Abstract
AIM: To investigate the effect of deleted pancreatic cancer locus 4 (DPC4) gene transfection on biological behaviors of human colorectal carcinoma cells and the role of DPC4 gene in colorectal carcinogenesis.
METHODS: PcDNA3.1-DPC4 plasmid was re-constructed by gene-recombination technology. SW620 cells, a human colorectal carcinoma cell line, were transfected with PcDNA3.1-DPC4 plasmid using lipofectamine transfecting technique. Transfected cells were selected with G418. Expression of Smad4 protein was detected in cells transfected with DPC4 gene by immunohistochemistry and Western blot. Biological characteristics of transfected cells were evaluated by population-doubling time and cloning efficiency. Alterations of percentage of S phage cells (S%) and apoptosis rate were determined by flow- cytometry.
RESULTS: PcDNA3.1-DPC4 plasmid was constructed successfully. SW620 cells transfected with PcDNA3.1-DPC4 plasmid (DPC4+-SW620 cells) showed a strong intracellular expression of Smad4 protein, and the positive signal was localized in cytoplasm and nuclei, mainly in cytoplasm, where the expressions of Smad4 protein in SW620 cells transfected with PcDNA3.1 plasmid (PcDNA3.1-SW620 cells) and non-transfected SW620 cells (SW620 cells) were weaker than those in DPC4+-SW620 cells. The population- doubling time in DPC4+-SW620 cells (116 h) was significantly longer than that in SW620 cells (31 h) and PcDNA3.1-Sw620 cells (29 h) (P<0.01). The cloning efficiencies of DPC4+-SW620 cells (12%) were markedly lower than those of SW620 cells (69%) and PcDNA3.1-Sw620 cells (67%) (P<0.01). Compared with SW620 cells and PcDNA3.1-Sw620 cells, the G0-G1% of DPC4+-SW620 cells was obviously higher and the S% was markedly lower (P<0.05). Apoptosis rate of DPC4+-SW620 cells was significantly higher than that of SW620 cells and PcDNA3.1-SW620 cells.
CONCLUSION: PcDNA3.1-DPC4 plasmid can be successfully re-constructed and stably transfected into human SW620 cells, thereby the cells can steadily express Smad4. DPC4 protein may regulate proliferation of colorectal carcinoma cells by inhibiting cell growth and inducing cell apoptosis.
Keywords: Colorectal Carcinoma, DPC4 gene, Transfection, Apoptosis
INTRODUCTION
TGF-β is a member of a large superfamily of structurally related growth and differentiation factors; these factors play an important role in modulating immune response, regulating cell differentiation and growth, stimulating extracellular matrix formation, etc[1]. TGF-β has been reported as a potent inhibitor of normal epithelial cell proliferation both in vitro and in vivo. However, epithelia-derived tumor cells often lose their sensitivity to TGF-β, and their growth inhibition, thereby forming tumors[2-4]. Smad4 is the central molecule of TGF-β signal transduction pathway, and all biological activities are the results of action between Smad4 and other Smads (Smad2, Smad3, and so on)[5-8]. Smad4 is produced by DPC4 gene (deleted in pancreatic carcinomas, locus 4). The loss of DPC4 activity is thought to be an important factor leading to the loss of growth inhibition, and the loss of DPC4 activity included 3 factors as follows: deletion of parts of chromosome including homozygous and heterozygous deletion gene mutation, for example, frameshift, nonsense and missense mutations; decline of gene expression[9].
Recent studies have demonstrated that colorectal carcinoma with invasion and metastasis shows an obviously higher frequency of DPC4 mutation than adenoma and intramucosal carcinoma[10,11]. Some findings strongly suggest a significant contribution of DPC4 gene inactivation in advanced stages, such as distant metastasis of human colorectal carcinogenesis[12-17].
A cell model that Smad4 was expressed stably and highly was achieved by transfecting DPC4 gene in our experiment, and we investigated the effect of DPC4 gene transfection on biological behaviors of human colorectal carcinoma cells and the role of DPC4 gene in colorectal carcinogenesis to put forward a new strategy of gene therapy for colorectal carcinoma.
