Abstract
AIM: To investigate the safety and efficacy of the formulation HD-03/ES capsules in the management of patients with chronic hepatitis B infection.
METHODS: A total of 25 patients were recruited to the study and were given HD-03/ES, two capsules twice daily for six months. Clinical assessment of symptoms and signs were done using the “clinical observation table” once a month before and after the treatment. Biochemical investigations of total bilirubin, ALT, AST, serum protein for liver function tests were done every month after initiating treatment. Serum was analyzed for HBV markers for HBsAg, HBeAg and HBV DNA at baseline, 4 and 6 mo after therapy using ELISA kits from Roche.
RESULTS: After 6 mo of therapy with HD-03/ES, a significant reduction of ALT values from 66.5 ± 11.1 to 39.1 ± 5.2 (P < 0.01) and a significant HBsAg loss (52%, P < 0.001), HBeAg loss (60%, P < 0.05) and HBV DNA loss (60%, P < 0.05) was observed. Adverse effects were mild and never warranted withdrawal of the drug.
CONCLUSION: The results of this pilot study indicate that HD-03/ES might be a safe and effective treatment for chronic hepatitis B infection and a long-term multicentric comparator trial is warranted and under way.
Keywords: HD-03/ES, Chronic hepatitis B, Liver function tests, Hepatitis B Virus markers, Clinical trial, HBsAg, HBeAg
INTRODUCTION
Hepatitis B virus (HBV) is a hepadnavirus that is noncytopathic and causes significant morbidity and mortality worldwide[1]. Chronic hepatitis B (CHB) affects an estimated 400 million people worldwide with over 50 000 fatalities each year[2]. 82% of the world’s 530 000 cases of liver cancer per year are caused by viral hepatitis infection, with 316 000 cases associated with hepatitis B infection[3]. According to a WHO report, India has intermediate endemicity of hepatitis B, with Hepatitis B surface antigen (HBsAg) prevalence between 2% and 7% among populations studied. The prevalence does not vary significantly by region in the country. The number of HBsAg carriers in India has been estimated to be over 40 million (4 crore). It has been estimated that, in India, of the 25 million infants born every year, over one million run the lifetime risk of developing chronic HBV infection. Every year over 100 000 Indians die due to illnesses related to HBV infection.
Ayurveda, an indigenous system of medicine in India, has a long tradition of treating liver disorders with plant drugs[4]. On the basis of leads available from folklore usage and recent experimental studies, HD-03/ES (a capsule formulation consisting of 125 mg each of hydroalcoholic extracts of the herbs Cyperus rotundus and Cyperus scariosus) was evolved to elicit hepatoprotective activity.
Surface antigen suppression and HBV elimination activities of herbal extract containing Cyperus rotundus and Cyperus scariosus were examined using two HBsAg expressing human hepatocellular carcinoma cell lines, PLC/PRF/5 and HepG2.2.215 polymerase chain reaction (PCR) for the study of amplification of DNA specific to HBV, reverse transcriptase inhibition assay, immunomodulatory effects and hepatoprotective ability against oxidative damage to hepatocytes were some of the other studies performed to evaluate the efficacy of the plant extract. The efficacy of the plant extract to eliminate the duck hepatitis B virus was assessed in experimentally infected Pekin ducks in a duck model study. Our investigations indicated that the extracts could reversibly inhibit cell growth and suppress HBsAg expression in both of the human hepatocellular carcinoma cell line models. Acute and sub-acute toxicity studies conducted in rats indicated that HD-03/ES is devoid of significant toxicity following acute and repeated administration in rats (Data on file).
A preliminary case study report indicated that there was significant reduction of HBsAg along with disappearance of viral DNA in a patient treated with HD-03/ES at a dosage of two capsules twice daily for a period of six months[5]. At the moment, there is no data to show whether HD-03/ES treatment is adequate for the treatment of HBV infection. We therefore, undertook this clinical study to evaluate the safety and efficacy of HD-03/ES in patients with chronic hepatitis B infection.
MATERIALS AND METHODS
Patients
An open prospective controlled clinical trial was carried out in the Department of Gastroenterology, Lifeline Rigid Hospitals, Chennai, Tamilnadu, India, between March 2005 and June 2006 to evaluate the safety and efficacy of HD-03/ES capsules alone in the management of chronic hepatitis B infection. Informed written consent was obtained from all study participants and the protocol of the study was approved by the ethical committee of the institute. The study in general was conducted in accordance with Declaration of Helsinki and GCP Guidelines issued by the Ministry of Health, Government of India.
