Abstract
Background
The paper mulberry (Broussonetia papyifera) is one of the multifunctional tree species in agroforestry system and is also commonly utilized in traditional medicine in China and other Asian countries. To identify the transcription factors (TFs) and comprehensively understand their regulatory roles in the growth of the paper mulberry, a global transcriptomics TF prediction and the differential expression analysis among root, shoot and leaf were performed by using RNA-seq.
Results
Results indicate that there is 1,337 TFs encoded by the paper mulberry and they belong to the 55 well-characterized TF families. Based on the phylogenetic analysis, the TFs exist extensively in all organisms are more conservative than those exclusively exist in plant and the paper mulberry has the closest relationship with the mulberry. According to the results of differential expression analysis, there are 627 TFs which exhibit the differential expression profiles in root, shoot and leaf. ARR-Bs, ARFs, NACs and bHLHs together with other root-specific and highly expressed TFs might account for the developed lateral root and unconspicuous taproot in the paper mulberry. Meanwhile, five TCPs highly expressed in shoot of the paper mulberry might negatively regulate the expression of 12 LBDs in shoot. Besides, LBDs, which could directly or indirectly cooperate with ARFs, bHLHs and NACs, seem to be the center knot involving in the regulation of the shoot development in the paper mulberry.
Conclusions
Our study provides the comprehensive transcriptomics identification of TFs in the paper mulberry without genome reference. A large number of lateral organ growth regulation related TFs exhibiting the tissue differential expression may entitle the paper mulberry the developed lateral roots, more branches and rapid growth. It will increase our knowledge of the structure and composition of TFs in tree plant and it will substantially contribute to the improving of this tree.
Electronic supplementary material
The online version of this article (doi:10.1186/s12870-014-0194-6) contains supplementary material, which is available to authorized users.
Keywords: Lateral organ development, Paper mulberry, Phylogeny, Root hair elongation, Transcription factors
Background
The paper mulberry belongs to the family of Moraceae and is naturally distributed in Eastern Asia and pacific countries. The paper mulberry has shallow roots with advanced lateral roots and without an obvious taproot. It is one of the multifunctional tree species in agroforestry systems [1], as well as being one of the traditional forages [2] and Chinese medicines in many countries of Asia [3]. Due to its fast growth and adaptability, the paper mulberry is commonly used for the ecological afforestation and landscape in both sides of highway, mined areas and on barren land [4]. It is the ideal tree species for ecological and gardening purposes, and can be widely used in papermaking, livestock, medicine and other industries [5]. Genetic diversity revealed by SRAP marker and cluster analysis show that there is a relationship between the genetic variation and geographical distribution [6]. These results provide reference for making genetic map and guide the breeding of the paper mulberry. However, because of lacking the knowledge of the genetic background, the molecular mechanism about strong adaptability and tolerance to biotic or abiotic stress of the paper mulberry has not been studied, which limits the exploitation of the paper mulberry.
TFs play important roles in plant development and environmental adaptation by regulating the expression of their target genes. TFs directly or indirectly involved in the response to plant hormones which control plant growth including cell division, elongation and differentiation. The identification and functional study of TFs are essential for the reconstruction of the transcriptional regulatory network in the development and ecological circumstances adaptation of plant. Many TF family proteins, such as bHLH [7], ERF [8], Dof [9], MYB [10], NAC [11] and WRKY [12], play regulatory roles in plants growth and development.
Many TFs have been reported to play roles in the vascular and xylem development [13]. Recent molecular studies of various trees have revealed that the coordinated gene expression during differentiation of these cells in wood and the presence of several TFs, such as ARF, HD-ZIP, MYB and NAC, which might govern the complex networks of transcriptional regulation in tree growth [14]. However, most studies about genome wide analysis of TFs in plants concentrate in a few species, such as Arabidopsis and kinds of crops. The universality of the mechanism is not explicit, especially in tree species. Because of low domestication, open-pollinated native populations and high levels of genetic variation, they are ideal organisms to unveil the molecular mechanism of population adaptive divergence in nature.
As nonclassical model plant, trees have gained much attention in recent years for environment adaptation, evolutionary and genomic studies. Overall study for each TF family has also been launched. Via the comprehensive analysis of NAC gene family in Populus, a total of 163 full-length NAC genes are identified, and they are phylogenetically clustered into 18 distinct subfamilies. Furthermore, 25 NAC genes are of tissue-specific expression patterns [15]. A total of 11 WOX TFs both mRNA and genomic DNA are isolated from Picea abies and further study shows that all major radiations within the WOX gene family taking place before the angiosperm-gymnosperm split and that there has been a recent expansion within the intermediate clade in the Pinaceae family [16]. However, there are little reports about the regulated network from the genome-scale under the control of TFs in tree species [13,17], especially as for those trees without genome information.
In our study, we performed a genome wide TF prediction using the transcriptome data. Additionally, we predicted the expressional pattern of the identified TF genes using a large amount of RNA-seq data which have just become available. A subset of TFs that are specifically expressed in particular tissues, including root, shoot and leaf, were thus identified. Our study provides a valuable resource of TF genes for further genetic and developmental studies in the paper mulberry.
Results
Identification and classification of TFs in the paper mulberry
To ascertain the TF families in the paper mulberry, sequences obtained from 3 libraries as mentioned in the materials and methods were assembled. After retrieving annotation results for every unigene, 1,337 TFs were identified and classified into 55 families (Table 1) based on their DNA-binding domains and other conserved motifs [18,19]. Of these TFs, 578 TFs belonged to 48 families with complete ORF (Table 2). The bHLH was the biggest family with 151 members and 69 of which have complete ORF. The following was WRKY (112), ERF (88) and other families. According to comparison of family size among the selected species, as shown in Table 2, most of families have been detected in the paper mulberry except for GeBP, HB-PHD, MIKC, and STAT families.
Table 1.
TF family in paper mulberry
| TF family | No. of TF | TF family | No. of TF | TF family | No. of TF | TF family | No. of TF |
|---|---|---|---|---|---|---|---|
| Alfin-like | 16 | CPP | 8 | HTH | 13 | S1Fa-like | 1 |
| AP2 | 25 | DBB | 5 | LBD | 26 | SAP | 2 |
| ARF | 26 | Dof | 28 | LFY | 2 | SBP | 21 |
| ARR-B | 33 | E2F/DP | 9 | LSD | 1 | SRS | 1 |
| B3 | 53 | EIL | 4 | M-type | 13 | TALE | 19 |
| BBR-BPC | 5 | ERF | 88 | MYB | 83 | TCP | 18 |
| BES1 | 6 | FAR1 | 50 | MYB-related | 33 | Trihelix | 13 |
| bHLH | 151 | G2-like | 29 | NAC | 79 | VOZ | 2 |
| BTF3 | 1 | GATA | 24 | NF-X1 | 2 | Whirly | 2 |
| bZIP | 35 | GRAS | 29 | NF-YA | 6 | WOX | 6 |
| C2H2 | 64 | GRF | 16 | NF-YB | 5 | WRKY | 112 |
| C3H | 67 | HD-ZIP | 35 | NF-YC | 3 | YABBY | 3 |
| CAMTA | 4 | HRT | 1 | Nin-like | 6 | ZF-HD | 11 |
| CO-like | 19 | HSF | 21 | RAV | 2 |
Table 2.
TF families with complete ORF in paper mulberry
| TF family | No. of TF | TF family | No. of TF | TF family | No. of TF | TF family | No. of TF |
|---|---|---|---|---|---|---|---|
| Alfin-like | 8 | CO-like | 9 | GRF | 4 | RAV | 1 |
| AP2 | 8 | CPP | 3 | HD-ZIP | 21 | SBP | 11 |
| ARF | 12 | DBB | 1 | HSF | 13 | SRS | 1 |
| ARR-B | 14 | Dof | 9 | LBD | 9 | TALE | 7 |
| B3 | 17 | E2F/DP | 6 | MADS-box | 4 | TCP | 8 |
| BBR-BPC | 4 | EIL | 3 | MYB | 30 | Trihelix | 8 |
| bHLH | 69 | ERF | 44 | MYB-related | 9 | VOZ | 2 |
| BTF3 | 1 | FAMILY | 1 | NAC | 21 | Whirly | 2 |
| bzip | 24 | FAR1 | 24 | NF-X1 | 2 | WOX | 3 |
| C2H2 | 19 | G2-like | 21 | NF-YA | 3 | WRKY | 33 |
| C3H | 41 | GATA | 14 | NF-YB | 4 | YABBY | 3 |
| CAMTA | 4 | GRAS | 19 | NF-YC | 1 | ZF-HD | 3 |
Phylogenetic analysis of TFs in the paper mulberry
Genetic distances were calculated according to the alignment of the conserved domain of the three TFs families chosen from 9 species including the paper mulberry and the phylogenetic trees were constructed using MEGA 5.0 program (Figure 1). As shown in Figure 1A, all of the CAMTAs from the selected species could be classified into six groups. Four BpaCAMTAs were listed in the group 1, 3, 4 and 6, respectively. All of the BpaCAMTAs were clustered with that from Morus notabilis, following as Cannabis sativa. There were two Whirly TFs in the paper mulberry and they were divided into two groups as that of other plants. One of them was clustered with that of M. notabilis and the other was clustered with Citrullus lanatus (Figure 1B). Two VOZs existed in the paper mulberry like as most of other plants and they had the highest identity with that of M. notabilis and C. sativa (Figure 1C).
