Abstract
AIM: Real-time and rapid identification of the malignant tissue can be performed during or before surgical operation. Here we aimed to detect in vivo and in situ colorectal cancer by using Fourier transform infrared (FTIR) spectroscopy and fiber-optic technology.
METHODS: A total of five patients with large intestine cancer were detected in vivo and in situ. Of them, three cases of colon cancer and one case of cecum cancer were detected intraoperatively and in vivo by using a FTIR spectrometer during surgical operation, and one case of rectum cancer was explored non-invasively and in vivo before the surgical operation. Normal and malignant colorectal tissues were detected in vivo and in situ using FTIR spectroscopy on the basis of fundamental studies.
RESULTS: There were significant differences between FTIR spectra of normal and malignant colorectal tissues detected in vivo and in situ. Experimental results revealed that the spectral characteristics of normal and malignant tissues found in vivo and in situ were similar to those obtained from in vitro measurement in our previous fundamental research.
CONCLUSION: FTIR fiber-optic attenuated total reflectance (ATR) spectroscopy can identify in situ and in vivo colorectal cancer. FTIR spectroscopic method with fiber optics is a non-invasive, rapid, accurate and in vivo cancer detection technique in clinical diagnosis.
Keywords: Colorectal cancer, Fourier transform infrared spectroscopy
INTRODUCTION
Cancer is one of the leading causes of death in the world. The mortality rate from malignant neoplasms has increased markedly in the last four decades. The important goal in cancer research is to develop an accurate, quick, convenient, and inexpensive cancer detection method to increase the survival probability.
Fourier transform infrared spectroscopy, as an effective tool for investigating chemical changes at molecular level, has been utilized to detect carcinoma[1-7]. This method has many advantages, for example, it possesses a promising perspective in detecting early cancer, which is very important for the survival of cancer patients. We have investigated the detection of malignant tissues, such as stomach, esophagus, gallbladder, colon, lung, liver, parotid gland carcinomas, etc. with FTIR spectroscopy since 1995[8-17]. Our research work consisted of three steps. The first step was to study the differences between malignant and normal tissues, which were stored in liquid nitrogen. The specimens were thawed at room temperature and measured in vitro using the FTIR method. In the second step of study, fresh samples obtained during surgical operation were measured by the FTIR method in vitro immediately. In these two steps of fundamental research, we demonstrated that the malignant tissues could be distinguished from the normal tissues measured in vitro using the FTIR method. The third step was the aim of this study, which was to measure the tumor in vivo and in situ using fiber optics with the FTIR method. FTIR fiber-optic technique can exhibit perspectives in cancer diagnosis in vivo and in situ because of its advantages[18].
Colorectal cancer is one of the most frequent cancers in Western countries[19]. It is the fourth leading cause of cancer deaths and tends to increase in China, especially in big cities like Shanghai. Reducing the mortality poses a big challenge for clinicians and researchers. It is of great importance to diagnose colorectal malignant tumors in vivo and in situ using the FTIR method and fiber optics with an ATR probe. Real-time and rapid identification of the malignant tissues would be performed during surgical operation. It is helpful for the surgeons to reduce the waiting time for the pathological results. Furthermore, it allows accurately and rapidly to determine the proper operative treatment, for example, the rapid determination of cut edges of surgically resected specimens, which is also a goal of surgery in the removal of neoplasms. The technique of FTIR with fiber optics has a promising perspective as a new non-invasive and early detection method of colorectal cancer.
MATERIALS AND METHODS
Patients and materials
Three colon cancer patients and one cecum cancer patient were measured in vivo using a FTIR spectrometer during surgical operation, and a rectum cancer patient was measured non-invasively in vivo and in situ before surgical operation in the Department of General Surgery, Third Hospital of Peking University, China. These five cases, including 4 males and 1 female, aged from 52 to 77 years (mean, 64.2 years), were analyzed in the present study. The consents of the patients were obtained before the experiments.
Spectral measurements
The spectra were measured in the Department of General Surgery, Third Hospital of Peking University by using a mobile WQF-500 FTIR spectrometer made in Beijing Second Optical Instrument Factory with a mid-IR fiber optics and ZnSe ATR probe (Spectra-Tech Corporation). A mercury cadmium telluride (MCT) detector was used and cooled by liquid nitrogen. Scans were performed with 4/cm resolution and 32 scans were co-added to increase the signal-to-noise ratio. Sterilization was strictly made at first.
