Abstract
Foxp3+ regulatory T cells (Treg cells) maintain immunological tolerance and their deficiency results in fatal multi-organ autoimmunity. Although heightened T cell receptor (TCR) signaling is critical for the differentiation of Treg cells, the role of TCR signaling in Treg cell function remains largely unknown. Here we demonstrate inducible ablation of the TCR results in Treg cell dysfunction which cannot be attributed to impaired Foxp3 expression, decreased expression of Treg cell signature genes or altered ability to sense and consume interleukin 2. Rather, TCR signaling was required for maintaining the expression of a limited subset of genes comprising 25% of the activated Treg cell transcriptional signature. Our results reveal a critical role for the TCR in Treg cell suppressor capacity.
Regulatory CD4+ T cells expressing the transcription factor Foxp3 play an essential role in maintaining immune tolerance1. In the thymus, increased affinity T cell receptor (TCR) engagement in immature CD4 single positive thymocytes is required for initiation of the Treg cell differentiation program and induction of Foxp3 expression2. As a consequence, Treg cells exported to the periphery exhibit a TCR repertoire skewed towards self-recognition3, 4. However, the requirement for TCR signaling in mediating Treg cell suppressive function in the periphery remains largely unclear.
In comparison to conventional CD4+ T cells, Treg cells exhibit impaired calcium flux, Akt activation and Erk phosphorylation upon TCR stimulation, and Foxp3 is known to potently repress at least some TCR-induced genes, as well as some genes involved in the TCR signaling pathway5–8. At the same time, Foxp3+ Treg cells have high basal expression of several cell surface molecules which are known to contribute to Treg cell function (such as CD25, CD39 and CTLA-4) and whose expression in conventional CD4+ T cells is dependent upon TCR stimulation9–14. Whether or not high affinity TCR interactions with self contribute to constitutive expression of these genes and consequently to Treg cell function is not known.
Treg cells—despite their intrinsically dampened response to TCR stimulation—acquire an activated phenotype and expand in response to their cognate antigens in settings of immune activation, such as infection and autoimmunity15, 16. These observations imply that self-antigen recognition helps maintain Treg cells of particular specificities and may potentiate their suppressive capacity during immune challenge17. Nevertheless, strict reliance on TCR expression for Treg cell activation—as opposed to preferential activation of antigen-specific Treg cells—has not been demonstrated, nor has TCR engagement in vivo been shown to be required for Treg cell function in any context.
We used inducible genetic ablation of cell surface TCR complexes to directly address the requirement of TCR expression for Treg cell immunosuppressive capacity. Notably, the TCR was largely dispensable for Foxp3 expression, lineage stability and for high expression of many Treg cell signature genes. Nevertheless, these features were not sufficient to preserve Treg cell function and to prevent immune activation. Loss of suppressor capacity in the absence of TCR was not due to impaired Treg cell ability to gain access to interleukin 2 (IL-2), and accordingly administration of exogenous IL-2 failed to rescue systemic autoimmunity. Instead, TCR expression was essential for Treg cell activation and maintenance of a limited set of genes which were found to be expressed almost exclusively in activated Treg cells. Among these genes, expression of the transcription factor IRF4 contributed to optimal Treg cell function and homeostasis. These results demonstrate an essential role for the TCR in eliciting the suppressor function of differentiated Treg cells.
Results
Maintenance of Treg cell identity in the absence of the TCR
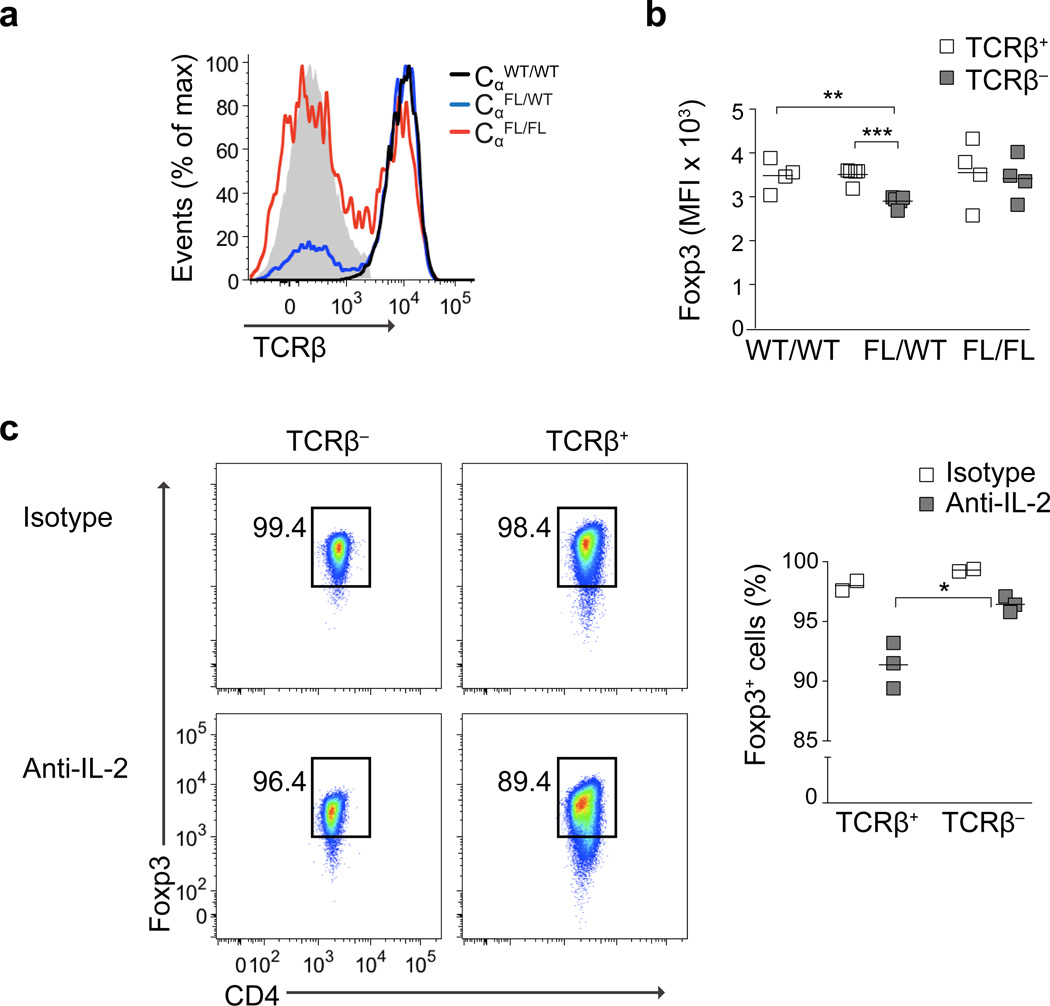
In order to investigate the consequences of TCR signaling in Treg cell function, we crossed Foxp3eGFP-CreERT2 mice to CαFL mice to allow inducible deletion of Cα encoding the TCRα constant chain specifically in Treg cells18, 19. In this model, Cre-induced loss of the conditional Cα allele upon tamoxifen administration eliminates TCRα expression, preventing cell surface TCRαβ heterodimer formation. Tamoxifen was administered via oral gavage on days 0 and 1 and mice were analyzed on day 9. Allelic exclusion at the Cα locus in heterozygous Foxp3CreERT2CαFL/WT mice yielded a minor population of TCR-deficient (TCR−) Treg cells (~25%), while in homozygous Foxp3CreERT2CαFL/FL mice the majority of Treg cells (~60–70%) lacked cell surface TCRβ (Fig. 1a). Although we cannot definitively exclude the possibility that few residual TCR complexes were present in minute amounts (below the detection limit of flow cytometric analyses) on the TCR− Treg cells, functional in vitro analyses confirmed loss of TCR crosslinking-dependent activation of TCR− Treg cells (Supplementary Fig. 1a–d).
Figure 1. Maintenance of Treg cell identity in the absence of the TCR.
(a, b) TCRβ expression (a) and Foxp3 median fluorescence intensity (MFI) (b) in CD4+Foxp3+ lymph node cells from 8–10 week old Foxp3CreERT2CαWT/WT (black line, WT/WT), Foxp3CreERT2CαFL/WT (blue line, FL/WT) and Foxp3CreERT2CαFL/FL (red line, FL/FL) mice gavaged on days 0 and 1 with tamoxifen and analyzed on day 9. The gray histogram in (a) shows TCRβ staining on CD4−TCRβ− cells. (c) Percentages of TCRβ+Foxp3+ and TCRβ−Foxp3+ T cells among CD4+YFP+ cells sorted to >99% purity from the spleens and lymph nodes of Foxp3CreERT2CαFL/WTRosaYFP mice on day 13 following treatment with tamoxifen on days 0 and 1 and i.p. injections of IL-2 neutralizing or control antibody on days 4 and 8. Data are representative of two independent experiments with four or more (a,b) or two or more (c,d) mice per group in each. ***, P < 0.001; **, P < 0.01; *, P < 0.05. P-values were calculated using an unpaired t-test.