Figure 1.
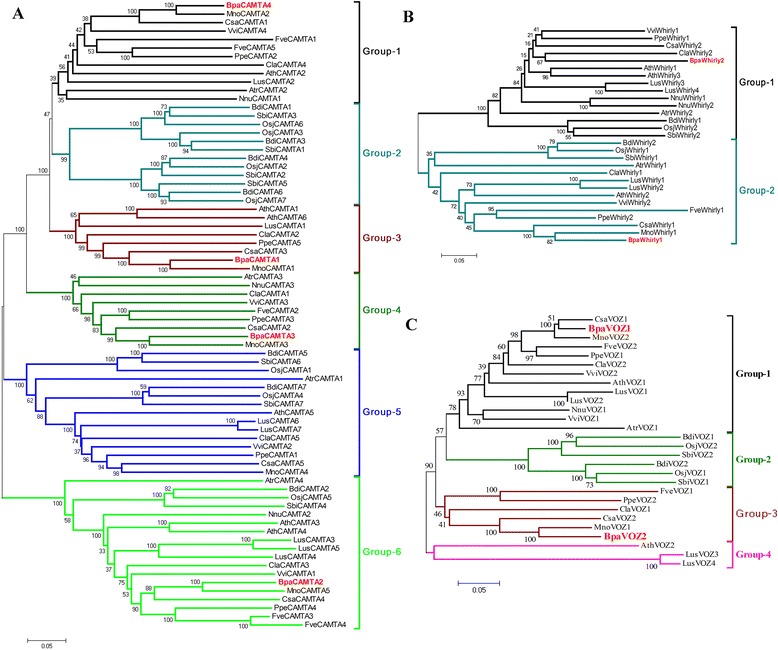
Evolutionary relationships revealed by phylogenetic analysis of CAMTA, Whirly and VOZ family. The evolutionary history was inferred using the Neighbor-Joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1500 replicates) was shown next to the branches. The tree was drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the p-distance method and were in the units of the number of amino acid differences per site. The analysis involved 74 CAMTAs (A), 29 Whirlys (B) and 27 VOZs (C) amino acid sequences and their correspondent accession numbers were list in Additional file 4: Table S6. All ambiguous positions were removed for each sequence pair. Evolutionary analyses were conducted in MEGA5.
The expression profile of the TFs from the paper mulberry
To identify the differentially expressed TFs between different samples, the expression level of all TFs were homogenized by using their RPKM values. Among the 1,337 TFs, the RPKM values of 1,104 TFs were distributed from 1 to 770 (see Additional file 1: Table S4). The unigene T6-23630 had the highest RPKM value 770 and belonged to the ERF family. Besides, there were 219 TFs which RPKM values were approximate to zero and belonged to the bHLH, WRKY and other families. They had a common characteristic of the short nucleotide length which distributed from 202 to 309 bp.
According to the RPKM value of each TF, there were 935, 1036 and 842 TFs expressed in the root, shoot and leaf, respectively (Figure 2A). A total of 771 TFs were co-expressed in three tissues. Meanwhile, there were 36, 132 and 26 TFs were specifically expressed in root, shoot and leaf, respectively.
Figure 2.
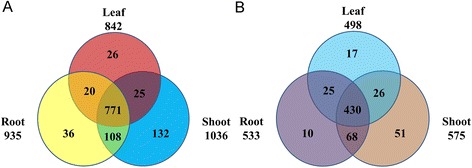
Venn diagram of TFs expressed and differently expressed in root, shoot and leaf. A Venn diagram of TFs expressed in root, shoot and leaf. B Venn diagram of differentially expressed TFs in root, shoot and leaf. TFs differentially expressed in root, shoot and leaf were identified. To be considered differentially expressed, the transcript must have RPKM ≥ 2 in at least one tissue, 2-fold or greater change between tissues, and P ≤ 0.05.
Differentially expressed TFs from the paper mulberry
The TFs with a RPKM value of more than or equal to 2 were chosen for the differential expressed analysis and The TFs with a ratio of RPKM between samples of more than 2 (Fold change ≥2) and an FDR ≤0.01 were considered to have the significant changes in expression. According to this rule, a total of 627 TFs were of the differential expressed characteristic among root, shoot and leaf (see Additional file 2: Figure S1 and Additional file 3: Table S5, Figure 2B) and belonged to AP2, CO-like, LBD and other 47 families (Figure 3 and see Additional file 2: Figure S1). There were 135, 296 and 196 TFs had the highest expression level in root, shoot and leaf, respectively (Figure 4A, B and C). Among of them, there were 10, 51 and 17 TFs were uniquely expressed in the root, shoot and leaf, respectively (Figure 2B). These expression patterns were validated by qPCR (Figure 5) and the error bars showed the corresponding standard deviation when three independent experiments were carried out. In addition, there were 332 TFs, belonged to 42 families, had complete ORFs among these tissue differential expressed TFs (see Additional file 4: Table S6).
Figure 3.
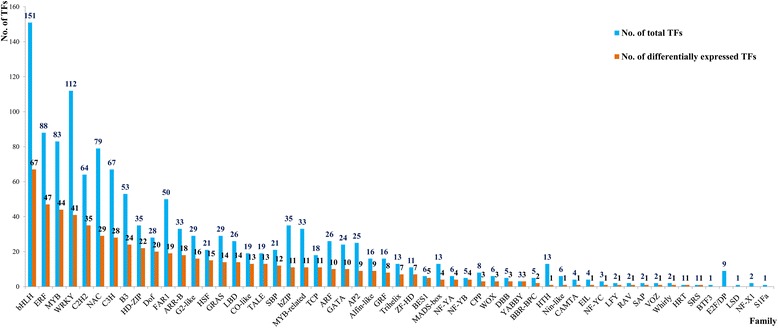
The differentially expressed TFs distributed in each family. According to the conserved domain, a total of 627 differentially expressed TFs could be classified into 50 families and most of them concentrated in bHLH, MYB, WRKY, C2H2, NAC, C3H, B3 and Dof family.
Figure 4.
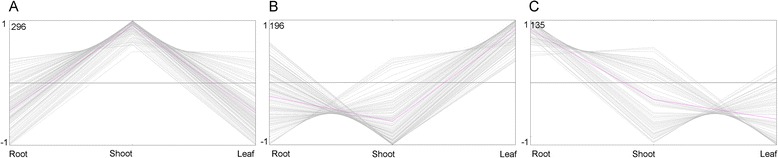
The cluster analysis of the differentially expressed TFs in root, shoot and leaf of the paper mulberry. A The TFs highly expressed in shoot than that in leaf and root. B The TFs highly expressed in leaf than that in shoot and root. C The TFs highly expressed in root than that in leaf and shoot. The pink line represented the expression trend of the cluster. The gray line represented the expression profile of every TF.
Figure 5.
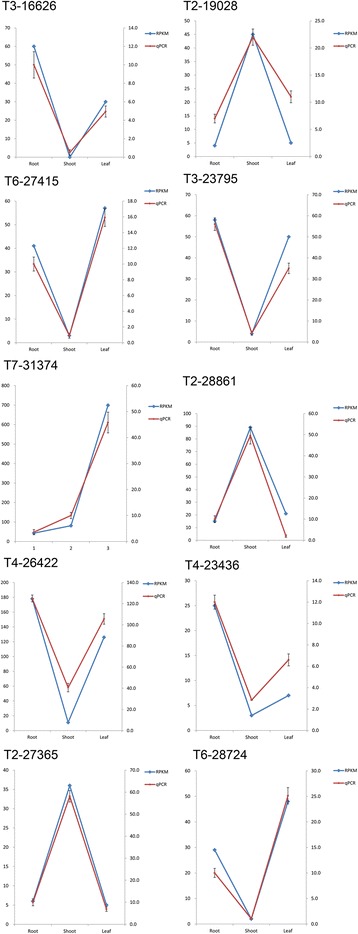
The expression profile of ten selected TFs validated by qPCR. The left axis represents the results of transcriptomics analysis while the right axis represents relative expression detected by qPCR, and the error bars represented the standard deviation (S.D.) values for three independent experiments, performed in triplicate.
