Abstract
AIM: To evaluate and compare the clinical usefulness of 13C-phenylalanine and 13C-methacetin breath tests in quantitating functional hepatic mass in patients with chronic liver disease and to further compare these results with those of conventional tests, Child-Pugh score and serum bile acid levels.
METHODS: One hundred and forty patients (50 HCV- related chronic hepatitis, 90 liver cirrhosis patients) and 40 matched healthy controls were studied. Both breath test and routine liver test, serum levels of cholic and chenodeoxycholic acid conjugates were evaluated.
RESULTS: Methacetin breath test, expressed as 60 min cumulative percent of oxidation, discriminated the hepatic functional capacity not only between controls and liver disease patients, but also between different categories of chronic liver disease patients. Methacetin breath test was correlated with liver function tests and serum bile acids. Furthermore, methacetin breath test, as well as serum bile acids, were highly predictive of Child-Pugh scores. The diagnostic power of phenylalanine breath test was always less than that of methacetin breath test.
CONCLUSION: Methacetin breath test represents a safe and accurate diagnostic tool in the evaluation of hepatic functional mass in chronic liver disease patients.
Keywords: Chronic hepatitis c, Liver cirrhosis, Breath Tests, Hepatic functional mass
INTRODUCTION
Evaluation of liver function is crucial in the overall management of patients with liver diseases[1]. In clinical practice, diagnosis of liver disease is based on the results of physical examination, imaging techniques (ultrasonography, computed tomography, magnetic resonance, etc.) and biochemical investigations[2]. As far as the latter is concerned, several tests are available, each reflecting a specific function of hepatocytes and/or a specific liver damage. However, these biochemical parameters are not sufficiently sensitive to evaluate the complex biological events occurring within the hepatocytes (biosynthesis, biotransformation and catabolism of xenobiotics, etc) as well as the alterations induced by the disease on these events. Furthermore, no single liver biochemical test of the liver is endowed with the diagnostic accuracy of tests used in the evaluation of other organs, such as creatinine for kidney function. In fact, no single biochemical test can be considered as a sensitive index of the overall hepatic function and is able alone to predict the severity and prognosis of hepatic diseases, whether acute or chronic[3].
To improve the diagnostic efficacy of biochemical tests, several quantitative tests have been proposed to measure the residual hepatic function and numerous substrates have been used in the assessment of liver function, such as sulfobromophthalein dyes, indocyanine green, and sorbitol[4] . However, these tests, although accurate in evaluating hepatic functional mass, have been shown to be unpractical in clinical setting for several reasons: need of repeated blood samples, need of prolonged catheterization, risk for anaphylactic reactions, elevated costs, etc. These problems have, in part, been overcome by the use of carbon-labeled compounds and by the evaluation of the kinetics of carbon excretion in breath[5]. The rate of 13C excretion in breath is determined by the rate-limiting step in the overall process used, and the rate limiting step is located at the site of the impaired organ or enzyme function[6].
Different substrates have been proposed, each exploring a specific hepatic function. Aminopyrine which was the first studied compound[7], is useful in the evaluation of hepatocyte microsomial function[8]. Other substrates include phenacetin[9], caffeine[10], lidocaine[11], methacetin[12] and erythromycin[13]. Phenylalanine[14] and galactose[15] are used to explore the cytosolic enzymatic activity, while methionine and ketoisocaproic acid have been proposed in the study of the mitochondrial function[5,16].
Although clinical application of breath test has been in use for several years, no general agreement has been reached concerning its application in the clinical setting[4,6,17,8].
As a consequence, the Child-Pugh classification[19,20], which was proposed several years ago and represents a concerted evaluation of clinical criteria and laboratory data, still remains the most widely accepted predictor of the severity of liver diseases.
Recently, the clinical utility of some breath tests, namely methacetin and phenylalanine breath tests, has been re-proposed[21,22], even if their diagnostic power did not completely discriminate between different types of chronic liver diseases.
