Abstract
Introduction
Unintentional weight gain is commonly observed in adult humans, often provoking intentional weight loss attempts followed by unintentional weight regain. This episodic variation in body weight over a period of time has been referred to as “weight cycling”. Over the last two decades, weight cycling has been associated with a number of morbid health conditions and increased mortality. This article provides a comprehensive evaluation of recent weight cycling evidence, looks to understand design differences between studies and study outcomes, assesses the need for further research on particular health outcomes and proposes alternative methodologies that will bridge the needs and capabilities of research.
Methods
Searches were conducted per PRISMA guidelines. Articles on weight cycling in the literature were initially identified using search strings in PubMed. Eligibility assessment of the remaining articles was performed independently by three reviewers to identify publications that presented direct evidence.
Results
Twenty human studies (in addition to seven animal studies) were selected and retained; 12 accounted for the intentionality of weight loss.
Discussion
Although weight regain following successful weight loss remains one of the most challenging aspects of body weight regulation, evidence for an adverse effect of weight cycling appears sparse, if it exists at all.
Keywords: weight cycling, mortality, morbidity, intentional weight loss
Introduction
Approximately 80% of individuals who intentionally achieve weight loss of ≥10% body weight will regain that weight within one year (1). It has been suggested that short periods of weight loss followed by weight gain (i.e. weight cycling) adversely affect health outcomes – particularly morbidity and mortality in humans. Discussion of the potential dangers of weight cycling and its relationship to mortality became prevalent in the scientific literature during the 1990s (2). It was proposed, based on initial animal studies (3) and human epidemiological reports (4), that weight cycling may be detrimental to health. Since then, a pervasive view found in many media outlets encouraged caution when embarking on weight-loss regimens, due to the “dangers” of weight cycling (2, 5). The messages are not unique to lay media outlets and can be found in various scientific writings. While many reports focus on health and disease patterns rather than mortality directly, the topic is of high interest and is a concern for those hoping to improve their health through weight reduction. Attributed primarily to the lack of clearly defined outcomes and the inability to randomize persons to levels of weight variability, recent epidemiological studies have challenged the contribution of weight cycling to mortality (6). To date, evidentiary support has precluded clarity(7).
Despite the commonly held belief that weight cycling increases morbidity and disease risk, evidence from rodent models is inconsistent, and that from humans even less compelling (2, 6-9). The lack of a standard definition for weight cycling, the complexity of weight cycling patterns, and the limitations in the design and methods of analysis of weight cycling studies make it difficult to compare risk and benefit across studies (2, 10-12). This has led to challenges in drawing definitive conclusions (2, 4, 7, 13).
By exploring evidence published in the past 5 years (the approximate time since the last published review on long-term weight loss and mortality (14) and morbidity (15, 16)) and discrepancies between findings, this review attempts to draw a consensus on the relationship between weight cycling and mortality and morbidity (e.g. dyslipidemia, dysglycemia, cancer). The focus of the review is on weight cycling in terms of weight loss followed by unintentional weight regain. Limitations and confounds in the design and analyses of recent studies and implications for clinical practice are also discussed. Finally, recommendations are offered for the design of a definitive study to help resolve the impact of weight cycling on morbidity and mortality.
Methods
Literature search
All searches were conducted per PRISMA guidelines. Articles on weight cycling in the literature were initially identified using search strings in PubMed. A union of the following descriptors was used to create a search string: “short term weight change”, “weight-cycling”, “yo-yo dieting”, (“weight patterns” AND cycling), “BMI fluctuation”, (“allostatic load of weight” and Tremblay), “weight cyclers”, (willett AND “weight cycling”), ((tremblay AND doucet), (“weight loss” AND regain)), (“frequency” and “intentional” or “unintentional” and “weight loss” and “risk factor”) and (“patterns of weight loss or regain” and (long-term or longitudinal)). Articles were restricted to a publication date from 2008-09-08 to 2013-09-06 and to the English language. The initial search string was entered in the PubMed builder on 6 September 2013 and is presented in the Appendix. Our focus was on the general able-bodied population and not people with physical disabilities or athletes. Hence, the search was narrowed to exclude articles focusing specifically on athletes, pregnant women or surgery. Studies from animal models were also included in the review. The narrowed search terms were related to (((“weight loss” AND “regain”) OR (“weight cycling”) OR (“yo yo”)) AND (diabetes OR cardiovascular OR cancer OR adiposity OR “body composition” OR mortality)). The final search string entered into the PubMed builder on 15 October 2013 is presented in the Appendix. The primary clinical aim of this review was to assess the level of evidence for any association of weight cycling with mortality and morbidity. Articles that were clearly unrelated to the clinical question, based on a review of titles and abstracts, were not included. Eligibility assessment of the remaining articles was performed independently by three reviewers to identify publications that presented direct evidence.
Data abstraction
At least one reviewer evaluated each manuscript independently to tabulate information on the: 1) primary outcome of the study; 2) age range of the sample; 3) length of follow-up of the study; 4) reference category when comparing the association of weight cycling with an outcome; 5) definition of intentional weight loss used and its accounting in the modeling; 6) definition of weight cycling definition used; 7) self-reported or measured weight data. A clear description in the text of what constituted weight cycling was required for inclusion. We considered that intentional weight loss (IWL) had been defined in the study if there was any explicit assessment of IWL, or the weight loss was followed as part of an intervention (e.g. diet) to reduce weight.
Results
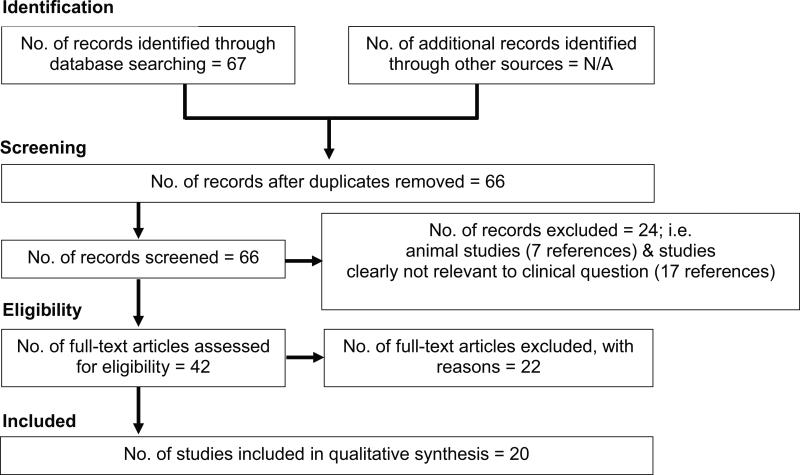
The literature search between the dates 2008-10-17 to 2013-10-15 yielded a total of 79 articles, of which 12 were excluded to eliminate publications specifically about athletes, pregnant women and surgery. Our interest was in reviewing the association or the effect of weight cycling on all-cause mortality, cardiometabolic condition or cancer. Of the remaining 67 articles, one was a duplicate, 17 were not considered relevant to the clinical question and were removed, and seven were from animal studies. Of the 42 remaining articles, only 20 were retained (Figure 1). Reasons for exclusion included (but were not limited to) vague or questionable definitions of weight cycle (17-19). For example, Yoo et al. (18) defined weight cycling as weight change that exceeded 5% of the initial body weight. Of the 20 human studies selected and retained (6, 20-38), 12 accounted for the intentionality of weight loss in their analyses (6, 20, 22, 28, 30-32, 34-38).