Discussion
The composition of TFs in the paper mulberry
TFs are usually classified into different families based on their DNA-binding domains and other conserved motifs [18,19]. As the model plants of dicots and monocots, the genomes of Arabidopsis and rice have been well discerned. The Arabidopsis genome encodes 2,296 TFs which can be classified into 58 families and account for 6.2% of its estimated total number of genes [18,20]. There are 2,408 TFs (1,859 loci) are identified and classified into 56 families in Oryza sativa subsp. Japonica. Furthermore, there are 4,288 TFs encoded by 2,455 genes accounting for about 6.4% of Poplar gene [18,21]. In our study, a total of 1,337 TFs identified from the transcriptome data of the paper mulberry could be classified into 55 families and 578 TFs of them had complete ORF. These TFs comprised of more than 3.5% of the genes of this plant [22]. Although the genome of paper mulberry has not been sequenced and its genes number might be underestimated, this ratio was much closed to that of other genome known plants, such as C. sativa, Fragaria vesca and Vitis vinifera (Figure 6) and it is less than that of Arabidopsis and rice. Besides, the TFs number of bHLH, AP2/ERF, MYB/MYB-related, NAC and WRKY family in the paper mulberry was 151, 114, 116, 79 and 112, respectively. They mostly made up half of the total TFs of the paper mulberry just as other plants (Table 3).
Figure 6.
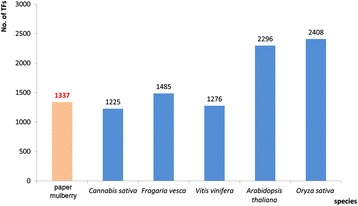
The total number of TFs in the selected species. The TF numbers of Arabidopsis thaliana, Cannabis sativa, Fragaria vesca, Oryza sativa subsp. Japonica and Vitis vinifera were obtained from Plant Transcription Factor Database (http://planttfdb.cbi.pku.edu.cn/index.php).
Table 3.
The comparison of family size among the selected species
| TF family | Oryza sativa | Arabidopsis thaliana | Vitis vinifera | Fragaria vesca | Cannabis sativa | Paper mulberry | TF family | Oryza sativa | Arabidopsis thaliana | Vitis vinifera | Fragaria vesca | Cannabis sativa | Paper mulberry |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AP2 | 22 | 30 | 19 | 17 | 16 | 25 | LBD | 39 | 50 | 44 | 36 | 26 | 26 |
| ARF | 48 | 37 | 17 | 17 | 13 | 26 | LFY | 2 | 1 | 1 | 4 | 1 | 2 |
| ARR-B | 11 | 21 | 12 | 7 | 5 | 33 | LSD | 12 | 12 | 3 | 3 | 3 | 1 |
| B3 | 65 | 77 | 29 | 77 | 60 | 53 | MIKC | 61 | 76 | 36 | 37 | 28 | - |
| BBR-BPC | 7 | 17 | 5 | 3 | 3 | 5 | M-type | 35 | 70 | 18 | 48 | 6 | 13 |
| BES1 | 6 | 14 | 6 | 6 | 8 | 6 | MYB | 130 | 168 | 138 | 110 | 81 | 83 |
| bHLH | 211 | 225 | 115 | 112 | 99 | 151 | MYB-related | 106 | 97 | 57 | 65 | 70 | 33 |
| bZIP | 140 | 127 | 47 | 52 | 54 | 35 | NAC | 170 | 138 | 71 | 127 | 75 | 79 |
| C2H2 | 135 | 116 | 64 | 83 | 62 | 64 | NF-X1 | 2 | 2 | 3 | 2 | 4 | 2 |
| C3H | 74 | 66 | 43 | 38 | 48 | 67 | NF-YA | 25 | 21 | 7 | 6 | 6 | 6 |
| CAMTA | 7 | 10 | 4 | 7 | 6 | 4 | NF-YB | 16 | 27 | 17 | 14 | 12 | 5 |
| CO-like | 21 | 22 | 6 | 7 | 10 | 19 | NF-YC | 19 | 21 | 8 | 9 | 8 | 3 |
| CPP | 20 | 9 | 6 | 6 | 3 | 8 | Nin-like | 15 | 17 | 8 | 10 | 4 | 6 |
| DBB | 13 | 14 | 7 | 6 | 5 | 5 | NZZ/SPL | - | 1 | 1 | 1 | - | |
| Dof | 37 | 47 | 22 | 23 | 27 | 28 | RAV | 4 | 7 | 1 | 5 | 2 | 2 |
| E2F/DP | 10 | 16 | 7 | 7 | 11 | 9 | S1Fa-like | 2 | 4 | 2 | 1 | 1 | 1 |
| EIL | 11 | 6 | 2 | 6 | 5 | 4 | SAP | - | 1 | 1 | 1 | 2 | |
| ERF | 163 | 139 | 80 | 92 | 59 | 88 | SBP | 29 | 30 | 19 | 14 | 18 | 21 |
| FAR1 | 133 | 26 | 18 | 82 | 36 | 50 | SRS | 6 | 16 | 5 | 5 | 7 | 1 |
| G2-like | 62 | 64 | 40 | 37 | 31 | 29 | STAT | 1 | 4 | 1 | 1 | 2 | - |
| GATA | 32 | 41 | 19 | 19 | 21 | 24 | TALE | 45 | 33 | 21 | 18 | 17 | 19 |
| GeBP | 13 | 23 | 1 | 6 | 5 | - | TCP | 23 | 33 | 15 | 18 | 17 | 18 |
| GRAS | 69 | 37 | 43 | 51 | 54 | 29 | Trihelix | 40 | 34 | 26 | 31 | 33 | 13 |
| GRF | 19 | 9 | 8 | 10 | 10 | 16 | VOZ | 2 | 3 | 2 | 2 | 2 | 2 |
| HB-other | 17 | 11 | 7 | 11 | 14 | - | Whirly | 2 | 4 | 2 | 1 | 3 | 2 |
| HB-PHD | 1 | 3 | 2 | 2 | 1 | - | WOX | 17 | 18 | 11 | 14 | 7 | 6 |
| HD-ZIP | 61 | 58 | 33 | 28 | 34 | 35 | WRKY | 128 | 90 | 59 | 58 | 49 | 112 |
| HRT-like | 1 | 2 | 1 | 3 | 2 | 1 | YABBY | 15 | 8 | 7 | 5 | 7 | 3 |
| HSF | 38 | 25 | 19 | 15 | 26 | 21 | ZF-HD | 15 | 18 | 10 | 9 | 8 | 11 |
Note: “-” means no TF presented in this family.
However, GeBP, HB-PHD, MIKC, and STAT families were not found in our transcriptome data (Table 3). Meanwhile, the MIKC-type, specific to plants and involved in floral organ identity determination, and NZZ/SPL TFs, playing a central role in regulating anther cell differentiation during the floral organ development, were also not appeared in the transcriptome data. This might mainly because that the fruit and flower were not included in this study. There might some new TF members would be presented when more transcriptome data could be obtained.
The phylogenetic relationship of the TFs in the paper mulberry
The mutation, expansion and functional diversification of gene family reflect the evolution process of plants to adapt to their differing external ecological circumstances. In our study, we chose three TF families, namely CAMTA, Whirly and VOZ, to illustrate the phylogenetic relationship of the TFs in the paper mulberry.
Investigations of CAMTAs in various organisms suggest a broad range of functions from sensory mechanisms to embryo development and growth control [23]. The CAMTAs have been shown to play an important role in the plant response to abiotic and biotic stresses [24]. Meanwhile, the CG-1, ANK and the IQ domain is very conservative from human to plant [23]. The phylogenetic tree of CAMTAs in our study showed that all of the BpaCAMTAs were clustered with that from M. notabilis, following was C. sativa (Figure 1A). The CAMTAs from A. trichopoda were located in the root position of group-1, 4, 5 and 6. This results was in accordance with that A. trichopoda was the single living species of the sister lineage and the most recent common ancestor to all other extant flowering plants [25]. In addition, the CAMTAs have evolved a novel clade in group 2 in B. distachyon, O. sativa and S. bicolor which confirmed that some gene family in monocots had rapidly evolved to adapt to the environment after the monocot-dicot divergence.
Whirly TFs throughout the plant kingdom are predicted to share the ability to bind to single-stranded DNA and they regulate defense gene expression as well as function in the chloroplast and in the nucleus [26]. Two Whirly TFs of the paper mulberry were divided into two groups as that of other plants (Figure 1B). One of them was clustered with that of M. notabilis. The other was clustered with that of C. lanatus. This suggested that the BpaWhirly1 was more conserved than BpaWhirly2.
VOZ is the plants specific one-zinc-finger type DNA-binding protein and is highly conserved in land plant evolution [27]. BpaVOZ1 was clustered with CsaVOZ1 while BpaVOZ2 was clustered with MnoVOZ1 (Figure 1C). This result showed that BpaVOZ1 had the higher identity with CsaVOZ1 other than MnoVOZ2 and implied that BpaVOZ1 and CsaVOZ1 have produced some similar mutation both in C. sativa and the paper mulberry.
C. sativa which has once been considered as one species of Moraceae in the Engler system [28] belongs to the Cannabaceae while C. lanatus belongs to the Cucurbitales (APG III Classification system). Even so, some TFs identified from the paper mulberry still had the higher identity with the TFs of them. These phylogenetic analyses suggested that the TFs existing in various organisms and playing the significant roles, such as CAMTAs, also were conserved in the paper mulberry. Meanwhile, the TFs which are specific to plants, for example VOZ and Whirly experienced a lower selection pressure, had more of the variation in the paper mulberry.