MATERIALS AND METHODS
Cell line and plasmid
SW620 cell line was purchased from Xiangya School of Medicine, Central South University, Changsha, China. PCMV5-DPC4 plasmid was a kind gift from professor Joan Massague. Hind III, BamH I, RPMI 1640 culture medium, 100 bp DNA marker, neomycin (G418) and lipofectamin were obtained from Gibco Co. Smad4Ab-1 was from Neomarkers Co. The cells were divided into three groups: SW620 cells, PcDNA3.1-Sw620 cells and DPC4+-SW620 cells.
Plasmid re-construction
Plasmids PCMV5-DPC4 and PcDNA3.1 were transferred into competent E.coli (JM109) respectively. Transferred E.coli (JM109) was cultured to amplify the two plasmids. Plasmids were digested and cut by Hind III and BamH I, and identified by electrophoresis (5400 bp, 4700 bp and 1700 bp); 1700 bp was the DPC4 sequence. The DPC4 sequence was massively extracted, purified, and cloned into PcDNA3.1 plasmid. A new plasmid, PcDNA3.1-DPC4, was re-constructed, and confirmed by direct sequencing. Empty vector DNA was used for control transfections.
Cell culture and gene transfer
SW620 cells were maintained in RPMI 1640 supplemented with antibiotics and fetal calf serum (FCS), transfected with PcDNA3.1-DPC4 and PcDNA3.1 plasmids respectively by a standard calcium phosphate co-precipitation method. Positive clones were selected with 0.5 g/L G418 for 3 d, and harvested after 4 wk of incubation in medium containing 0.25 g/L G418. The G418 resistant clones were passaged. The single clone was isolated with cloning cylinders and expanded for analysis of Western blot and growth parameters.
Western blot analysis
Expression of DPC4 gene was analyzed by Western blotting. Briefly, cells were lysed in lysis buffer containing a proteinase inhibitor cocktail. Total proteins were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to immobile membranes. Expression of Smad4 protein was detected using anti-smad4 monoclonal antibody (1:50) by streptavidin peroxidase (SP) conjugated method .
Immunocytochemistry
Cells were seeded and cultured to reach the cell density of 106 cells/well, and then the cells were digested and centrifuged. The cell sediment was fixed with formalin (40 g/L) for 24 h at 4 °C. After centrifuged once again, the cell sediment was wrapped with agar (20 g/L) and embedded in paraffin and sliced. Anti-Smad4 monoclonal antibody (1:50) was used to detect the expression of Smad4 protein by SP method. For negative control, primary antibody was replaced by PBS.
In vitro growth inhibition
When the three groups of cells were seeded and each reached to the cell density of 106 cells/well respectively, the cells were digested and seeded in 24-well plates (4×104 cells/well) respectively. Cells of three wells from each group were counted daily. The experiment was continued for 7 d and repeated three times. Then the growth curve was drawn and cell doubling time was calculated by using the formula: TD = t×log2/ (logNt-logNo). For analysis of cloning efficiency, 50, 100 and 200 cells were seeded in 6-well plates, respectively. When cell clones appeared, they were fixed with formalin (40 g/L) for 15 min and stained with Giemsa for 20 min. Finally, the results were evaluated.
Alterations of S% and apoptosis rate
Alterations of S% and apoptosis rate were determined by flow-cytometry. For analysis of TGF-β-mediated growth inhibition, 3×105 cells were incubated into 60-mm dishes with serum medium added with TGF-β 1 (5×10-6 g/L) one day after plating. Apoptosis rate was evaluated by flow-cytometry.
Statistical method
Student’s t test and χ2 test were carried out to compare the state. P<0.05 was considered statistically significant.
RESULTS
Expression of Smad4 protein
Although a same quantity of protein (80×10-6g) was extracted from the SW620 cells, PcDNA3.1-Sw620 and DPC4+-SW620 cells respectively, Western blot analysis showed that the expression of Smad4 protein was the strongest in DPC4+-SW620 cells (clone 2) (Figure 1). DPC4+-SW620 cells showed a strong intracellular expression of Smad4 protein, and the positive signal was localized in cytoplasm and nuclei, mainly in cytoplasm. The expressions of Smad4 protein in PcDNA3.1-Sw620 and SW620 cells were obviously weaker than those in DPC4+-SW620 cells (Figure 2).
Figure 1.
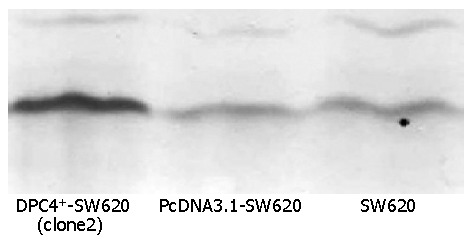
Strongest expression of Smad4 protein in clone2 detected by Western blot analysis.
