Abstract
Background
Remote ischemic conditioning (RIC) is a powerful endogenous mechanism whereby a sublethal ischemic stimulus confers a protective benefit against a subsequent severe ischemic insult. RIC has significant potential clinical implications for the prevention of delayed ischemic neurological deficit (DIND) after aneurysmal subarachnoid hemorrhage (aSAH). While RIC has been extensively investigated in animal models, it has not been fully evaluated in humans.
Objective
To assess the feasibility and safety of RIC for aSAH in a phase I clinical trial.
Methods
Consecutive patients hospitalized for treatment of an aSAH who met the inclusion/exclusion criteria were approached for consent. Enrolled patients received up to four RIC sessions on non-consecutive days. Primary endpoints were (1) the development of a symptomatic deep venous thrombosis (DVT), bruising or injury to the limb and, (2) request to stop by the patient or surrogate. The secondary endpoints were the development of new neurological deficits or cerebral infarct, demonstrated by brain imaging after enrollment, and neurological deficit and condition at follow-up.
Results
Twenty patients were enrolled and underwent 76 RIC sessions, 75 of which were completed successfully. One session was discontinued when the patient became confused. No patient developed a DVT or injury to the preconditioned limb. No Patient developed DIND during their enrollment. At follow-up, median modified Rankin Scale was 1 and Glasgow Outcome Score was 5.
Conclusion
The RIC procedure was well tolerated and did not cause any injury. RIC for aSAH warrants investigation in a subsequent pivotal clinical trial.
Keywords: Clinical Trial, Remote Ischemic Conditioning, Subarachnoid Hemorrhage
Introduction
Ischemic preconditioning is a powerful endogenous mechanism whereby a mild ischemic stress to a tissue renders it resistant to subsequent severe ischemia. This protective mechanism has also been demonstrated when a low-risk organ, such as a limb, is used to induce the ischemic transient stress yet still confers a protective benefit to more critical organs such as the heart or brain, a phenomenon known as remote ischemic conditioning (RIC).1–6 RIC has potential clinical applications in the prevention of cerebral ischemia, as it can be applied without exposing the brain to unnecessary risk. The mechanism of ischemic protection underlying RIC is likely multifactorial and includes down-regulation of genes associated with inflammation,7,8 reduction of glutamatergic excitotoxicity,9,10 blockage of apoptotic neuronal pathways,11,12 and increase in blood flow to the ischemic penumbra.13
So far, the majority of clinical applications of ischemic preconditioning have focused on the prevention of myocardial infarction, showing potential protective benefit before coronary bypass and percutaneous angioplasty and stenting procedures.1,14,15 There have been a limited number of attempts to apply RIC for cerebral protection, the results of which have been inconsistent.5,16
To successfully translate the powerful effects of RIC observed in animal studies into clinical practice, several challenges need to be overcome in a systematic manner: 1) A remote stimulus that can generate transient but effective ischemia must be identified and demonstrated to induce ischemia; 2) It must be applied to a condition in which the time window of likely ischemia allows for the use of preconditioning; 3) The physiological effects associated with the RIC maneuvers should be evaluated; 4) The technique selected for induction of remote preconditioning should be tested for safety and tolerability; and 5) Finally, the effects of RIPC should be compared with standard management of that condition in a randomized trial. We have performed preliminary studies addressing the first three steps of this translational strategy. Our prior work has demonstrated that lower limb transient ischemia is feasible and produces local metabolic changes consistent with sublethal ischemia.17 The rationale for selecting patients with aneurysmal subarachnoid hemorrhage to prevent delayed ischemic neurological deficit (DIND) is based on the well-defined time course of vasospasm and DIND after aneurysmal rupture and has been extensively discussed.18 In a pilot study, we demonstrated cerebral vascular and metabolic effects of the RIC maneuvers with lower limb ischemia.19 Finally, to test the hypothesis that RIC by induction of transient lower limb ischemia is a feasible and safe strategy in patients hospitalized for the treatment of aSAH, we present here the results of a prospective phase I clinical trial to test the safety and feasibility of RIC induced by transient lower limb ischemia in patients with aSAH.
Methods
Study Design and Patients
This was a single-center, Phase I study to assess feasibility and safety of RIC as a prophylactic treatment for DIND. Patients with aSAH confirmed by CT, MR, or catheter angiography and single or multiple aneurysms protected by endovascular coiling or surgical clipping and who met the inclusion and exclusion criteria (Table 1) were considered for enrollment. While the intention was to only enroll patients within 72 hours of hemorrhage, many patients are admitted after this window. For this reason the decision was made to extend the enrollment widow to 14 days post-hemorrhage. Consecutive eligible patients, or their legal representatives, were approached for consent. This study and the materials used for enrollment and consent were approved by the university’s institutional review board.
Table 1.