Furthermore, it has been suggested[21] that methacetin might be preferable to aminopyrine because of its rapid metabolism and lack of toxicity in small doses[23]. The test dosage is lower than therapeutic levels and no adverse reactions have been reported.
The aims of this study were to comparatively explore the clinical usefulness of breath tests using these two different substrates, 13C-phenylalanine and 13C-methacetin breath tests (PBT and MBT), in the assessment of functional hepatic reserve or function in patients with chronic liver disease and to verify the presence of a relationship, in terms of disease severity, between PBT and MBT results and those obtained with conventional liver function tests, Child-Pugh score, and serum bile acid levels. The latter comparison was performed since serum bile acid levels, especially serum levels of primary bile acids, are considered a sensitive index of liver function and disease prognosis[24,25].
MATERIALS AND METHODS
Study population
A total of 140 patients with chronic liver diseases were studied: 50 with histologically diagnosed hepatitis C virus (HCV)-related chronic hepatitis and 90 with clinically or histologically diagnosed liver cirrhosis.
Patients with liver diseases of unknown etiology and with cancer or who were heavy smokers and those aged <18 years or >80 years were not taken into consideration. The presence of factors known to potentially influence endogenous carbon dioxide production (no recent food ingestion and physical activity, respiratory diseases, thyroid dysfunctions, fever)[26] was investigated and, if found positive, those patients were excluded from the study. Furthermore, patients having recently used drugs being potentially able to interfere with hepatic cytochrome P450, such as corticosteroids, cimetidine, benzodiazepines, and omeprazole, were also excluded from the study[27].
For control purposes, 40 subjects with no clinical and biochemical evidence of hepatic, gastro-intestinal, endocrine or respiratory diseases, and no history of chronic alcohol consumption or drug use, were enrolled.
Demographic and clinical characteristics of the study population are shown in Table 1.
Table 1.
Patient characteristics.
| CTR n = 40 | CH n = 50 | LCA n = 30 | LCB n = 30 | LCC n = 30 | |
| Gender male/female | 25/15 | 27/23 | 23/7 | 20/10 | 22/8 |
| Age mean (range) | 50 (30-80) | 57 (38-78) | 60 (33-80) | 63 (32-80) | 59 (39-76) |
| BSA Mean | 1.76 | 1.75 | 1.78 | 1.74 | 1.72 |
| Etiology Viral/Alcoholic | 0 | 50/0 | 22/8 | 25/5 | 24/6 |
| Child score Mean (range) | 0 | 0 | 5.38 (5-6) | 8.0 (7-9) | 10.54 (10-15) |
CTR: Controls; CH: Chronic hepatitis; LCA: Child A cirrhosis; LCB: Child B; LCC: Child C; BSA: Body surface area.
The study was approved by the local ethics committees and all individuals provided written informed consent prior to enrollment in the study.
Biochemical and ultrasonographic evaluation of the liver
Liver function tests and hepatic ultrasonography (US) were performed on the first day of the study period, prior to carrying out the breath test. Routine liver function tests [alanine and aspartate transaminase, total proteins, serum albumin and gamma-globulins, prothrombin activity, γ-glutamyltransferase (γ-GT), total and conjugated bilirubin, alkaline phosphatase and blood ammonia] were performed. Furthermore, serum levels of cholic acid (CCA) and chenodeoxycholic acid (CDCA) conjugates were evaluated using the enzyme-linked immunosorbent assay ( ELISA)[28]
US was performed in all the patients in order to detect the presence of parenchymal structural alterations and/or ascites. Portal echo-colour Doppler and endoscopy of the upper gastrointestinal tract were performed in cirrhotic patients in order to assess the portal hypertension grade and the presence of oesophageal varices. Child Pugh scores were calculated for cirrhotic patients[19,20]. Patients were classified by their score into either class A (scores 5-6), class B (scores 7-9) or class C (scores 10-15).