Figure 2.
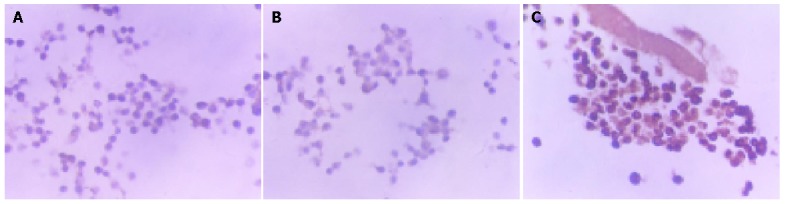
Expression of smad4 protein. A: Weak intracellular expression of Smad4 protein in SW620 cells. The positive staining for Smad4 was localized in cytoplasm; B: Weak intracellular expression of Smad4 protein in PcDNA3.1-SW620 cells. The positive staining for Smad4 was localized in cytoplasm; C: Strong intracellular expression of Smad4 protein in DPC4+-SW620 cells (clone2). The positive staining for Smad4 was localized in cytoplasm and nucleus, mainly in cytoplasm.
In vitro growth property of DPC4+-SW620 cells
The doubling time of DPC4+-SW620 cells (116 h) was prolonged obviously, compared with SW620 (31 h) and PcDNA3.1-Sw620 cells (29 h). The cloning efficiency of DPC4+-SW620 cells (12%) dropped obviously compared to SW620 (69%) and PcDNA3.1-Sw620 cells (67%) (over 50 cells were thought as a clone) (Figure 3).
Figure 3.
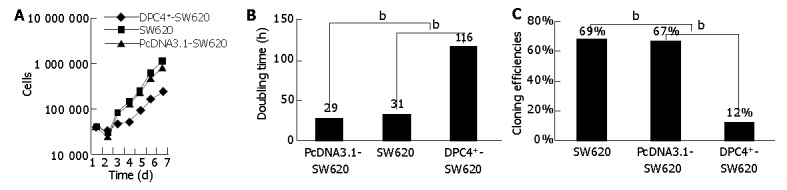
Growth property of different groups. A: Growth curve of cells of different groups; B: Doubling time of cells of different groups; C: Cloning efficiencies in different groups. bP<0.01 vs DPC4+-SW620.
Alterations of S% and apoptosis rate
Compared with SW620 and PcDNA3.1-Sw620 cells, the G0-G1% of DPC4+-SW620 cells was significantly higher and the S% was markedly lower (Figure 4A). The apoptosis rate of DPC4+-SW620 cells was significantly higher than that of SW620 and PcDNA3.1-Sw620 cells (Figure 4B). After stimulated by TGF-β1, the apoptosis rate of PcDNA3.1-SW620 and SW620 cells increased, and that of DPC4+-SW620 cells declined.
Figure 4.
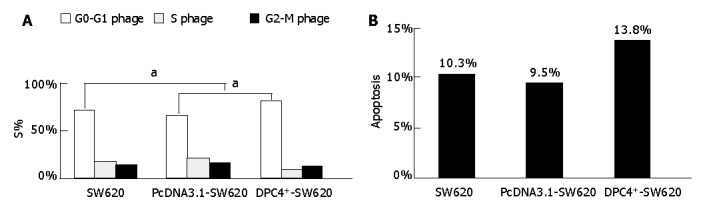
Alterations of S% (A) and apoptosis rate (B) detected by flow- cytometry. aP<0.05 vs DPC4+-SW620.
DISCUSSION
A high frequency of loss of heterozygosity (LOH) on chromosome 18q has been recognized in the progression of carcinomas of colon and other tissues, including pancreas[18,19]. Since DPC4 gene was isolated from the same region as a tumor suppressor gene for pancreatic cancer, mutation analysis of this gene has been carried out on cancers in various other organs[20,21]. DPC4 plays an important role in TGF-β signal transduction pathway, and has been found to be functionally inactivated in about 50% of pancreatic carcinomas[22,23], 30% of colon carcinomas and a subset of biliary tract carcinomas[24,25]. DPC4 has been thought as a tumor suppressor gene[26,27]. Despite an extensive knowledge of the biochemical properties of Smad4 proteins, little is known about how the loss of Smad4 function contributes to the tumorigenic process.
