Abstract
AIM: Local recurrence after curative surgical resection for rectal cancer remains a major problem. Several studies have shown that incomplete removal of cancer deposits in the distal mesorectum contributes a great share to this dismal result. Clinicopathologic examination of distal mesorectum in lower rectal cancer was performed in the present study to assess the incidence and extent of distal mesorectal spread and to determine an optimal distal resection margin in sphincter-saving procedure.
METHODS: We prospectively examined specimens from 45 patients with lower rectal cancer who underwent curative surgery. Large-mount sections were performed to microscopically observe the distal mesorectal spread and to measure the extent of distal spread. Tissue shrinkage ratio was also considered. Patients with involvement in the distal mesorectum were compared with those without involvement with regard to clinicopathologic features.
RESULTS: Mesorectal cancer spread was observed in 21 patients (46.7%), 8 of them (17.8%) had distal mesorectal spread. Overall, distal intramural and/or mesorectal spreads were observed in 10 patients (22.2%) and the maximum extent of distal spread in situ was 12 mm and 36 mm respectively. Eight patients with distal mesorectal spread showed a significantly higher rate of lymph node metastasis compared with the other 37 patients without distal mesorectal spread (P = 0.043).
CONCLUSION: Distal mesorectal spread invariably occurs in advanced rectal cancer and has a significant relationship with lymph node metastasis. Distal resection margin of 1.5 cm for the rectal wall and 4 cm for the distal mesorectum is proper to those patients who are arranged to receive operation with a curative sphincter-saving procedure for lower rectal cancer.
Keywords: Lower rectal cancer, Mesorectal cancer spread, Sphincter-saving procedure, Lymph node metastasis
INTRODUCTION
With the recent development in double-stapling devices and techniques, more and more sphincter-saving procedures and coloanal anastomoses have been employed in very low rectal cancers[1,2]. However, the increased use of these techniques causes many debates about the extent of distal resection margin. Although the resection at 5 cm from the distal edge of tumors has been conventionally accepted as a rule, recent evidence indicates that shorter resection margins of 3 to 1 cm or even less are also safe[3-5]. Surgeons encouraged by these findings may seek a much shorter resection margin, which would run a risk of cancer cells remaining in the anastomosis sites or pelvic sidewalls. Many investigators have studied cancer spread to define distal resection margins, but most of these studies focused on the distal intramural spread[3,4]. Since a series of studies reported that total mesorectal excision (TME) could remarkably decrease local recurrence[6-9], there has been increasing interest in the mesorectum known as “oncologically dangerous” tissues that surround the rectum. Scott et al[10] and Tocci et al[11] demonstrated that distal mesorectal spread usually exceeded intramural spread of rectal cancer, showing that examination for microscopic spread in the distal mesorectum is more essential for a surgical decision. Therefore, the main purpose of the present study was to assess the incidence and extent of distal mesorectal spread and to provide pathological proofs for colorectal surgeons to determine an optimal distal resection margin in sphincter-saving procedure.
MATERIALS AND METHODS
Patients
We prospectively studied 45 consecutive patients with lower rectal cancer who were treated with curative resection of the tumor in the Department of Gastrointestinal Surgery of West China Hospital, Sichuan University, Chengdu, China, between November 2000 and June 2001. Lower rectal cancer was defined as the tumor at or below the peritoneal reflection. Twenty-seven of the 45 patients, were males (60.0%) with a mean age of 62.8±10.7 years. Thirteen patients underwent abdominoperineal resection (APR) and 32 had low anterior resection (LAR). No patient received preoperative radiotherapy and chemotherapy. All patients were operated by a surgeon on the basis of the principles of total mesorectal excision described by Heald et al[12] and MacFarlane et al[13]. The tumor mass and mesorectum enveloped within the visceral endopelvic fascia were removed as an intact unit.
Specimen management
The excised specimen was opened along the antimesenteric border and shaped to same dimensions as in situ, and then carefully pinned flat to a corkboard under a certain tension. The macroscopical observations, including the distance of distal resection margin, were recorded in detail. Two knots were sutured at intervals of 5 cm on the longitudinal bowel wall. After the specimen was fixed in 10 mL/L buffered formalin for 48 h, the distance of 5 cm was re-measured. Tissue shrinkage ratio was calculated by dividing this length after fixation by 5 cm. Finally, serial longitudinal stripes of tissue were made at 5 mm intervals from the proximal mesorectum to the distal resection margin in continuity. Each stripe, including the full thickness of the tumor, bowel wall and mesorectum, was embedded in paraffin, sliced to 10 μm large-mount sections on a microtome, and then stained with hematoxylin and eosin.