Because binding sites for the transcription factors NFAT and c-Rel have been identified within the Foxp3 locus, and TCR engagement-driven NF-κB signaling is critical for induction of Foxp3 expression, we speculated that the TCR might be essential to maintain Foxp3 expression20–22. However, Foxp3 expression was only marginally reduced in TCR− Treg cells in Foxp3CreERT2CαFL/WT mice, and was not reduced at all in TCR− Treg cells in Foxp3CreERT2CαFL/FL mice, compared to TCR+ Treg cells present in the same mice and in Foxp3CreERT2CαWT/WT animals (Fig. 1b). Similarly, the expression of several Treg cell signature molecules, including CD25, GITR, CD39 and CD73, was largely unaffected in TCR− Treg cells from both Foxp3CreERT2CαFL/WT and Foxp3CreERT2CαFL/FL mice (Supplementary Fig. 1e). These results indicates that, at steady state, continuous TCR-mediated recognition of self does not contribute significantly to Foxp3-dependent maintenance of expression for these genes11, 23. In contrast, CTLA-4 expression was notably diminished in TCR− Treg cells in Foxp3CreERT2CαFL/WT mice, although not in TCR− Treg cells in Foxp3CreERT2CαFL/FL mice, compared to TCR+ Treg cells in these mice and in Foxp3CreERT2CαWT/WT animals (Supplementary Fig. 1e).
Compared to Foxp3CreERT2CαWT/WT mice, percentages and absolute numbers of Foxp3+ cells in the spleens and lymph nodes of Foxp3CreERT2CαFL/WT and Foxp3CreERT2CαFL/FL mice were unaltered (Supplementary Figure 1f). However, to address the possibility that a portion of Treg cells completely lost Foxp3 expression upon ablation of the TCR and that these “former” Treg cells were not accounted for in this experimental setup, we crossed Foxp3CreERT2CαFL/WT mice with mice expressing the recombination reporter Rosa26YFP allele. TCRβ and Foxp3 expression was assessed in CD4+YFP+ cells sorted from the spleens and lymph nodes of Foxp3CreERT2CαFL/WTRosaYFP mice on day 9 or 50 following tamoxifen administration on two consecutive days. YFP-expressing CD4+TCRβ− and CD4+ TCRβ+ cell subsets contained similarly minor frequencies of Foxp3− cells at both time points (data not shown). Furthermore, following in vivo IL-2 neutralization – a condition known to promote loss of Foxp3 expression – in Foxp3CreERT2CαFL/WTRosaYFP mice, CD4+YFP+TCRβ− cells retained a higher percentage of Foxp3+ cells than did CD4+YFP+TCRβ+ cells. Together, these data indicate that TCR signaling is dispensable for the maintenance of the Treg cell phenotype and lineage stability, and moreover, that TCR signaling can drive the loss of Foxp3 when IL-2 amounts are limiting (Fig. 1c)19.
Requirement for the TCR in Treg cell effector differentiation
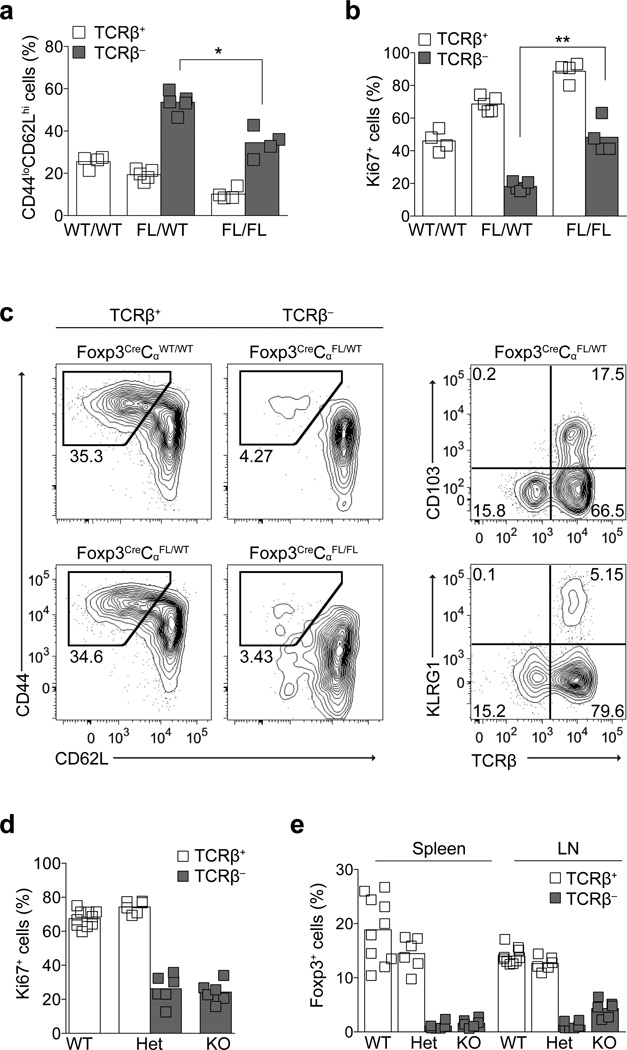
Although the Treg cell phenotype was largely preserved upon ablation of the TCR, we observed a relative enrichment of naïve-like CD44loCD62Lhi cells among TCR− Treg cells in lymph nodes and spleens of Foxp3CreERT2CαFL/WT and Foxp3CreERT2CαFL/FL mice (Fig. 2a and Supplementary Fig. 1g). Treg cell proliferation is almost exclusively restricted to the CD44hi subset, and in part, the enrichment we observed appeared to be a consequence of the severely impaired proliferative capacity of CD44hi Treg cell in the absence of the TCR (Fig. 2b)24. The minor population of TCR− Treg cells in Foxp3CreERT2CαFL/WT mice was predominantly non-dividing: these cells showed minimal expression of Ki67, failed to incorporate BrdU over a 24 hour labeling period and contained the largest percentage of CD44loCD62Lhi cells among all TCR+ and TCR− Treg cell populations in Foxp3CreERT2CαWT/WT, Foxp3CreERT2CαFL/WT, and Foxp3CreERT2CαFL/FL mice (Fig 2a,b and Supplementary Fig. 1h). In Foxp3CreERT2CαFL/FL mice, however, TCR− Treg cells exhibited considerable proliferative activity, albeit reduced in comparison to TCR+ Treg cells present in the same mouse (Fig 2a). Increased Ki67 staining in CD44hi Treg cells inversely correlated with a decreased percentage of CD44loCD62Lhi cells within all TCR+ and TCR− Treg cell populations present in Foxp3CreERT2CαWT/WT, Foxp3CreERT2CαFL/WT, and Foxp3CreERT2CαFL/FL animals (Fig 2a, b).
Figure 2. Requirement for the TCR in Treg cell differentiation and expansion.
(a, b) CD44 and CD62L expression on TCR+ and TCR− CD4+Foxp3+ (a) and Ki67 expression on CD44hi CD4+Foxp3+ (b) lymph node cells isolated on day 9 from Foxp3CreERT2CαWT/WT, Foxp3CreERT2CαFL/WT, and Foxp3CreERT2CαFL/FL mice treated with tamoxifen on days 0 and 1. Data in (a) and (b) are representative of two independent experiments with four or more mice per group in each. (c) CD44 and CD62L expression on TCRβ+ and TCRβ− CD4+Foxp3+ lymph node cells in 2.5 week old Foxp3CreCαWT/WT (WT/WT), Foxp3CreCαFL/WT (FL/WT) and Foxp3CreCαFL/FL (FL/FL) mice (left) and differentiation marker expression on TCRβ+ and TCRβ− CD4+Foxp3+ lymph node cells in 2.5 week old Foxp3CreCαFL/WT mice (right). Data is representative of three independent experiments involving a total of six or more mice in each group. (d,e) Ki67 expression in lymph node TCRβ+ and TCRβ− CD4+Foxp3+ cells (d) and percentages of TCRβ+ and TCRβ− CD4+Foxp3+ cells among total CD4+ cells (e) in 2.5 week old Foxp3CreCαWT/WT (WT), Foxp3CreCαFL/WT (Het), and Foxp3CreCαFL/FL (KO) mice. The data in (d) and (e) represent an aggregate of three independent experiments with a total of six or more mice per group. **, P < 0.001; *, P < 0.01. P-values were calculated using an unpaired t-test.
To address the possibility that continuous peripheral differentiation of naïve-like Treg cells into CD44hi cells was impeded in the absence of TCR expression and that such a differentiation block contributed to the predominantly CD44loCD62Lhi phenotype of TCR− Treg cells, we bred CαFL mice to Foxp3YFP-Cre mice to induce ablation of the TCR in newly generated, “naïve” Treg cells25, 26. We reasoned that if the TCR was critical for Treg cell effector differentiation in the periphery, TCR− Treg cells in these mice would retain a CD44loCD62Lhi naïve-like phenotype.