Some scholars have suggested a significant contribution of DPC4 gene inactivation in advanced stages[28], such as distant metastasis of human colorectal carcinogenesis. They believe the presence of DPC4 gene mutation in primary carcinoma is a prognostic marker[29-33].
In order to attain the goal of our experiments, the PcDNA3.1-DPC4 plasmid was re-constructed successfully using PCMV5-DPC4 plasmid, and the re-constructed plasmid was cut and confirmed by direct sequencing. After selected by G418, the positive clones of SW620 cells transfected with PcDNA3.1-DPC4 plasmid were obtained. The expression of Smad4 protein was the strongest in DPC4+-SW620 cells detected by Western blot and immunocytochemistry. SW620 cells were successfully transfected with PcDNA3.1-DPC4 plasmid. A cell model steadily expressing Smad4 was obtained.
Candidate tumor suppressor gene DPC4 could inhibit growth of pancreatic carcinoma[34-39], but little information is available on whether DPC4 can inhibit growth of SW620 cells and how it affects the proliferation of SW620 cells transfected with DPC4 gene and the biological behaviors of human colorectal carcinoma cells. In order to further elucidate these questions, we carried out this study by dividing the cells into three groups: SW620 cells, PcDNA3.1-Sw620 cells and DPC4+-SW620 cells. Compared to SW620 and PcDNA3.1-Sw620 cells, the doubling time of DPC4+-SW620 cells was obviously prolonged and their cloning efficiency was dropped. The growth of colorectal carcinoma cells was inhibited by Smad4. Flow-cytometry showed that G0-G1% of DPC4+-SW620 cells was much higher and S% was much lower compared with SW620 and PcDNA3.1-Sw620 cells. The expression of Smad4 protein inhibited the growth of colorectal carcinoma by arresting cells from G1 phase to S phase. In addition, the apoptosis rate of DPC4+-SW620 cells was much higher than that of SW620 and PcDNA3.1-SW620 cells, suggesting that Smad4 can inhibit the growth of colorectal carcinoma by inducing apoptosis of colorectal carcinoma cells.
TGF-β signal transduction pathway is an efficient channel for inhibiting cell growth, proliferatiation and differentiation, and Smad4 is the central molecule in this signal pathway. The apoptosis rates of SW620 and PcDNA3.1-SW620 cells were increased after stimulated by TGF-β1. It is essential to pay attention to the phenomenon that the apoptosis rate of DPC4+-SW620 cells declined after stimulated by TGF-β1. This result suggests that DPC4 inhibits cell growth, not only by TGF-β signal transduction pathway, but also by other pathways. In fact, some scholars have revealed that over-expression of wild-type DPC4 may re-construct a new pathway of negative regulation of cell proliferation besides TGF-β signal transduction pathway[40,41].
At present, gene therapy is considered as an effective channel in treating malignant tumors, and many scholars in medical science agree on this point. This study suggests that increase of Smad4 protein could restore or strengthen TGF-β signal transduction pathway and inhibit growth of colorectal carcinoma, which provides some experimental foundations for gene treatment of tumors.
Invasion and metastasis are important characteristics of malignant tumors, and also the important reasons for the death of tumor patients. Which genes or proteins participate in invasion and metastasis of tumor is the central task of tumor molecular biological researches. If inactivation of DPC4 gene is relevant with invasion and metastasis of tumors, is DPC4 a metastasis suppressor gene? What are the downstream target genes? These questions should be studied deeply.
ACKNOWLEDGEMENTS
We thank professor Joan Massague for providing PCMV5-DPC4 plasmids.