TFs involved in the tissue growth of the paper mulberry
A TF that expresses exclusively in a special tissue may play a central role in regulating tissue development [29]. Expression patterns contain important information to infer the functions of TFs. Transcriptome-wide identification of tissue-specific TFs across tissues can help to understand of the molecular mechanisms of tissue development. The plantlet of the paper mulberry was in seedling stage with vigorously vegetative growth and without reproductive growth. So the key TFs involved in the regulation of root, shoot and leaf development could be identified by detecting the expression profile and screening the tissue-specific expression.
Root growth
The paper mulberry has developed horizontal, strong lateral and densely tangled fibrous root which can effectively absorb the water and nutrients existing in the topsoil to accommodate the poor soil and harsh environmental conditions. According to our results, a total of 135 TFs belonged to 40 families specifically higher expressed in the root than that in shoot and leaf. It included ARR-B (8), bHLH (15), CO-like (6), G2-like (2), GATA (3), and MYB (8) and so on (see Additional file 2: Figure S1, Additional file 3: Table S5 and Figures 4 and 7).
Figure 7.
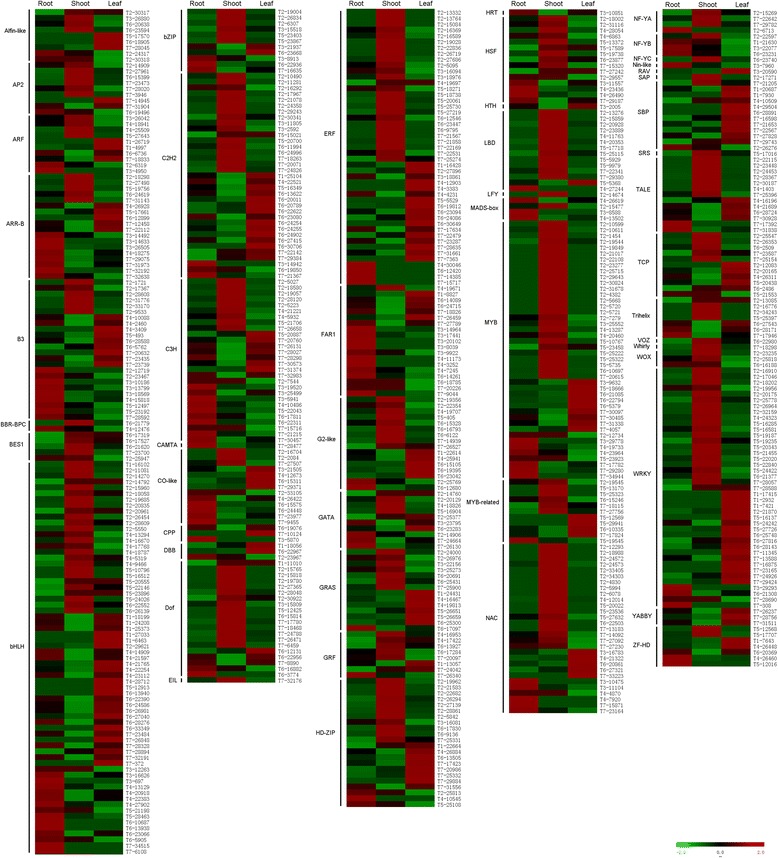
Heat map of expression profiles of TFs involved in the differential expression among root, shoot and leaf. Red indicates high expression, black indicates intermediate expression, and green indicates low expression. To be considered differentially expressed, the transcript must have RPKM ≥ 2 in at least one tissue, 2-fold or greater change between tissues, and P ≤ 0.05. TFs have been grouped by family.
Investigations on the growth and development of plant roots mainly lie in the top of the regulation of root apical meristem, lateral roots and root hairs growth and development. ARFs promote lateral root growth via an auxin-responsive regulatory network [30] while NAC1 down-regulate auxin signals for Arabidopsis lateral root development [31]. Auxin targets elongating epidermal cells during the gravitropic response and also regulates cell division in the meristem and stem cell niche [32]. Two ARFs and 6 NACs highly expressed in the root (see Additional file 3: Table S5) might be the candidate gene that control the growth of lateral root and root tip in the paper mulberry. In addition, COL3 as a positive regulator of photomorphogenesis can promote lateral root development independently of COP1 and also function as a day length-sensitive regulator of shoot branching [33]. Six CO-likes highly expressed in the root and four of them showed the root-specific expression, which was thought to promote the lateral root development of the paper mulberry.
Genetic analyses suggest that AtMYC2 belongs to bHLH family and is a common TF involving in light, ABA, and JA signaling pathways. It acts as a negative regulator of blue light-mediated photomorphogenic growth and blue and far-red-light–regulated gene expression, meanwhile it functions as a positive regulator of lateral root formation [34]. MYC3, another bHLH TF, directly interactes with JAZs via its N-terminal region and regulate JA responses. The transgenic plants with overexpression of MYC3 exhibit hypersensitivity in JA-inhibitory root elongation and seedling development [35]. A bHLH TF, RSL4 is sufficient to promote postmitotic cell growth in Arabidopsis root-hair cells. Loss of RSL4 function resulted in the development of very short root hairs. In contrast, constitutive RSL4 expression programs constitutive growth and results in the formation of very long root hairs. Hair-cell growth signals, such as auxin and low phosphate availability, modulate hair cell extension by regulating RSL4 transcript and its protein levels [36]. A total of 15 highly expressed bHLHs in the root implied their function in the lateral root formation as well as the root hairs development via the perception of auxin and other circumstance signals in the paper mulberry.
Cross-talk exists among phytohormones signaling pathways. For example, root meristem size and root growth are mediated mainly by the interplay between cytokinin and auxin. Cytokinin activates ARR-B TFs which promote the expression of SHY2 and affects auxin signaling pathway [37]. ARR10 and ARR12 have been proved that they are involved in the AHK-dependent signaling pathway that negatively regulates the protoxylem specification in root vascular tissues [38]. Twelve ARR-Bs highly expressed in the root and 8 of them showed the root-specific expression (see Additional file 3: Table S5) in the paper mulberry. Thus we proposed that those ARR-B TFs redundantly played pivotal roles in response to cytokinin and interacted with the auxin signaling pathway in root growth of the paper mulberry.
Ethylene regulates cell division in quiescent center and auxin biosynthesis in columella cells, which is likely to be involved in root meristem maintenance. In the ethylene signaling pathway, the activated EIN2 promotes the activation of EIN3 and EIN3-like (EIL) TFs, which induces the expression of ERF which is another TF implicated in the activation of a subset of ethylene response genes [32]. Thus, 4 EILs expressed in the root might induce the expression of ERFs which expression level was higher than leaf or shoot, and then activated a series of downstream genes to regulate the root meristem maintenance.
Alfin-like TF is involved in the root growth and controls the target genes which are crucial for the root hair elongation [39]. Two Alfin-likes showed the highly expressed in the root, suggesting their function in the root hair growth of the paper mulberry.
Although G2-like (GOLDEN2-LIKE) TFs are required for chloroplast development and have been reported to co-regulate and synchronize the expression of a suite of nuclear photosynthetic genes and thus act to optimize photosynthetic capacity in varying environmental and developmental conditions, two G2-likes were root-specifically expressed and other six G2-likes also showed the higher expression characteristic, which implied that those G2-likes involved in controlling of root growth and suggested that their functional diverse in the regulation of plant development.
Besides, many other TFs, such as GATAs, GRASs, HSFs, NF-YBs, Trihelix and ZF-HD also showed the root-specific expression or highly expressed in root than in leaf or shoot, suggesting their complicated cross-talk in the regulation of root growth in the paper mulberry.
Many root-specific expressed and highly expressed TFs belonged to the ARR-B, ARF, NAC and bHLH family, which might play key roles in the lateral root development under the interaction with kinds of plant hormones and other TFs, though lest specifically expressed TFs were identified in the root compared with shoot and leaf. This might account for the developed lateral root and without an obvious taproot in the paper mulberry (Figure 8).
Figure 8.
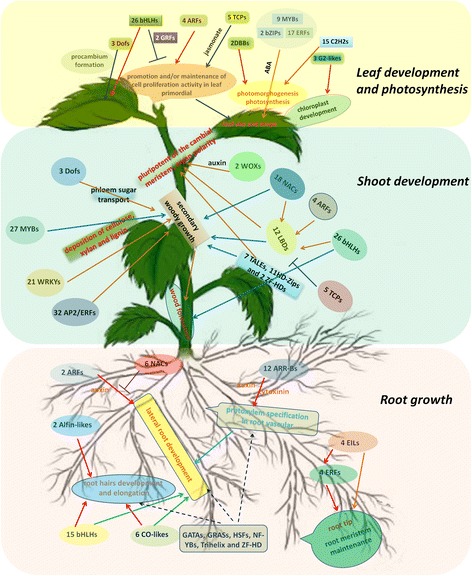
The proposed TFs involved in tissues development and growth of the paper mulberry. The arrow lines stand for the effect of activation. The “T” lines stand for the effects of inhibition. The dotted lines stand for the unknown effects.