Immature HSAhiCD4+Foxp3+ cells in the thymi of Foxp3CreCαFL/FL mice showed similar cell surface expression of TCR complexes as their wild type counterparts, while more mature HSAloCD4+Foxp3+ thymocytes showed only slightly reduced TCR expression (Supplementary Fig. 2a.). Among Foxp3+ cells present in the spleens and lymph nodes of Foxp3CreCαFL/WT mice, ~5–15% were TCRβ− whereas ~80% of Foxp3+ cells in Foxp3CreCαFL/FL mice lacked surface TCRβ expression (Fig 2e and Supplementary Fig. 2b). We again observed a slight decrease in the amount of Foxp3 protein in the TCR− Treg cells in Foxp3CreCαFL/WT mice, but not in Foxp3CreCαFL/FL mice, while expression of Treg cell signature genes was variably affected in the TCR− Treg cell populations in both mice as compared to TCR+ Treg cells in Foxp3CreCαWT/WT, Foxp3CreCαFL/WT, and Foxp3CreCαFL/FL mice (Supplementary Fig. 2c,d).
Despite their generally intact Treg cell surface phenotype, nearly all TCR− Treg cells in healthy Foxp3CreCαFL/WT mice had a naïve-like CD62LhiCD44lo phenotype and lacked expression of all Treg cell differentiation markers tested, including KLRG1, CD103 and CXCR3 (Fig. 2c). Notably, this pattern was also observed under severe inflammatory conditions in Foxp3CreCαFL/FL mice, which were moribund by three weeks of age (Fig. 2c and Supplementary Fig. 3e,f). Lack of CD44hi cells among TCR− populations in Foxp3CreCαFL/WT and Foxp3CreCαFL/FL mice correlated with decreased proliferation and markedly diminished percentages and numbers of TCR− Treg cells in lymph nodes and, particularly, in the spleens and tissues such as liver and lung as compared to TCR+ Treg cells in the same mice and in Foxp3CreCαWT/WT animals (Fig. 2d,e and Supplementary Fig. 2g,h). Together, these data are consistent with an absolute requirement for TCR expression—loss of which cannot be compensated for even in conditions of extreme immune activation—for peripheral effector differentiation of naïve-like Treg cells and acquisition of an activated CD44hi phenotype.
TCR-dependent effector function of mature Treg cells
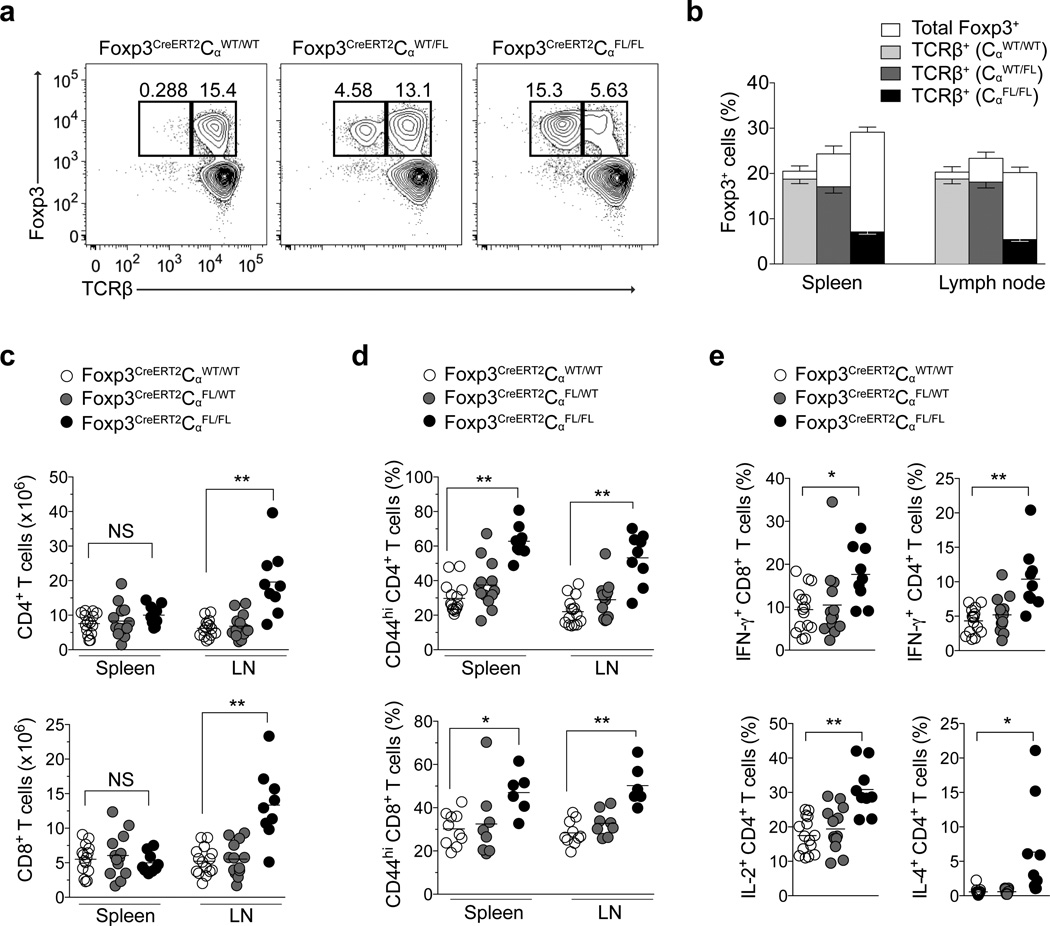
Foxp3 expression and Treg cell expansion are facilitated by IL-2R signaling27–29. The increase in Foxp3 protein expression and proliferative activity of TCR− Treg cells in Foxp3CreERT2CαFL/FL mice vs. Foxp3CreERT2CαFL/WT mice led us to suspect that ablation of TCR expression, even on mature Treg cells, might precipitate immune activation and elevate production of IL-2 and other cytokines by activated CD4+ T cells. Indeed, analysis of Foxp3CreERT2CαFL/FL mice treated twice with tamoxifen and analyzed on day 9 post-treatment revealed increased percentages of CD44hi T cells and increased numbers of IL-2-producing CD4+ T cells compared to tamoxifen-treated Foxp3CreERT2CαWT/WT and Foxp3CreERT2CαFL/WT mice (data not shown).
To more rigorously assess the role for TCR expression in mature Treg cell function, we administered four doses of tamoxifen to mice (on days 0, 3, 7 and 10) in order to maximize Cre-ERT2-mediated recombination. On day 13, we noted deletion of TCR expression in ~75–80% of Treg cells in Foxp3CreERT2CαFL/FL and ~25–30% in Treg cells in Foxp3CreERT2CαFL/WT mice (Fig. 3a,b). Despite normal or even increased percentages of total Foxp3+ cells in the lymph nodes and spleens of Foxp3CreERT2CαFL/FL mice, elevated numbers of CD4+Foxp3− and CD8+ T cell were found in the lymph nodes, and increased percentages of CD44hi CD4+ and CD8+ T cells were found in the lymph nodes and spleens of these mice (Fig. 3ca–d). CD8+ T cells and CD4+ T cells from Foxp3CreERT2CαFL/FL mice produced increased amounts of interferon-γ (IFN-γ) and IFN-γ, IL-2, IL-4, IL-13, IL-5 and IL-17, respectively, when compared to T cells from Foxp3CreERT2CαWT/WT and Foxp3CreERT2CαFL/WT mice (Fig. 3e and data not shown).
Figure 3. TCR-dependent effector function of mature Treg cells in adult mice.
(a,b) TCRβ and Foxp3 expression among lymph node CD4+ cells (a) and percentages of Foxp3+ cells among lymph node and splenic CD4+ cells (b) in Foxp3CreERT2CαWT/WT, Foxp3CreERT2CαFL/WT and Foxp3CreERT2CαFL/FL mice on day 13 following tamoxifen treatment on days 0, 3, 7 and 10. In (b), percentages of TCRβ+Foxp3+ cells among CD4+ cells in Foxp3CreERT2CαWT/WT (light gray bars), Foxp3CreERT2CαFL/WT (dark gray bars) and Foxp3CreERT2CαFL/FL (black bars) are shown together with percentages of total Foxp3+ cells among CD4+ cells (white bars) within each genotype. Error bars in (b) indicate s.e.m. Data in (a) is representative of three experiments with three mice or more per group in each. Data in (b) represent an aggregate of three experiments with a total of nine mice or more mice per group. (c–d) Numbers of (c) and CD44 expression (d) and cytokine production (e) by CD4+Foxp3− and CD8+ T cells in Foxp3CreERT2CαWT/WT (white circles), Foxp3CreERT2CαFL/WT (gray circles) and Foxp3CreERT2CαFL/FL (black circles) mice on day 13 following tamoxifen treatment on days 0, 3, 7 and 10 in spleens and lymph nodes for (c,d) and spleens for (e). Data represent an aggregate of three experiments with nine or more mice per group. ***, P < 0.001; **, P < 0.01; *, P < 0.05. P-values were calculated using an unpaired t-test.