Microscopically pathologic examination
All sections were examined by one pathologist. The mesorectum proximal, deep and distal to the tumor was entirely investigated. Cancer spread patterns in the mesorectum were classified as direct infiltration, lymph node metastasis, vessel invasion, perineural invasion, lymphatic permeation and isolated foci (Figure 1). Distal cancer spread occurred in layers of the mesorectum and rectal wall. The extent of distal spread was measured with a micrometer from the microscopically distal mucosal portion of the tumor. The actually maximum extent in situ was figured out as the above-mentioned extent divided by tissue shrinkage ratio. The “donuts” included in the stapler device were also examined microscopically, none of them was found with residual cancer cells.
Figure 1.
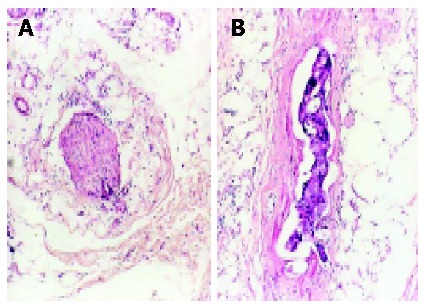
Microscopic findings of cancer spread in mesorectum. A: Perineural invasion (H&E, original magnification, ×40); and B: Vessel invasion (H&E, original magnification, ×40).
Follow-up schedule
All patients were followed up every 3 mo after surgery during the first year and every 6 mo thereafter. Each one was evaluated by physical examination, blood cell count, serum carcinoembryonic antigen level and chest radiography. Endoscopy, abdominal-pelvic ultrasonography and computed tomography were performed yearly. Local recurrence and distant metastasis were recorded during follow up.
Statistical analysis
Chi-square test or Fisher’s exact probability test for differences in frequencies and Student’s t test for differences in mean±SD were carried out by the SPSS 10.0 software package. P<0.05 was considered statistically significant.
RESULTS
Incidence of cancer spread in distal mesorectum
Mesorectal cancer spread was observed in 21 patients (46.7%). Spread towards one direction was identified in 9 patients (20.0%), two directions in 9 (20.0%) and three directions in 3 (6.7%). Overall, cancer spread was observed in 8 patients (17.8% of all) in distal mesorectum, compared with 17 patients (37.8%) in mesorectum underlying the tumor and 11 patients (24.4%) in proximal mesorectum (Table 1). The main pattern of mesorectal cancer spread was direct infiltration. However, lymph node metastasis was most frequently found in distal mesorectum of 7 patients (15.6%). Among these 8 patients, there were 7 patients (15.6%) with TNM stage III disease, 1 (2.2%) with stage IV disease.
Table 1.
Site and frequency of cancer spread in mesorectum.
| Site of spread | n | % |
| Proximal | 3 | 14.3 |
| Deep | 5 | 23.8 |
| Distal | 1 | 4.8 |
| Proximal+deep | 5 | 23.8 |
| Deep+distal | 4 | 19 |
| Proximal+deep+distal | 3 | 14.3 |
| Total | 21 | 100 |
Comparison of clinicopathologic characteristics
We compared the clinicopathologic parameters between the 8 patients with involvement in distal mesorectum and the other 37 patients without distal mesorectal spread, and the results are shown in Table 2. Lymph node metastasis occurred at a significantly higher rate in the patients with distal mesorectal spread compared to those without distal mesorectal spread (P = 0.043). No significant difference was found between the two groups in other parameters.
Table 2.