Footnotes
Assistant Editor Guo SY Edited by Kumar M and Wang XL
References
- 1.Wei HS, Li DG, Lu HM, Zhan YT, Wang ZR, Huang X, Zhang J, Cheng JL, Xu QF. Effects of AT1 receptor antagonist, losartan, on rat hepatic fibrosis induced by CCl(4) World J Gastroenterol. 2000;6:540–545. doi: 10.3748/wjg.v6.i4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang X, Li C, Herrera PL, Deng CX. Generation of Smad4/Dpc4 conditional knockout mice. Genesis. 2002;32:80–81. doi: 10.1002/gene.10029. [DOI] [PubMed] [Google Scholar]
- 3.Miyaki M, Kuroki T. Role of Smad4 (DPC4) inactivation in human cancer. Biochem Biophys Res Commun. 2003;306:799–804. doi: 10.1016/s0006-291x(03)01066-0. [DOI] [PubMed] [Google Scholar]
- 4.Cattaneo M, Orlandini S, Beghelli S, Moore PS, Sorio C, Bonora A, Bassi C, Talamini G, Zamboni G, Orlandi R, et al. SEL1L expression in pancreatic adenocarcinoma parallels SMAD4 expression and delays tumor growth in vitro and in vivo. Oncogene. 2003;22:6359–6368. doi: 10.1038/sj.onc.1206665. [DOI] [PubMed] [Google Scholar]
- 5.Grady WM, Markowitz SD. Genetic and epigenetic alterations in colon cancer. Annu Rev Genomics Hum Genet. 2002;3:101–128. doi: 10.1146/annurev.genom.3.022502.103043. [DOI] [PubMed] [Google Scholar]
- 6.Schwarte-Waldhoff I, Schmiegel W. Smad4 transcriptional pathways and angiogenesis. Int J Gastrointest Cancer. 2002;31:47–59. doi: 10.1385/IJGC:31:1-3:47. [DOI] [PubMed] [Google Scholar]
- 7.Lin X, Liang M, Liang YY, Brunicardi FC, Melchior F, Feng XH. Activation of transforming growth factor-beta signaling by SUMO-1 modification of tumor suppressor Smad4/DPC4. J Biol Chem. 2003;278:18714–18719. doi: 10.1074/jbc.M302243200. [DOI] [PubMed] [Google Scholar]
- 8.Lin X, Liang M, Liang YY, Brunicardi FC, Feng XH. SUMO-1/Ubc9 promotes nuclear accumulation and metabolic stability of tumor suppressor Smad4. J Biol Chem. 2003;278:31043–31048. doi: 10.1074/jbc.C300112200. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy DM, Hruban RH, Argani P, Howe JR, Conlon KC, Brennan MF, Zahurak M, Wilentz RE, Cameron JL, Yeo CJ, et al. Role of the DPC4 tumor suppressor gene in adenocarcinoma of the ampulla of Vater: analysis of 140 cases. Mod Pathol. 2003;16:272–278. doi: 10.1097/01.MP.0000057246.03448.26. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi A. Genetic changes in liver metastasis of colorectal cancer and their clinical application. Nihon Geka Gakkai Zasshi. 2001;102:370–375. [PubMed] [Google Scholar]
- 11.Miyaki M, Iijima T, Konishi M, Sakai K, Ishii A, Yasuno M, Hishima T, Koike M, Shitara N, Iwama T, et al. Higher frequency of Smad4 gene mutation in human colorectal cancer with distant metastasis. Oncogene. 1999;18:3098–3103. doi: 10.1038/sj.onc.1202642. [DOI] [PubMed] [Google Scholar]
- 12.Mikami T, Ookawa K, Shimoyama T, Fukuda S, Saito H, Munakata A. KAI1, CAR, and Smad4 expression in the progression of colorectal tumor. J Gastroenterol. 2001;36:465–469. doi: 10.1007/s005350170069. [DOI] [PubMed] [Google Scholar]
- 13.Maitra A, Molberg K, Albores-Saavedra J, Lindberg G. Loss of Dpc4 expression in colonic adenocarcinomas correlates with the presence of metastatic disease. Am J Pathol. 2000;157:1105–1111. doi: 10.1016/S0002-9440(10)64625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Had ija MP, Kapitanović S, Radosević S, Cacev T, Mirt M, Kovacević D, Cacev T, Hadzija M, Spaventi R, Pavelić K. Loss of heterozygosity of DPC4 tumor suppressor gene in human sporadic colon cancer. J Mol Med (Berl) 2001;79:128–132. doi: 10.1007/s001090000179. [DOI] [PubMed] [Google Scholar]
- 15.Taketo MM, Takaku K. Gastrointestinal tumorigenesis in Smad4 (Dpc4) mutant mice. Hum Cell. 2000;13:85–95. [PubMed] [Google Scholar]