Shoot development
The shoot of the paper mulberry is the tissue of elongation growth and shoot apical meristem, lateral meristem development. Being rich in branches, the shoot of the paper mulberry grows quickly, especially during secondary growth. In our study, a total of 296 TFs belonged to 42 families specifically higher expressed in the shoot than that in root and leaf. It included bHLH (26), Dof (15), ERF (26), LBD (12) and WOX (2) and so on (see Additional file 3: Table S5 and Figure 6). These TFs might govern the complex networks of transcriptional regulation during the shoot development in the paper mulberry.
Indeed, transcriptional profiling indicates that many genes encoding TFs are expressed preferentially during wood formation in various plant species and specific TFs might regulate their expression in a coordinated fashion [14]. Many TFs have been reported to play roles in vascular and xylem development, maintenance of procambium in stem [40,41]. For example, a total of 439 TFs are differentially expressed during shoot development in Populus, including MYBs, NACs, and ERFs [13].
Diverse MYB TFs may participate in the development of vascular tissues and the tension wood response. PttMYB21a expression is much higher in secondary cell wall formation zone of xylem and phloem fibers than in other developmental zones. Transgenic expression lines show the reduced growth and had fewer internodes compared to the wild-type, suggesting that PttMYB21a might function as a transcriptional repressor in shoot growth [42]. Overexpression of PtrMYB3 or PtrMYB20 increases deposition of cellulose, xylan and lignin in Arabidopsis. Besides, expression of PtrMYB3 and PtrMYB20 is directly activated by PtrWND2, a NAC TF which preferentially expressed in developing wood [40]. Out of the expressed 69 MYBs in shoot of the paper mulberry, more than 47.8% showed the shoot growth associated expression patterns. Similarly to MYBs, 18 NACs were higher expressed in shoot (Figure 7). These data together suggest that the enriched NAC and MYB TFs in the shoot implied their function in regulating wood formation in the paper mulberry.
Although WRKY TFs are mainly implicated in regulating defense signal [43], they have also been identified to be highly expressed in Arabidopsis stem of secondary growth and xylem tissue [44]. There were 21 WRKY members that were highly expressed in the shoot of the paper mulberry (Figure 6). The functional study of those WRKYs would help to expand knowledge of the diversity of WRKY developmental functions in this tree.
There were 32 AP2/ERFs which was the largest TF family that highly expressed in the shoot of the paper mulberry (see Additional file 5: Table S2). AP2/ERF family members are known to be involved in integration of jasmonic acid and ethylene signals in plant defense [8,45,46], but also have members that affect cell expansion, proliferation and differentiation pathways in Arabidopsis [47,48]. It has also been identified in aspen as differentially expressed at phloem localized in secondary tissue [49].
The role of Dof TFs, a group of plant-specific TFs, recently emerged as part of the transcriptional regulatory networks acting on the formation and functioning of the vascular tissues. Some of the Dof TF genes (AtDof2.4, AtDof5.8 and AtDof5.6/HCA2) are reported to be expressed specifically in cells at an early stage of vascular tissue development [9]. Besides, AtDof TFs also potentially control the phloem sugar transport. Therefore, 3 Dofs high expressed in the shoot implied their important and diverse functions in the vascular tissue development of the the paper mulberry shoot.
The TALE, HD-Zip, WOX and ZF-HD homeodomain containing TFs have been associated with processes related to meristem function, organ polarity, and vascular development in several species [13]. There were TALE (4), HD-Zip (8), WOX (2) and ZF-HD (2) showed the shoot-specific expression in the paper mulberry.
The maintenance of the pluripotent identity of the cambium is crucial for the continuous meristem activity. Current evidence indicates that a similar molecular mechanism regulating shoot apical meristem (SAM) and root apical meristem (RAM) is likely applicable in cambial meristems. The WOX TFs function have been identified in SAM and RAM by a dynamic feedback loop involving the CLAVATA3 (CLV3) peptide ligand and the CLV1 receptor in SAM [50]. Two WOXs (T2-25818 and T2-23235) exhibited shoot-specific expression, which suggested their key roles in the maintenance of the pluripotent of the cambial meristem during the shoot development of the paper mulberry.
There were 12 LBDs highly expressed in shoot than that in leaf and root. LBD family is plant-specific TF and has been implicated in plant development. Two members of the Arabidopsis LBD family, AS2-LIKE19 (ASL19)/LBD30 and ASL20/LBD18 were expressed in immature tracheary elements (TEs), and the expression was dependent on VND6 and VND7, which are NAC TFs required for TE differentiation. ASL20 appears to be involved in a positive feedback loop for VND7 expression that regulates TE differentiation-related gene [51]. Dominant-negative suppression of PtaLBD1 via translational fusion with the repressor SRDX domain caused decreased diameter growth and highly irregular phloem development. In wild-type plants, LBD1 was most highly expressed in the phloem and cambial zone. These results suggested that a broader regulatory role of LBD during secondary woody growth in Poplar [52]. Four LBD genes downstream of ARFs, LBD16, LBD17, LBD18 and LBD29, are rapidly and dramatically induced by callus-inducing medium, LBD as key regulators in the callus induction process, thereby establishing a molecular link between auxin signaling and the plant regeneration program [53].
In addition, bHLH048 regulates the function of LOB (namely LBD TF) at lateral organ boundaries [54]. However, TCP TFs play a pivotal role in the control of morphogenesis of shoot organs by negatively regulating the expression of boundary specific genes, including LBDs [55]. Five TCPs highly expressed in shoot of the paper mulberry, which might negatively regulated the expression of LBDs in shoot and this was consistent with that, except T7-29380, the all of shoot-specific LBDs RPKM was relatively lower.
Besides, ARF (4), bHLH (26) and NAC (18) were shoot-specific expression, which might directly or indirectly involve in the shoot development via the regulation of LBD in the paper mulberry. It seemed that LBD TFs were the center link in the regulation of shoot development in the paper mulberry (Figure 8).
Leaf development and photosynthesis
Leaves are photosynthetic organs. Thus, the shapes and sizes of leaves are very important factors influencing the success of plants. In our study, a total of 196 TFs belonged to 33 families were specifically higher expressed in the leaf than shoot and root. It included bHLHs (26), C2H2 (15), ERF (17) and TCP (5) and so on (see Additional file 3: Table S5 and Figure 7). These TFs might have the important regulated effect on the photosynthesis and leaf development in the paper mulberry.
Atgrf5 mutants exhibit narrow-leaf phenotypes due to decreases in cell number. Conversely, cell proliferation in leaf primordia is enhanced and leaves grow larger than normal when AtGRF5 is overexpressed. These results suggest that AtGRF5 is required for the development of appropriate leaf size and shape through the promotion and/or maintenance of cell proliferation activity in leaf primordial [56]. The SPT gene, encoding a bHLH TF, functions as a repressor of leaf growth and acts independently from another set of cell proliferation dependent organ size regulators, AN3 and AtGRF5 [7]. So, 4 ARFs and 26 bHLHs might play the major roles in the development of appropriate leaf size and shape of the paper mulberry.
A loss-of function of the ANT gene, a member of the AP2/ERF family, typically results in small leaves with fewer cells of larger volume as compared with wild-type cells. In contrast, ANT overexpression in petals causes an increase in cell number without a change in cell size [57]. A total of 17 ERFs highly expressed in the leaf might play different roles from the shoot-specific expressed members, involving the leaf development of the paper mulberry.
BBX proteins are key factors in regulatory networks controlling growth and developmental processes that include seedling photomorphogenesis, photoperiodic regulation of flowering, shade avoidance, and responses to biotic and abiotic stresses [58]. Their functions are not totally redundant, as judged by the fact that some DBBs were apparently implicated in light signal transduction in a negative manner, whereas others were implicated in a positive manner with regard to light-induced inhibition of elongation of hypocotyls [59]. For instance, BBX25 and BBX24 function as transcriptional co-repressors forming inactive heterodimers with HY5 (a bZIP TF) down regulating BBX22 expression for the fine-tuning of light-mediated seedling development [60]. Therefore, two leaf-specific expressed DBBs were considered to involve in the photomorphogenesis of the paper mulberry.
AtDof control the procambium formation during leaf development [9] and its homologous in the paper mulberry may function in the formation of procambium. TCP3, a model of CIN-like TCPs of Arabidopsis, plays important roles in the signaling pathways that generate different leaf forms without having any lethal effects on shoots by directly activating the expression of miR164, AS1, IAA3/SHY2, and SAUR [61]. In addition, analysis of tcp9 and tcp20 mutants exhibits an antagonistic function of TCP9 and TCP20 proteins in the control of leaf development via the jasmonate signaling pathway [62]. Recent study reveal that TIE1, as a major modulator of TCP activities during leaf development, may interact with both TCPs and TPL/TPRs to form transcriptional repressor complexes to repress the expression of TCP target genes, thus preventing the cells in young leaves from undergoing differentiation. In mature leaves, TIE1 expression is decreased and the activities of TCP proteins may not be inhibited by TIE1. Therefore, the downstream genes of TCPs are activated to promote cell differentiation. Overexpression of TIE1 leads to hyponastic and serrated leaves, whereas disruption of TIE1 causes epinastic leaves [63]. So, 5 highly expressed TCPs in the leaf might involve in the regulation of leaf forms of the paper mulberry (Figure 8).