Inclusion and exclusion criteria for enrollment in the trial
| Inclusion Criteria |
|---|
| Patient age 18–80 |
| Patient capable of providing consent or accompanied by appropriate surrogate for consent |
| SAH confirmed on CT or LP with aneurysm origin confirmed by CTA or angiography |
| Aneurysm has been protected by clipping or coiling |
| Time of enrollment within 72 hours of SAH |
| Informed consent signed by patients or surrogate |
| Exclusion Criteria |
| Unprotected intracranial aneurysm or aneurysms |
| History of peripheral vascular disease, lower extremity bypass of physical exam findings of vascular disease |
| History of deep vein thrombosis or physical exam findings of deep vein thrombosis |
| Pregnancy |
| Parenchymal or intracerebral hemorrhage |
| History of peripheral neuropathy or physical exam findings of peripheral neuropathy |
The enrollment goal for this trial was guided by the Confidence Interval Approach described by Thabane et al.20 A safety concern for RIC is the development of a deep-venous thrombosis (DVT) from the repeated lower limb compression. However, DVTs can develop as a side effect of routine ICU care and, therefore, confound the assessment of safety. The sample size calculation was based on a 95% confidence interval for the proportion of patients who develop a DVT with an upper bound of 0.25. The estimated incidence of DVT for unscreened patients with aSAH was based on a reported value of 12%,21 giving a required sample size of 15 patients.
Preconditioning protocol
Patients underwent RIC sessions on non-consecutive days until any one of the following three conditions were met: 4 sessions had been completed, the patient reached a clinical endpoint (development of a DVT or request to stop), or the patient was discharged. The leg that had not received any catheter treatments (catheter angiogram or venous cooling catheter devices) was designated for preconditioning. In preparation for each session the dorsalis pedis artery was identified with the aid of a pulse Doppler and marked with a permanent marker. A large blood pressure cuff was placed around the leg, inflated to 20 mm Hg above the patient’s systolic blood pressure, and the connecting tubes were clamped using locking forceps. The pedal pulse Doppler was then used to verify that the signal had been obliterated, confirming cessation of blood flow. If the pulse was detected, the blood pressure cuff was inflated further within the comfort level of the patient. After successful induction of ischemia, the cuff pressure was maintained for 5 minutes, while the absence of a pedal pulse was periodically verified. After 5 minutes of ischemia the blood pressure cuff was deflated and the limb allowed to reperfuse. Reperfusion was confirmed with the pedal pulse Doppler. After 5 minutes, the cycle of ischemia/reperfusion was repeated an additional 3 times. After the completion of 4 cycles of ischemia and reperfusion, the cuff was removed and the leg inspected for bruising or other injury. A complete preconditioning session is defined as 4 rounds of verified ischemia and perfusion.
Primary and secondary endpoints
The primary endpoints to assess the feasibility and safety of the RIC for aSAH were (1) the development of a symptomatic DVT in the limb used for preconditioning that is temporally related to the procedure or bruising/damage to the limb during or after the preconditioning treatment, and (2) request by the patient or surrogate to cease the RIPC treatment.
The secondary endpoints were the development of new neurological deficits or cerebral infarct demonstrated by brain imaging after enrollment and neurological deficit and condition at follow-up (modified Rankin score and Glasgow Outcome score).
Physiologic data collection
Vital signs and additional monitoring data (Table 2) were collected at baseline and at each subsequent inflation and deflation, for a total of nine measurements for each variable per RIC session. In addition to routine monitoring, patients received continuous bilateral transcranial Doppler (TCD) monitoring during the entirety of the preconditioning session when possible. If the patient had an external ventricular drain placed, it was closed prior to the start of the session and opened if the ICP exceeded the designated clinical goal.
Table 2.
Vital signs and additional monitoring measurements
| Vital Sign | Units |
|---|---|
| Heart Rate | beats/minute |
| Systolic Blood Pressure | mmHg |
| Diastolic Blood Pressure | mmHg |
| Mean Arterial Pressure | mmHg |
| Respiratory Rate | beats/minute |
| Temperature | Degrees Celsius |
| Additional Monitoring | |
| Central Venous Pressure | mmHg |
| Pain | 0 – 10, ordinal |
| Intracranial Pressure | mmHg |
| RMCA Mean Cerebral Blood Flow Velocity | cm/second |
| LMCA Mean Cerebral Blood Flow Velocity | cm/second |
| RMCA Pulsatility Index | - |
| LMCA Pulsatiliy Index | - |
Systolic and diastolic blood pressure, mean arterial pressure, central venous pressure, intracranial pressure, heart rate, and respiratory rate were collected from the bedside monitors. Cerebral blood flow velocity and pulsatility indices of both middle cerebral arteries were measured using TCD. Measurements were taken from the readings of the bedside monitors and TCD machine at the midpoint of each period of ischemia (2.5 min after cuff inflation) and reperfusion (2.5 min after cuff deflation). Data were manually recorded.
Statistical methods
All rational vital signs and additional monitoring data were analyzed using a repeated measures analysis of variance (rANOVA). The three rANOVA treatments considered were inflation, timepoint (baseline, 1st inflation, 1st deflation…), and session (1st session, 2nd session…). Because the pain scale was ordinal, changes were assessed by using a Wilcoxon Signed-Rank test to compare the median pain scale for each patient between inflation and deflation. To account for the repeated comparisons, the Holm-Bonferroni correction was applied to an alpha of 0.05 for all statistical tests. All statistical calculations were performed using IBM SPSS Statistics 64-bit edition Version 21 Release 21.0.0.0.