Immune activation in Foxp3CreERT2CαFL/FL mice was milder than that resulting from complete Treg cell depletion in Foxp3DTR mice, which express the human diphtheria toxin receptor (DTR) concomitantly with Foxp330. Thus, it was possible that the large numbers of TCR− Treg cells in Foxp3CreERT2CαFL/FL mice retained measurable TCR-independent suppressor capacity and were still capable of immune regulation. Alternatively, the minor population of remaining TCR-sufficient Treg cells in these mice might have limited the activation of effector T cells and the associated autoimmunity to some degree. To address these possibilities, we attempted to reduce the Treg cell percentages among CD4+ cells in Foxp3DTR mice to approximate the percentages of the residual TCR-sufficient Treg cells in Foxp3CreERT2CαFL/FL mice following four doses of tamoxifen31. We reasoned that if TCR− Treg cells were capable of significant suppression, autoimmunity in Foxp3DTR mice subjected to only partial depletion of Treg cells would be more severe than in Foxp3CreERT2CαFL/FL mice. As diphtheria toxin (DT) injection depletes Treg cells within 24 hours, while tamoxifen-induced Cre-ERT2 mediated recombination progressively increases over a four day period, we treated Foxp3DTR, Foxp3CreERT2CαWT/WT and Foxp3CreERT2CαFL/FL mice each with DT four days after their first dose of tamoxifen (which like DT was administered to mice of all genotypes) in order to account for the time needed for complete Cre-ERT2-mediated deletion of Cα (Supplementary Fig. 3a,b and data not shown). Partial depletion of the Treg cell compartment in Foxp3DTR mice resulted in CD4+Foxp3− T cell activation and cytokine production grossly comparable to that observed in Foxp3CreERT2CαFL/FL mice harboring populations of TCR-sufficient Treg cells of a similar or even larger size (Supplementary Fig. 3b–d). Together, these results demonstrate that Treg cells require continuous TCR expression for the effective elaboration of their suppressor function, and suggest that TCR− Treg cells, which are abundant in Foxp3CreERT2CαFL/FL mice, are grossly devoid of detectable suppressor capacity.
TCR− Treg cell dysfunction is not secondary to impaired IL-2R signaling
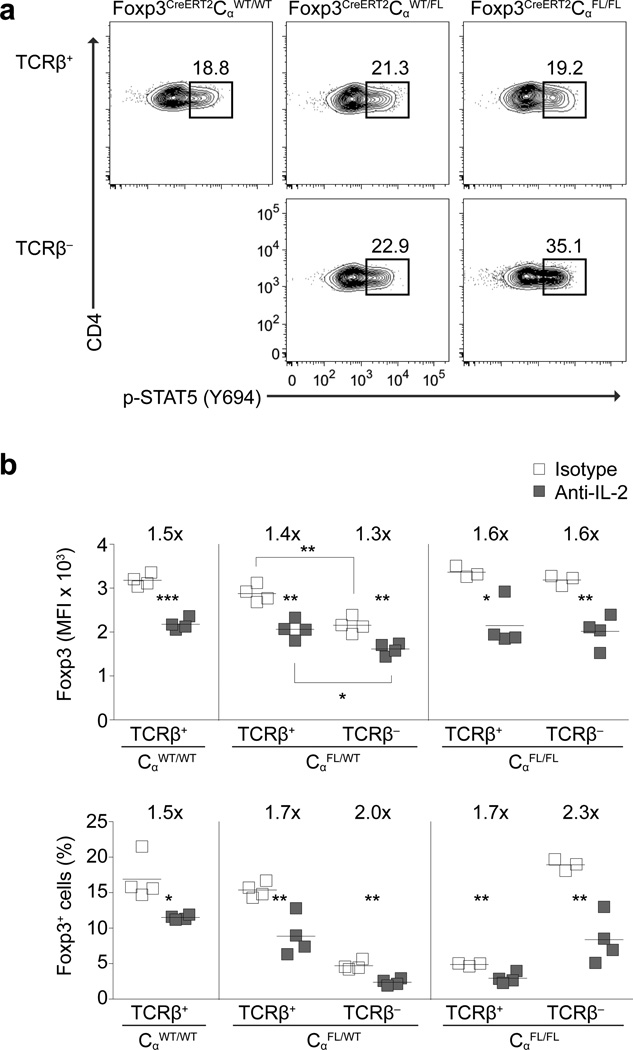
We considered that the apparent loss of Treg cell suppressive capacity in the absence of the TCR might be an indirect consequence of an impaired ability to localize in a TCR- and antigen-dependent manner to sites of CD4+ T cell activation and to, thereby, acquire IL-2, a cytokine known to be critical for Treg cell function and homeostasis. This scenario would explain the decreased expression of Foxp3 and minimal proliferation of TCR− Treg cells in healthy Foxp3CreERT2CαFL/WT mice, in which IL-2 amounts were not elevated and would not be able to partially remedy these defects32.
However, direct ex vivo analysis of phosphorylation of Stat5—which occurs downstream of IL-2 signaling in Treg cells—in spleen and lymph nodes showed at least equivalent proportions of p-Stat5 in TCR− and TCR+ Treg cells in both Foxp3CreERT2CαFL/WT and Foxp3CreERT2CαFL/FL mice (Fig. 4a). In Foxp3CreERT2CαFL/FL mice, TCR− Treg cells had increased p-Stat5 compared to TCR+ Treg cells, mirroring expression of CD25 and CD62L, which remained high on TCR− cells, but was decreased on the residual, activated TCR+ Treg cells present in these mice (Fig. 4a and Supplementary Figure 1e,f). These results are consistent with the observation that p-Stat5+ Treg cells are largely found within the CD62LhiCD44lo (and CD25hi) Treg cell subset which, in contrast to the activated CD44hiCD62Llo (and CD25int) Treg cell subset, rely on IL-2R signaling rather than co-stimulatory receptor engagement for their maintenance24.
Figure 4. Treg cell TCR expression is dispensable for IL-2R signaling in vivo.
(a) p-Stat5 expression in lymph node TCRβ+ and TCRβ− CD4+Foxp3+ cells in Foxp3CreERT2CαWT/WT, Foxp3CreERT2CαFL/WT and Foxp3CreERT2CαFL/FL mice on day 9 following tamoxifen administration on days 0 and 1. Data is representative of three experiments with three or more mice per group in total. (b) Foxp3 median fluorescence intensity (MFI) in TCRβ+ and TCRβ− Foxp3+ cells (upper panel) and percentages of TCRβ+ and TCRβ− Foxp3+ cells among CD4+ cells (lower panel) in lymph nodes of Foxp3CreERT2CαWT/WT, Foxp3CreERT2CαFL/WT and Foxp3CreERT2CαFL/FL mice on day 13 following tamoxifen treatment on days 0, 3, 7 and 10 and i.p. injections of IL-2 neutralizing or isotype antibody on days 4 and 8. The data in (b) is representative of two experiments with two or more mice per group each. ***, P < 0.001; **, P < 0.01; *, P < 0.05. P-values were calculated using an unpaired t-test.
In vitro analysis confirmed that lack of TCR expression did not significantly influence Stat5 phosphorylation in response to IL-2, nor impair Treg cell ability to capture and deplete IL-2 from culture media, suggesting that in the absence of TCR expression Treg cell-mediated IL-2 deprivation may not be a significant mechanism of immune suppression (Supplementary Fig. 4a,b). Furthermore, treatment of Foxp3CreERT2CαWT/WT, Foxp3CreERT2CαFL/WT and Foxp3CreERT2CαFL/FL mice with neutralizing anti-IL-2 (or isotype control) antibody reduced Foxp3 expression and decreased cell percentages among total CD4+ cells comparably between TCR+ and TCR− Treg cells (Fig. 4b). Together, these data suggest that TCR− Treg cells efficiently capture IL-2 during immune activation and at steady state. Although it remains to be determined what signal(s) drive the proliferation of TCR− Treg cells selectively in diseased Foxp3CreERT2CαFL/FL mice, we observed increased CD80 and CD86 expression on lymph node dendritic cells (DCs) in Foxp3CreERT2CαFL/FL mice, and activated DCs were able to induce limited proliferation of TCR− Treg cells in vitro (Supplementary Fig. 4c,d).
Lastly, administration of IL-2-anti-IL-2 complexes to Foxp3CreERT2CαFL/FL mice did not diminish to any measurable extent the activation and lymphoproliferation of effector T cells caused by loss of TCR expression in Treg cells (Supplementary Fig. 5). Conversely, IL-2 depletion did not further exacerbate autoimmunity (data not shown). Notably, this was the case in spite of a 1.5-fold expansion of TCR−, but not TCR+, Treg cells following IL-2 administration (a likely consequence of higher CD25 expression and heightened IL-2 responsiveness by TCR− Treg cells) and a greater than two-fold reduction in TCR− Treg cells following IL-2 depletion (Fig. 4b and Supplementary Fig. 5b). These observations further confirm that TCR− Treg cells—even when present in elevated numbers—possess minimal suppressive capacity. Together, these results indicate that neither TCR-dependent interactions with antigen presenting cells nor continuous TCR-mediated localization within lymphoid organs are required for Treg cell acquisition of IL-2, and that Treg cell dysfunction in the absence of TCR cannot be attributed to altered IL-2R signaling.