Figure 1.
Selection process and final number of human studies included in the analysis
Results from animal studies
Across the seven animal studies (8, 9, 39-43), sample sizes were small, with all studies performed in one species or strain (four rodent studies used the C57BL/6J inbred strain of mice), in one biological sex, and for a relatively short duration (≤1 year, other than List et al.(8)) (Table 1). Exploiting the shorter lifespan of many laboratory animal models, to date no studies have reported associations between mortality and intentional weight cycling (8). Recent evidence associating weight cycling with risk factors for bone and metabolic disorders were identified. In rats, weight cycling was associated with significantly lower bone mineral content, compared with non-weight cycling rats (39) albeit in the context of a calcium phosphate-deficient diet. Elevated fasting glucose and impaired glucose tolerance, along with adipose tissue insulin sensitivity impairment, were present in rodents following weight cycling, compared with weight-matched obese rodents that gained weight but did not undergo cycling (40, 42). However, these glucose alterations were primarily in the context of diet cycling (switching between low-fat and high-fat / high-sugar diets), and weight cycling did not consistently impair glucose metabolism to a greater extent than higher body weight-matched controls.
Table 1.
Design of animal weight-cycling studies and outcome types (published 2008-09-08 to 2013-09-06)
| Study | Primary Outcome | Sex, strain | Length of follow-up | Weight loss | Sample size | Findings | Limitations |
|---|---|---|---|---|---|---|---|
| List et al. 20138 | Mortality | Male, C57BL/6 J mice | Lifespan | Three groups: 1) low-fat diet (LFD); 2) high-fat diet (HFD); 3) weight cyclers | n = 10, 9, 9 for HF, LF and weight cycling (WC) groups respectively | -No difference in lifespan WC compared with LF, but weighed more and ate HF for half their lifespan -HF decreased lifespan vs LF and WC mice |
Diet cycling |
| Bogden et al. 200839 | Bone density | Female, Sprague-Dawley rats | 21 weeks | Six groups (3×2): 1)weight maintenance/ gain; 2)one weight cycle; 3) two weight cycles | n = 84 (14 rats/ group) | -Weight cyclers had lower bone mineral content and marginally lower bone mineral density; resistant starch reduced the loss | All groups had calcium deficient diets |
| Anderson et al. 201340 | Glucose tolerance | Male, C57BL/6 J mice | 27 weeks | Three groups: 1)27 wks LFD 2)9 wks HFD/ 9 wks LFD/ 9 wks/ HFD 3)9 wks LFD/ 18 wks HFD |
Variable (n = 4–17 per group) | -Systemic glucose tolerance decreased and impaired adipose tissue (AT) insulin sensitivity compared with non-WC -WC increases adaptive immune response |
Diet cycling |
| Barbosa-da-Silva et al. 201242 | Adipose inflammation | Male, C57BL/6 mice | 6 months | 2 months WL (standard chow (SC)), 2 months WG (high-fat (HF) chow), 2 months WL (e.g. SC, HF, SC; HF, SC, HF), no overt restriction | 10 groups, n = 8/ group | -At 6 months, less weight loss with SC, more gain with HF | Diet cycling |
| Barbosa-da-Silva et al. 201341 | Liver | Male, C57BL/6 mice | 6 months | SC6 only standard chow (SC), LFD 10% kcal fat, 6 months; (ii) HF6 group only HFD, 60% kcal fat, (iii) SC2/HF2/SC2 - LFD 2 months, HFD 2 months, LFD 2 months; and (iv) HF2/SC2/HF2 – HFD 2 months, LFD 2 months, HFD 2 months | 4 groups, n = 8/group | HF liver steatosis | Diet cycling |
| Nagaoka et al. 201043 | Body weight/fat | Female, adult beagles | Unclear | 19 weeks obesity induction (pecan cookies with food), intentional restriction, 17 weeks regain | n = 9 | -Less time and fewer calories to re-induce same obesity -Increased food efficiency |
Lack of age-matched controls |
| McMillen et al. 20139 | Atherosclerosis and cardiac | LDL receptor-deficient mice | 29 weeks | 3 groups: 1)Weight gain (HFD) 2)Alternating HFD and normal rodent chow (4 wks HFD × 4 wks normal rodent chow (LFD), then 5 wks × 5 wks, then 7 wks × 4 wks) 3)little weight gain (LFD of rodent chow) |
n = 4–8/group | -WC improved glucose homeostasis during WL -WC reduced atherosclerosis versus HFD, but increased compared with LFD |
Diet cycling |
Results from human studies
Across the 20 human studies that met the eligibility criteria (Table 2), there was no consistent definition used for weight cycling (examples are given in Table 3) (6, 20-38). A tabulated summary of the major findings is included in the Appendix.
Table 2.