Results
Patient demographics and admission status
Twenty patients were enrolled and received RIC treatment. Of these patients, 6 (30%) were male and 14 (70%) were female. The age range was 22–77 years of age (Mean 53, STD 9.27). Complete patient demographics are detailed in Table 3.
Table 3.
Patient demographics and past medical history
| Patient Demographics and Past Medical History | ||
|---|---|---|
| Mean Age | 53 | |
| Ethnicity | ||
| Hispanic | 7 | |
| Not-Hispanic | 13 | |
| Race | ||
| White | 13 | |
| Black | 3 | |
| Asian | 4 | |
| Pacific Islander | 0 | |
| Native American | 0 | |
| Past Medical History | ||
| Diabetes | 3 | |
| Hypertension | 12 | |
| Hyperlipidemia | 2 | |
| Past Social History | ||
| Smoking | 7 | |
| Alcohol | 6 | |
| Unknown | 1 | |
Enrolled patients had a median Hunt and Hess score of 3 and a median Fisher score of 4 at admission (Table 4).
Table 4.
Admission status
| Admission Status | ||
|---|---|---|
| Hunt and Hess | ||
| 1 | 2 | |
| 2 | 4 | |
| 3 | 5 | |
| 4 | 4 | |
| 5 | 5 | |
| Fisher | ||
| 1 | 0 | |
| 2 | 2 | |
| 3 | 5 | |
| 4 | 13 | |
Adherence to the protocol
A total of 8 patients (40%) were enrolled within 72 hours post-hemorrhage, and 18 (90%) patients were enrolled within 7 days post-hemorrhage. Two patients (10%) were not enrolled within 7 days (9 and 14 days). Protocol adherence is summarized in Table 5.
Table 5.
Protocol adherence
| Protocol Adherence | ||
|---|---|---|
| Days to Enrollment Post Hemorrhage | ||
| 1 | 0 | |
| 2 | 2 | |
| 3 | 6 | |
| 4 | 2 | |
| 5 | 2 | |
| 6 | 3 | |
| 7 | 3 | |
| 7 or more | 2 | |
| Number of Sessions Completed | ||
| 1 | 0 | |
| 2 | 1 | |
| 3 | 3 | |
| 4 | 16 | |
| Number of Sessions Not Completed | ||
| 1 | 1 | |
Primary endpoints
During the course of the preconditioning treatment, no patient developed a symptomatic DVT or bruising/injury related to the RIC procedure. Two patients eventually developed symptomatic DVTs 11 and 28 days after the completion of the final conditioning session. Both patients had catheters placed in the ipsilateral femoral vein for treatment of hyperthermia after the conclusion of the RIC treatments and prior to the development the DVTs.
In general, the RIC procedure was well tolerated by the patients. A total of 76 RIC sessions were attempted for the enrolled patients. Of these, 75 were completed and one was discontinued early due to an episode of delirium in the patient, which limited cooperation with the procedure. Three patients (15%) only received three RIC sessions – one patient died from complications related to the initial aSAH (Hunt & Hess Grade 5, Fisher Grade 4) and two were discharged before all four sessions could be completed (Table 6).
Table 6.
Primary endpoints
| Primary Endpoints | |||
|---|---|---|---|
| Endpoint | Notes | ||
| Deep Vein Thrombosis | 2 | 11 and 28 days after final session | |
| Bruising or Damage to Limb | 0 | ||
| Request to Stop Preconditioning | 0 | ||
Secondary endpoints
Of the enrolled patients, 5 developed DIND. Two patients suffered cerebral infarcts prior to enrollment and 3 others suffered cerebral infarcts beyond 48 hours after the completion of the final conditioning session. After discharge, patients were followed as part of their routine care. The mean follow-up time was 5.7 months with a standard deviation of 6.9 months. At follow-up, patients had a median mRS of 1 and a median GOS of 5 (Table 7).
Table 7.