In several land plants, G2-like TFs are required for chloroplast development. Double mutants of glk1 and glk2 Arabidopsis accumulate abnormal levels of chlorophyll precursors and constitutive GLK gene expression leads to increased accumulation of transcripts for antenna proteins and chlorophyll biosynthetic enzymes. GLK genes help to co-regulate and synchronize the expression of a suite of nuclear photosynthetic genes and thus act to optimize photosynthetic capacity in varying environmental and developmental conditions [64]. Three G2-likes were leaf-specific expression and its three paralogous were shoot-specific expression which suggested their different functions during chloroplast development in the leaf and shoot of the paper mulberry. However, there were also 5 G2-likes that exhibited the root-specific expression, which implied their different roles in the root growth of the paper mulberry and the essentiality of further study.
Collectively, our results indicated that leaf-specific expression TFs were focus on the families which played significant roles in the leaf development, such as AP2/EREBP, bHLH, GRFs, TCPs, as well as the families functioned in photomorphogenesis and photosynthesis, like DBBs, G2-like and so on. Together with C2H2, MYB, NAC and WRKY, all of these leaf-specific expressed TFs corporately regulated the leaf development, photosynthesis and carbohydrate metabolism in the paper mulberry (Figure 8).
Conclusion
Our study is the comprehensive transcriptome-wide identification of TFs in the paper mulberry without genome information as reference. More importantly, a large numbers of TFs regulated the lateral organ growth are tissue-specific expression, which may contribute for the developed lateral roots, more branches and rapid growth of the paper mulberry. Of course, the more specific functional differentiation of those TFs need further study. Transcriptomics-based identification of these TFs, particularly the tissue-specific expression TFs genes, provides important information for understanding the development and transcriptional regulation of the paper mulberry and leads to potential applications in the development of genetically modified with the paper mulberry.
Methods
Plant material and RNA extraction
Plantlets were cultured on MS culture media in an artificial climatic chamber kept at 26°C and a 14/10 h photoperiod (day/night). In this study, a mixed sampling strategy was adopted to eliminate differences between individuals.
Total RNA was isolated with TRIzol® Reagent (Life technologies, Shanghai, China) from each sample according to the manufacturer’s instruction. It was treated with RNase-free DNase I (Takara, Dalian, China) to remove the residual DNA. RNA quality and purity were assessed with OD260/230 ratio and RNA integrity number (RIN) by using the NanoDrop 2000 (Thermo Fisher, Waltham, USA) and the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, USA), respectively.
Sequence retrieval, identification, classification and expression analysis of TFs
Raw sequence data were generated by Illumina pipeline and have been available in NCBI’s Short Read Archive (SRA) database (http://www.ncbi.nlm.nih.gov/Traces/sra/sra.cgi) under accession number SRP029966. All of the Illumina reads generated from different cDNA libraries were de novo assembled with Trinity program to form the global transcriptome of the paper mulberry [22]. For the functional annotation, unigenes were firstly aligned by Blastx to protein databases nr (E ≤ 1e-5), retrieving proteins with the highest sequence similarity to the given unigenes along with their functional annotations. After getting annotation result for every unigene, all of the TFs of the paper mulberry were identified and classified into different families based on their DNA-binding domains and other conserved motif [18]. Based on the alignment results of orthologous in the NCBI using the Blastx tool, the TFs would be determined whether they contained the complete ORF. In addition, all of the TF families’ abbreviations presented in this paper were referenced to Plant TFDB3.0 [18].
For gene expression analysis, the expression level of each TF in each sample was calculated by quantifying the number of Illumina reads that mapped to transcriptome of the paper mulberry. The raw gene expression counts were normalized using the RPKM method (Reads per kb per million reads).
Phylogenetic analysis of TF families
TFs with the completed ORF of CAMTA, VOZ and Whirly families were used to do phylogenetic analysis. The TF family protein sequences of Amborella trichopoda, Arabidopsis thaliana, Brachypodium distachyon, Cannabis sativa, Citrullus lanatus, Fragaria vesca, Linum usitatissimum, Nelumbo nucifera, Oryza sativa subsp. Japonica, Prunus persica, Sorghum bicolor and Vitis vinifera were downloaded from Plant Transcription Factor Database (http://planttfdb.cbi.pku.edu.cn/index.php). The TFs of Morus notabilis were searched from the Morus Genome Database (http://morus.swu.edu.cn/morusdb/). The information of these TFs was listed in Additional file 6: Table S1. Phylogenetic and molecular evolutionary analyses were conducted using MEGA (version 5.0) with pairwise distance and Neighbor- Joining algorithm. The evolutionary distances were computed using the p-distance method and were used to estimate the number of amino acid substitutions per site. The reliability of each tree was established by conducting 1500 bootstrap sampling steps.
Identification of differentially expressed TFs
For screening of differentially expressed TFs, p value corresponds to differentially expressed genes (DEGs) was obtained via a general Chi squared test that was performed by using IDEG6 (http://telethon.bio.unipd.it/bioinfo/IDEG6/). The threshold of p value in multiple tests was checked through manipulating the false discovery rate (FDR) value. The TF with ratio of RPKM between samples of more than 2 (Fold change ≥2) and the FDR ≤ 0.01 was taken as the significantly difference expressed TF. The MeV (Multiexperiment Viewer, v4.9) was used to make the heat map and expressing pattern classification.
Validation by qPCR
Real time PCR was adopted to validate the DEGs identified in analysis of the RNA-seq data. Ten TFs were chosen for verification (see Additional file 5: Table S2). RNA used for validation was the same as that isolated for RNA-seq. First-strand cDNA for each sample was made from 1 μg of total RNA using SuperScript II reverse transcriptase (Takara, Dalian, China) following the manufacturer’s recommendations and diluted 3 times before use in PCR. Gene-specific primers based on the selected considerate unigenes were subsequently designed using the Primer premier 5.0 program and are list in Additional file 5: Table S2. Real-Time PCR reaction condition and volume was performed as described by in our former study [22]. Relative transcript levels for each sample were obtained using the comparative cycle threshold method using the cycle threshold value of the GAPDH gene for each sample as a standard.
Acknowledgements
This work was supported by Knowledge Innovation Program through the Chinese academy (KZCX2-YW-359-2) and the National Natural Science Foundation of China (31270653).
Additional files
The expression profile of all the TFs in paper mulberry.
The differentially expressed TFs distributed in every family.
The expression profile of the TFs with differential expression among root, shoot and leaf.
Differentially expressed TF families with complete ORF in paper mulberry.
The primers designed for the selected TFs and used for qPCR.
The accession number and the sequence information of the TFs from the selected species. TFs of CAMTA, VOZ and Whirly families of Amborella trichopoda, Arabidopsis thaliana, Brachypodium distachyon, Cannabis sativa, Citrullus lanatus, Fragaria vesca, Linum usitatissimum, Nelumbo nucifera, Oryza sativa subsp. Japonica, Prunus persica, Sorghum bicolor and Vitis vinifera were downloaded from Plant Transcription Factor Database (http://planttfdb.cbi.pku.edu.cn/index.php). The TFs of Morus notabilis were searched from the Morus Genome Database (http://morus.swu.edu.cn/morusdb/). All the TFs were re-named for the convenience of phylogenetic analysis.
Footnotes
Competing interests
We declare that we have no competing interests.
Authors' contributions
PXJ performed the RNA-seq experiments including all statistical and bioinformatic analyses. WYC and HRP performed the expression profile cluster analyses. ZML contributed to the transcriptomic and qPCR analyses. SSH was responsible for the overall concept and experimental designs, data integration, analysis and interpretation, and manuscript preparation. All authors read and approved the final manuscript.
Contributor Information
Xianjun Peng, Email: pengxianjun@ibcas.ac.cn.
Yucheng Wang, Email: wangyucheng@ibcas.ac.cn.
Ruiping He, Email: heruipinging@126.com.
Meiling Zhao, Email: zml@ibcas.ac.cn.
Shihua Shen, Email: shshen@ibcas.ac.cn.