Diagnostic criteria
Patients with a history of hepatitis B or HBsAg carriers for at least 6 mo, who still had symptoms and signs of hepatitis as well as abnormal liver function and positive HBsAg, were diagnosed as having CHB infection in the present study.
Criteria for enrollment
Patients, aged 18-60 years, with their serum alanine aminotransferase (ALT) level being 41-200 IU/L and who had positive serum HBsAg, were enrolled.
Criteria for exclusion
Patients aged over 60 years or less than 18 years, pregnant or lactating women, patients who had hepatitis C or other hepatic viral infection, autoimmune hepatitis and drug-induced hepatitis or alcoholic hepatitis; patients with severe complications of the cardiovascular, renal or hematopoietic system; and patients with mental diseases, were excluded. Patients were excluded if they had decompensated liver disease (defined by serum albumin ≤ 360 g/L, bilirubin ≥ 150 g/L, prothrombin time ≥ 2 s prolonged, or a history of ascites, variceal hemorrhage or hepatic encephalopathy), pancytopenia (defined as hemoglobin < 110 g/L, white cell count < 4000/mm3 or platelets < 105/mm3). Patients with a history of using interferon or anti-viral agents or corticosteroids or immunosuppressive drugs were also excluded.
Treatment
Each patient was asked to take two capsules of HD-03/ES (The Himalaya Drug Company, Bangalore, India) twice daily, two capsules in the morning and two capsules at bedtime after food for a period of six months.
Recording and observation of symptoms and signs
The symptoms and signs of patients were recorded in detail using the “Clinical Observation Table” once a month before and during the treatment.
Etiological markers of hepatitis B
Serum samples collected from patients were stored at -20°C until analysis. Serum was assayed for HBsAg, hepatitis B e-Antigen (HBeAg), and HBV DNA at baseline, four and six months after therapy using commercially available enzyme-linked immunosorbent assay kits from Roche.
Liver function
The patients had liver function examinations every month during the treatment, including contents of serum proteins, total bilirubin (TB) and activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
Safety analysis
Safety analysis included data for all treated patients during dosing. The primary safety end point was discontinuation of study medication because of adverse events. Other safety evaluations included incidence of adverse effects.
End points
The primary end point was HBsAg clearance. Secondary endpoints included HBV DNA levels and ALT normalization to 40 IU/L at the end of treatment.
Statistical analysis
The intention-to-treat analysis included all randomized patients who were HBsAg-positive at baseline and received at least one dose of the study medication. Data were expressed as mean ± SD. One-way ANOVA with Bonferroni’s multiple comparison test or Dunnett's multiple comparison test was performed wherever appropriate using GraphPad Prism version 4.00 for Windows, GraphPad Software, San Diego California USA. A P value of < 0.05 was taken as statistically significant.
RESULTS
Twenty five patients (22 males and 3 females) aged between 20 and 45 years with a mean age of 33.7 years participated in this open study. Their baseline characteristics are shown in Table 1.
Table 1.
Demographic data
| Characteristic | HD-03/ES | |
| Age (yr) | Mean (SD) | 33.7 (6.6) |
| Median (range) | 36 (20-45) | |
| Sex | Male | 22 |
| Female | 3 | |
| Body weight (kg) | Mean (SD) | 56 (10) |
Clinical response
Six months of therapy with HD-03/ES capsules was markedly effective in the majority of the patients as it resulted in disappearance or alleviation of chief clinical symptoms such as abdominal pain and poor appetite. The effect of six months of treatment with HD-03/ES on liver function tests are shown in Table 2. As shown in the table, there was a trend towards normalization of liver function tests in all patients treated with HD-03/ES.
Table 2.
