. doi: 10.1097/01.psy.0000073871.67679.d8. [DOI] [PubMed] [Google Scholar]
- Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;60:549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- Harris JR, Magnus P, Tambs K. The Norwegian Institute of Public Health Twin Panel: a description of the sample and program of research. Twin Res. 2002;5:415–423. doi: 10.1375/136905202320906192. [DOI] [PubMed] [Google Scholar]
- Hartge P. Participation in population studies. Epidemiology. 2006;17:252–254. doi: 10.1097/01.ede.0000209441.24307.92. [DOI] [PubMed] [Google Scholar]
- Hiser Patricia L. Concerns of Multiparas During the Second Postpartum Week. Journal of Obstetric, Gynecologic, & Neonatal Nursing. 1987;16(3):195–203. doi: 10.1111/j.1552-6909.1987.tb01457.x. [DOI] [PubMed] [Google Scholar]
- Hoek HW. Incidence, prevalence and mortality of anorexia nervosa and other eating disorders. Curr Opin Psychiatry. 2006;19(4):389–394. doi: 10.1097/01.yco.0000228759.95237.78. [DOI] [PubMed] [Google Scholar]
- Irgens LM, Bergsjø P, Lie RT. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand. 2000;79:435–439. [PubMed] [Google Scholar]
- Knoph C, Von Holle A, Zerwas S, Torgersen L, Tambs K, Stoltenberg C, Reichborn-Kjennerud T. Course and predictors of maternal eating disorders in the postpartum period. Int J Eat Disord. 2013;46(4):355–368. doi: 10.1002/eat.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: The Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35:1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- Mazzeo SE, Slof-Op’t Landt MC, Jones I, Mitchell K, Kendler KS, Neale MC, Bulik CM. Associations among postpartum depression, eating disorders, and perfectionism in a population-based sample of adult women. Int J Eat Disord. 2006;39(3):202–211. doi: 10.1002/eat.20243. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Knight JR, Teter CJ, Wechsler H. Non-medical use of prescription stimulants among US college students: prevalence and correlates from a national survey. Addiction. 2005;100(1):96–106. doi: 10.1111/j.1360-0443.2005.00944.x. [DOI] [PubMed] [Google Scholar]
- Meltzer-Brody S, Zerwas S, Leserman J, Holle AV, Regis T, Bulik C. Eating disorders and trauma history in women with perinatal depression. J Womens Health (Larchmt) 2011;20(6):863–870. doi: 10.1089/jwh.2010.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C, McPartlan L, Sines J, Waller G. Accuracy of self-reported weight and height: Relationship with eating psychopathology among young women. Int J Eat Disord. 2008 doi: 10.1002/eat.20618. [DOI] [PubMed] [Google Scholar]
- Micali N, De Stavola B, dos-Santos-Silva I, Steenweg-de Graaff J, Jansen PW, Jaddoe VW, Tiemeier H. Perinatal outcomes and gestational weight gain in women with eating disorders: a population-based cohort study. Bjog. 2012;119(12):1493–1502. doi: 10.1111/j.1471-0528.2012.03467.x. [DOI] [PubMed] [Google Scholar]
- Micali N, Simonoff E, Treasure J. Risk of major adverse perinatal outcomes in women with eating disorders. Br J Psychiatry. 2007;190:255–259. doi: 10.1192/bjp.bp.106.020768. [DOI] [PubMed] [Google Scholar]
- Micali N, Treasure J. Biological effects of a maternal ED on pregnancy and foetal development: a review. Eur Eat Disord Rev. 2009;17(6):448–454. doi: 10.1002/erv.963. [DOI] [PubMed] [Google Scholar]
- Nilsen RM, Vollset SE, Gjessing HK, Skjaerven R, Melve KK, Schreuder P, Magnus P. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23(6):597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- Onyango AW, Nommsen-Rivers L, Siyam A, Borghi E, de Onis M, Garza C Group, W. H. O. Multicentre Growth Reference Study. Post-partum weight change patterns in the WHO Multicentre Growth Reference Study. Matern Child Nutr. 2011;7(3):228–240. doi: 10.1111/j.1740-8709.2010.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. Retrieved from http://www.R-project.org. [Google Scholar]
- Reichborn-Kjennerud T, Bulik CB, Tambs K, Harris JR. Genetic and environmental influences on binge eating in the absence of compensatory behaviours: a population-based twin study. Int J Eat Disord. 2004;36:307–314. doi: 10.1002/eat.20047. [DOI] [PubMed] [Google Scholar]
- Reichborn-Kjennerud T, Bulik CM, Kendler KS, Maes H, Roysamb E, Tambs K, Harris JR. Gender differences in binge-eating: A population-based twin study. Acta Psychiatr Scand. 2003;108:196–202. doi: 10.1034/j.1600-0447.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- Reichborn-Kjennerud T, Bulik CM, Kendler KS, Roysamb E, Tambs K, Harris JR, Torgeson S. Influence of weight on self-evaluation: A population-based study of gender differences. Int J Eat Disord. 2004;35:123–132. doi: 10.1002/eat.10252. [DOI] [PubMed] [Google Scholar]
- Reichborn-Kjennerud T, Bulik CM, Sullivan PF, Tambs K, Harris JR. Psychiatric and medical symptoms in binge eating in the absence of compensatory behaviors. Obes Res. 2004;12:1445–1454. doi: 10.1038/oby.2004.181. [DOI] [PubMed] [Google Scholar]
- SAS/STAT® 9.2 User’s Guide. Cary, NC: SAS Institute Inc; 2008. [Google Scholar]
- Siega-Riz AM, Haugen M, Meltzer HM, Von Holle A, Hamer R, Torgersen L, Bulik CM. Nutrient and food group intakes of women with and without bulimia nervosa and binge eating disorder during pregnancy. Am J Clin Nutr. 2008;87(5):1346–1355. doi: 10.1093/ajcn/87.5.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobal J, Stunkard AJ. Socioeconomic status and obesity: a review of the literature. Psychol Bull. 1989;105(2):260–275. doi: 10.1037/0033-2909.105.2.260. [DOI] [PubMed] [Google Scholar]
- Stein A, Fairburn CG. Eating habits and attitudes in the postpartum period. Psychosom Med. 1996;58:321–325. doi: 10.1097/00006842-199607000-00004. [DOI] [PubMed] [Google Scholar]
- Stuebe AM, Kleinman K, Gillman MW, Rifas-Shiman SL, Gunderson EP, Rich-Edwards J. Duration of lactation and maternal metabolism at 3 years postpartum. J Womens Health (Larchmt) 2010;19(5):941–950. doi: 10.1089/jwh.2009.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss Robert E. Modeling Longitudinal Data. New York, NY: Springer-Verlag; 2005. [Google Scholar]
- White MA, Masheb RM, Grilo CM. Accuracy of self-reported weight and height in binge eating disorder: misreport is not related to psychological factors. Obesity (Silver Spring) 2010;18(6):1266–1269. doi: 10.1038/oby.2009.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO; W. H. Organization, editor. Indicators for assessing breastfeeding practices. Geneva: 1991. WHO/CDD/SER/91.14, Corr. 1. [Google Scholar]