The spectra of four colon and cecum cancer patients were measured in vivo and in situ during surgical operation. After strict sterilization, the ATR probe was put on the surface of the detected tissues by the surgeons and one spectrum was recorded for about one minute using a FTIR spectrometer. The spectra of the samples were collected from paired carcinomas and adjacent normal tissues in colorectal cancer patients. The FTIR measurement was non-invasive and harmless to the patients.
One rectum cancer case was measured non-invasively and in vivo before surgical operation. After strict sterilization, the fiber optics with ATR probe was inserted into the rectum through anus and put on the cancerous tissue 3 cm away from the anus. It took about one minute to measure the spectrum non-invasively. For the comparative analysis, the spectrum of the fresh tissue sample from the same site obtained during surgery was measured in vitro immediately.
Each FTIR analysis result was compared with the corresponding histological result, that is to say, the double blind method was used for FTIR and biopsy measurements.
RESULTS
For the systematic report of these colorectal cancer detection research projects, the detection of malignant colorectal tumor samples in vitro was introduced first. In our previous fundamental study[20-24], a large amount of frozen samples stored in liquid nitrogen and fresh tissues during surgical operation immediately were measured in vitro by using a FTIR spectrometer. The research results showed that there were obvious differences between the FTIR spectra of malignant and normal tissues measured in vitro. Taking the colon tissue specimens measured in vitro as examples, the spectra are shown in Figure 1. Through the spectral analysis, the spectral characteristics of malignancy were as follows. The bands in the C-H stretching vibration in the region 2800-3000/cm and C = O band near 1700-1750/cm became weak and even disappeared. The peak of amide I band shifted to a lower wave number. The intensity of the amides II bands became weak, and the intensity of bands near 1400/cm was stronger than that of the bands near 1460/cm. The variations of the FTIR spectra between the normal and malignant colon tissues provided a basis and an opportunity for clinical application.
Figure 1.
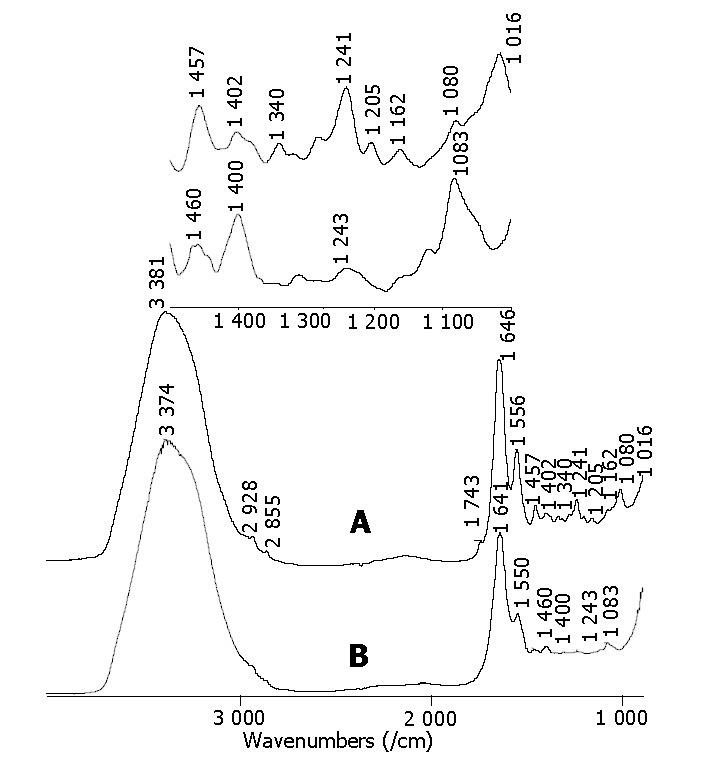
Typical FTIR spectra of colon tissues measured in vitro. A: spectrum of normal colon tissue sample; B: spectrum of malignant colon tissue sample.