Effect of six months treatment with HD-03/ES on liver function tests (mean ± SD)
| Parameter | d 0 | Four months | Six months |
| Alanine aminotransferase (ALT) (IU/L) | 66.5 ± 11.1 | 41.6 ± 05.1 | 39.1 ± 5.2 |
| Aspartate aminotransferase (AST) (IU/L) | 47.5 ± 9.5 | 42.4 ± 10.7 | 40.2 ± 10.1 |
| Serum albumin (g%) | 3.5 ± 0.8 | 3.5 ± 0.7 | 3.6 ± 0.7 |
| Serum globulin (g%) | 2.9 ± 0.3 | 3.1 ± 0.2 | 3.2 ± 0.2 |
| Total protein (g%) | 6.2 ± 0.7 | 6.5 ± 0.7 | 6.5 ± 0.7 |
| Serum bilirubin (mg%) | 1.3 ± 0.6 | 1.2 ± 0.5 | 1.1 ± 0.5 |
| Alkaline phosphatase (IU/L) | 155.5 ± 9.8 | 140.2 ± 9.0 | 127.0 ± 7.5 |
ALT normalization
Levels of ALT before and after six months of treatment with HD-03/ES are shown in Table 3. After six months of treatment, the levels of ALT were decreased from initial value of 66.5 ± 11.1 to 39.1 ± 5.2, and this reached levels of statistical significance (P < 0.01). In 14 of the 25 patients (56%), ALT levels were normalized. Although ALT levels were not normalized in the remaining 11 patients, there was a trend towards reduction and in none of the patients was a rise in ALT levels seen (Figure 1).
Table 3.
Biochemical and serological response to 6 mo of HD-03/ES therapy
| Variable | HD-03/ES (No. of patients) | ||
| ALT normalization (%) | Four months | 32% | |
| Six months | 56% | ||
| Positive | Negative | ||
| HBsAg | d 0 | 25 | 0 |
| Four months | 19 | 6 | |
| Six months | 12 | 13b | |
| HBeAg | |||
| d 0 | 16 | 9 | |
| Four months | 11 | 14 | |
| Six months | 10 | 15a | |
| HBV DNA | d 0 | 16 | 9 |
| Four months | 11 | 14 | |
| Six months | 10 | 15a | |
P < 0.05; vs d 0;
P < 0.001 vs d 0.
Figure 1.
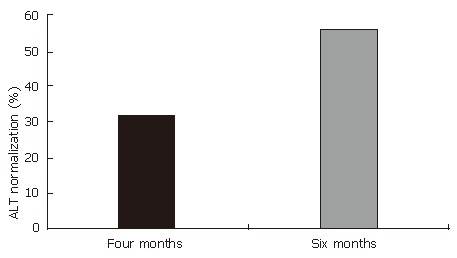
Improvement in ALT normalization after treatment with HD-03/ES.
Virological response
The effect of 6 mo of treatment with HD-03/ES treatment on virological response is shown in Table 3. Thirteen of the 25 patients (52%) at the end of treatment, who were treated with HD-03/ES, had undetectable HBsAg. This difference was statistically significant (P < 0.001)
(Figure 2). HBeAg loss (60%, P < 0.001) and HBV DNA loss (60%, P < 0.05) also occurred during treatment with HD-03/ES in that 6 patients who were positive for both HBeAg and HBV DNA initially, but were negative for the same at the end of therapy (Figure 3 and Figure 4).
Figure 2.
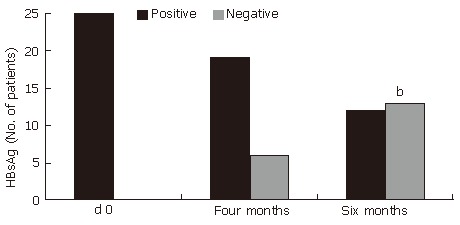
Improvement in HBsAg after treatment with HD-03/ES. bP < 0.001 vs d 0.
Figure 3.
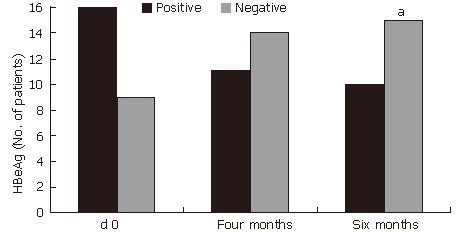
Improvement in HBeAg after treatment with HD-03/ES. aP < 0.05 vs d 0.
Figure 4.
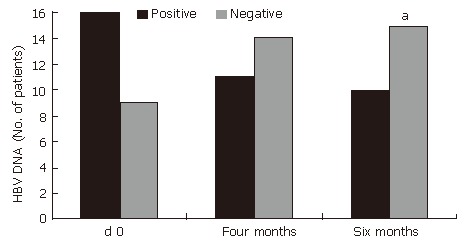
Improvement in HBV DNA after treatment with HD-03/ES.aP < 0.05 vs d 0
Adverse events
HD-03/ES was well tolerated in this study. No patient was withdrawn from therapy either for adverse effects or for other reasons. The adverse events observed during therapy are shown in Table 4. Most of the observed side-effects were mild (fatigue, headache and insomnia) in nature. The most common adverse event was abdominal discomfort. No serious biochemical abnormalities were experienced by any patient.