Secondary endpoints
| Secondary Endpoints | ||
|---|---|---|
| Stroke | ||
| Before enrollment | 2 | |
| During RIPC + 48 hours | 0 | |
| > 48 After RIPC completion | 3 | |
| Modified Rankin Scale | ||
| 0 – No symptoms | 7 | |
| 1 – No significant disability | 6 | |
| 2 – Slight disability | ||
| 3 – Moderate disability | ||
| 4 – Moderately severe disability | 2 | |
| 5 - Severe disability | 4 | |
| 6 – Dead | 1 | |
| Glasgow Outcome Scale | ||
| 1 – Death | 1 | |
| 2 – Persistent vegetative state | 1 | |
| 3 – Severe disability | 5 | |
| 4 – Moderate disability | ||
| 5 – Low disability | 13 | |
| Follow up time (months) | ||
| Mean | 5.7 | |
| Standard deviation | 6.9 | |
Vital signs and physiological monitoring
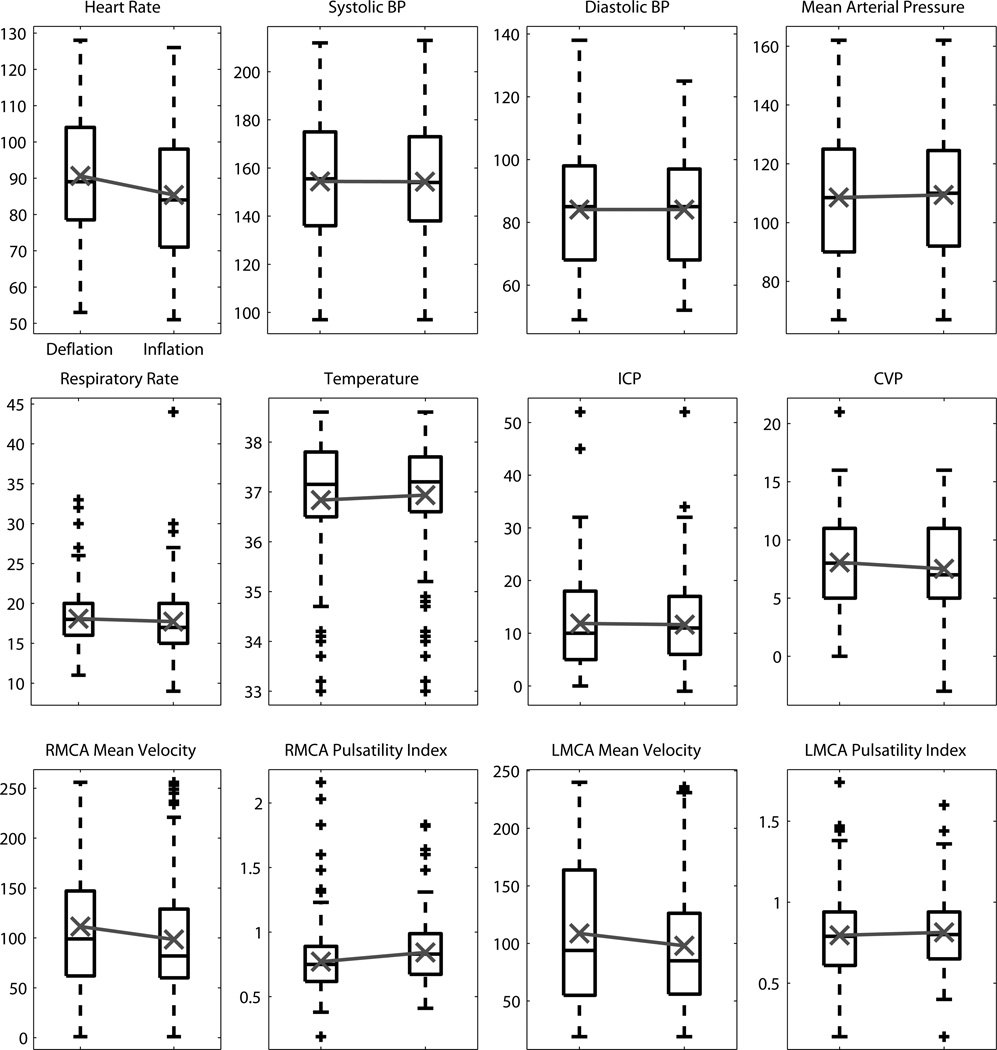
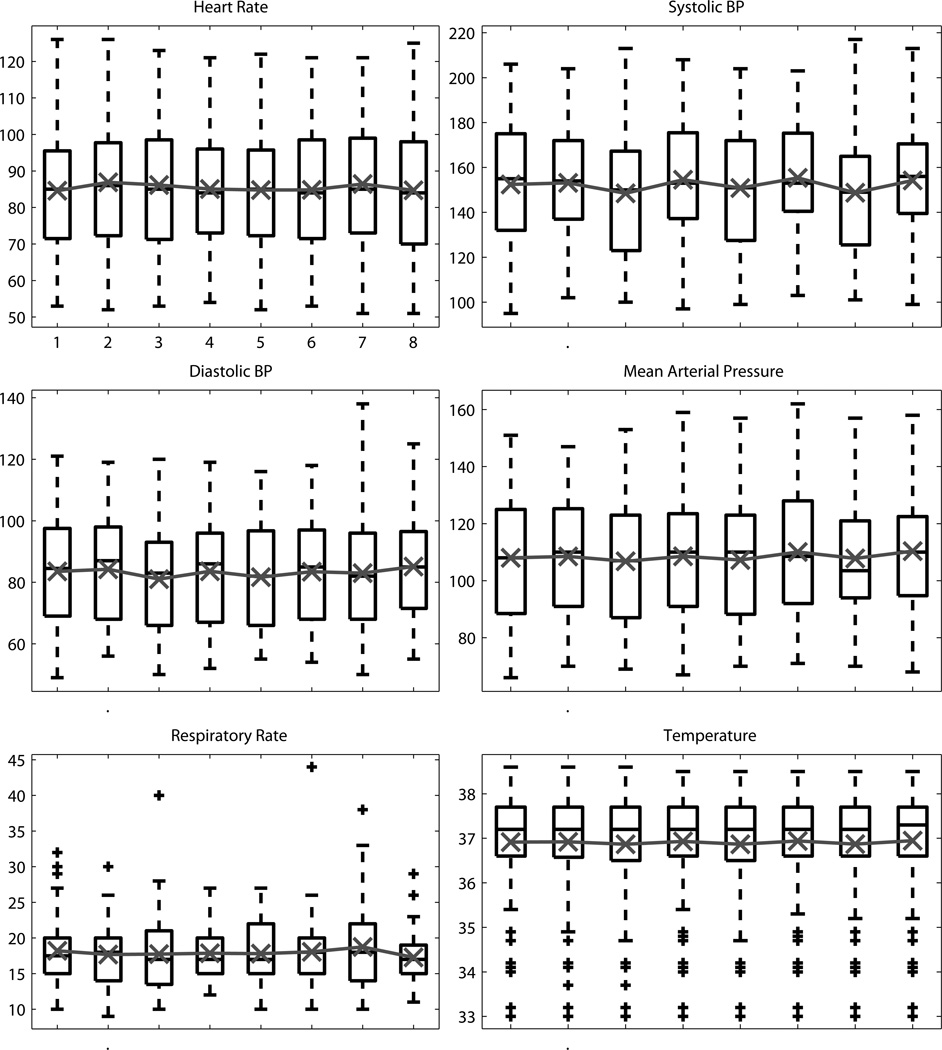
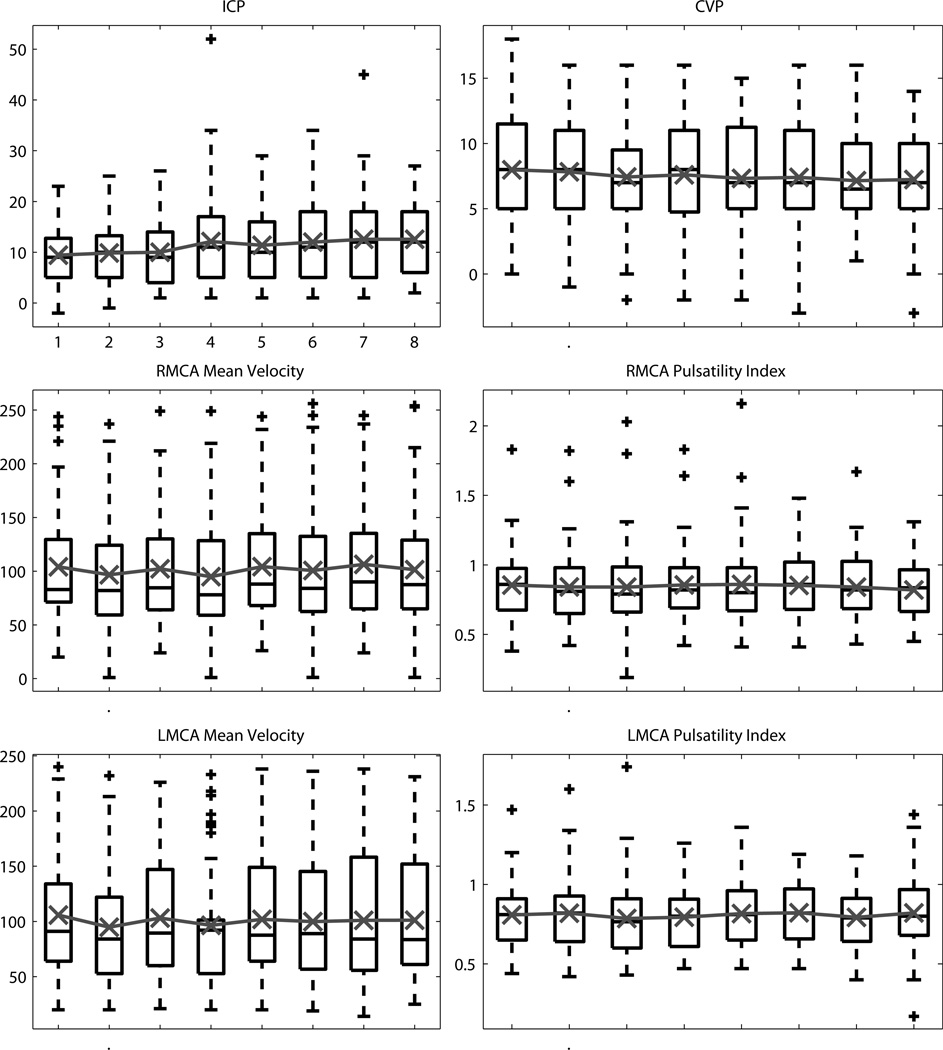
Analysis of the vital signs and additional monitoring data found no statistically significant change for any of the three rANOVA treatments (inflation, timepoint, session). The time-course of the vital signs and additional monitoring data are shown for inflation in Figure 1 and for timepoint in Figures 2&3.
Figure 1.