On the basis of the fundamental research, the FTIR spectra of malignant colon and cecum tissues measured in vivo and in situ during surgical operation were investigated. All the spectra of the measured colon and cecum cancerous tissues are shown in Figure 2. We could see that the relative intensity of I1456/I1413 became smaller in the spectra of the malignant colon and cecum tissues. In addition, there was a weak amide II band near 1550/cm, a shift of the amide I band to a lower wavenumber near 1640/cm in the spectra of the cancerous colon and cecum tissues. The peaks of 2924/cm, 2989/cm, assigned to CH2 and CH3 vibration bands, and the band near 1740/cm related to C = O vibrations, became weak and even disappeared in the spectra of the malignant colon and cecum tissues. These spectral features indicated the malignancy of the detected tissues. The spectral characteristics and FTIR analysis results of all these cases are listed in Table 1. After the FTIR measurement, the detected colorectal tissues were resected and histologically verified as colorectal carcinomas. The FTIR analysis results were in agreement with the pathological results. Experimental results revealed that the spectral characteristics of normal and malignant colorectal tissues measured in vivo and in situ were similar to those obtained from in vitro measurement.
Figure 2.
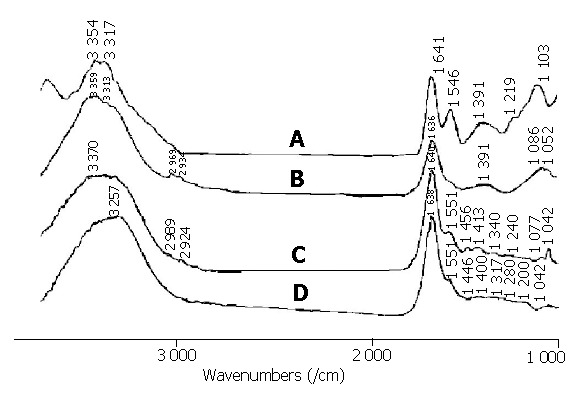
FTIR spectra of malignant cecum and colon tissues measured in vivo and in situ during surgical operation. A: spectrum of malignant cecum tissue sample; B, C and D: spectra of malignant colon tissue samples.
Table 1.
Results of FTIR spectral analysis and corresponding pathological detection of measured colorectal tissues.
| Case number | Tissue | Sex | Age (yr) | Measurement date | FTIR measurement mode | Characteristics of FTIR spectra | FTIR analysis results | Pathological results |
| The bands of C-H stretching | ||||||||
| 1 | Cecum | F | 69 | 2000-7-21 | in vivo during | vibration and C = O vibration | Adenocarcinoma | |
| surgical operation | disappeared; peak position of | Cecum cancer | of cecum | |||||
| amide I = 1641/cm; Iamide II (middle | ||||||||
| weak); I1393/cm>I1460/cm | ||||||||
| I2969/cm (weak) , I2934/cm | Moderately | |||||||
| 2 | Sigmoid colon | M | 63 | 2003-7-22 | in vivo during | (weak); peak position of | Colon cancer | differentiated |
| surgical operation | amide I = 1636/cm; Iamide II | adenocarcinoma of | ||||||
| (very weak); I1391 /cm>I1460/cm | sigmoid colon | |||||||
| I2989/cm (weak) , I2924/cm | ||||||||
| 3 | Sigmoid colon | M | 52 | 2003-11-3 | in vivo during | (weak); peak position of | Colon cancer | Carcinoma |
| surgical operation | amide I = 1640/cm; Iamide II | of sigmoid | ||||||
| (very weak); I1413/cm>≈I1456/cm | ||||||||
| The bands of C-H stretching | ||||||||
| 4 | Transverse colon M | 77 | 2003-12-5 | in vivo during | vibration and C = O vibration | Colon cancer | Adenocarcinoma | |
| surgical operation | disappear; peak position of amide | of transverse colon | ||||||
| I = 1638/cm; Iamide II (very weak); | ||||||||
| I1400/cm>I1446/cm | ||||||||
| I2924/cm (weak), I2850/cm (weak), | Moderately | |||||||
| 5 | Rectum | M | 60 | 2003-7-22 | in vivo before | I1731 cm-1 (weak); peak position of | Rectum cancer | differentiated |
| surgical operation | amideI = 1641/cm; Iamide II (weak); | adenocarcinoma | ||||||
| I1402 /cm>I1469/cm | of rectum |
In addition, one rectum cancer patient was measured non-invasively and in vivo before surgical operation. The spectrum before surgical operation was similar to that of the fresh tissue from the same site measured in vitro immediately (Figure 3). The C = O band near 1740/cm, nearly disappeared in the spectrum as shown in Figure 3A. The band of amide I of protein was located at a lower wavenumber near 1640/cm. The intensity of amide II bands located at -1556/cm in the malignant tissue samples was less intense. The intensity of 1469/cm band decreased. These spectral characteristics of the FTIR spectrum of malignant rectum tissue measured in vivo before surgical operation were consistent with those measured in vitro. The result of the FTIR detection for the rectum tumor was consistent with the biopsy test.