Comparison of clinicopathologic characteristics between patients with and without in distal mesorectum (mean±SD, n, %).
| Parameters | With involvement (n = 8) | Without involvement (n = 37 ) | P value |
| Age, mean±SD (yr) | 61.7±19.4 | 63.4±12.5 | NS |
| Gender | NS | ||
| Male | 6 (75.0) | 23 (62.2) | |
| Female | 2 (25.0) | 14 (37.8) | |
| Tumor location1 | NS | ||
| Anterior | 2 (25.0) | 13 (35.1) | |
| Lateral | 3 (37.5) | 10 (27.0) | |
| Posterior | 1 (12.5) | 7 (18.9) | |
| Circumferential | 2 (25.0) | 7 (18.9) | |
| Distance of tumor from dentate line (cm) | NS | ||
| ≤5 | 5 (62.5) | 26 (70.3) | |
| > 5 | 3 (37.5) | 11 (29.7) | |
| Patterns of tumor growth | NS | ||
| Infiltrating | 6 (75.0) | 28 (75.7) | |
| Pushing | 0 (0.0) | 4 (10.8) | |
| Mixed | 2 (25.0) | 5 (13.5) | |
| Tumor maximal diameter (cm) | NS | ||
| ≤5 | 5 (62.5) | 22 (59.5) | |
| >5 | 3 (37.5) | 15 (40.5) | |
| Tumor differentiation | NS | ||
| Well | 2 (25.0) | 9 (24.3) | |
| Moderate | 3 (37.5) | 21 (56.8) | |
| Poor | 3 (37.5) | 7 (18.9) | |
| TNM stage2 | NS | ||
| I | 0 (0.0) | 6 (16.2) | |
| II | 0 (0.0) | 11 (29.7) | |
| III | 7 (87.5) | 17 (45.9) | |
| IV | 1 (12.5) | 3 (8.1) | |
| Depth of tumor invasion | NS | ||
| T1 | 0 (0.0) | 3 (8.1) | |
| T2 | 0 (0.0) | 11 (29.7) | |
| T3 | 7 (87.5) | 21 (56.8) | |
| T4 | 1 (12.5) | 2 (5.4) | |
| Lymph node metastasis | 0.043 | ||
| Present | 7 (87.5) | 15 (45.9) | |
| Absent | 1 (12.5) | 22 (54.1) | |
| Distant metastasis during follow up | NS | ||
| Present | 1 (12.5) | 1(2.7) | |
| Absent | 7 (87.5) | 36 (97.3) |
NS = not significant.
Confirmation of tumor location according to more than two-thirds of tumor area within any side of rectal wall.
According to the American Joint Committee on Cancer Staging Manual[14].
Distal resection margin and maximum extent of distal spread
In the series, the mean distance of the tumor from the distal resection margin was 3.2 cm (range, 1.5-5 cm). Twenty-eight patients (62.2%) had a distal resection margin of more than 3.5 cm and 17 patients (37.8%) had a distal resection margin of less than 3.5 cm. We examined the distal mesorectal spread as well as the distal intramural spread. Distal intramural spread was observed in 5 patients (11.1%), 3 (6.7%) of them had both types of spread. As a whole, distal cancer spread was found in 10 patients (22.2%) (Table 3). The mean tissue shrinkage ratio was 85% (range, 72-98%). After converted by tissue shrinkage ratio, the maximum extent of distal mesorectal and intramural spread in situ was 36 mm and 12 mm, respectively.
Table 3.
Mode of distal mesorectal and intramural spread of lower rectal cancer.
| Number | Pattern ofgrowth | Tumor Differentiation | pTNMstage1 | Layer ofspread | Mode ofspread | Maximum extentin situ (mm) | Distal resection Margin (mm) | Outcome after2.5 yr |
| 1 | Infiltrating | Poor | pT2N1M0 | Mesorectum | LN | 11 | 45 | Disease free |
| 2 | Infiltrating | Poor | pT2N2M0 | Submucosa | LN | 8 | 33 | Disease free |
| 3 | Mixed | Moderate | pT2N1M0 | Mesorectum | LN | 13 | 35 | Local recurrence |
| 4 | Infiltrating | Moderate | pT3N2M0 | MP | ly | 10 | 40 | Disease free |
| Mesorectum | LN | 25 | ||||||
| 5 | Infiltrating | Moderate | pT2N0M0 | Submucosa | D | 7.5 | 40 | Disease free |
| 6 | Infiltrating | Poor | pT3N1M0 | Mesorectum | LN | 36 | 38 | Disease free |
| 7 | Mixed | Moderate | pT3N2M1 | MP | D | 5 | 25 | Distant metastasis |
| Mesorectum | LN | 14 | ||||||
| 8 | Infiltrating | Well | pT2N2M0 | Mesorectum | ly | 8 | 43 | Disease free |
| 9 | Infiltrating | Moderate | pT2N0M0 | Mesorectum | D | 6 | 23 | Disease free |
| 10 | Infiltrating | Moderate | pT3N2M0 | MP | vi | 4 | 28 | Disease free |
| Mesorectum | LN | 9 |
1According to the American Joint Committee on Cancer Staging Manual[14]; LN = lymph node metastasis; ly = lymphatic permeation; vi = vessel invasion; D = direct infiltration; MP = muscularis propria.