Human weight-cycling studies that consider intentional weight loss in their design (published 2008-09-08 to 2013-09-06)
| Study reference | Outcome type | Intentional weight loss | Sample size | Age | Sex | Length of follow-up (years) | Reported # cycles | Weight loss per cycle | Cycle type (degree of energy balance)* | |
|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes (#) | |||||||||
| Field et al. 200920 | Mortality | Yes | 44,876 | 30–55 | Female | 12 | Repeated intentional weight losses reported (≥3 times in the past 4 years) | ‘Severe’ weight cyclers: ≥9.1 kg (≥20 lbs) ‘Mild’ weight cyclers: ≥4.5 kg (10 lbs) |
Precise cycle type unclear | |
| Strychar et al. 200922 | CVD risk factors (e.g. dyslipidemia, dysglycemia) | Yes | 121 | Post-menopausal | Female | Cross-sectional | Repeated intentional weight losses reported (never, once, 2–3 times, ≥4 times in their lifetime) | >10.0 kg | Precise cycle type unclear | |
| Cereda et al. 201128 | Body composition | Yes | 914 | 51.1 ± 14.2 | Male and female | Cross-sectional | ≥5 intentional weight losses reported followed by rapid return to pre-diet/higher body weight (time period not specified) | ≥5.0 kg (≥11 lbs) | Repeated cycles of negative energy balance to neutral or positive energy balance | |
| Blüher et al. 201230 | Dysglycemia, dyslipidemia | Yes | 322 | 40–65 | Male and female | 2 | Rapid weight loss phase (0-6 months), then partial/complete reversal phase (i.e. weight maintenance or regain; 7–24 months) | Weight loss during dietary intervention | Remains in negative energy balance or possibly shifts to neutral or positive energy balance | |
| Neiberg et al. 201231 | Dysglycemia and dyslipidemia | Yes | 2,438 | 45–76 | Male and female | 4 | Weight change trajectory of weight loss followed by slight regain (i.e. slightly U-shaped) during the first year of lifestyle intervention | Weight loss during lifestyle intervention | Remains in negative energy balance | |
| Stevens et al. 2012 (Cancer Epidemiol Biomarkers Prev)32 | Cancer | Yes | 38,148 | 50–74 | Female | 15 | Lifetime number of times that weight was intentionally lost and later regain of as much weight as was lost | ≥4.5 kg (≥10 lbs) | Repeated cycles of negative energy balance to neutral or possibly shifts to positive energy balance | |
| Stevens et al. 2012 (Am J Epidemiol)6 | Mortality | Yes | 122,638 | 50–74 | Male and female | 16 | Lifetime number of times that weight was intentionally lost and then later regain of as much weight as was lost | ≥4.5 kg (≥10 lbs) | Repeated cycles of negative energy balance to neutral or possibly shifts to positive energy balance | |
| Ambeba et al. 201334 | CVD risk factors (e.g. inflammatory cytokines) | Yes | 66 | 48.4 ± 7.3 | Male and female | 2 | Loss and then regain of weight during a 24-month weight loss intervention | ≥4.5 kg (≥10 lbs) | Negative energy balance to neutral energy balance or possibly shifts to positive energy balance | |
| Bosy-Westphal et al. 201335 | Body composition | Yes | 103 | 22-45 | Male and female | 13-week diet intervention, 6 month follow-up | Weight regainers: Weight loss during 13 week intervention followed by weight regain of ≥30% of weight loss during 6 month follow-up (Weight stable was weight change±20% of weight loss) |
Weight loss during 13 week intervention | Remains in negative energy balance, or possibly shifts to neutral or positive energy balance | |
| Haufe et al. 201336 | Dysglycemia | Yes | 50 | 46 ± 9 | Male and female | 2 | Difference between body weight at follow-up and body weight after the active dietary intervention period at 6 months (mean 24 months follow-up; mean weight regain not complete at follow-up) | Weight loss during 6 month intervention | Remains in negative energy balance (based on mean weight regain) | |
| Mauriège et al. 201337 | Body composition | Yes | 19 | 35 – 48 | Male and female | 15-week weight loss program; 17–22 month follow-up | Body weight loss during 15-week weight loss program and then regain to baseline at 17–22-month follow-up (mean weight regain complete at follow-up) | Weight loss during 15-week intervention | Negative energy balance to neutral energy balance (based on mean weight regain) | |
| Nagle et al. 201338 | Cancer | Yes | 2936 | 18–79 | Female | Cross-sectional | ≥1 episodes intentional weight loss and then a regain of most of that weight within 12 months | >9 kg (>20 lbs) | Remains in negative energy balance, or possibly shifts to neutral or positive energy balance | |
Energy balance descriptions are based on interpretation of the study design and are not noted in the publications
Table 3.
Examples of weight-cycling definitions reported in human studies (synonyms: weight fluctuation, weight variation, yo-yo dieting, weight instability)
| Source | Definition |
|---|---|
| Thompson & McTiernan 201112 | Weight cycling: Typical pattern is weight loss and then regain in a repetitive cycle. |
| Waring et al. 201027 | Weight cycling: For sensitivity analysis, reflects weight cycles of ~2 kg/m2 or more. |
| Arnold et al. 201023 | Weight cycling: Loss and gain of ≥5% of body weight over time during yearly assessments of weight changes (ie, comparing current weight against weight in the previous year and the initial study year). |
| Lee et al. 201026 | Weight cycling: Weight loss of ≥3% of baseline weight for the first-year follow-up period, with regain to within ±3% of baseline weight by the second-year follow-up. |
| Yoo et al. 201018 | Weight cycling: Weight change >5% of initial body weight within the previous 2 years. |
| Field et al. 200920 | Severe weight cyclers: Women who reported they had intentionally lost ≥9.1 kg (20 lbs) at least 3 times. Mild weight cyclers: Women who had intentionally lost ≥4.5 kg (10 lbs) at least 3 times, but did not meet the criteria for severe weight cycling. Information was provided from the Nurses’ Health Study on intentional weight losses between a 20-year period (1972 to 1992). |
| Stevens et al. 20126 | Weight cycle: An intentional loss of ≥10 pounds (≥4.5kg) followed by regain of that weight. The lifetime number of weight cycles was reported on a questionnaire. |
| Kataja-Tuomola et al. 201024 | Weight fluctuation: Root mean square error (RMSE) using annual weight deviations from the corresponding estimated regression line (divided into quintiles). |
| Taing et al. 201133 | Weight cyclers: Those who experienced both a period of weight gain and loss (≥5%) based on weight changes during early, mid, and late adulthood. |
| Cereda et al. 201128 | Weight cycling: ≥5 intentional weight loss episodes of ≥5 kg followed by rapid return to pre-diet or higher body weight, with no limit on the length of time for weight loss and regain. |
| Osborn et al. 201129 | Weight cyclers: Those providing a response of “once” to “five times or more” to question 9 of the Questionnaire on Eating and Weight Patterns Revised (QEWP-R): “How many times (approximately) have you lost 20 lbs (9.1 kg) or more when you weren't sick and then gained it back?” |
| Mason et al. 201319 | Weight cyclers: In answer to the question “Since you were 18 years old, how many different times did you lose each of the following amounts of weight on purpose (excluding pregnancy or illness): 5–9 lbs, 10–19 lbs, 20–49 lbs, 50–79 lbs, 80–99 lbs, ≥100 lbs?”. Severe cyclers reported losing ≥20 lbs (≥9.9 kg) on three or more occasions; moderate cyclers reported losing ≥10 lbs (≥4.5 kg) on three or more occasions and do not classify as severe. Weight regain was not quantified after each loss. Since all participants were overweight/obese at baseline, at least partial weight regain was assumed, particularly among those with ≥3 episodes. |
| Hooper et al. 201017 | Overweight or obese women were asked “Within the last 20 years, when you were not pregnant or sick, did you ever lose 10 pounds or more on purpose?”, and reported how often they had lost between 10 and 19 pounds, between 20 and 49 pounds, and 50 pounds or more. Since participants were overweight or obese, weight regain over the past 20 years was assumed. |
Mortality
Four publications focused on mortality as an outcome (6, 20, 23, 33). Of these, three (6, 23, 33) evaluated mortality in middle-aged to older women, and two (6, 20) accounted for the intentionality of weight loss in some manner. In three studies (6, 20, 33), no statistically significant association was observed between weight cycling and elevated mortality, independent of body mass index (BMI). Only Arnold et al. (23) including older adult males and females, reported a positive association between weight cycling and mortality after adjusting for race and sex. However, this analysis did not consider the intentionality of the weight loss in the modeling. Given the age of the population (>65 years) this may represent a substantial area of confounding.