Figure 3.
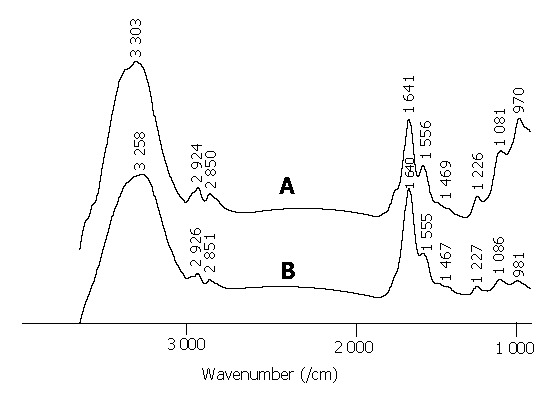
FTIR spectra of malignant rectum tissues. A: spectrum measured non-invasively, in vivo before surgical operation; B: spectrum of fresh resected rectum tissue from the same site measured in vitro.
DISCUSSION
FTIR spectroscopy can provide information of molecular structure and composition. The development of cancer is always along the following sequences. Gene mutation is the first event, the second step is the alternation of biomolecules in both composition and molecular structure aspects. After that, the variation on cells and morphology of biological tissues will take place and can be detected by iconographic and pathological techniques. Vibrational spectroscopic methods, which are sensitive to the chemical changes at molecular level, may be developed as a powerful method to detect cancer at the second step of its development process, prior to most cancer diagnostic methods available today[25].
FTIR spectra are sensitive to the changes of biomolecules so as to diagnose the cancerous tissues[26]. The spectra of normal tissues often have a stretching vibration of carbonyl located near 1745/cm, and the symmetry and asymmetry stretching vibrations of methylene located around 2 852/cm and 2930/cm, as well as methyl at 2873/cm and 2958/cm. However, these peaks mentioned above often decrease, even disappear in the spectra of malignant tissues, because triglyceride contains a large proportion of methyl, methylene and carbonyl, and the fat in the region of the malignant tissue is consumed because of the increased nutritional and energy requirement in the development of carcinoma. The bands near 1645/cm assigned to amide I of protein and deformation vibration of water molecule are located at a higher wavenumber in the normal colorectal tissue than those in the malignant tissue. The peak of amide II bands located at 1545/cm in the malignant colorectal tissue is less intense and much broader than that in the normal tissue.
In conclusion, FTIR fiber-optic ATR spectroscopy can identify colorectal cancers in situ and in vivo. It provides real- time results for operating surgeons. FTIR spectroscopy can be applied to in vivo and in situ detection of not only colorectal cancer but also other malignant tissues of the digestive system. In addition, with further research, the technique of FTIR with fiber optics may exhibit its potential for non-invasive, in situ and in vivo detection of cancerous tissues before surgical operation. FTIR spectroscopic method with fiber optics possesses a promising perspective to be a non-invasive, rapid, accurate and in vivo cancer detection technique.