TCR expression promotes Treg cell adhesive properties in vitro
The in vitro suppressive capacity of Treg cells requires TCR engagement, possibly involving pathways independent of Zap70 catalytic activity—which is essential for conventional T cell effector function—but dependent on membrane proximal inside-out activation of integrins and subsequent enhancement of Treg cell interactions with antigen presenting cells8, 33, 34. To test this possibility, we cultured TCR+ or TCR− Treg cells isolated from Foxp3CreERT2CαWT/WT, Foxp3CreERT2CαFL/WT or Foxp3CreERT2CαFL/FL mice with DCs and assessed DC-Treg cell conjugate formation. We did not detect any differences in conjugate formation between DCs and TCR+ or TCR− Treg cells following 30 min of incubation (data not shown). However, TCR− Treg cells isolated from Foxp3CreERT2CαFL/WT or Foxp3CreERT2CαFL/FL mice were less efficient than TCR+ Treg cells at forming conjugates with DCs (3.7% vs. 7.5% of Treg cells) following overnight culture (Supplementary Fig. 6a). Conjugate formation was unaffected by the presence or absence of MHC class II molecules on DCs, which may imply that the increased adhesion of TCR+ vs. TCR− Treg cells in this assay was not a result of MHC class II-TCR interactions and may instead have been a consequence of the overall heightened activation status of TCR+ vs. TCR− Treg cells (Supplementary Fig. 6a). As LFA-1 expression is higher on CD44hi Treg cells in comparison to CD44loCD62Lhi Treg cells, it is possible that superior conjugate formation by TCR+ Treg cells, which are enriched for CD44hi cells compared to TCR− Treg cells, may be due, at least in part, to increased expression of this integrin (Supplementary Figure 6b). Although further work is necessary to determine precisely how TCR engagement in vivo affects signaling pathways to modulate Treg cell adhesive properties, our results indicate that TCR expression contributes to optimal Treg cell contact-dependent interactions with APCs, which may contribute to TCR-dependent immunosuppressive function.
TCR signaling modulates the effector Treg cell transcriptional signature
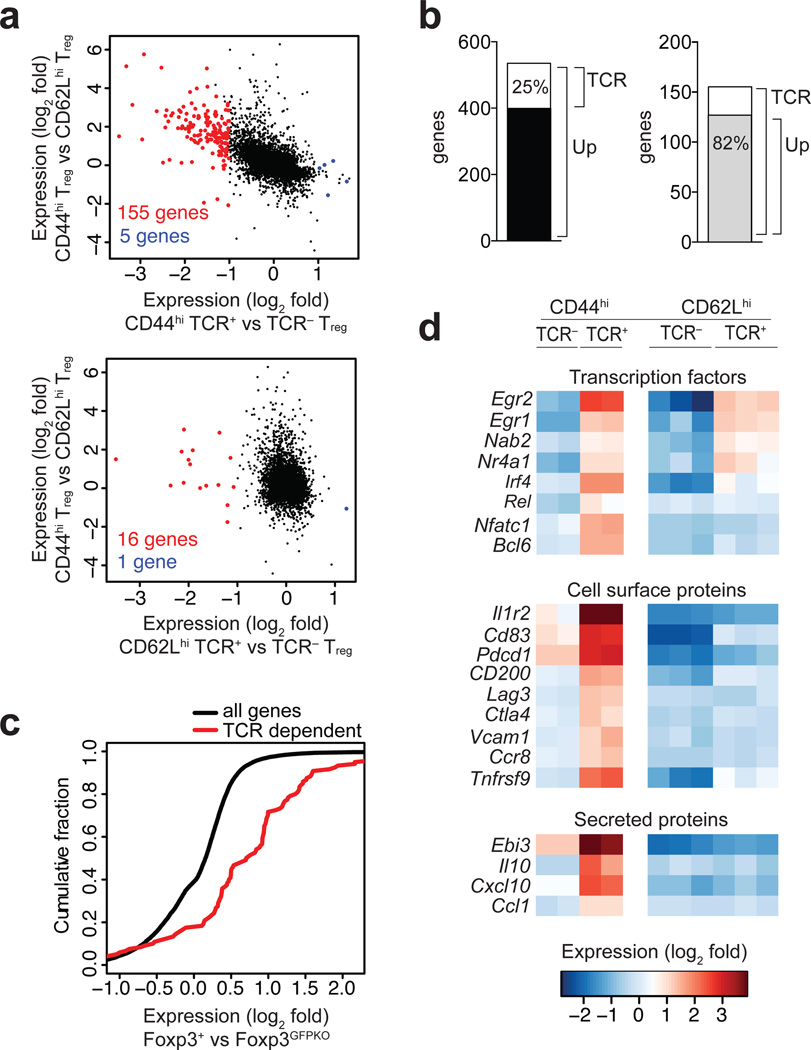
In order to explore whether TCR signals, apart from influencing Treg cell adhesion, might drive transcriptional events to license suppressor function in vivo, we conducted gene expression analysis of TCR+ and TCR− Treg cells. Flow cytometric analysis showed that loss of TCR expression had a stronger effect on effector-like CD44hiCD62Llo Treg cells compared to naïve-like CD44loCD62Lhi Treg cells (data not shown) prompting us to investigate the gene expression profiles of these two populations separately within the TCR+ and TCR− Treg cell populations isolated from healthy Foxp3CreERT2CαFL/WT mice (to avoid confounding effects of immune activation). We found 155 genes were significantly down-regulated, and only five genes were significantly up-regulated, by at least 2-fold in effector-like CD44hiCD62Llo TCR− Treg cells compared to CD44hiCD62Llo TCR+ Treg cells (Fig. 5a). Sixteen genes were significantly down-regulated in the naïve-like CD44loCD62Lhi TCR− Treg cells compared to CD44loCD62Lhi TCR+ Treg cells (all of them also down-regulated in CD44hiCD62Llo TCR− vs. TCR+ Treg cells), whereas one gene was up-regulated (Fig 5a). 535 genes showed higher expression (2-fold or greater) in effector-like CD44hiCD62Llo TCR+ Treg cells compared to naïve-like CD44loCD62Lhi TCR+ Treg cells, and expression of 136 of them (25%) was TCR-dependent (Fig. 5b). Importantly, 127 of the 155 genes (82%) down-regulated in the absence of TCR in CD44hiCD62Llo Treg cells showed at least 2-fold higher expression in effector-like vs. naïve-like Treg cells.
Figure 5. TCR signaling maintains the effector Treg cell transcriptional signature.
(a) Genes differentially expressed in CD44hiCD62Llo vs. CD44loCD62Lhi TCRβ+ Treg cells compared to genes differentially expressed in CD44hiCD62Llo TCRβ− vs. CD44hiCD62Llo TCRβ+ Treg cells (two replicates, upper panel) or CD44loCD62Lhi TCRβ− vs. CD44loCD62Lhi TCRβ+ Treg cells (three replicates, lower panel). The indicated cell subpopulations were sorted on day 14 from Foxp3CreERT2CαFL/WT mice (five or more mice per replicate) treated with tamoxifen on days 0, 1 and 3 using an Aria II flow cytometer. Gene expression was analyzed using Affymetrix 430 2.0 mouse gene expression arrays. Genes down- or up-regulated in the absence of TCR are shown in red or blue, respectively. The number of genes significantly differentially expressed by 2-fold or more are indicated, q-value < 0.01. (b) Genes down-regulated 2-fold or more in CD44hiCD62Llo TCRβ− vs. TCRβ+ Treg cells (‘TCR’) among genes up-regulated 2-fold or more in CD44hiCD62Llo vs. CD44loCD62Lhi Treg cells (‘Up’) are shown as white portion of the black bar (left) and ‘Up’ genes among ‘TCR’ genes are shown as the gray portion of the white bar (right). (c) Cumulative distribution function plot of TCR-dependent genes vs. all genes differentially expressed in Foxp3GFPKO vs. Foxp3+ CD4+ T cells. P < 10−20, two-sample Kolmogorov-Smirnov test. (d) Treg cell TCR-dependent genes encoding transcription factors, cell surface, and secreted molecules.
Foxp3 has been proposed to solidify and amplify a transcriptional program initiated in Treg cell precursors by TCR engagement9, 10, 35, 36. We compared expression of the 155 genes identified above as being maintained by TCR in Treg cells (which we call TCR-dependent genes) to Foxp3-dependent genes, identified as upregulated in wild-type Treg cells compared to Foxp3null-expressing T cells from Foxp3GFPKO mice (which express a Foxp3 reporter null allele).36 We found that a significantly enriched percentage of the TCR-dependent genes, compared to all genes, were also Foxp3-dependent (Fig. 5c). These observation suggest that the TCR-driven transcriptional program in Treg cells is enhanced by Foxp3 expression, but that Foxp3 alone is not sufficient to maintain the full effector Treg cell transcriptional signature.
Examination of the TCR-dependent genes identified several transcription factors that were upregulated in TCR+ compared to TCR− CD44hiCD62Llo Treg cells as well as compared to TCR+ and TCR− CD44loCD62Lhi Treg cells, including NFATc1, c-Rel, Bcl6 and IRF4 the latter two of which were previously shown to be important for Treg cell effector differentiation and function (Fig 5d).37–39 Of the 155 TCR-dependent genes, we identified only one gene encoding an adhesion molecule, Vcam1, which was similarly upregulated in CD44hiCD62Llo Treg cells compare to CD44loCD62Lhi Treg cells in a manner than depended upon TCR expression (Fig. 5d). Several genes encoding potential effector molecules were also found to be upregulated in CD44hiCD62Llo vs. CD44loCD62Lhi Treg cells in a TCR-dependent manner, including IL-1R2, a decoy receptor for IL-1, and the immune inhibitory molecules CD83, CD200, and LAG-3, as well as IL-10 and EBI3, a subunit of the cytokines IL-27 and IL-35, all of which have been implicated in Treg cell function26, 40–45. In addition, the chemokine-encoding genes Cxcl10 and Ccl1, as well as Ccr8 encoding the receptor for CCL1, were significantly down-regulated in TCR− compared to TCR+ Treg cells, suggesting that Treg cells may signal each other through the expression of chemokines and their corresponding receptors, or may recruit into close proximity the targets of their suppressive acitivity46.