Cardiometabolic risk/biomarkers (cardiovascular disease- and diabetes-related)
There were 14 publications related to cardiometabolic conditions (21, 22, 24-31, 34-37), which included diabetes/insulin resistance, dyslipidemia/cardiovascular disease (CVD) and body composition (fat and lean mass). Ten articles examined the association of weight cycling with outcomes or biomarkers related to diabetes or CVD (21, 22, 24, 25, 27, 29-31, 34, 36), and four focused on body composition (26, 28, 35, 37). Of those related to diabetes or CVD, three examined CVD-related factors specifically (25, 29, 34) and seven studied associations related to diabetes (21, 22, 24, 27, 30, 31, 36). Seven publications focused on the association of weight cycling to an outcome related to dyslipidemia and CVD (21, 22, 25, 29-31, 34), of which three presented evidence indicative of a deleterious association between cardiovascular health and weight cycling (21, 25, 30). Lee et al. (25) found that the greatest fluctuation in BMI (in adults, interpreted as weight) was associated with greater risk of coronary artery calcification, compared with the reference category of the quintile of least weight fluctuation. Blüher et al. (30) found that a biomarker profile of insulin, triglycerides, leptin, chemerin, monocyte chemoattractant protein 1, and retinol-binding protein corresponded to changes in body weight; a trend towards decreased levels was observed during the first 6 months corresponding with the weight-loss phase (i.e. the shift indicating health improvement) and a trend of increasing levels in months 7–24 during the weight maintenance/weight-regain phase (i.e. the shift indicating health decline). Finally, Kim et al. (21) reported that frequent weight cycling was associated with shorter telomere length, which is a risk factor for several comorbidities including CVD. Two of these studies (21, 30) had relatively small sample sizes.
Of the seven studies that examined the association of weight cycling with outcomes related to diabetes and/or insulin resistance (21, 22, 24, 27, 30, 31, 36), one demonstrated a deleterious association (24), while two demonstrated a statistically significant beneficial association (30, 36). Kataja-Tuomola et al. (24) reported that, in Finnish male smokers, a group with large weight fluctuations was associated with an increased risk of diabetes compared with one showing small weight fluctuations. While this study had a large sample size (~21,000), intentionality of weight change was not accounted for. Interestingly, the one study that accounted for the intentionality of weight loss showed beneficial associations of weight cycling with diabetes-related outcomes (30)., The Blüher et al. (30) weight-loss intervention study investigated biomarkers related to cardiometabolic risk, and found two differing patterns in relation to weight cycling. The first pattern was characterized by biomarker levels of insulin, triglycerides, leptin, chemerin, monocyte chemoattractant protein 1, and retinol-binding protein that decreased with weight reduction in the weight-loss period, and increased, returning towards baseline levels, during the weight regain/maintenance phase. However, a contrasting pattern emerged for other biomarkers: high-density lipoprotein cholesterol (HDL-C), high-sensitivity C-reactive protein (hsCRP), high molecular weight (HMW) adiponectin, fetuin-A, progranulin and vaspin levels were significantly improved, even after partial weight regain. Although the weight loss in this study was intentional, the generalizability of the findings to free-living conditions is limited because of the unique workplace intervention setting it used, in which all food dishes were coded according to diet type and served daily in the cafeteria over a 2-year span.
Out of the four studies with body composition as the outcome, we found that only two studies were associated with an adverse outcome (26, 28). In a cross-sectional study, Cereda et al. (28) reported that weight cycling was associated with excess body weight and abdominal fat accumulation. Lee et al. (26) found that in older men and women (70–79 years old) lean mass lost during the weight-loss period was not fully regained during the weight-regain period. Also, there was a net gain in fat mass, which was statistically significant. In contrast, they also found that among race-matched women, weight-stable controls did not report any significant decline in lean mass or fat mass. However, in the race-matched weight-stable men, there was a net loss in lean mass and gain in fat mass. Bosy-Westphal et al. (35) presented results for overweight and obese adults whose measurements were collected at baseline, after weight-loss intervention (of 13 weeks approximately) and in a follow-up 6 months after the end of the weight-loss intervention. They demonstrated that intentional weight loss followed by regain did not adversely affect body fat distribution. However, weight regainers showed a statistically significant decrease in resting energy expenditure (REE) with weight loss, possibly indicating a loss of lean mass or other physiological adaptations to weight loss that persist even in the follow-up phase when weight is regained. However, when comparing the phase-specific changes between weight regainers and weight-stable groups, the decline in the REE of weight regainers was not significantly different from the decline in the REE of the weight-stable group.
Cancer risk
In the two publications identified that related to weight cycling and cancer, endometrial cancer was specifically referenced (32, 38). Nagle et al. (38) reported a significant association between weight cycling and both endometrial as well as non-endometrial cancer. However, the study by Stevens et al. (32) observed that, compared with weight stable individuals and independently of BMI, weight cyclers were not at increased risk of endometrial cancer. The study by Stevens et al. had a substantially larger sample size (N = ~35,000) than that of Nagle et al. (N < 3000), and was a longitudinal versus a cross-sectional case–control study.
Discussion
Messaging related to intentional weight loss has often been muddled with the apparent risk of weight cycling. Most weight-loss interventions fail when the adherence level drops. In this review, we have qualitatively synthesized the associations between health outcomes and intentional weight cycling, based on articles published in the last five years.