13C-Methacetin and 13C phenylalanine breath tests
Breath tests were carried out using 100 mg of 13C-phenylalanine (99%13C, Cambridge Isotope Laboratories, Andover, MA, USA) and 75 mg of 13C-methacetin (99%13C, Cambridge Isotope Laboratories, Andover, MA, USA) on two different days in fasting subjects. Duplicate baseline breath samples were collected before administration of the substrates, which were dissolved in 50 mL of water. Duplicate breath collections were also taken every 10 min for 2 h using glass vacutainers. Breath samples were stored at 4 °C until analysis, which was performed within 15 d. During the tests, subjects were required to stay at rest, without eating, drinking and smoking.
Analytical methods
The 13CO2 enrichment in breath, expressed as cumulative percent of oxidation of a dispensed dose, was measured with a stable isotope mass spectrometer (Europe Scientific Tracermass, Crewe, UK).
To quantify the rate of hepatic substrate oxidation, analytical data were expressed as percentages of the 13CO2 recovery per hour using an area under curve (AUC) method, assuming a CO2 production rate of 5 mmol/min/m2 body surface area, as described by Schoeller et al[29].
Analysis of the elimination kinetics of 13C-labeled isotope (expressed as parts per million) related to time (expressed as minutes) allowed the study of other parameters, such as the isotopic peak excretion (parts per million), and the AUC of max. percent of oxidation.
Statistical analysis
Categorical variables were summarized as means of frequencies and proportions, continuous variables were summarized as mean±SD. Differences in PBT and MBT scores and in CCA and CDCA serum levels between controls, chronic hepatitis and cirrhotic patients were compared with one way analysis of variance (ANOVA). Data was expressed as mean and 95% confidence interval (95% CI).
Multiple linear regression was performed. The dependent variable was the Child score, and independent variables were the first hour percent of oxidation of MBT and PBT, CCA and CDCA serum levels and liver function tests. Ability to discriminate between the different groups (controls, chronic hepatitis and cirrhosis patients) was quantified by using the area under the receiver operating characteristic curve (ROC area)[30]. The ROC area was a reliable measure to summarize the discriminative power of a diagnostic model. A test that correctly classified all subjects had an area of 1.0 (perfect discrimination) and a test with no discriminatory value had an area of 0.5 or less. A value of 0.7-0.8 was considered to represent reasonable discrimination, and a value >0.8 to represent good discrimination[31].
All two-tailed P-values less than 0.05 were considered statistically significant. Statistical calculations were carried out using the statistical software Stata (Release 7, Santa Monica, CA, USA).
RESULTS
No side effects were observed after administration of the isotopes.
Results of MBT are shown in Figure 1A. MBT percent of oxidation at 60 min significantly discriminated between controls and patients with chronic hepatitis and cirrhosis of all Child-Pugh classes (P<0.001). Statistically significant differences were also found between patients with chronic hepatitis and those with Child-Pugh A cirrhosis and between the latter and Child-Pugh B and C cirrhosis (P<0.001). The same results were obtained by evaluating the cumulative percent of oxidation after 120 min.
Figure 1.
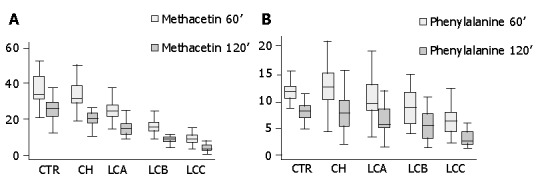
Methacetin and phenylalanine breath tests: cumulative percent of oxidation at 60 and 120 min. A: Methacetin breath test: cumulative percent of oxidation at 60 and 120 min. CTR = controls; CH = chronic hepatitis; LCA = liver cirrhosis Child A; LCB = liver cirrhosis Child B; LCC = liver cirrhosis Child C. CTR vs CH, LCA, LCB, LCC: P<0.001 CH vs LCA, LCB, LCC: P<0.001 LCA vs LCB, LCC: P<0.001 LCB vs LCC: P<0.001. B: Phenylalanine breath test: cumulative percent oxidation at 60 and 120 min. CTR = controls; CH = chronic hepatitis; LCA = liver cirrhosis Child A; LCB = liver cirrhosis Child B; LCC = liver cirrhosis Child C. CTR vs LCB, LCC: P<0.001 CH vs LCB, LCC: P<0.001 LCA vs LCC: P<0.05.