Follow-up outcome
The median follow-up time was 31 (range, 29-37) months. Five of the 45 patients (11.1%) developed local recurrence and distant metastasis during the follow up. The outcomes of 10 patients with distal mesorectal and/or intramural spread are summarized in Table 3. Local recurrence was detected in 1/10 patients (patient 3) at the site of pelvis floor and distant metastasis was found in one patient (patient 7) in liver.
DISCUSSION
Since Heald et al[12] first reported the presence of microscopic deposits in mesorectum and subsequently proposed a new surgical procedure for rectal cancer called total mesorectal excision (TME), many investigators have described the pathologic features of cancer spread in mesorectum. According to the published data[7,15-18], the incidence of mesorectal involvement of rectal cancer is quite high, varying from 27% to 83.1%. In the present study, cancer spread in mesorectum was found in 21 (46.7%) of the 45 patients, further confirming the high frequency of mesorectal involvement. This fact shows that failure to perform total mesorectal excision might leave a lot of tumor deposits in the remaining mesorectum and as a result, cause high local recurrences. The distal mesorectum, undoubtedly contributes a great share to these high recurrences. Many authors have reported that the distal spread in mesorcectum is found in 6% to 35.1% of patients with rectal cancer. A review of recent studies in this field is shown in Table 4.
Table 4.
Review of distal mesorectal spread of rectal cancer.
| References | Cases (n) | With DMS (n) | Frequency of DMS (%) | Pattern of DMS | Maximum extent of DMS (cm) | Suggested DCM (cm) |
| Heald et al.[12] | - | 5 | - | ly, vi, LN | 4 | TME |
| Williams et al.[19] | 50 | 3 | 6 | LN | 1.3 | <5 |
| Scott et al.[10] | 20 | 4 | 20 | ly, D | 3 | 3 to 5 |
| Shirouzu et al.[20] | 610 | 44 | 7.2 | ly, D, LN | ≤2 | 1 |
| Reynolds et al.[7] | 50 | 12 | 24 | LN, foci | 5 | TME |
| Hida et al.[21] | 198 | 40 | 20.2 | LN | 4 | 2 cm (lower rectal cancer) |
| Tocci et al.[11] | 53 | 19 | 35.1 | LN, foci | - | TME |
| Ono et al.[5] | 40 | 3 | 7.5 | LN | 2.4 | 3 |
DMS = distal mesorectal spread; DCM= distal resection margin; LN = lymph node metastasis; ly = lymphatic permeation; vi = vessel invasion; D = direct infiltration; foci = neoplastic microfoci.
Our pathologic examination showed that 8 of 45 patients (17.8%) were involved in distal mesorectum, 17 patients (37.8%) in mesorectum underlying the tumor and 11 patients (24.4%) in proximal mesorectum. The value was almost similar to that described by Scott et al[10] (20%) and Hida et al[21] (20.2%), but significantly lower than that reported by Tocchi et al[11]. In this series, the maximum extent of distal mesorectal spread in situ was 36 mm. Scott et al[9], however, described that a discontinuous mesorectal deposit was present at 5 cm below the tumor mass. The length of 5 cm may be the greatest microscopic extent reported in the published documents up to now. Distal intramural spread occurred in 5/10 patients and spread of more than 1 cm was rare in the present study. The frequency and extent of distal intramural spread were less than those of distal mesorectal spread. These findings are in agreement with several studies[10-12], suggesting that 1.5 cm distal resection margin of the distal rectal wall and 4 cm distal resection margin of the mesorectum might be appropriate and safe for a curative sphincter-saving surgery for lower rectal cancer. In fact, this resection process could be achieved by the so-called “denudation” or “muscularization” of rectal wall, which needs a longer or complete removal of the distal mesorectum and a “close shave” of the rectal wall[22].