The animal models used for studying the impact of weight cycling were primarily rodent models (8, 9, 39-42). While rodents are a primary pre-clinical research model, extrapolation from animal studies to the clinical setting should be undertaken with caution due to translational limitations. Nevertheless, animal models continue to provide mechanistic insight regarding the impact of dietary manipulations of body weight and body composition into metabolic, physiologic and pathologic outcomes. Our review did not find any report of elevated mortality risk associated with weight cycling in animals when comparisons were made with a stable-weight obese group. In fact, the sole longevity study of weight cycling found no significant difference between weight cycling mice and low-fat fed controls; both weight cyclers and low-fat fed mice survived longer than high-fat fed animals (8). Thus, although the weight cycling mice had a higher average body weight and greater caloric intake than their smaller, low-fat fed counterparts, they exhibited no apparent mortality risk elevation. Some studies reported deleterious effects of weight cycling, such as risk factors for bone and metabolic disorders, compared with weight-matched animals that had never weight cycled (9, 39, 40, 42). However, these studies are often confounded by the diet regimen provided (changing between diet compositions) in that the outcomes cannot be specifically attributable to the weight cycle given the conflated diet cycling. Furthermore, the limited study durations (6 months or less), low number of weight cycling events, the need for additional control group comparisons (e.g. weight-matched, non-cycling controls), low sample sizes utilized and the lack of sex comparisons leave many questions to be answered regarding the robustness of current findings (see Table 1).
Human studies were primarily epidemiological studies. The majority assessed participants at multiple time points (6, 20, 23-27, 30-37) and a few studies were cross-sectional (21, 22, 28, 29, 38). Only Arnold et al. (23) reported a deleterious association of weight cycling with mortality. The differences between the findings of Arnold et al. (23) and others could be due to the population chosen (individuals were older in the Arnold et al. study) as well as the analytic choices – such as consideration of the intentionality of weight loss, and adjusting for BMI at baseline. For example, sensitivity analyses were conducted that included only those participants who self-reported good to excellent health in 1998–1999 in order to reduce the likelihood of recording weight changes due to poor health. In addition, the model chosen in Arnold et al. (23) did not adjust for baseline BMI, which could confound the risk of weight cycling with mortality. This confounding due to baseline BMI was evident in the study by Stevens et al. (6), in which models not adjusted for baseline BMI reported a harmful association of weight cycling with mortality. After accounting for BMI, an association was no longer apparent. Several studies evaluated the association between weight cycling and cardiometabolic markers (21, 22, 24-31, 34-37). Most of these studies were relatively small in sample size (N < 1000). In a large study, Kataja-Tuomola et al. (24) reported that weight fluctuation did increase the risk of diabetes in Finnish males. However, the intentionality of weight loss was not considered and the participants were smokers, who have a higher mortality relative to non-smokers with greatest mortality in those with very high or very low BMI (44). Evidence for an adverse effect between weight cycling and cardiometabolic markers appears sparse, if it exists at all. Evidence for a harmful association between weight cycling and body composition was primarily present in two studies (26, 28) with strong caveats. While the study by Cereda et al. (28) is a cross-sectional study, the study by Lee et al. (26) is based on an older cohort of subjects. In Lee et al. (26), no between-group comparisons were made between weight cyclers and the weight stable group, limiting our ability to draw any conclusions about an association between weight cycling and body composition when compared to the weight stable group. Results from Bosy-Westphal et al. (35), which compared the phase-specific changes in REE between weight regainers and weight-stable subjects suggests that weight cycling was not adversely associated with REE when compared to the weight-stable group. The two studies we reviewed that had examined the relationship between weight cycling and cancer yielded conflicting findings, and clearly additional studies are required before conclusions can be made regarding this outcome (32, 38).
Although no study is without its limitations, there are multiple advantages to using randomized controlled experiments with animals in weight cycling studies. For instance, much is known about the impact of diet composition on body weight gain and fat mass accrual across multiple strains of mice and rats. By leveraging this information, a randomized study design that includes rodents of both sexes and focuses on weight cycling (i.e. weight loss through energy restriction and regain through restraint relief), rather than “diet cycling” (high-fat diet to low-fat diet), would improve understanding of the impact on morbidity and mortality outcomes. Such a design would require adequate sample sizes (n > 40 per group), sufficient control groups (with appropriate levels of body weight based on feeding the same diet at varying levels for low body weight, together with non-restricted obese controls), and longitudinal or multiple measures of primary metabolic outcomes. Furthermore, weight cycling studies in humans have largely focused on adult populations, whereas many previous animal studies have either utilized young animals that are often very lean at study initiation, or have investigated repetitive, short-duration weight cycles across the lifespan. By initiating animal studies of weight cycling in an established obese state – such as in mature adult models – a clearer understanding of the impact on metabolic and physiologic outcomes will be possible.
In animal studies, control over diet provision can have positive and negative effects on study design and applicability of results. While such control allows for accurate and reliable assessment of energy balance, providing a fixed macronutrient composition at every feeding may not adequately reflect human dietary patterns. Furthermore, studies that vary macronutrient compositions to alter body weight and adiposity in the weight-gain and weight-loss phases are confounded by contribution of diet quality to health outcomes layered onto the weight-cycling phenotype (e.g. high fat diet has greater adverse health effects in the diet-induced obesity mouse model) (45). Animal studies designed to accommodate even several diet compositions are not generalizable to “uncontrolled” human feeding behavior and consequential changes in weight, body composition and disease risk. Future studies using animal models should attempt to incorporate a larger sample size and multiple/intermediate measures and tests for mediator effects in relation to longevity outcomes.
There are several challenges involved in comparing evidence across human weight cycling studies and in drawing any conclusions with respect to morbidity and mortality. One important challenge was the varied and sometimes questionable definitions used for weight cycling (Table 3). Some studies, for example, assumed that participants were weight cyclers if they had purposely and repeatedly lost weight (e.g. ≥10 pounds) in the preceding 20 years but were nevertheless currently overweight or obese despite this (17, 19). Moreover, several studies did not consider intentionality of weight loss while defining weight cycling (23-27, 29, 33). In this review, we focus on weight cycling in terms of weight loss followed by unintentional weight regain. Intentionality is an important factor, as unintentional and/or unexplained loss of body weight in human and rodent models is associated with impending mortality (46). Even within studies where weight cycling was defined in terms of intentional weight loss, all the weight loss was attributed to the intention (or intervention). This may be inadequate as previous work has demonstrated that an expression of intent to lose weight does not necessarily validate subsequent weight loss (47, 48), such as that resulting from a diet or lifestyle change. Analytic approaches often varied between studies, for example in the accounting for baseline BMI or other variables that potentially lie in the causal path between weight cycling and mortality. It has also been proposed that weight cycling may be defined in the context of energy balance (e.g. weight cycling where weight is lost, and some of it is regained, versus when weight is lost, regained, and exceeds baseline weight). This too, presents some limitations. For example, negative energy balance can only be sustained for some finite period, and overall, positive energy balance is present in most organisms across the duration of a healthy lifespan, with weight loss (negative energy balance) accompanying or preceding morbidity and/or mortality. In this context, the adverse effects of greater fluctuations in weight (as in the Lee study (25)) on health outcomes may reside in the degree of obesity, and thus an indirect association may have been spurious. Finally, the populations under study varied; for example, some studies were limited to older age groups or to smokers. More definitive, individual-level meta-analytic studies are required (49), especially when assessing the risk of cardiometabolic outcomes in weight cycling. Study design and methodological considerations to address in future studies include: 1) statistical rigour and confounds, 2) measured versus self-reported variables, 3) limitations of epidemiologic studies, 4) short-term (acute) improvement/ biomarker detection versus long-term benefit to morbidity and mortality 5) patterns of weight loss and regain, including the time frame of weight loss and regain (e.g. if weight loss is maintained over a longer period) and 6) intentional versus unintentional weight loss (or no assessment of intention (50-52)) versus weight stabilization (2, 20, 53-59).