The distribution across deflation and inflation for each vital sign and physiologic signal monitored. Black box plots show the median, 25th and 75th percentiles, range, and outliers ‘+’. Overlaid in gray is the mean value.
Figure 2.
The distribution across timepoints during the RIC sessions for vital signs. Black box plots show the median, 25th and 75th percentiles, range, and outliers ‘+’. Overlaid in gray is the mean value.
Figure 3.
The distribution across timepoints during the RIC sessions for other physiologic monitoring measurements (intracranial pressure, central venous pressure, bilateral cerebral blood flow velocities and pulsatility indicies). Black box plots show the median, 25th and 75th percentiles, range, and outliers ‘+’. Overlaid in gray is the mean value.
Discussion
The goal of this phase I clinical trial was to determine whether RIC was safe and feasible as a prophylactic treatment for DIND in patients with aSAH. Patients who met the inclusion and exclusion criteria and were consented to participate in the study received up to four sessions of RIC every other day or until discharge. We found that no patient developed bruising or injury to the conditioned limb, no patient developed symptomatic DVTs that were spatially or temporally related to the preconditioning procedure, and no patient requested that the preconditioning procedure be stopped. These results suggest that the RIC is safe and feasible for patients with acute aSAH.
RIC is a powerful endogenous mechanism where a transient, sublethal ischemia to one tissue can confer protective benefits to another tissue. Through a multifactorial process, RIC induces anti-ischemia mechanisms such as the down-regulation of proinflammatory genes, the upregulation of anti-inflammatory genes,7 and the upregulation of genes associated with cytoprotection, DNA repair, growth, and metabolism.22 The systemic effect of these anti-ischemia mechanisms, through humoral and neural pathways, makes RIC a promising potential prophylactic treatment for acute ischemia to the brain.
To effectively investigate clinical application of RIC, it was necessary to (1) obtain evidence that the proposed procedure was capable of creating effective, but sublethal, ischemia in the limb, (2) to identify a time window at which a patient is at an elevated risk of stroke, and (3) demonstrate that the procedure could induce a detectable physiological change. To address the first point, we conducted a preliminary study using muscle microdialysis during the RIC procedure.17 We found that after the prolonged lower limb ischemia there was a significant increase in the lactate concentration and lactate/pyruvate ratio, both markers of ischemia. Moreover, there was no significant variation of glycerol, indicating the ischemia did not cause any appreciable permanent cell damage.
With a RIC procedure capable of producing sub-lethal ischemia, it was then necessary to identify a condition and time window where the procedure has the potential to provide a benefit. Following an aSAH there is a well-defined period when patients are at an elevated risk of DIND and may benefit from RIC as a prophylactic measure.18 Additionally, the controlled conditions and close monitoring afforded by their treatment in the neurocritical care unit were ideal to investigate the safety and feasibility of RIC for aSAH.
To assess whether the RIC procedure induced any physiological change, we collected intracranial pressure (ICP) and transcranial Doppler (TCD) waveforms as well as brain microdialysis.19 With this data we demonstrated that during the preconditioning procedure the ICP and TCD waveform changes were consistent with those observed during hypercapnic vasodilation.
The next step was to test the hypothesis that RIC is safe and feasible in the setting of aSAH. In this study, 20 patients were enrolled within a median of 4–5 days post-aSAH (range 1–13). While only 8 patients were enrolled prior to the beginning of the window of elevated risk of DIND (day 3), 18 were enrolled prior to the period of maximum DIND risk (days 7–10). The two patients who were enrolled later than day seven were both transferred from outside hospitals.
In the cohort reported here, no patient reached the first primary endpoint: bruising or damage to the limb or symptomatic DVT formation that could be temporally related to the RIC sessions. Two patients did eventually develop symptoms of DVT that were confirmed with venous Doppler. However, these events occurred more than one week after the completion of all four RIC sessions and after cooling catheters had been applied to the ipsilateral femoral vein as part of the patients’ treatment unrelated to the study. The reported association between endovascular cooling treatments and an increased risk of deep venous23,24 and inferior vena cava25 thrombus formation supports that the DVTs were not related to the RIC procedure. However, this is a point to consider for the clinical application of RIC, as critical and, specifically, aSAH patients have an increased incidence of DVT.21,26
The second endpoint of this study addressed the patients’ tolerability of the procedure. As reported above, 75 of the attempted 76 RIC sessions were completed successfully. One session was aborted early when it was observed that the patient was experiencing confusion and became non-cooperative. In the other 75 RIC sessions, no conscious patient requested that the procedure be stopped, though some did express that the procedure caused them a mild but tolerable level of discomfort. A previous clinical trial of RIC for aSAH by Koch et al. also found that the procedure was tolerated by the patients and that no patient developed any neurovascular injury.6 However, the results reported by Koch et al. are limited. In the study by Koch et al., the preconditioning was performed by inflating a large blood pressure cuff around the limb to 200 mmHg without confirming the cessation of blood flow to the limb (e.g. by pedal Doppler). In patients with atherosclerotic vessels, those who are obese, or those with elevated blood pressure as part of their treatment, 200 mmHg may have been insufficient to produce ischemia. By not confirming that ischemia was produced in the limb, the study by Koch et al. did not fully assess the safety and tolerability of RIC.