References
- 1.Saito K, Linquist B, Keobualapha B, Shiraiwa T, Horie T. Broussonetia papyrifera (paper mulberry): its growth, yield and potential as a fallow crop in slash-and-burn upland rice system of northern Laos. Agrofor Syst. 2009;76(3):525–532. doi: 10.1007/s10457-009-9206-1. [DOI] [Google Scholar]
- 2.Zhai X, Zeng H, Liu Y, Liu F. Change of nutrients and shape of Broussonetia papyrifera leaves from different clones. J Northeast Forestry Univ. 2012;11:011. [Google Scholar]
- 3.Sun J, Peng X, Fan W, Tang M, Liu J, Shen S. Functional analysis of BpDREB2 gene involved in salt and drought response from a woody plant Broussonetia papyrifera. Gene. 2013;535(2):140–149. doi: 10.1016/j.gene.2013.11.047. [DOI] [PubMed] [Google Scholar]
- 4.Yan J, Wu P, Du H, Zhang Q. First report of black spot caused by Colletotrichum gloeosporioides on paper Mulberry in China. Plant Dis. 2011;95(7):880–880. doi: 10.1094/PDIS-03-11-0186. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Liu J, Peng X, Ni Z, Wang G, Shen S. Applied hybrid paper mulberry in ecological virescence of the coastal saline. Tianjin Agric Sci. 2014;20(02):95–101. [Google Scholar]
- 6.Liu Z, Fan W, Shen S. SRAP marker in Broussonetia papyrifera. Scientia Silvae Sinicae. 2009;45(12):54–58. [Google Scholar]
- 7.Ichihashi Y, Horiguchi G, Gleissberg S, Tsukaya H. The bHLH transcription factor SPATULA controls final leaf size in Arabidopsis thaliana. Plant Cell Physiol. 2010;51(2):252–261. doi: 10.1093/pcp/pcp184. [DOI] [PubMed] [Google Scholar]
- 8.Peng X, Zhang L, Zhang L, Liu Z, Cheng L, Yang Y, Shen S, Chen S, Liu G. The transcriptional factor LcDREB2 cooperates with LcSAMDC2 to contribute to salt tolerance in Leymus chinensis. Plant Cell Tissue Organ Cult. 2013;113:245–256. doi: 10.1007/s11240-012-0264-0. [DOI] [Google Scholar]
- 9.Le Hir R, Bellini C. The plant-specific Dof transcription factors family: new players involved in vascular system development and functioning in Arabidopsis. Front Plant Sci. 2013;4:164. doi: 10.3389/fpls.2013.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu J, Verslues PE, Zheng X, Lee BH, Zhan X, Manabe Y, Sokolchik I, Zhu Y, Dong C-H, Zhu J-K. HOS10 encodes an R2R3-type MYB transcription factor essential for cold acclimation in plants. Proc Natl Acad Sci U S A. 2005;102(28):9966–9971. doi: 10.1073/pnas.0503960102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.He XJ, Mu RL, Cao WH, Zhang ZG, Zhang JS, Chen SY. AtNAC2, a transcription factor downstream of ethylene and auxin signaling pathways, is involved in salt stress response and lateral root development. Plant J. 2005;44(6):903–916. doi: 10.1111/j.1365-313X.2005.02575.x. [DOI] [PubMed] [Google Scholar]
- 12.Berri S, Abbruscato P, Faivre-Rampant O, Brasileiro A, Fumasoni I, Satoh K, Kikuchi S, Mizzi L, Morandini P, Pè M. Characterization of WRKY co-regulatory networks in rice and Arabidopsis. BMC Plant Biol. 2009;9(1):120. doi: 10.1186/1471-2229-9-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dharmawardhana P, Brunner AM, Strauss SH. Genome-wide transcriptome analysis of the transition from primary to secondary stem development in Populus trichocarpa. BMC Genomics. 2010;11(1):150. doi: 10.1186/1471-2164-11-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demura T, Fukuda H. Transcriptional regulation in wood formation. Trends Plant Sci. 2007;12(2):64–70. doi: 10.1016/j.tplants.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Hu R, Qi G, Kong Y, Kong D, Gao Q, Zhou G. Comprehensive analysis of NAC domain transcription factor gene family in Populus trichocarpa. BMC Plant Biol. 2010;10(1):145. doi: 10.1186/1471-2229-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedman H, Zhu T, von Arnold S, Sohlberg JJ. Analysis of the WUSCHEL-RELATED HOMEOBOX gene family in the conifer picea abies reveals extensive conservation as well as dynamic patterns. BMC Plant Biol. 2013;13(1):89. doi: 10.1186/1471-2229-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du J, Groover A. Transcriptional regulation of secondary growth and wood formation. J Integr Plant Biol. 2010;52(1):17–27. doi: 10.1111/j.1744-7909.2010.00901.x. [DOI] [PubMed] [Google Scholar]
- 18.Jin J, Zhang H, Kong L, Gao G, Luo J. PlantTFDB 3.0: a portal for the functional and evolutionary study of plant transcription factors. Nucleic Acids Res. 2013;42(D1):D1182–D1187. doi: 10.1093/nar/gkt1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pérez-Rodríguez P, Riaño-Pachón DM, Corrêa LGG, Rensing SA, Kersten B, Mueller-Roeber B. PlnTFDB: updated content and new features of the plant transcription factor database. Nucleic Acids Res. 2010;38(suppl 1):D822–D827. doi: 10.1093/nar/gkp805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yilmaz A, Mejia-Guerra MK, Kurz K, Liang X, Welch L, Grotewold E. AGRIS: the Arabidopsis gene regulatory information server, an update. Nucleic Acids Res. 2011;39(suppl 1):D1118–D1122. doi: 10.1093/nar/gkq1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuskan GA, Difazio S, Jansson S, Bohlmann J, Grigoriev I, Hellsten U, Putnam N, Ralph S, Rombauts S, Salamov A. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray) Science. 2006;313(5793):1596–1604. doi: 10.1126/science.1128691. [DOI] [PubMed] [Google Scholar]
- 22.Peng X, Teng L, Wang X, Wang Y, Shihua S. De Novo assembly of expressed transcripts and global transcriptomic analysis from seedlings of the Paper Mulberry (Broussonetia kazinoki x Broussonetia papyifera) PLoS One. 2014;9(5):e97487. doi: 10.1371/journal.pone.0097487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finkler A, Ashery-Padan R, Fromm H. CAMTAs: calmodulin-binding transcription activators from plants to human. FEBS Lett. 2007;581(21):3893–3898. doi: 10.1016/j.febslet.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 24.Yang T, Peng H, Whitaker BD, Conway WS. Characterization of a calcium/calmodulin-regulated SR/CAMTA gene family during tomato fruit development and ripening. BMC Plant Biol. 2012;12(1):19. doi: 10.1186/1471-2229-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albert VA, Barbazuk WB, Der JP, Leebens-Mack J, Ma H, Palmer JD, Rounsley S, Sankoff D, Schuster SC, Soltis DE. The Amborella genome and the evolution of flowering plants. Science. 2013;342(6165):1241089. doi: 10.1126/science.1241089. [DOI] [PubMed] [Google Scholar]
- 26.Desveaux D, Maréchal A, Brisson N. Whirly transcription factors: defense gene regulation and beyond. Trends Plant Sci. 2005;10(2):95–102. doi: 10.1016/j.tplants.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Nakai Y, Nakahira Y, Sumida H, Takebayashi K, Nagasawa Y, Yamasaki K, Akiyama M, Ohme‐Takagi M, Fujiwara S, Shiina T. Vascular plant one-zinc-finger protein 1/2 transcription factors regulate abiotic and biotic stress responses in Arabidopsis. Plant J. 2013;73(5):761–775. doi: 10.1111/tpj.12069. [DOI] [PubMed] [Google Scholar]
- 28.McPartland JM, Guy GW. The evolution of Cannabis and coevolution with the cannabinoid receptor-a hypothesis. Med Use Cannabis Cannabinoids. 2004;4:71–101. [Google Scholar]
- 29.Jiang Y, Zeng B, Zhao H, Zhang M, Xie S, Lai J. Genome‐wide transcription factor gene prediction and their expressional tissue‐specificities in MaizeF. J Integr Plant Biol. 2012;54(9):616–630. doi: 10.1111/j.1744-7909.2012.01149.x. [DOI] [PubMed] [Google Scholar]
- 30.Marin E, Jouannet V, Herz A, Lokerse AS, Weijers D, Vaucheret H, Nussaume L, Crespi MD, Maizel A. miR390, Arabidopsis TAS3 tasiRNAs, and their AUXIN RESPONSE FACTOR targets define an autoregulatory network quantitatively regulating lateral root growth. Plant Cell. 2010;22(4):1104–1117. doi: 10.1105/tpc.109.072553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo H-S, Xie Q, Fei J-F, Chua N-H. MicroRNA directs mRNA cleavage of the transcription factor NAC1 to downregulate auxin signals for Arabidopsis lateral root development. Plant Cell. 2005;17(5):1376–1386. doi: 10.1105/tpc.105.030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ubeda-Tomás S, Beemster GT, Bennett MJ. Hormonal regulation of root growth: integrating local activities into global behaviour. Trends Plant Sci. 2012;17(6):326–331. doi: 10.1016/j.tplants.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Datta S, Hettiarachchi G, Deng X-W, Holm M. Arabidopsis CONSTANS-LIKE3 is a positive regulator of red light signaling and root growth. Plant Cell. 2006;18(1):70–84. doi: 10.1105/tpc.105.038182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yadav V, Mallappa C, Gangappa SN, Bhatia S, Chattopadhyay S. A basic helix-loop-helix transcription factor in Arabidopsis, MYC2, acts as a repressor of blue light–mediated photomorphogenic growth. Plant Cell. 2005;17(7):1953–1966. doi: 10.1105/tpc.105.032060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng Z, Sun L, Qi T, Zhang B, Peng W, Liu Y, Xie D. The bHLH transcription factor MYC3 interacts with the jasmonate ZIM-domain proteins to mediate jasmonate response in Arabidopsis. Mol Plant. 2011;4(2):279–288. doi: 10.1093/mp/ssq073. [DOI] [PubMed] [Google Scholar]