While we acknowledge that extrapolating results from animal studies to humans is somewhat difficult and should be undertaken with caution, the present research paradigm in science relies heavily on pre-clinical findings in laboratory models (both cell and animal) to inform our understanding of human outcomes. It remains highly unlikely that any long-term human study – particularly as it relates to morbidity and mortality outcomes – will ever be performed by a randomized control trial of weight cycling. Thus, animal studies can be important to: 1) update the scientific community about recent findings; 2) point out the progress and remaining limitations in the animal work; 3) suggest approaches for future work to move beyond these current barriers in knowledge and 4) reinforce the growing consensus from human observational studies that there is no strong justification for concern regarding weight cycling on morbidity, and mortality particularly. Several methodological opportunities exist for strengthening the evidence related to weight cycling. Coffey and colleagues have presented a statistical modelling framework where the intentional weight-loss effect is treated as a latent variable problem, which relies on the assumption of random assignment to a group intending to lose weight and group not intending to lose weight (47, 48). This assumption is unlikely to be met in most human studies. To address this challenge of lack of randomization in human weight cycling studies, non-traditional designs such as Mendelian randomization (60) may be worth exploring. In addition, genetic markers may explain the propensity for non-adherence to behavioral adaptations following intentional weight loss that leads to weight regain. These genetic markers could then be used as instruments for randomizing groups of study participants into weight cyclers or non-weight cyclers. Furthermore, predictive modelling techniques such as energy balance models that predict body composition changes and energy intake based on weight changes and other parameters, can be used to identify and define patterns of weight cycling that are clinically relevant (61, 62).
In conclusion, there is no evidence that weight cycling is causally associated with early mortality compared to remaining overweight or obese. Very few studies report an adverse health effect of weight cycling on morbidity. In fact, morbidity may contribute to the relationship such that weight cycling in the disease state often differs from that for individuals without comorbidities. Because findings are confounded by intentionality of weight loss, disease state and methodological limitations – as well as the absence of a uniform or standard definition of weight cycling – clinical implications are difficult to ascertain. The evidence, at least for all-cause mortality, suggests that weight-loss intervention should not be discouraged because of a person's propensity to be a weight cycler. However, monitoring cardiometabolic clinical markers regularly when intentional weight loss is pursued is advisable especially in light of unintended weight regain leading to weight cycling. Most studies attempting to identify an association between weight cycling and mortality or morbidity demonstrate no effect at all. Therefore, evidence for an adverse effect of weight cycling appears sparse, if it exists at all.
Acknowledgements
The authors would like to acknowledge Geula Bernstein of AXON Communications for providing editorial assistance in the development of this manuscript. Editorial assistance was sponsored by Novo Nordisk.
Footnotes
Potential conflicts of interest: The sponsors had no role in design and conduct of the study;the collection, management, analysis, and interpretation of the data; or the preparation, review,or approval of the manuscript. TM has consulted with GJORDING FOUSER PLLC and wassupported by a UAB Doctoral Training Grant in Obesity and Nutrition funded by Kraft Foods.Funding to DLS was through the UAB by grants received currently or in the past from NIH,Ellison Medical Foundation, and The Obesity Society. Authors received editorial assistance fromGeula Bernstein of AXON Communications in the development of this manuscript. Editorialassistance was sponsored by Novo Nordisk.
References
- 1.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323–41. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson RL, Dietz WH, Foreyt JP, et al. Weight cycling. National Task Force on the Prevention and Treatment of Obesity. JAMA. 1994;272:1196–202. [PubMed] [Google Scholar]
- 3.Brownell KD, Greenwood MR, Stellar E, Shrager EE. The effects of repeated cycles of weight loss and regain in rats. Physiol Behav. 1986;38:459–64. doi: 10.1016/0031-9384(86)90411-7. [DOI] [PubMed] [Google Scholar]
- 4.Lissner L, Odell PM, D'Agostino RB, et al. Variability of body weight and health outcomes in the Framingham population. N Engl J Med. 1991;324:1839–44. doi: 10.1056/NEJM199106273242602. [DOI] [PubMed] [Google Scholar]
- 5.Sygo J. [April 10, 2014];National Post: weighing in on the weight loss debate. [WWW document] 2012 URL http://life.nationalpost.com/2012/02/14/weighing-in-on-the-weight-loss-debate/.