While the design of our study was not intended to assess the efficacy of RIC in preventing DIND after aSAH, the preliminary findings were promising. No patient developed permanent DIND within 3 days of a successful RIC session. Three patients did go on to develop cerebral infarcts 3 and 5 days after their final RIC session. These findings agree with previous reports that the effect of ischemic conditioning decreases over time and has a limited duration.27 This result also suggests that the RIC protocol may be extended to cover the entire time window where patients are at an increased risk of DIND. The other secondary endpoint was the patient outcome at follow-up. At an average follow-up time of 5.7 months, patients enjoyed a median GOS of 5 and mRS of 1. While these findings are encouraging, the cohort was too small and heterogeneous to draw any specific conclusion.
As many of the patients enrolled in this study were sedated, comatose, or otherwise unable to provide feedback about any discomfort created by the procedure, we also recorded the patients’ vital signs and other physiological monitoring information. We found that there was no significant change in any monitoring modality in response to the RIC. While not associated with a specific endpoint in this study, these findings add support to the safety and feasibility of applying RIC in unconscious patients as was reported in a study of RIC before carotid endarterectomy by Walsh et al.16
Taken together, these results demonstrate that RIC is safe and feasible for patients hospitalized for treatment of aSAH.
Limitations
This study was limited by the pre-specified number of RIC sessions. While we were able to demonstrate the feasibility and safety of RIC for aSAH over a period of seven days, this does not necessarily extrapolate to the feasibility and safety over the entire period of elevated risk of DIND. However, this design permitted the identification of the durability of a potential protective effect, which appears to be 48 hours following each session. Future studies on the clinical application of RIC for aSAH should address this by continuing the non-consecutive RIC sessions throughout the duration of the patients’ stay in the ICU, along with a continued evaluation of the safety and tolerability of the procedure.
Conclusion
We conducted a Phase I clinical trial to assess the feasibility and safety of RIC as a prophylactic treatment for DIND after aSAH. We found that the RIC procedure was well tolerated by patients and did not cause any injury. No patient developed DIND during their enrollment in the trial and patients had promising outcomes at follow-up. RIC for aSAH should be investigated in a subsequent pivotal clinical trial.
Acknowledgements
This work is supported by the Ruth and Raymond Stotter Endowed Chair in Neurosurgery and the National Institutes of Health National Institute of Neurological Disorders and Stroke award K23NS079477.
Footnotes
Disclosure: The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
References
- 1.Cheung MMH, Kharbanda RK, Konstantinov IE, et al. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006;47(11):2277–2282. doi: 10.1016/j.jacc.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 2.Kharbanda RK, Mortensen UM, White PA, et al. Transient Limb Ischemia Induces Remote Ischemic Preconditioning In Vivo. Circ. 2002;106(23):2881–2883. doi: 10.1161/01.cir.0000043806.51912.9b. [DOI] [PubMed] [Google Scholar]
- 3.Ren C, Gao X, Steinberg GK, Zhao H. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. [Accessed January 16, 2014];Neuroscience. 2008 151(4):1099–1103. doi: 10.1016/j.neuroscience.2007.11.056. Available at: http://www.sciencedirect.com/science/article/pii/S0306452207015576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen HA, Loukogeorgakis S, Yannopoulos F, et al. Remote ischemic preconditioning protects the brain against injury after hypothermic circulatory arrest. Circulation. 2011;123(7):714–721. doi: 10.1161/CIRCULATIONAHA.110.986497. [DOI] [PubMed] [Google Scholar]
- 5.Meng R, Asmaro K, Meng L, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2012;79(18):1853–1861. doi: 10.1212/WNL.0b013e318271f76a. [DOI] [PubMed] [Google Scholar]
- 6.Koch S, Katsnelson M, Dong C, Perez-Pinzon M. Remote ischemic limb preconditioning after subarachnoid hemorrhage: a phase Ib study of safety and feasibility. Stroke. 2011;42(5):1387–1391. doi: 10.1161/STROKEAHA.110.605840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konstantinov IE, Arab S, Kharbanda RK, et al. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics. 2004;19(1):143–150. doi: 10.1152/physiolgenomics.00046.2004. [DOI] [PubMed] [Google Scholar]