Peak MBT excretion (Table 2) was different in controls with respect to all patients (P<0.001). Differences were observed between patients with chronic liver diseases and those with Child-Pugh B and C cirrhotic patients (P<0.001), but not with Child-Pugh A cirrhotic patients. Similar results were obtained when MBT area under the curve of the max. percent of oxidation was considered (Table 2).
Table 2.
13CO2-peak and area under the curve (AUC) of 13CO2 maximal scores in all groups of subjects studied.
| Tests | CTR Mean (95% CI) | CH Mean (95% CI) | LCA Mean (95% CI) | LCB Mean (95% CI) | LCC Mean (95% CI) |
| 13C- phenylalanine | |||||
| 13CO2-peak | 12.68 (10.94-14.42) | 16.11 (13.58-18.63) | 11.85 (9.41-14.29) | 10.45 (8.07- 12.83) | 7.11 (2.74-11.47) |
| AUC13CO2max | 5.40 (2.32-7.62) | 6.85 (2.83-12.80) | 5.81 (2.64-10.36) | 4.60 (2.13-9.25) | 5.40 (2.77-10.65) |
| 13C-methacetin | |||||
| 13CO2-peak | 31.03b (25.61-36.46) | 22.80d (20.74-24.85) | 19.19f (15.60-22.77) | 10.18 (8.52-11.83) | 7.02 (5.03-9.00) |
| AUC13CO2 max | 16.76h (7.35-28.04) | 14.69d (5.41-31.27) | 9.44e (2.76-29.01) | 4.91 (3.13-8.83) | 5.32 (2.87-7.17) |
CTR: Controls, CH: Chronic hepatitis, LCA: Child A cirrhosis, LCB: Child B, LCC: Child C bP<0.001 vs All groups dP<0.001 vs Child B,C. fP<0.01 vs Child B,C. hP<0.001 vs Child A,B,C. eP<0.05 vs Child B.
Results of PBT are shown in Figure 1B. PBT cumulative percent of oxidation calculated at 60 min was able to discriminate between controls and chronic hepatitis patients with respect to Child-Pugh B and C patients (P<0.001), but not between chronic hepatitis patients and Child-Pugh A cirrhotic patients (Figure 1B); the same results were obtained by evaluating the cumulative percent of PBT oxidation 120 min after ingestion of the labeled substrates. No statistical differences were found between the studied groups when PBT peak excretion and AUC of max percent of oxidation was considered (Table 2).
Serum CCA and CDCA levels are shown in Figure 2. Both CCA and CDCA levels were significantly higher in liver disease patients than in controls; furthermore, differences were observed between chronic hepatitis patients and cirrhotic patients, as well as between Child-Pugh A, B and C cirrhotic patients (P<0.001).
Figure 2.
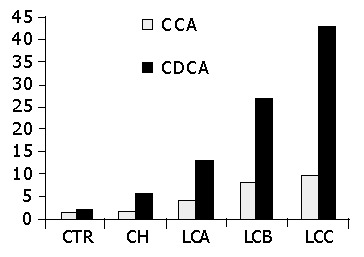
Serum levels of cholic acid (CCA) and chenodeoxycholic acid (CDCA) conjugates. CTR = controls; CH = chronic hepatitis; LCA = liver cirrhosis Child A; LCB = liver cirrhosis Child B; LCC = liver cirrhosis Child C. aP<0.05 vs CH, LCA, LCB, LCC bP<0.001 vs LCA, LCB, LCC dP<0.001 vs LCB, LCC.