- 6.Stevens VL, Jacobs EJ, Sun J, et al. Weight cycling and mortality in a large prospective US study. Am J Epidemiol. 2012;175:785–92. doi: 10.1093/aje/kwr378. [DOI] [PubMed] [Google Scholar]
- 7.Casazza K, Fontaine KR, Astrup A, et al. Myths, presumptions, and facts about obesity. N Engl J Med. 2013;368:446–54. doi: 10.1056/NEJMsa1208051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.List EO, Berryman DE, Wright-Piekarski J, Jara A, Funk K, Kopchick JJ. The effects of weight cycling on lifespan in male C57BL/6J mice. Int J Obes (Lond) 2013;37:1088–94. doi: 10.1038/ijo.2012.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMillen TS, Minami E, Leboeuf RC. Atherosclerosis and cardiac function assessment in low-density lipoprotein receptor-deficient mice undergoing body weight cycling. Nutr Diabetes. 2013;3:e79. doi: 10.1038/nutd.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cutter G, St Jeor S, Brunner R, et al. Methodological issues in weight cycling. Ann Behav Med. 1996;18:280–9. doi: 10.1007/BF02895290. [DOI] [PubMed] [Google Scholar]
- 11.Panarotto D, Bosi GR, Neumann M, de Braga GL, Hickmann S, Marcki CR. Reliability and internal consistency of questionnaire for evaluating weight cycling in Southern Brazil. J Clin Nurs. 2012;23:385–93. doi: 10.1111/jocn.12021. [DOI] [PubMed] [Google Scholar]
- 12.Thompson HJ, McTiernan A. Weight cycling and cancer: weighing the evidence of intermittent caloric restriction and cancer risk. Cancer Prev Res (Phila) 2011;4:1736–42. doi: 10.1158/1940-6207.CAPR-11-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lissner L, Andres R, Muller DC, Shimokata H. Body weight variability in men: metabolic rate, health and longevity. Int J Obes. 1990;14:373–83. [PubMed] [Google Scholar]
- 14.Poobalan AS, Aucott LS, Smith WC, Avenell A, Jung R, Broom J. Long-term weight loss effects on all cause mortality in overweight/obese populations. Obes Rev. 2007;8:503–13. doi: 10.1111/j.1467-789X.2007.00393.x. [DOI] [PubMed] [Google Scholar]
- 15.Aucott LS. Influences of weight loss on long-term diabetes outcomes. Proc Nutr Soc. 2008;67:54–9. doi: 10.1017/S0029665108006022. [DOI] [PubMed] [Google Scholar]
- 16.Strohacker K, McFarlin BK. Influence of obesity, physical inactivity, and weight cycling on chronic inflammation. Front Biosci (Elite Ed) 2010;2:98–104. doi: 10.2741/e70. [DOI] [PubMed] [Google Scholar]
- 17.Hooper LE, Foster-Schubert KE, Weigle DS, Sorensen B, Ulrich CM, McTiernan A. Frequent intentional weight loss is associated with higher ghrelin and lower glucose and androgen levels in postmenopausal women. Nutr Res. 2010;30:163–70. doi: 10.1016/j.nutres.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoo HJ, Kim BT, Park YW, Park KH, Kim CW, Joo NS. Difference of body compositional changes according to the presence of weight cycling in a community-based weight control program. J Korean Med Sci. 2010;25:49–53. doi: 10.3346/jkms.2010.25.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason C, Foster-Schubert KE, Imayama I, et al. History of weight cycling does not impede future weight loss or metabolic improvements in postmenopausal women. Metabolism. 2013;62:127–36. doi: 10.1016/j.metabol.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Field AE, Malspeis S, Willett WC. Weight cycling and mortality among middle-aged or older women. Arch Intern Med. 2009;169:881–6. doi: 10.1001/archinternmed.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S, Parks CG, DeRoo LA, et al. Obesity and weight gain in adulthood and telomere length. Cancer Epidemiol Biomarkers Prev. 2009;18:816–20. doi: 10.1158/1055-9965.EPI-08-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strychar I, Lavoie ME, Messier L, et al. Anthropometric, metabolic, psychosocial, and dietary characteristics of overweight/obese postmenopausal women with a history of weight cycling: a MONET (Montreal Ottawa New Emerging Team) study. J Am Diet Assoc. 2009;109:718–24. doi: 10.1016/j.jada.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Arnold AM, Newman AB, Cushman M, Ding J, Kritchevsky S. Body weight dynamics and their association with physical function and mortality in older adults: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2010;65:63–70. doi: 10.1093/gerona/glp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kataja-Tuomola M, Sundell J, Mannisto S, et al. Short-term weight change and fluctuation as risk factors for type 2 diabetes in Finnish male smokers. Eur J Epidemiol. 2010;25:333–9. doi: 10.1007/s10654-010-9444-6. [DOI] [PubMed] [Google Scholar]
- 25.Lee DH, Steffes MW, Gross M, et al. Differential associations of weight dynamics with coronary artery calcium versus common carotid artery intima-media thickness: The CARDIA Study. Am J Epidemiol. 2010;172:180–9. doi: 10.1093/aje/kwq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JS, Visser M, Tylavsky FA, et al. Weight loss and regain and effects on body composition: the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2010;65:78–83. doi: 10.1093/gerona/glp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waring ME, Eaton CB, Lasater TM, Lapane KL. Incident diabetes in relation to weight patterns during middle age. Am J Epidemiol. 2010;171:550–6. doi: 10.1093/aje/kwp433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cereda E, Malavazos AE, Caccialanza R, Rondanelli M, Fatati G, Barichella M. Weight cycling is associated with body weight excess and abdominal fat accumulation: a cross-sectional study. Clin Nutr. 2011;30:718–23. doi: 10.1016/j.clnu.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Osborn RL, Forys KL, Psota TL, Sbrocco T. Yo-yo dieting in African American women: weight cycling and health. Ethn Dis. 2011;21:274–80. [PMC free article] [PubMed] [Google Scholar]
- 30.Blüher M, Rudich A, Kloting N, et al. Two patterns of adipokine and other biomarker dynamics in a long-term weight loss intervention. Diabetes Care. 2012;35:342–9. doi: 10.2337/dc11-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neiberg RH, Wing RR, Bray GA, et al. Patterns of weight change associated with long-term weight change and cardiovascular disease risk factors in the Look AHEAD Study. Obesity (Silver Spring) 2012;20:2048–56. doi: 10.1038/oby.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens VL, Jacobs EJ, Sun J, et al. Weight cycling and risk of endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2012;21:747–52. doi: 10.1158/1055-9965.EPI-12-0038. [DOI] [PubMed] [Google Scholar]
- 33.Taing KY, Ardern CI, Kuk JL. Effect of the timing of weight cycling during adulthood on mortality risk in overweight and obese postmenopausal women. Obesity (Silver Spring) 2012;20:407–13. doi: 10.1038/oby.2011.207. [DOI] [PubMed] [Google Scholar]