Rectal cancer can spread into distal mesorectum through a variety of routes. Retrograde lymph node metastases are the most widely documented. Recently, Ono et al[5] reported that 3 of 40 patients (7.5%) with rectal cancer had distal mesorectal spread and all were caused by lymph node metastasis. In our study, this pattern of distal mesorectal spread was observed in 7 of 45 patients (15.6%). Furthermore, all these 7 patients showed more advanced features, 6 patients with TNM stage III and 1 with stage IV disease. According to our data, a significant relationship is found between lymph node metastasis and distal mesorectal spread, that is, the more frequently the lymph node metastasis occurs, the more likely the distal mesorectal spread develops. A probable reason is that while an advanced rectal cancer exists, upward lymphatic flow is blocked and then turns downward to the distal mesorectum. Therefore, lymph node metastasis seems to be an important risk factor for distal mesorectal spread.
In the present study, all patients were followed up for at least 29 mo. One of the 8 patients with distal mesorectal spread developed local recurrence. Several authors have reported the relationship between distal resection margin and local recurrence[4,19,20,23]. They hold that the extent of distal resection margin less than 1 cm has no statistical difference to local recurrence, and that a distal resection margin of 1 cm from the tumor is an appropriate clearance for most rectal cancers. The value of 1 cm could not be estimated in our series because all patients had a distal resection margin of more than 1.5 cm. We also found one of the 8 patients (12.5%) developed distant metastasis. This rate is far lower than that reported previously (60%)[10,19], but could be explained by our relatively small series and a short time of follow-up. Shirouzu et al[20] examined 610 patients and followed up them from 1982 to 1994. Their follow-up results showed that most of the 61 patients with distal spread died of distant metastasis rather than local recurrence. A similar conclusion was drawn by Williams et al[19] implying that distal spread should be regarded as a more systemic spread than a regional lesion. It seems reasonable to apply neoadjuvant chemoradiation to these patients with distal spread, but still multicentre studies for a larger patient population are required to confirm this supposition.
Footnotes
Supported by the Key Project of National Outstanding Youth Foundation of China, No. 39925032
Edited by Kumar M and Wang XL
References
- 1.Laxamana A, Solomon MJ, Cohen Z, Feinberg SM, Stern HS, McLeod RS. Long-term results of anterior resection using the double-stapling technique. Dis Colon Rectum. 1995;38:1246–1250. doi: 10.1007/BF02049147. [DOI] [PubMed] [Google Scholar]
- 2.Nakagoe T, Sawai T, Tuji T, Nanashima A, Yamaguchi H, Yasutake T, Ayabe H. Avoidance of rectovaginal fistula as a complication after low anterior resection for rectal cancer using a double-stapling technique. J Surg Oncol. 1999;71:196–197. doi: 10.1002/(sici)1096-9098(199907)71:3<196::aid-jso11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 3.Kirwan WO, Drumm J, Hogan JM, Keohane C. Determining safe margin of resection in low anterior resection for rectal cancer. Br J Surg. 1988;75:720. doi: 10.1002/bjs.1800750734. [DOI] [PubMed] [Google Scholar]
- 4.Andreola S, Leo E, Belli F, Lavarino C, Bufalino R, Tomasic G, Baldini MT, Valvo F, Navarria P, Lombardi F. Distal intramural spread in adenocarcinoma of the lower third of the rectum treated with total rectal resection and coloanal anastomosis. Dis Colon Rectum. 1997;40:25–29. doi: 10.1007/BF02055677. [DOI] [PubMed] [Google Scholar]
- 5.Ono C, Yoshinaga K, Enomoto M, Sugihara K. Discontinuous rectal cancer spread in the mesorectum and the optimal distal clearance margin in situ. Dis Colon Rectum. 2002;45:744–749. doi: 10.1007/s10350-004-6290-1. [DOI] [PubMed] [Google Scholar]