All together, these data indicate that, under physiologic conditions, a substantial portion of the effector—but not the naïve-like—Treg cell transcriptional program characterized by elevated expression of several potential Treg cell effector molecules, is maintained by continuous TCR signaling.
IRF4 expression promotes Treg cell function and homeostasis
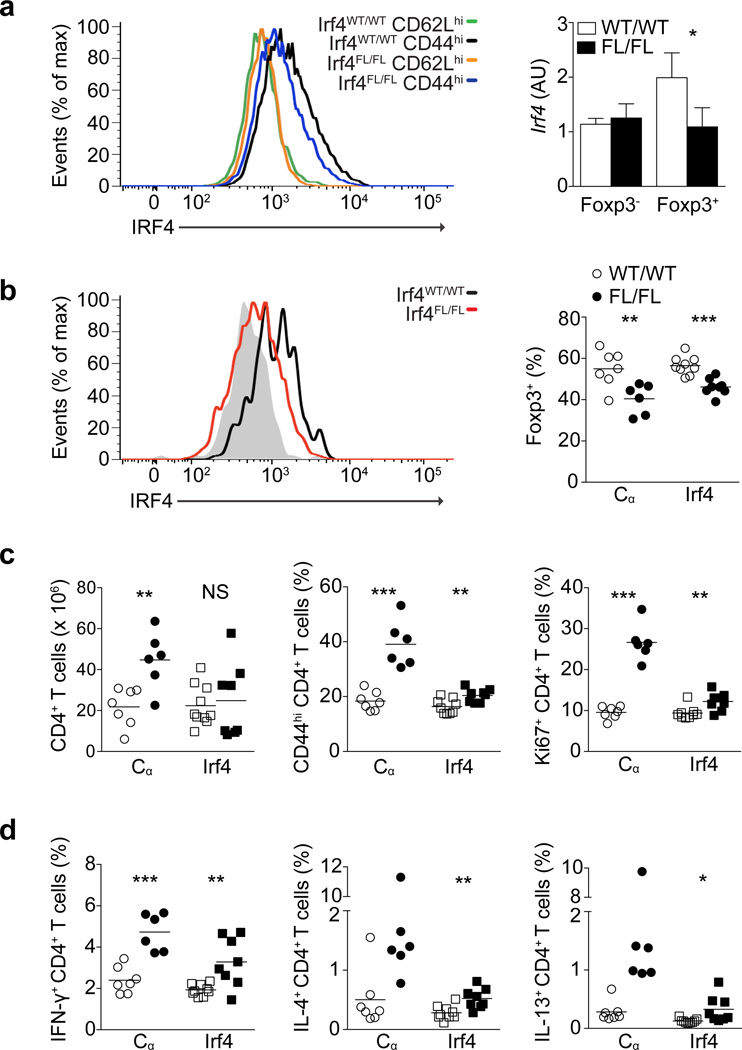
To begin to assess the importance of the TCR-dependent transcriptional program for continuous Treg cell function in vivo, we focused on IRF4 as a downstream target of the TCR signaling pathway in Treg cells. We confirmed that elevated IRF4 expression in Treg cells was restricted to CD44hi cells and was reduced to basal levels upon TCR ablation in both Foxp3CreERT2CαFL/WT and Foxp3CreERT2CαFL/FL mice (Supplementary Fig. 7a). Foxp3YFP-CreIrf4FL/- mice, in which IRF4 is constitutively deleted in Treg cells, have been shown to develop a severe TH2 cytokine-dominated autoimmunity by 8 weeks of age37. Treg cells in Irf4−/− mice were previously demonstrated to have an almost exclusively naïve-like phenotype, and we similarly found that Treg cells in Foxp3YFP-CreIrf4FL/FL mice were largely CD44loCD62Lhi even in the context of severe inflammation (data not shown), suggesting impaired differentiation, survival and/or expansion of effector Treg cells38. In order to determine whether fully differentiated Treg cells require IRF4 expression downstream of TCR engagement for their in vivo suppressive function, we administered tamoxifen on days 0, 3, 7 and 10 to Foxp3CreERT2Irf4FL/FL and Foxp3CreERT2Irf4WT/WT littermates47. On day 13, we observed a slight but reproducible decrease in IRF4 protein expression in lymph node CD44hi Treg cells in Foxp3CreERT2Irf4FL/FL mice compared to Foxp3CreERT2Irf4WT/WT mice, and an increase in GFP expression in the Treg cells as a sum of fluorescence of the Irf4-deletion GFP reporter (whose expression is switched on in the Irf4 locus upon Cre-mediated deletion of the Irf4FL allele) and the GFP-CreERT2 fusion protein expressed concomitantly with Foxp3 (Fig. 6a and Supplementary Fig. 7b. qPCR analysis indicated a ~50% reduction in Irf4 mRNA transcript amount in Treg cells sorted from pooled spleens and lymph nodes of tamoxifen-treated Foxp3CreERT2Irf4FL/FL compared to Foxp3CreERT2Irf4WT/WT mice (Fig. 6a).
Figure 6. IRF4 expression contributes to optimal Treg cell suppressive capacity and homeostasis.
(a) IRF4 expression in lymph node CD44hiCD62Llo (‘CD44hi’) and CD44loCD62Lhi (‘CD62Lhi’) CD4+Foxp3+ cells in Foxp3CreERT2Irf4WT/WT and Foxp3CreERT2Irf4FL/FL mice (left) and qPCR analysis of IRF4 mRNA transcript amounts in CD4+eGFP− and CD4+eGFP+ cells sorted from pooled spleens and lymph nodes (right). qPCR is representative of two experiments with four or more mice per group each (b) IRF4 expression in colonic lamina propria CD4+Foxp3+ cells in Foxp3CreERT2Irf4WT/WT and Foxp3CreERT2Irf4FL/FL mice (left); gray histogram represents CD4+Foxp3− cells. Percent Foxp3+ among CD4+ cells in the large intestine lamina propria in Foxp3CreERT2CαWT/WT and Foxp3CreERT2Irf4WT/WT (white circles) and Foxp3CreERT2CαFL/FL and Foxp3CreERT2Irf4FL/FL (black circles) mice, as indicated (right). (c,d) Numbers, percent CD44hi, percent Ki67+ (c) and percent cytokine-producing cells (d) of CD4+Foxp3− T cells in lymph nodes (c) and spleens (d) of Foxp3CreERT2CαWT/WT (white circles), Foxp3CreERT2Irf4WT/WT (white squares), Foxp3CreERT2CαFL/FL (black circles), and Foxp3CreERT2Irf4FL/FL (black squares) mice. All mice were analyzed on day 13 following tamoxifen treatment on days 0, 3, 7 and 10. Histograms (a,b) are representative of two experiments with four or more mice per group each. All other data in (b–d) is combined from two experiments with four or more mice per group each. ***, P < 0.001; **, P < 0.01; *, P < 0.05. P-values were calculated using an unpaired t-test.
We hypothesized that the suboptimal ~50% reduction in mRNA amount and the only slight reduction in IRF4 protein expression in the spleens and lymph nodes of Foxp3CreERT2Irf4FL/FL compared to Foxp3CreERT2Irf4WT/WT mice might have resulted from a competitive disadvantage of Treg cells lacking IRF4 protein, which might lead to preferential expansion of IRF4-sufficient Treg cells remaining in Foxp3CreERT2Irf4FL/FL mice. This would be consistent with the reported observation that the survival and expansion of strongly antigen-stimulated CD8+ T cells was greatly impaired in the absence of IRF448. In order to assess whether IRF4 might similarly contribute to the maintenance of Treg cells that have been strongly activated, we looked in the colonic lamina propria, in which nearly all Treg cells were CD44hi and likely had recently experienced TCR engagement based on robust IL-10 production (data not shown). Indeed, we observed decreased percentages of colonic lamina propria Treg cells in Foxp3CreERT2Irf4FL/FL compared to Foxp3CreERT2Irf4WT/WT mice, similar to the decrease in Treg cell percentages seen in the colonic lamina propria of Foxp3CreERT2CαFL/FL compared to Foxp3CreERT2CαWT/WT mice (Fig. 6b). As opposed to spleen and lymph node Treg cells, and consistent with their decreased percentages, colonic lamina propria Treg cells in Foxp3CreERT2Irf4FL/FL mice exhibited a substantial reduction in IRF4 protein expression compared to Treg cells in Foxp3CreERT2Irf4WT/WT mice (Fig. 6b). We hypothesized that the reportedly low influx of circulating Treg cells into the colonic lamina propria at steady state may have precluded IRF4-sufficient cells from becoming activated and repopulating to wild type percentages the Treg cell niche in this tissue24.