- 8.Wei D, Ren C, Chen X, Zhao H. The chronic protective effects of limb remote preconditioning and the underlying mechanisms involved in inflammatory factors in rat stroke. PLoS One. 2012;7(2):e30892. doi: 10.1371/journal.pone.0030892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Qian H, Zhao P, Hong S-S, Xia Y. Rapid hypoxia preconditioning protects cortical neurons from glutamate toxicity through delta-opioid receptor. Stroke. 2006;37(4):1094–1099. doi: 10.1161/01.STR.0000206444.29930.18. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y-X, Zhang M, Liu L-Z, Cui X, Hu Y-Y, Li W-B. The role of glutamate transporter-1a in the induction of brain ischemic tolerance in rats. Glia. 2012;60(1):112–124. doi: 10.1002/glia.21252. [DOI] [PubMed] [Google Scholar]
- 11.Ding Z-M, Wu B, Zhang W-Q, et al. Neuroprotective Effects of Ischemic Preconditioning and Postconditioning on Global Brain Ischemia in Rats through the Same Effect on Inhibition of Apoptosis. Int J Mol Sci. 2012;13(5):6089–6101. doi: 10.3390/ijms13056089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang N, Yin Y, Han S, et al. Hypoxic preconditioning induced neuroprotection against cerebral ischemic injuries and its cPKCγ-mediated molecular mechanism. Neurochem Int. 2011;58(6):684–692. doi: 10.1016/j.neuint.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura H, Katsumata T, Nishiyama Y, Otori T, Katsura K, Katayama Y. Effect of ischemic preconditioning on cerebral blood flow after subsequent lethal ischemia in gerbils. [Accessed January 13, 2014];Life Sci. 2006 78(15):1713–1719. doi: 10.1016/j.lfs.2005.08.008. Available at: http://www.sciencedirect.com/science/article/pii/S0024320505009859. [DOI] [PubMed] [Google Scholar]
- 14.Thielmann M, Kottenberg E, Kleinbongard P, et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. 2013;382(9892):597–604. doi: 10.1016/S0140-6736(13)61450-6. [DOI] [PubMed] [Google Scholar]
- 15.Hoole SP, Heck PM, Sharples L, et al. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: a prospective, randomized control trial. Circulation. 2009;119(6):820–827. doi: 10.1161/CIRCULATIONAHA.108.809723. [DOI] [PubMed] [Google Scholar]
- 16.Walsh SR, Nouraei SA, Tang TY, Sadat U, Carpenter RH, Gaunt ME. Remote Ischemic Preconditioning for Cerebral and Cardiac Protection During Carotid Endarterectomy: Results From a Pilot Randomized Clinical Trial. Vasc Endovascular Surg. 2010;44(6):434–439. doi: 10.1177/1538574410369709. [DOI] [PubMed] [Google Scholar]
- 17.Bilgin-Freiert A, Dusick JR, Stein NR, Etchepare M, Vespa P, Gonzalez NR. Muscle microdialysis to confirm sublethal ischemia in the induction of remote ischemic preconditioning. Transl Stroke Res. 2012;3(2):266–272. doi: 10.1007/s12975-012-0153-1. [DOI] [PubMed] [Google Scholar]
- 18.Koch S, Gonzalez N. Preconditioning the human brain: proving the principle in subarachnoid hemorrhage. Stroke. 2013;44(6):1748–1753. doi: 10.1161/STROKEAHA.111.000773. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez NR, Hamilton R, Bilgin-Freiert A, et al. Cerebral hemodynamic and metabolic effects of remote ischemic preconditioning in patients with subarachnoid hemorrhage. Acta Neurochir Suppl. 2013;115:193–198. doi: 10.1007/978-3-7091-1192-5_36. [DOI] [PubMed] [Google Scholar]
- 20.Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10(1):1. doi: 10.1186/1471-2288-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray WZ, Strom RG, Blackburn SL, Ashley WW, Sicard GA, Rich KM. Incidence of deep venous thrombosis after subarachnoid hemorrhage. J Neurosurg. 2009;110(5):1010–1014. doi: 10.3171/2008.9.JNS08107. [DOI] [PubMed] [Google Scholar]
- 22.Konstantinov IE, Arab S, Li J, et al. The remote ischemic preconditioning stimulus modifies gene expression in mouse myocardium. J Thorac Cardiovasc Surg. 2005;130(5):1326–1332. doi: 10.1016/j.jtcvs.2005.03.050. [DOI] [PubMed] [Google Scholar]
- 23.Simosa HF, Petersen DJ, Agarwal SK, Burke PA, Hirsch EF. Increased risk of deep venous thrombosis with endovascular cooling in patients with traumatic head injury. [Accessed March 21, 2014];Am Surg. 2007 73(5):461–464. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17520999. [PubMed] [Google Scholar]
- 24.Prunet B, Lacroix G, Bordes J, Poyet R, D’Aranda E, Goutorbe P. Catheter related venous thrombosis with cooling and warming catheters: two case reports. Cases J. 2009;2:8857. doi: 10.4076/1757-1626-2-8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau E, Bajzer C, Menon V. Inferior vena cava thrombus associated with intravascular cooling catheter. Resuscitation. 2010;81(11):1457–1458. doi: 10.1016/j.resuscitation.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Marik PE. The Incidence of Deep Venous Thrombosis in ICU Patients. CHEST J. 1997;111(3):661. doi: 10.1378/chest.111.3.661. [DOI] [PubMed] [Google Scholar]
- 27.Guo Y, Wu WJ, Qiu Y, Tang XL, Yang Z, Bolli R. Demonstration of an early and a late phase of ischemic preconditioning in mice. [Accessed March 21, 2014];Am J Physiol. 1998 275(4 Pt 2):H1375–H1387. doi: 10.1152/ajpheart.1998.275.4.H1375. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3701297&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]