- 16.Ohtaki N, Yamaguchi A, Goi T, Fukaya T, Takeuchi K, Katayama K, Hirose K, Urano T. Somatic alterations of the DPC4 and Madr2 genes in colorectal cancers and relationship to metastasis. Int J Oncol. 2001;18:265–270. doi: 10.3892/ijo.18.2.265. [DOI] [PubMed] [Google Scholar]
- 17.Shawler DL, Bartholomew RM, Garrett MA, Trauger RJ, Dorigo O, Van Beveren C, Marchese A, Ferre F, Duffy C, Carlo DJ, et al. Antigenic and immunologic characterization of an allogeneic colon carcinoma vaccine. Clin Exp Immunol. 2002;129:99–106. doi: 10.1046/j.1365-2249.2002.01863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahm KB, Lee KM, Kim YB, Hong WS, Lee WH, Han SU, Kim MW, Ahn BO, Oh TY, Lee MH, et al. Conditional loss of TGF-beta signalling leads to increased susceptibility to gastrointestinal carcinogenesis in mice. Aliment Pharmacol Ther. 2002;16 Suppl 2:115–127. doi: 10.1046/j.1365-2036.16.s2.3.x. [DOI] [PubMed] [Google Scholar]
- 19.Biasi F, Tessitore L, Zanetti D, Cutrin JC, Zingaro B, Chiarpotto E, Zarkovic N, Serviddio G, Poli G. Associated changes of lipid peroxidation and transforming growth factor beta1 levels in human colon cancer during tumour progression. Gut. 2002;50:361–367. doi: 10.1136/gut.50.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barberá VM, Martín M, Mariñoso L, Munné A, Carrato A, Real FX, Fabre M. The 18q21 region in colorectal and pancreatic cancer: independent loss of DCC and DPC4 expression. Biochim Biophys Acta. 2000;1502:283–296. doi: 10.1016/s0925-4439(00)00054-5. [DOI] [PubMed] [Google Scholar]
- 21.Schneider G, Schmid RM. Genetic alterations in pancreatic carcinoma. Mol Cancer. 2003;2:15. doi: 10.1186/1476-4598-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowgill SM, Muscarella P. The genetics of pancreatic cancer. Am J Surg. 2003;186:279–286. doi: 10.1016/s0002-9610(03)00226-5. [DOI] [PubMed] [Google Scholar]
- 23.Moore PS, Beghelli S, Zamboni G, Scarpa A. Genetic abnormalities in pancreatic cancer. Mol Cancer. 2003;2:7. doi: 10.1186/1476-4598-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tascilar M, Skinner HG, Rosty C, Sohn T, Wilentz RE, Offerhaus GJ, Adsay V, Abrams RA, Cameron JL, Kern SE, et al. The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2001;7:4115–4121. [PubMed] [Google Scholar]
- 25.Pizzi S, Azzoni C, Bassi D, Bottarelli L, Milione M, Bordi C. Genetic alterations in poorly differentiated endocrine carcinomas of the gastrointestinal tract. Cancer. 2003;98:1273–1282. doi: 10.1002/cncr.11621. [DOI] [PubMed] [Google Scholar]
- 26.Schwarte-Waldhoff I, Klein S, Blass-Kampmann S, Hintelmann A, Eilert C, Dreschers S, Kalthoff H, Hahn SA, Schmiegel W. DPC4/SMAD4 mediated tumor suppression of colon carcinoma cells is associated with reduced urokinase expression. Oncogene. 1999;18:3152–3158. doi: 10.1038/sj.onc.1202641. [DOI] [PubMed] [Google Scholar]
- 27.Schwarte-Waldhoff I, Volpert OV, Bouck NP, Sipos B, Hahn SA, Klein-Scory S, Lüttges J, Klöppel G, Graeven U, Eilert-Micus C, et al. Smad4/DPC4-mediated tumor suppression through suppression of angiogenesis. Proc Natl Acad Sci USA. 2000;97:9624–9629. doi: 10.1073/pnas.97.17.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iacobuzio-Donahue CA, Wilentz RE, Argani P, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Dpc4 protein in mucinous cystic neoplasms of the pancreas: frequent loss of expression in invasive carcinomas suggests a role in genetic progression. Am J Surg Pathol. 2000;24:1544–1548. doi: 10.1097/00000478-200011000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Salovaara R, Roth S, Loukola A, Launonen V, Sistonen P, Avizienyte E, Kristo P, Järvinen H, Souchelnytskyi S, Sarlomo-Rikala M, et al. Frequent loss of SMAD4/DPC4 protein in colorectal cancers. Gut. 2002;51:56–59. doi: 10.1136/gut.51.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohtaki N, Yamaguchi A, Goi T, Fukaya T, Takeuchi K, Katayama K, Hirose K, Urano T. Somatic alterations of the DPC4 and Madr2 genes in colorectal cancers and relationship to metastasis. Int J Oncol. 2001;18:265–270. doi: 10.3892/ijo.18.2.265. [DOI] [PubMed] [Google Scholar]