Despite the only modest decrease in IRF4 expression in Treg cells isolated from the spleens and lymph nodes of Foxp3CreERT2Irf4FL/FL mice, we were able to detect a very mild, but statistically significant increase in the percentage of CD44hi and Ki67+ lymph node Foxp3−CD4+ T cells as well as increased IFN-γ, IL-4 and IL-13 production by splenic Foxp3−CD4+ T cells, suggesting that IRF4 expression downstream of TCR signaling in Treg cells contributes to Treg cells suppressive function (Fig. 6c,d). An increase in the production of TH2 cytokines was consistent with the phenotype of mice with constitutive ablation of IRF4 in Treg cells, whereas the increased IFN-γ was likely a consequence of the substantial TH1 bias in the C56B/L6 adult mice prior to induced Irf4 deletion37. As a control, we confirmed that in the absence of tamoxifen treatment, CD4+Foxp3− T cell expression of CD44, Ki67, IFN-γ, IL-4 and IL-13 in Foxp3CreERT2Irf4FL/FL and Foxp3CreERT2Irf4WT/WT mice were indistinguishable, as were Treg cell percentages in the colonic lamina propria (data not shown), suggesting that the modest differences we observed between Foxp3CreERT2Irf4FL/FL and Foxp3CreERT2Irf4WT/WT mice upon tamoxifen treatment were not a consequence of the Irf4FL allele itself. Together, these data indicate that even partial loss of IRF4 expression downstream of TCR engagement in Treg cells interferes with optimal suppressive function of these cells.
Discussion
Despite major progress in understanding the molecular mechanisms of TCR engagement-driven differentiation of Treg cells, the role of the TCR in Treg cell function in vivo has remained unclear. Here, we demonstrate that TCR signaling in differentiated Treg cells is dispensable for the maintenance of Foxp3 expression and for expression of many Treg cell characteristic markers. Although the bulk of the Treg cell-specific gene signature was also preserved in the absence of TCR, suppressor function was critically dependent on the TCR.
Given that antigen-activated CD4+Foxp3− T cells in lymphoid organs are thought to produce IL-2 in a spatially restricted manner, we considered the possibility that Treg cells might analogously require their TCRs to correctly position themselves to gain preferential access to IL-2, which might elicit their suppressive function by stimulating IL-2R. However, our in vivo and in vitro data suggests that Treg cells acquire and likely effectively deplete IL-2 in a TCR-independent manner, and thus may instead rely predominantly on CCR7 expression to ensure sufficient IL-2 exposure, as recently proposed24.
Our observation that newly generated Treg cells in Foxp3CreCαFL/WT and Foxp3CreCαFL/FL mice remained naïve-like upon loss of TCR and did not populate non-lymphoid tissues suggests that effector differentiation and expansion are TCR- and likely antigen-dependent processes. This finding may help explain the observation that distinct Treg cell TCR repertoires are displayed by Treg cells populations found in distinct lymphoid organs and tissues in adult mice49, 50. Furthermore, the fact that inducible ablation of the TCR resulted in a far more pronounced change in gene expression in the effector-like vs. the naïve-like Treg cell subset suggests that continuous TCR signaling may be selectively driving the homeostasis and suppressor function of effector-like Treg cells. As inducible deletion of the TCR in differentiated Treg cells precipitated autoimmunity, our data may suggest that all—or the bulk—of Treg cell suppressor function in vivo is mediated by the CD44hi effector-like Treg cell subset.
We found that partial inducible ablation of Irf4, expressed downstream of TCR engagement in CD44hi Treg cells, resulted in a very mild, but highly reproducible immune activation. Although this result suggests that IRF4 expression is important for TCR-dependent Treg cell function, given the suboptimal Irf4 deletion and modest immune activation, to what extent and how TCR-dependent IRF4 induction in Treg cells contributes to their ability to suppress spontaneous autoimmunity remains to be determined. IRF4 may predominantly function to control maintenance of highly activated Treg cells, which was particularly evident in the colonic lamina propria and which, when altered, may affect the ability of the Treg cell pool to suppress. Alternatively, IRF4 may play a more direct role in promoting Treg cell suppressive activity, perhaps by driving expression of certain Treg cell effector molecules. Although we observed decreased ICOS expression on colonic Treg cells lacking IRF4, the remaining IRF4-sufficient Treg cells present in lymphoid organs of Foxp3CreERT2Irf4FL/FL mice complicate a more rigorous identification of IRF4 targets (data not shown).
It is important to note, however, that the fact that naïve-like Treg cells do not express IRF4 and are overwhelmingly unaffected by TCR deletion on a transcriptional level is not necessarily an indication that these cells are non-functional or that they are not experiencing TCR engagement. Indeed, several genes including Egr1, Egr2 and Nr4a1 were found to be down-regulated in this Treg cell subset in the absence of TCR.
Future experiments will be necessary to elucidate the contributions of other individual Treg cell TCR-dependent genes to maintenance of immune tolerance in the steady state, and to the restraint of immune responses directed against commensal microorganisms, food and environmental antigens and pathogens. In this regard, it is noteworthy that while IL-10 production by Treg cells has been implicated in the control of inflammatory responses at environmental interfaces such as the gut, lungs and skin, it has also been shown to be dispensable for Treg cell control over systemic autoimmunity26. Likewise, we found that while constitutive deletion of calcineurin B1 in Foxp3CreCnb1FL/FL mice (which eliminated calcineurin-dependent NFAT activation in Treg cells) resulted in lethal early onset autoimmunity, highly efficient inducible deletion in adult lymphoreplete Foxp3CreERT2Cnb1FL/FL mice had no detectable adverse consequences on Treg cell function (data not shown). Together, these findings suggest that TCR engagement on Treg cells may drive a focused transcriptional program, select aspects of which are required in a context dependent manner for mediation of a broad range of Treg cell immunosuppressive functions.
Online Methods
Mice
Mice were bred and housed in the pathogen-free animal facility at Memorial Sloan-Kettering Cancer Center and were used in accordance with institutional guidelines. Unless otherwise noted, 8–10 week old mice were used for all experiments. Foxp3YFP-Cre, Foxp3eGFP-CreERT2, Rosa26YFP, Foxp3DTR, and Irf4FL mice have been previously described19, 26, 30, 47. We thank Marc Schmidt-Supprian and Klaus Rajewsky for kindly providing CαFL mice. For tamoxifen administration, 40mg tamoxifen were dissolved in 100ul ethanol and subsequently in 900uL olive oil (Sigma-Aldrich) and sonicated 4 × 30 seconds in a Bioruptor Twin (Diagenode). Mice were orally gavaged with 200 µl tamoxifen emulsion per treatment. For diphtheria toxin (DT) injections, DT was dissolved in PBS and 200 µl of indicated doses were injected i.p per mouse. For in vivo IL-2 depletion, mice were gavaged with tamoxifen on days 0 and 1 or on days 0, 3, 7 and 10 and injected i.p. on days 4 and 8 with a 0.5mg 1:1 mix of IL-2 neutralizing JES6- 1A12 and S4B6-1 antibodies or IgG2a isotype (BioX-Cell). For administration of IL-2-anti-IL2 complexes, 1ug recombinant mouse IL-2 (R&D Systems) was incubated for 10 minutes at room temperature with 5 µg JES6-1 (BioXCell) and diluted to 200 µl in PBS immediately before i.p injection. Mice were gavaged with taxomofen on days 0, 3, 7 and 10 and received IL-2-anti-IL-2 complexes or PBS on days 5, 7, 9 and 11.
FACS staining and cell isolation
Cells were stained with LIVE/DEAD Fixable Yellow Dead Cell Stain (Molecular Probes) and antibodies listed in Supplementary Table 1. For BrdU experiments, mice were injected i.p. with 1mg BrdU in 1mL PBS and staining was performed using the BD Pharmingen BrdU Flow Kit. Flow cytometric analysis was performed using an LSRII flow cytometer (BD Bioscience) and FlowJo software (Tree Star). For cell isolation, CD4+ T cells were purified from pooled spleen and lymph node cell suspensions using magnetic Dynabeads (Invitrogen) and further sorted using an Aria II cell sorter (BD Bioscience). Intracellular staining was performed using eBioscience Fixation Permeabilization buffers. For cytokine staining lymph node and spleen cells were stimulated with soluble anti-CD3 clone 2C11 (5ug/ml) and anti-CD28 clone 37.51 (5ug/ml) in the presence of 1ug/mL brefeldin A for 4–6 hours at 37°C, 5% CO2.
In vitro proliferation assay
Dendritic cells (DCs) were expanded in vivo by subcutaneous injection of B16 melanoma cells secreting Flt3 ligand into the left hind flank of B6 mice. Once tumors were visible, spleens from injected animals were dissociated in RPMI 1640 medium containing 1.67 units/mL liberase TL (Roche) and 50 µg/mL DNAse I (Roche) for 20 min at 37 °C with shaking. EDTA was added at a final concentration of 5 mM to stop digestion and the resulting homogenate was processed for CD11c+ cell isolation using the MACS mouse CD11c (N418) purification kit (Miltenyi Biotec). FACS purified CD4+eGFP+ cells from tamoxifen treated Foxp3CreERT2CαFL/WT mice were labeled with CellTrace Violet (Molecular Probes) according to the manufacturer’s instructions and plated in triplicate in 96-well flat bottom plates (5 × 104 cells/well) in media containing 25 U/mL human recombinant IL-2 (PeproTech) with or without equal numbers of DCs, with or without 100ng/mL LPS (E. coli 0111:B4, Sigma-Aldrich.)