Table 4.
List of adverse effects
| Adverse effect | HD-03/ES |
| Abdominal discomfort | 3 |
| Fatigue | 2 |
| Headache | 1 |
| Insomnia | 1 |
DISCUSSION
High morbidity and mortality have been found in Asia among HBsAg-positive patients, even in the absence of overt liver disease[6,7]. The goals of treatment in CHB infection are sustained viral suppression, normalization of ALT levels and improvement in liver histology leading to long-term reduction in the risk of cirrhosis and hepatocellular carcinoma[8]. Loss of HbsAg, HBeAg and normalization of ALT levels and improvement in liver histology are the usual short-term end points of therapy[9]. The results of this preliminary study indicate that short-term therapy with HD-03/ES is effective in the management of CHB. Although the initial results of this study are promising, it remains to be seen whether virological response will be sustained during chronic dosing and whether relapse rates after cessation of therapy would be low unlike conventional therapies whose relapse rates are high after treatment cessation[10].
The ultimate endpoint of antiviral therapy for CHB infection is loss of HBsAg, which is accompanied by disease remission in terms of ALT normalization[11]. In this study, HBsAg loss was observed in 52% of the patients after 6 mo of therapy with HD-03/ES. This is in contrast to several clinical trials of lamivudine or adefovir where HBsAg loss was not reported[12,13] or tends to occur later than 24 wk as with interferon therapy[14]. Although six months of therapy is limited and not capable of inducing pronounced viral suppression, five patients lost their HBV DNA after six months of therapy, which is highly encouraging.
Loss of HBeAg either spontaneously or following therapy significantly improves the clinical outcome and survival in chronic HBV patients. Therefore, HBeAg loss has remained as a major end point of antiviral therapy in chronic HBV infection. Monotherapy with alpha interferon for 16 to 26 wk is associated with loss of serum HBeAg in 20%-40% of the patients[15]. Our results (55%) are slightly better.
The possible mechanisms of action as studied using HBsAg expressing human hepatocellular carcinoma cell lines PLC/PRF/5 and HepG2.2.2.15 indicate to HBsAg suppression by binding to the antigen, and HBV elimination by reverse transcriptase inhibition. Immunomodulatory effects occur by causing the release of nitric oxide (NO) by macrophages and cytokines like TNF-α. It was found to have an hepatoprotective effect by reversing the oxidative damage caused by hepatocytes.
A strong correlation was found between HBV DNA levels and histology activity index scores in HBeAg negative patients[16]. As ALT levels are consistent with histological activity index scores, the findings in the present study of ALT normalization, HBsAg loss together with loss of DNA during short-term treatment with HD-03/ES indicates that patients treated with HD-03/ES may lose their infectivity faster and relapse rates would be low.
Although the initial results of this study are promising, it remains to be seen whether virological response will be sustained during chronic dosing and whether relapse rates after cessation of therapy would be low unlike conventional therapies whose relapse rates are high after treatment cessation[17]. Our study has several obvious limitations and among these we should consider the small sample size.
In summary, this trial demonstrated that 24 wk of HD-03/ES treatment resulted in clinically significant virological and biochemical benefits in patients with CHB infection. Hence to conclude the potential benefit of HD-03/ES in the management of CHB, HD-03/ES should be studied in long-term comparative trials with standard drugs with extended duration of follow-up.
ACKNOWLEDGMENTS
We thank all the trial participants for their consent and participation in this study. We thank the management Lifeline Rigid Hospitals, Kilpauk, Chennai-600010, India, for kind permission to conduct the study and publish the results.