- 6.Heald RJ, Karanjia ND. Results of radical surgery for rectal cancer. World J Surg. 1992;16:848–857. doi: 10.1007/BF02066981. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds JV, Joyce WP, Dolan J, Sheahan K, Hyland JM. Pathological evidence in support of total mesorectal excision in the management of rectal cancer. Br J Surg. 1996;83:1112–1115. doi: 10.1002/bjs.1800830826. [DOI] [PubMed] [Google Scholar]
- 8.McCall JL. Total mesorectal excision: evaluating the evidence. Aust N Z J Surg. 1997;67:599–602. doi: 10.1111/j.1445-2197.1997.tb04605.x. [DOI] [PubMed] [Google Scholar]
- 9.Wiig JN, Carlsen E, Søreide O. Mesorectal excision for rectal cancer: a view from Europe. Semin Surg Oncol. 1998;15:78–86. doi: 10.1002/(sici)1098-2388(199809)15:2<78::aid-ssu4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Scott N, Jackson P, al-Jaberi T, Dixon MF, Quirke P, Finan PJ. Total mesorectal excision and local recurrence: a study of tumour spread in the mesorectum distal to rectal cancer. Br J Surg. 1995;82:1031–1033. doi: 10.1002/bjs.1800820808. [DOI] [PubMed] [Google Scholar]
- 11.Tocchi A, Mazzoni G, Lepre L, Liotta G, Costa G, Agostini N, Miccini M, Scucchi L, Frati G, Tagliacozzo S. Total mesorectal excision and low rectal anastomosis for the treatment of rectal cancer and prevention of pelvic recurrences. Arch Surg. 2001;136:216–220. doi: 10.1001/archsurg.136.2.216. [DOI] [PubMed] [Google Scholar]
- 12.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613–616. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 13.MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341:457–460. doi: 10.1016/0140-6736(93)90207-w. [DOI] [PubMed] [Google Scholar]
- 14.Fleming ID. 1997. AJCC cancer staging manual. 1sted. Philadelphia Lippincott Raven; pp. 85–86. [Google Scholar]
- 15.Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;2:996–999. doi: 10.1016/s0140-6736(86)92612-7. [DOI] [PubMed] [Google Scholar]
- 16.Zheng YC, Zhou ZG, Zheng XL, Li L, Lei WZ, Wang TC, Deng YL, Chen DY, Liu WP. Anatomic pathology of tumor cell spread through lymph nodes in the mesorectum of rectal cancer. Shijie Huaren Xiaohua Zazhi. 2004;12:570–573. [Google Scholar]
- 17.Ueno H, Mochizuki H, Tamakuma S. Prognostic significance of extranodal microscopic foci discontinuous with primary lesion in rectal cancer. Dis Colon Rectum. 1998;41:55–61. doi: 10.1007/BF02236896. [DOI] [PubMed] [Google Scholar]
- 18.Ratto C, Ricci R, Rossi C, Morelli U, Vecchio FM, Doglietto GB. Mesorectal microfoci adversely affect the prognosis of patients with rectal cancer. Dis Colon Rectum. 2002;45:733–742; discussion 742-743. doi: 10.1007/s10350-004-6288-8. [DOI] [PubMed] [Google Scholar]
- 19.Williams NS, Dixon MF, Johnston D. Reappraisal of the 5 centimetre rule of distal excision for carcinoma of the rectum: a study of distal intramural spread and of patients' survival. Br J Surg. 1983;70:150–154. doi: 10.1002/bjs.1800700305. [DOI] [PubMed] [Google Scholar]
- 20.Shirouzu K, Isomoto H, Kakegawa T. Distal spread of rectal cancer and optimal distal margin of resection for sphincter-preserving surgery. Cancer. 1995;76:388–392. doi: 10.1002/1097-0142(19950801)76:3<388::aid-cncr2820760307>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 21.Hida J, Yasutomi M, Maruyama T, Fujimoto K, Uchida T, Okuno K. Lymph node metastases detected in the mesorectum distal to carcinoma of the rectum by the clearing method: justification of total mesorectal excision. J Am Coll Surg. 1997;184:584–588. [PubMed] [Google Scholar]
- 22.Karanjia ND, Schache DJ, North WR, Heald RJ. 'Close shave' in anterior resection. Br J Surg. 1990;77:510–512. doi: 10.1002/bjs.1800770512. [DOI] [PubMed] [Google Scholar]
- 23.Vernava AM, Moran M, Rothenberger DA, Wong WD. A prospective evaluation of distal margins in carcinoma of the rectum. Surg Gynecol Obstet. 1992;175:333–336. [PubMed] [Google Scholar]