In vitro IL-2 stimulation and pSTAT5 detection
Foxp3CreERT2CαFL/WT mice were treated with tamoxifen on days 0 and 1, and on day 9 CD4+ T cells were purified from pooled spleen and lymph node cell suspensions using magnetic Dynabeads (Invitrogen). Cells were stained with anti-CD4 and anti-TCRβ, washed, and plated in 96-well V-bottom plates (1 × 106 cells/well) in RPMI containing 10% FBS with or without increasing concentrations of IL-2 for 20 minutes at 37oC. Cells were subsequently processed using BD Phosflow Lyse/Fix Buffer and Perm Buffer III (BD Biosciences) and stained with anti-p-STAT5 Y694 antibody according to the manufacturer’s instructions. Treg cells were identified by eGFP expression. For ex vivo p-STAT5 staining, spleen and lymph nodes were dissociated at 4°C in PBS (0.5% BSA) containing anti-CD4 and anti-TCRβ antibodies, stained for 10 minutes on ice, and washed twice before fixation.
In vitro IL-2 depletion
Foxp3CreERT2CαFL/WT mice were treated with tamoxifen on days 0 and 1, and on day 9 pooled spleens and lymph nodes were enriched for CD4+ cells and subsequently sorted to >99% purity into eGFP+TCRβ+, eGFP+TCRβ−, and eGFP−TCRβ+ populations. Each population was divided among 8 wells of a V-bottom 96-well plate (250,000 cells/well) in 25uL RPMI(10%) with or without increasing doses of recombinant human IL-2 for 2 hours at 37°C. IL-2 depletion from the media was assessed using the BD Cytometric Bead Array and Human IL-2 Enhanced Sensitivity Flex Set (BD Biosciences) according to the manufacturer’s instructions.
In vitro conjugation assay
Foxp3CreERT2CαWT/WT, Foxp3CreERT2CαFL/WT, and Foxp3CreERT2CαFL/FL mice were treated with tamoxifen on days 0 and 1 and on day 9, CD4+ T cells were isolated from spleen and lymph nodes using the Dynabeads Untouched Mouse CD4 Cells negative selection kit (Invitrogen.) TCR+ Treg cells were sorted from Foxp3CreERT2CαWT/WT mice based on eGFP expression alone. TCR− Treg cells were sorted from Foxp3CreERT2CαFL/WT and Foxp3CreERT2CαFL/FL mice as eGFP+ TCRβ−. Treg cells were subsequently labeled with CFSE and Flt3L-expanded DCs from Ab (MHC class II)+/+ and Ab (MHC class II)−/− mice were labeled with CellTrace Violet. 104 Treg cells and 6 × 104 DCs were cultured together in a 96-well round bottom plate in RPMI 10% supplemented with 500 U/mL IL-2. Concanavalin A was used at a final concentration of 2.5 ug/mL. Following a 10 hr culture at 37°C, cells were gently resuspended before flow cytometric analysis.
Gene expression analysis
eGFP+ TCRβ+ and TCRβ− CD44hiCD62Llo (two replicates) and CD44loCD62Lhi (three replicates) cell populations were sorted from tamoxifen-treated Foxp3CreERT2CαFL/WT mice (five or more mice per replicate) using an Aria II flow cytometer. Complementary DNA (cDNA) libraries were amplified and hybridized to Affymetrix 430 2.0 chips. Arrays were normalized using RMA, and genes were considered differentially expressed if they had a q value <0.01 after Benjamini-Hochberg FDR correction. Differential gene expression in Treg cells from Foxp3GFPKO vs. Foxp3+ mice has been previously described.
Supplementary Material
Footnotes
Author contributions
A.G.L. and A.Y.R. designed the experiments, and A.G.L conducted experiments and wrote the manuscript. A.Y.R. supervised the research and edited the manuscript. W.J. prepared samples for microarray analysis and A.A. and A.G.L. conducted the analysis.
Competing financial interests
The authors declare no competing financial interests.
Accession codes. GEO: microarray data, GSE61077
References
- 1.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annual review of immunology. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee HM, Bautista JL, Scott-Browne J, Mohan JF, Hsieh CS. A broad range of self-reactivity drives thymic regulatory T cell selection to limit responses to self. Immunity. 2012;37:475–486. doi: 10.1016/j.immuni.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nature immunology. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh CS, et al. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nature immunology. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 6.Ouyang W, et al. Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature. 2012;491:554–559. doi: 10.1038/nature11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marson A, et al. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 2007;445:931–935. doi: 10.1038/nature05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Au-Yeung BB, et al. A genetically selective inhibitor demonstrates a function for the kinase Zap70 in regulatory T cells independent of its catalytic activity. Nature immunology. 2010;11:1085–1092. doi: 10.1038/ni.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gavin MA, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 10.Lin W, et al. Regulatory T cell development in the absence of functional Foxp3. Nature immunology. 2007;8:359–368. doi: 10.1038/ni1445. [DOI] [PubMed] [Google Scholar]
- 11.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nature immunology. 2007;8:277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 12.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 13.Borsellino G, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 14.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nature immunology. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 15.Shafiani S, et al. Pathogen-specific Treg cells expand early during mycobacterium tuberculosis infection but are later eliminated in response to Interleukin-12. Immunity. 2013;38:1261–1270. doi: 10.1016/j.immuni.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samy ET, Parker LA, Sharp CP, Tung KS. Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+CD25+ regulatory T cells in the regional lymph node. The Journal of experimental medicine. 2005;202:771–781. doi: 10.1084/jem.20041033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polic B, Kunkel D, Scheffold A, Rajewsky K. How alpha beta T cells deal with induced TCR alpha ablation. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8744–8749. doi: 10.1073/pnas.141218898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubtsov YP, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nature reviews. Immunology. 2012;12:157–167. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- 21.Zheng Y, et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruan Q, et al. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009;31:932–940. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 24.Smigiel KS, et al. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. The Journal of experimental medicine. 2014;211:121–136. doi: 10.1084/jem.20131142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisson S, et al. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. The Journal of experimental medicine. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Tang Q, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nature immunology. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 29.Webster KE, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. The Journal of experimental medicine. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nature immunology. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 31.Tian L, et al. Foxp3(+) regulatory T cells exert asymmetric control over murine helper responses by inducing Th2 cell apoptosis. Blood. 2011;118:1845–1853. doi: 10.1182/blood-2011-04-346056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou T, et al. Cutting edge: IL-2 signals determine the degree of TCR signaling necessary to support regulatory T cell proliferation in vivo. J Immunol. 2012;189:28–32. doi: 10.4049/jimmunol.1200507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. The Journal of experimental medicine. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JK, et al. Impact of the TCR signal on regulatory T cell homeostasis, function, and trafficking. PloS one. 2009;4:e6580. doi: 10.1371/journal.pone.0006580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohkura N, et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37:785–799. doi: 10.1016/j.immuni.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Samstein RM, et al. Foxp3 exploits a pre-existent enhancer landscape for regulatory T cell lineage specification. Cell. 2012;151:153–166. doi: 10.1016/j.cell.2012.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng Y, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cretney E, et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nature immunology. 2011;12:304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- 39.Sawant DV, et al. Bcl6 controls the Th2 inflammatory activity of regulatory T cells by repressing Gata3 function. J Immunol. 2012;189:4759–4769. doi: 10.4049/jimmunol.1201794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collison LW, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 41.Colotta F, et al. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993;261:472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- 42.Huang CT, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Petermann KB, et al. CD200 is induced by ERK and is a potential therapeutic target in melanoma. The Journal of clinical investigation. 2007;117:3922–3929. doi: 10.1172/JCI32163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reinwald S, et al. CD83 expression in CD4+ T cells modulates inflammation and autoimmunity. J Immunol. 2008;180:5890–5897. doi: 10.4049/jimmunol.180.9.5890. [DOI] [PubMed] [Google Scholar]
- 45.Hall AO, et al. The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 2012;37:511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoelzinger DB, et al. Blockade of CCL1 inhibits T regulatory cell suppressive function enhancing tumor immunity without affecting T effector responses. J Immunol. 2010;184:6833–6842. doi: 10.4049/jimmunol.0904084. [DOI] [PubMed] [Google Scholar]
- 47.Klein U, et al. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nature immunology. 2006;7:773–782. doi: 10.1038/ni1357. [DOI] [PubMed] [Google Scholar]
- 48.Man K, et al. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nature immunology. 2013;14:1155–1165. doi: 10.1038/ni.2710. [DOI] [PubMed] [Google Scholar]
- 49.Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh CS. Antigen-specific peripheral shaping of the natural regulatory T cell population. The Journal of experimental medicine. 2008;205:3105–3117. doi: 10.1084/jem.20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lathrop SK, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.