Footnotes
S- Editor Liu Y L- Editor Alpini GD E- Editor Wang HF
References
- 1.Asmuth DM, Nguyen HH, Melcher GP, Cohen SH, Pollard RB. Treatments for hepatitis B. Clin Infect Dis. 2004;39:1353–1362. doi: 10.1086/425010. [DOI] [PubMed] [Google Scholar]
- 2.Lim SG, Ng TM, Kung N, Krastev Z, Volfova M, Husa P, Lee SS, Chan S, Shiffman ML, Washington MK, et al. A double-blind placebo-controlled study of emtricitabine in chronic hepatitis B. Arch Intern Med. 2006;166:49–56. doi: 10.1001/archinte.166.1.49. [DOI] [PubMed] [Google Scholar]
- 3.Lai CL, Ratziu V, Yuen MF, Poynard T. Viral hepatitis B. Lancet. 2003;362:2089–2094. doi: 10.1016/S0140-6736(03)15108-2. [DOI] [PubMed] [Google Scholar]
- 4.De S, Ravishankar B, Bhavsar GC. Plants with hepatoprotective activity: A review. Ind Drugs. 1993;30:355–363. [Google Scholar]
- 5.Kulkarni KS, Dixit MN, Bhagwat , V G, Meru AK, Babu UV, Ekta Saxena, Mitra SK. Reduction of HBsAg with decrease in viral load with herbal formulation, HD-03/ES: A case study. Med Update. 2002;7:61–63. [Google Scholar]
- 6.Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942–1956. doi: 10.1002/1097-0142(19880515)61:10<1942::aid-cncr2820611003>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Sakuma K, Takahara T, Okuda K, Tsuda F, Mayumi M. Prognosis of hepatitis B virus surface antigen carriers in relation to routine liver function tests: a prospective study. Gastroenterology. 1982;83:114–117. [PubMed] [Google Scholar]
- 8.Jacobson IM. Therapeutic options for chronic hepatitis B: considerations and controversies. Am J Gastroenterol. 2006;101 Suppl 1:S13–S18. doi: 10.1111/j.1572-0241.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- 9.Yuen MF, Lai CL. Current and future antiviral agents for chronic hepatitis B. J Antimicrob Chemother. 2003;51:481–485. doi: 10.1093/jac/dkg122. [DOI] [PubMed] [Google Scholar]
- 10.Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, Lu ZM, Piratvisuth T, Germanidis G, Yurdaydin C, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351:1206–1217. doi: 10.1056/NEJMoa040431. [DOI] [PubMed] [Google Scholar]
- 11.Flink HJ, van Zonneveld M, Hansen BE, de Man RA, Schalm SW, Janssen HL. Treatment with Peg-interferon alpha-2b for HBeAg-positive chronic hepatitis B: HBsAg loss is associated with HBV genotype. Am J Gastroenterol. 2006;101:297–303. doi: 10.1111/j.1572-0241.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- 12.Hadziyannis SJ, Papatheodoridis GV, Dimou E, Laras A, Papaioannou C. Efficacy of long-term lamivudine monotherapy in patients with hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2000;32:847–851. doi: 10.1053/jhep.2000.17915. [DOI] [PubMed] [Google Scholar]
- 13.Santantonio T, Mazzola M, Iacovazzi T, Miglietta A, Guastadisegni A, Pastore G. Long-term follow-up of patients with anti-HBe/HBV DNA-positive chronic hepatitis B treated for 12 months with lamivudine. J Hepatol. 2000;32:300–306. doi: 10.1016/s0168-8278(00)80076-8. [DOI] [PubMed] [Google Scholar]
- 14.Brunetto MR, Oliveri F, Coco B, Leandro G, Colombatto P, Gorin JM, Bonino F. Outcome of anti-HBe positive chronic hepatitis B in alpha-interferon treated and untreated patients: a long term cohort study. J Hepatol. 2002;36:263–270. doi: 10.1016/s0168-8278(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 15.van Nunen AB, Hansen BE, Suh DJ, Löhr HF, Chemello L, Fontaine H, Heathcote J, Song BC, Janssen HL, de Man RA, et al. Durability of HBeAg seroconversion following antiviral therapy for chronic hepatitis B: relation to type of therapy and pretreatment serum hepatitis B virus DNA and alanine aminotransferase. Gut. 2003;52:420–424. doi: 10.1136/gut.52.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng J, Luo K, Zhu Y, Guo Y, Zhang L, Hou J. Clinical and histological characteristics of chronic hepatitis B with negative hepatitis B e-antigen. Chin Med J (Engl) 2003;116:1312–1317. [PubMed] [Google Scholar]
- 17.Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, Jeffers L, Goodman Z, Wulfsohn MS, Xiong S, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808–816. doi: 10.1056/NEJMoa020681. [DOI] [PubMed] [Google Scholar]