Footnotes
Supported by the National Natural Science Foundation of China, No. 30371604 and State Key Project of China, No. 2002CCA01900
Assistant Editor Guo SY Edited by Kumar M and Wang XL
References
- 1.Rigas B, Morgello S, Goldman IS, Wong PT. Human colorectal cancers display abnormal Fourier-transform infrared spectra. Proc Natl Acad Sci USA. 1990;87:8140–8144. doi: 10.1073/pnas.87.20.8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koenig F, Larne R, Enquist H, McGovern FJ, Schomacker KT, Kollias N, Deutsch TF. Spectroscopic measurement of diffuse reflectance for enhanced detection of bladder carcinoma. Urology. 1998;51:342–345. doi: 10.1016/s0090-4295(97)00612-2. [DOI] [PubMed] [Google Scholar]
- 3.Rahman J, Hewlett A, Taylor DR, Xiao SY, Wu JG, Soloway RD. Reproducible, rapid quantitation of hepatic fibrosis in human liver biopsies using Fourier transform infrared (FTIR) spectroscopy. Gastroenterology. 2002;122:A303. [Google Scholar]
- 4.Wang HP, Wang HC, Huang YJ. Microscopic FTIR studies of lung cancer cells in pleural fluid. Sci Total Environ. 1997;204:283–287. doi: 10.1016/s0048-9697(97)00180-0. [DOI] [PubMed] [Google Scholar]
- 5.Huleihel M, Salman A, Erukhimovitch V, Ramesh J, Hammody Z, Mordechai S. Novel spectral method for the study of viral carcinogenesis in vitro. J Biochem Biophys Methods. 2002;50:111–121. doi: 10.1016/s0165-022x(01)00177-4. [DOI] [PubMed] [Google Scholar]
- 6.Fung Kee Fung M, Senterman M, Eid P, Faught W, Mikhael NZ, Wong PT. Comparison of Fourier-transform infrared spectroscopic screening of exfoliated cervical cells with standard Papanicolaou screening. Gynecol Oncol. 1997;66:10–15. doi: 10.1006/gyno.1997.4724. [DOI] [PubMed] [Google Scholar]
- 7.Argov S, Ramesh J, Salman A, Sinelnikov I, Goldstein J, Guterman H, Mordechai S. Diagnostic potential of Fourier-transform infrared microspectroscopy and advanced computational methods in colon cancer patients. J Biomed Opt. 2002;7:248–254. doi: 10.1117/1.1463051. [DOI] [PubMed] [Google Scholar]
- 8.Wang JS, Shi JS, Xu YZ, Duan XY, Zhang L, Wang J, Yang LM, Weng SF, Wu JG. FT-IR spectroscopic analysis of normal and cancerous tissues of esophagus. World J Gastroenterol. 2003;9:1897–1899. doi: 10.3748/wjg.v9.i9.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng Q, Soloway RD, Wu JG, Zhou XS, Zhao DW, Xu DF, Fu W, Wang SF. Infrared spectroscopic features of colon cancer cells differ from those of normal colonic cells from the same patient. Gastroenterology. 1996;110:A576. [Google Scholar]
- 10.Li WH, Peng Q, Soloway RD, De La Pe-a O, Sun B, Weng SF, Xu Z, Zhao DW, Zhou XS, Wu JG. Surface proteins of cancer and normal epithelium from the same patients differ as assessed by their primary and second structure determined by Fourier transform infrared (FTIR) spectroscopy. Gastroenterology. 1997;112:A604. [Google Scholar]
- 11.Wang JS, Shi JS, Xu YZ, Duan XY, Zhang L, Zhu AJ, Weng SF, Wu JG. A study on different tissues of gallbladder by Fourier transform infrared spectroscopy. Zhonghua Gandan Waike Zazhi. 2003;9:657–660. [Google Scholar]
- 12.Ren Y, Xu YZ, Wang J, Zhang YF, Wang F, Shi JS, Wu JG. FTIR spectroscopic and statistical studies on the lung tissues. Guangpuxue Yu Guangpu Fenxi. 2003;23:681–684. [Google Scholar]