- 36.Yi K, Menand B, Bell E, Dolan L. A basic helix-loop-helix transcription factor controls cell growth and size in root hairs. Nat Genet. 2010;42(3):264–267. doi: 10.1038/ng.529. [DOI] [PubMed] [Google Scholar]
- 37.Muraro D, Byrne H, King J, Voß U, Kieber J, Bennett M. The influence of cytokinin–auxin cross-regulation on cell-fate determination in Arabidopsis thaliana root development. J Theor Biol. 2011;283(1):152–167. doi: 10.1016/j.jtbi.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 38.Yokoyama A, Yamashino T, Amano Y-I, Tajima Y, Imamura A, Sakakibara H, Mizuno T. Type-B ARR transcription factors, ARR10 and ARR12, are implicated in cytokinin-mediated regulation of protoxylem differentiation in roots of Arabidopsis thaliana. Plant Cell Physiol. 2007;48(1):84–96. doi: 10.1093/pcp/pcl040. [DOI] [PubMed] [Google Scholar]
- 39.Chandrika NNP, Sundaravelpandian K, Yu SM, Schmidt W. ALFIN-LIKE 6 is involved in root hair elongation during phosphate deficiency in Arabidopsis. New Phytol. 2013;198(3):709–720. doi: 10.1111/nph.12194. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Elo A, Helariutta Y. Arabidopsis as a model for wood formation. Curr Opin Biotechnol. 2011;22(2):293–299. doi: 10.1016/j.copbio.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Plomion C, Leprovost G, Stokes A. Wood formation in trees. Plant Physiol. 2001;127(4):1513–1523. doi: 10.1104/pp.010816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karpinska B, Karlsson M, Srivastava M, Stenberg A, Schrader J, Sterky F, Bhalerao R, Wingsle G. MYB transcription factors are differentially expressed and regulated during secondary vascular tissue development in hybrid aspen. Plant Mol Biol. 2004;56(2):255–270. doi: 10.1007/s11103-004-3354-5. [DOI] [PubMed] [Google Scholar]
- 43.He H, Dong Q, Shao Y, Jiang H, Zhu S, Cheng B, Xiang Y. Genome-wide survey and characterization of the WRKY gene family in Populus trichocarpa. Plant Cell Rep. 2012;31(7):1199–1217. doi: 10.1007/s00299-012-1241-0. [DOI] [PubMed] [Google Scholar]
- 44.Zhao C, Craig JC, Petzold HE, Dickerman AW, Beers EP. The xylem and phloem transcriptomes from secondary tissues of the Arabidopsis root-hypocotyl. Plant Physiol. 2005;138(2):803–818. doi: 10.1104/pp.105.060202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xianjun P, Xingyong M, Weihong F, Man S, Liqin C, Alam I, Lee B-H, Dongmei Q, Shihua S, Gongshe L. Improved drought and salt tolerance of Arabidopsis thaliana by transgenic expression of a novel DREB gene from Leymus chinensis. Plant Cell Rep. 2011;30(8):1493–1502. doi: 10.1007/s00299-011-1058-2. [DOI] [PubMed] [Google Scholar]
- 46.Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim Biophys Acta. 2012;1819(2):86–96. doi: 10.1016/j.bbagrm.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 47.Marsch-Martinez N, Greco R, Becker JD, Dixit S, Bergervoet JH, Karaba A, de Folter S, Pereira A. BOLITA, an Arabidopsis AP2/ERF-like transcription factor that affects cell expansion and proliferation/differentiation pathways. Plant Mol Biol. 2006;62(6):825–843. doi: 10.1007/s11103-006-9059-1. [DOI] [PubMed] [Google Scholar]
- 48.Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T, Zhang L, Hattori J, Liu C-M, van Lammeren AA, Miki BL. Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell. 2002;14(8):1737–1749. doi: 10.1105/tpc.001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Raemdonck D, Pesquet E, Cloquet S, Beeckman H, Boerjan W, Goffner D, El Jaziri M, Baucher M. Molecular changes associated with the setting up of secondary growth in aspen. J Exp Bot. 2005;56(418):2211–2227. doi: 10.1093/jxb/eri221. [DOI] [PubMed] [Google Scholar]
- 50.Van der Graaff E, Laux T, Rensing SA. The WUS homeobox-containing (WOX) protein family. Genome Biol. 2009;10(12):248. doi: 10.1186/gb-2009-10-12-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soyano T, Thitamadee S, Machida Y, Chua N-H. Asymmetric leaves2-like19 lateral organ boundaries domain30 and Asl20/Lbd18 regulate tracheary element differentiation in Arabidopsis. Plant Cell. 2008;20(12):3359–3373. doi: 10.1105/tpc.108.061796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yordanov YS, Regan S, Busov V. Members of the LATERAL ORGAN BOUNDARIES DOMAIN transcription factor family are involved in the regulation of secondary growth in Populus. Plant Cell. 2010;22(11):3662–3677. doi: 10.1105/tpc.110.078634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan M, Xu C, Xu K, Hu Y. LATERAL ORGAN BOUNDARIES DOMAIN transcription factors direct callus formation in Arabidopsis regeneration. Cell Res. 2012;22(7):1169–1180. doi: 10.1038/cr.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Husbands A, Bell EM, Shuai B, Smith HM, Springer PS. LATERAL ORGAN BOUNDARIES defines a new family of DNA-binding transcription factors and can interact with specific bHLH proteins. Nucleic Acids Res. 2007;35(19):6663–6671. doi: 10.1093/nar/gkm775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koyama T, Furutani M, Tasaka M, Ohme-Takagi M. TCP transcription factors control the morphology of shoot lateral organs via negative regulation of the expression of boundary-specific genes in Arabidopsis. Plant Cell. 2007;19(2):473–484. doi: 10.1105/tpc.106.044792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horiguchi G, Kim GT, Tsukaya H. The transcription factor AtGRF5 and the transcription coactivator AN3 regulate cell proliferation in leaf primordia of Arabidopsis thaliana. Plant J. 2005;43(1):68–78. doi: 10.1111/j.1365-313X.2005.02429.x. [DOI] [PubMed] [Google Scholar]
- 57.Mizukami Y, Fischer RL. Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proc Natl Acad Sci. 2000;97(2):942–947. doi: 10.1073/pnas.97.2.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gangappa SN, Botto JF: The BBX family of plant transcription factors.Trends Plant Sci 2014. doi:10.1016/j.tplants.2014.01.010. [DOI] [PubMed]
- 59.Kumagai T, Ito S, Nakamichi N, Niwa Y, Murakami M, Yamashino T, Mizuno T. The common function of a novel subfamily of B-Box zinc finger proteins with reference to circadian-associated events in Arabidopsis thaliana. Biosci Biotechnol Biochem. 2008;72(6):1539–1549. doi: 10.1271/bbb.80041. [DOI] [PubMed] [Google Scholar]
- 60.Gangappa SN, Crocco CD, Johansson H, Datta S, Hettiarachchi C, Holm M, Botto JF. The Arabidopsis B-box protein BBX25 interacts with HY5, negatively regulating BBX22 expression to suppress seedling photomorphogenesis. Plant Cell. 2013;25(4):1243–1257. doi: 10.1105/tpc.113.109751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koyama T, Mitsuda N, Seki M, Shinozaki K, Ohme-Takagi M. TCP transcription factors regulate the activities of ASYMMETRIC LEAVES1 and miR164, as well as the auxin response, during differentiation of leaves in Arabidopsis. Plant Cell. 2010;22(11):3574–3588. doi: 10.1105/tpc.110.075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Danisman S, Van der Wal F, Dhondt S, Waites R, de Folter S, Bimbo A, van Dijk AD, Muino JM, Cutri L, Dornelas MC. Arabidopsis class I and class II TCP transcription factors regulate jasmonic acid metabolism and leaf development antagonistically. Plant Physiol. 2012;159(4):1511–1523. doi: 10.1104/pp.112.200303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tao Q, Guo D, Wei B, Zhang F, Pang C, Jiang H, Zhang J, Wei T, Gu H, Qu L-J. The TIE1 transcriptional repressor links TCP transcription factors with TOPLESS/TOPLESS-RELATED corepressors and modulates leaf development in Arabidopsis. Plant Cell Online. 2013;25(2):421–437. doi: 10.1105/tpc.113.109223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waters MT, Wang P, Korkaric M, Capper RG, Saunders NJ, Langdale JA. GLK transcription factors coordinate expression of the photosynthetic apparatus in Arabidopsis. Plant Cell. 2009;21(4):1109–1128. doi: 10.1105/tpc.108.065250. [DOI] [PMC free article] [PubMed] [Google Scholar]