- 31.Xu X, Brodie SG, Yang X, Im YH, Parks WT, Chen L, Zhou YX, Weinstein M, Kim SJ, Deng CX. Haploid loss of the tumor suppressor Smad4/Dpc4 initiates gastric polyposis and cancer in mice. Oncogene. 2000;19:1868–1874. doi: 10.1038/sj.onc.1203504. [DOI] [PubMed] [Google Scholar]
- 32.Beghelli S, Orlandini S, Moore PS, Talamini G, Capelli P, Zamboni G, Falconi M, Scarpa A. Ampulla of vater cancers: T-stage and histological subtype but not Dpc4 expression predict prognosis. Virchows Arch. 2002;441:19–24. doi: 10.1007/s00428-002-0625-x. [DOI] [PubMed] [Google Scholar]
- 33.Holloway S, Davis M, Jaber R, Fleming J. A clinically relevant model of human pancreatic adenocarcinoma identifies patterns of metastasis associated with alterations of the TGF-beta/Smad4 signaling pathway. Int J Gastrointest Cancer. 2003;33:61–69. doi: 10.1385/IJGC:33:1:61. [DOI] [PubMed] [Google Scholar]
- 34.Schutte M. DPC4/SMAD4 gene alterations in human cancer, and their functional implications. Ann Oncol. 1999;10 Suppl 4:56–59. [PubMed] [Google Scholar]
- 35.Narai S, Watanabe M, Hasegawa H, Nishibori H, Endo T, Kubota T, Kitajima M. Significance of transforming growth factor beta1 as a new tumor marker for colorectal cancer. Int J Cancer. 2002;97:508–511. doi: 10.1002/ijc.1631. [DOI] [PubMed] [Google Scholar]
- 36.Koyama M, Ito M, Nagai H, Emi M, Moriyama Y. Inactivation of both alleles of the DPC4/SMAD4 gene in advanced colorectal cancers: identification of seven novel somatic mutations in tumors from Japanese patients. Mutat Res. 1999;406:71–77. doi: 10.1016/s1383-5726(99)00003-5. [DOI] [PubMed] [Google Scholar]
- 37.Iacobuzio-Donahue CA, Klimstra DS, Adsay NV, Wilentz RE, Argani P, Sohn TA, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Dpc-4 protein is expressed in virtually all human intraductal papillary mucinous neoplasms of the pancreas: comparison with conventional ductal adenocarcinomas. Am J Pathol. 2000;157:755–761. doi: 10.1016/S0002-9440(10)64589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramachandra M, Atencio I, Rahman A, Vaillancourt M, Zou A, Avanzini J, Wills K, Bookstein R, Shabram P. Restoration of transforming growth factor Beta signaling by functional expression of smad4 induces anoikis. Cancer Res. 2002;62:6045–6051. [PubMed] [Google Scholar]
- 39.Fralix KD, Zhao S, Venkatasubbarao K, Freeman JW. Rap1 reverses transcriptional repression of TGF-beta type II receptor by a mechanism involving AP-1 in the human pancreatic cancer cell line, UK Pan-1. J Cell Physiol. 2003;194:88–99. doi: 10.1002/jcp.10192. [DOI] [PubMed] [Google Scholar]
- 40.Yan Z, Kim GY, Deng X, Friedman E. Transforming growth factor beta 1 induces proliferation in colon carcinoma cells by Ras-dependent, smad-independent down-regulation of p21cip1. J Biol Chem. 2002;277:9870–9879. doi: 10.1074/jbc.M107646200. [DOI] [PubMed] [Google Scholar]
- 41.Fink SP, Swinler SE, Lutterbaugh JD, Massagué J, Thiagalingam S, Kinzler KW, Vogelstein B, Willson JK, Markowitz S. Transforming growth factor-beta-induced growth inhibition in a Smad4 mutant colon adenoma cell line. Cancer Res. 2001;61:256–260. [PubMed] [Google Scholar]