- 34.Ambeba EJ, Styn MA, Kuller LH, Brooks MM, Evans RW, Burke LE. Longitudinal effects of weight loss and regain on cytokine concentration of obese adults. Metabolism. 2013;62:1218–22. doi: 10.1016/j.metabol.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bosy-Westphal A, Schautz B, Lagerpusch M, et al. Effect of weight loss and regain on adipose tissue distribution, composition of lean mass and resting energy expenditure in young overweight and obese adults. Int J Obes (Lond) 2013;37:1371–7. doi: 10.1038/ijo.2013.1. [DOI] [PubMed] [Google Scholar]
- 36.Haufe S, Haas V, Utz W, et al. Long-Lasting Improvements in Liver Fat and Metabolism Despite Body Weight Regain After Dietary Weight Loss. Diabetes Care. 2013;36:3786–92. doi: 10.2337/dc13-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mauriège P, Imbeault P, Doucet E, et al. Weight loss and regain in obese individuals: a link with adipose tissue metabolism indices? J Physiol Biochem. 2013;69:497–505. doi: 10.1007/s13105-013-0237-8. [DOI] [PubMed] [Google Scholar]
- 38.Nagle CM, Marquart L, Bain CJ, et al. Impact of weight change and weight cycling on risk of different subtypes of endometrial cancer. Eur J Cancer. 2013;49:2717–26. doi: 10.1016/j.ejca.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Bogden JD, Kemp FW, Huang AE, et al. Bone mineral density and content during weight cycling in female rats: effects of dietary amylase-resistant starch. Nutr Metab (Lond) 2008;5:34. doi: 10.1186/1743-7075-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson EK, Gutierrez DA, Kennedy A, Hasty AH. Weight cycling increases T-cell accumulation in adipose tissue and impairs systemic glucose tolerance. Diabetes. 2013;62:3180–8. doi: 10.2337/db12-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barbosa-da-Silva S, da Silva NC, Aguila MB, Mandarim-de-Lacerda CA. Liver damage is not reversed during the lean period in diet-induced weight cycling in mice. Hepatol Res. 2013;44:450–9. doi: 10.1111/hepr.12138. [DOI] [PubMed] [Google Scholar]
- 42.Barbosa-da-Silva S, Fraulob-Aquino JC, Lopes JR, Mandarim-de-Lacerda CA, Aguila MB. Weight cycling enhances adipose tissue inflammatory responses in male mice. PLoS One. 2012;7:e39837. doi: 10.1371/journal.pone.0039837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagaoka D, Mitsuhashi Y, Angell R, Bigley KE, Bauer JE. Re-induction of obese body weight occurs more rapidly and at lower caloric intake in beagles. J Anim Physiol Anim Nutr (Berl) 2010;94:287–92. doi: 10.1111/j.1439-0396.2008.00908.x. [DOI] [PubMed] [Google Scholar]
- 44.Koster A, Leitzmann MF, Schatzkin A, et al. The combined relations of adiposity and smoking on mortality. Am J Clin Nutr. 2008;88:1206–12. doi: 10.3945/ajcn.2008.26298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reed GW, Hill JO. Weight cycling: a review of the animal literature. Obes Res. 1993;1:392–402. doi: 10.1002/j.1550-8528.1993.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 46.Ray MA, Johnston NA, Verhulst S, Trammell RA, Toth LA. Identification of markers for imminent death in mice used in longevity and aging research. J Am Assoc Lab Anim Sci. 2010;49:282–8. [PMC free article] [PubMed] [Google Scholar]
- 47.Coffey CS, Gadbury GL, Fontaine KR, Wang C, Weindruch R, Allison DB. The effects of intentional weight loss as a latent variable problem. Stat Med. 2005;24:941–54. doi: 10.1002/sim.1964. [DOI] [PubMed] [Google Scholar]
- 48.Gadbury GL, Supapakorn T, Coffey CS, Keith SW, Allison DB. Application of potential outcomes to an intentional weight loss latent variable problem. Stat Interface. 2008;1:87–97. doi: 10.4310/sii.2008.v1.n1.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:c221. doi: 10.1136/bmj.c221. [DOI] [PubMed] [Google Scholar]
- 50.French SA, Folsom AR, Jeffery RW, Zheng W, Mink PJ, Baxter JE. Weight variability and incident disease in older women: the Iowa Women's Health Study. Int J Obes Relat Metab Disord. 1997;21:217–23. doi: 10.1038/sj.ijo.0800390. [DOI] [PubMed] [Google Scholar]
- 51.Dyer AR, Stamler J, Greenland P. Associations of weight change and weight variability with cardiovascular and all-cause mortality in the Chicago Western Electric Company Study. Am J Epidemiol. 2000;152:324–33. doi: 10.1093/aje/152.4.324. [DOI] [PubMed] [Google Scholar]
- 52.Rzehak P, Meisinger C, Woelke G, Brasche S, Strube G, Heinrich J. Weight change, weight cycling and mortality in the ERFORT Male Cohort Study. Eur J Epidemiol. 2007;22:665–73. doi: 10.1007/s10654-007-9167-5. [DOI] [PubMed] [Google Scholar]
- 53.Gregg EW, Gerzoff RB, Thompson TJ, Williamson DF. Intentional weight loss and death in overweight and obese U.S. adults 35 years of age and older. Ann Intern Med. 2003;138:383–9. doi: 10.7326/0003-4819-138-5-200303040-00007. [DOI] [PubMed] [Google Scholar]
- 54.Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: The SCALE Maintenance randomized study. Int J Obes (Lond) 2013;37:1443–51. doi: 10.1038/ijo.2013.120. [DOI] [PubMed] [Google Scholar]
- 55.Field AE, Manson JE, Taylor CB, Willett WC, Colditz GA. Association of weight change, weight control practices, and weight cycling among women in the Nurses' Health Study II. Int J Obes Relat Metab Disord. 2004;28:1134–42. doi: 10.1038/sj.ijo.0802728. [DOI] [PubMed] [Google Scholar]
- 56.Williamson DF, Thompson TJ, Thun M, Flanders D, Pamuk E, Byers T. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care. 2000;23:1499–504. doi: 10.2337/diacare.23.10.1499. [DOI] [PubMed] [Google Scholar]
- 57.Williamson DF, Pamuk E, Thun M, Flanders D, Byers T, Heath C. Prospective study of intentional weight loss and mortality in never-smoking overweight US white women aged 40-64 years. Am J Epidemiol. 1995;141:1128–41. doi: 10.1093/oxfordjournals.aje.a117386. [DOI] [PubMed] [Google Scholar]
- 58.Sorensen TI, Rissanen A, Korkeila M, Kaprio J. Intention to lose weight, weight changes, and 18-y mortality in overweight individuals without co-morbidities. PLoS Med. 2005;2:e171. doi: 10.1371/journal.pmed.0020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vasselli JR, Weindruch R, Heymsfield SB, et al. Intentional weight loss reduces mortality rate in a rodent model of dietary obesity. Obes Res. 2005;13:693–702. doi: 10.1038/oby.2005.78. [DOI] [PubMed] [Google Scholar]
- 60.Sheehan NA, Didelez V, Burton PR, Tobin MD. Mendelian randomisation and causal inference in observational epidemiology. PLoS Med. 2008;5:e177. doi: 10.1371/journal.pmed.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hall KD, Chow CC. Estimating changes in free-living energy intake and its confidence interval. Am J Clin Nutr. 2011;94:66–74. doi: 10.3945/ajcn.111.014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomas D, Das SK, Levine JA, et al. New fat free mass - fat mass model for use in physiological energy balance equations. Nutr Metab (Lond) 2010;7:39. doi: 10.1186/1743-7075-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]