- 13.Zhang L, Wang JS, Yang ZL, Xu YZ, Weng SF, Shi JS, Wu JG. The difference of FTIR spectroscopic studies on normal and malignant tissues of lung. Gaodeng Xuexiao Huaxue Xuebao. 2003;24:2173–2176. [Google Scholar]
- 14.Ling XF, Xu YZ, Weng SF, Li WH, Zhi X, Hammaker RM, Fateley WG, Wang F, Zhou XS, Soloway RD, et al. Investigation of normal and malignant tissue samples from the human stomach using Fourier transform Raman spectroscopy. Appl Spectrosc. 2002;56:570–573. [Google Scholar]
- 15.Yang LM, Xu Z, Zhang YF, Xu YZ, Zhou S, Zhang NW, Li QB, Liu Z, Wang LX, Zhou WJ, et al. The application of mid-IR fiber optics in diagnosis of colon cancer in vivo and in situ. Guangpuxue Yu Guangpu Fenxi. 2003;23:883–884. [Google Scholar]
- 16.Xu YZ, Zhang YF, Yang LM, Peng X, Sun KH, Li QB, Zhou WJ, Wu HZ, Yu GY, Xu YZ, et al. In vivo and rapid detection of the malignancy tumor of oral tissues using Mid-FTIR fiber optics spectroscopy. Gaodeng Xuexiao Huaxue Xuebao. 2004;25:348–350. [Google Scholar]
- 17.Wu JG, Xu YZ, Sun CW, Soloway RD, Xu DF, Wu QG, Sun KH, Weng SF, Xu GX. Distinguishing malignant from normal oral tissues using FTIR fiber-optic techniques. Biopolymers. 2001;62:185–192. doi: 10.1002/bip.1013. [DOI] [PubMed] [Google Scholar]
- 18.Sukuta S, Bruch R. Factor analysis of cancer Fourier transform infrared evanescent wave fiberoptical (FTIR-FEW) spectra. Lasers Surg Med. 1999;24:382–388. doi: 10.1002/(sici)1096-9101(1999)24:5<382::aid-lsm9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 19.Blum HE. Colorectal cancer: future population screening for early colorectal cancer. Eur J Cancer. 1995;31A:1369–1372. doi: 10.1016/0959-8049(95)00215-5. [DOI] [PubMed] [Google Scholar]
- 20.Wang F, Ling XF, Yang ZL, Xu Z, Ren Y, Li WH, Weng SF, Wu JG. FTIR spectroscopic study of normal and malignant tissues of rectum. GuangPuXue Yu GuangPu FenXi. 2003;23:498–501. [PubMed] [Google Scholar]
- 21.Weng SF, Lin XF, Yang LM, Soloway RD, Xu YZ, Tian W, Yang ZL, Kang N, Yan WF, Hu XB, et al. Use of mid-infrared fiber optics to determine the extent of spread of gastric and colonic cancer. Gastroenterology. 2000;118:A6436. [Google Scholar]
- 22.Xu YZ, Soloway RD, Lin XF, Zhi X, Weng SF, Wu QG, Shi JS, Sun WX, Zhang TX, Wu JG, et al. Fourier transform infrared (FTIR) mid-IR spectroscopy separates normal and malignant tissue from the colon and stomach. Gastroenterology. 2000;118:A6438. [Google Scholar]
- 23.Sun XJ, Su YL, Soloway RD, Zhang L, Wang JS, Ren Y, Yang LM, Zheng AG, Zhang YF, Xu YZ, et al. Rapid, intraoperative detection of malignancy using attenuated total reflectance (ATR) and mobile Fourier transform infrared (FTIR) spectroscopy. Gastroenterology. 2003;124:A420–A421. [Google Scholar]
- 24.Peng Q, Xu Y, Li W, Wu J, Zhou X. [FTIR study on the normal and tumor gastrointestinal tissues] Guang Pu Xue Yu Guang Pu Fen Xi. 1998;18:528–531. [PubMed] [Google Scholar]
- 25.Zhang L, Sun KH, Soloway RD, Ling XF, Xu YZ, Wu QG, Weng SF, Yang ZL, Zhang TL, Yao GQ, et al. Intraoperative Fourier transform infrared spectroscopy can guide individual resections in patients with gastric cancer. Gastroenterology. 2004;126:A626. [Google Scholar]
- 26.Wong PTT, Lacelle S, Fung Kee Fung M, Senterman M, Mikhael NZ. Characterization of exfoliated cells and tissues from human endocervix and ectocervix by FTIR and ATR/FTIR spectroscopy. Biospectroscopy. 1995;1:357–364. [Google Scholar]