Comparison between MBT and PBT results and liver function tests failed to reveal any significant correlation in chronic liver disease patients, while significant correlations were present in cirrhotic patients. In fact, the percent of PBT oxidation at 60 min was significantly related to serum levels of albumin (r = 0.35, P<0.01), total (r =-0.40, P<0.001) and conjugated bilirubin (r =-0.33, P<0.05), and to serum CCA (r =-0.33, P<0.05) and CDCA levels (r = -0.28, P<0.05). No significant correlation was found between PBT and prothrombin time, AST, ALT or alkaline phosphatase. The same correlations were found when 120-min cumulative percent of PBT oxidation was considered. No correlation was found between PBT, expressed as excretion peak and maximal AUC percent of oxidation and liver function tests.
As far as 60-min percent of MBT oxidation was concerned, it was significantly related to prothrombin time (r = 0.43, P<0.001), total (r = -0.47, P<0.001) and direct bilirubin (r = -0.49, P<0.01), serum albumin (r = 0.41, P<0.001), serum CCA (r = -0.50, P<0.001) and CDCA levels (r = -0.44, P<0.01). No significant correlation was found between MBT and transaminase or alkaline phosphatase levels. The same correlations were documented when 120-min MBT oxidation was considered. Moreover, MBT oxidation was significantly correlated (r = -0.52, P<0.001) with portal vein calibre measured at US. No correlation was found between MBT, expressed as excretion peak and maximal AUC percent of oxidation, and liver function tests.
The results of the areas under the ROC curves for PBT and MBT evaluated to discriminate between chronic hepatitis and cirrhotic patients, depending on collecting time, are summarized in Table 3. The best results were obtained at 60 min for PBT and MBT (area under the ROC curve of 0.72 and 0.93 respectively).
Table 3.
Areas under receiver operating characteristic (ROC) curves for phenylalanine breath test (PBT) and methacetin breath test (MBT) in chronic hepatitis and cirrhotic patients.
| PBT collecting time | Area | SE | MBT collecting time | Area | SE |
| 10 min | 0.45 | 0.07 | 10 min | 0.83 | 0.04 |
| 20 min | 0.54 | 0.07 | 20 min | 0.86 | 0.04 |
| 30 min | 0.59 | 0.07 | 30 min | 0.89 | 0.03 |
| 40 min | 0.64 | 0.06 | 40 min | 0.91 | 0.03 |
| 50 min | 0.69 | 0.06 | 50 min | 0.92 | 0.03 |
| 60 min | 0.72 | 0.05 | 60 min | 0.93 | 0.03 |
| 70 min | 0.7 | 0.06 | 70 min | 0.93 | 0.02 |
| 80 min | 0.7 | 0.06 | 80 min | 0.93 | 0.02 |
| 90 min | 0.7 | 0.06 | 90 min | 0.93 | 0.02 |
| 100 min | 0.71 | 0.05 | 100 min | 0.93 | 0.02 |
| 110 min | 0.72 | 0.06 | 110 min | 0.92 | 0.03 |
| 120 min | 0.72 | 0.05 | 120 min | 0.92 | 0.03 |
The comparative evaluation between MBT and PBT in terms of areas under the ROC curves documented higher and significant values for MBT in all the comparisons, except for that related to controls and chronic hepatitis patients (Table 4). Figures 3A and 3B illustrate the behaviors of 60-min MBT and PBT, in comparison between controls and liver disease patients, and chronic hepatitis and Child-Pugh A cirrhotic patients, respectively. MBT always showed higher values with respect to PBT, thus confirming its higher diagnostic power.
Table 4.
Comparison between area under the receiver operating characteristic (ROC) curves for methacetin breath test (MBT) and phenylalanine breath test (PBT).
| Group | Time (min) |
MBT |
PBT |
χ2 | ||
| Area | SE | Area | SE | |||
| CTR vs all patient | 60 | 0.86 | 0.04 | 0.73 | 0.04 | 0.02 |
| CH vs C | 60 | 0.89 | 0.03 | 0.69 | 0.05 | 0.003 |
| CTR vs CH | 60 | 0.67 | 0.07 | 0.56 | 0.008 | ns |
| CH vs C-Child A | 60 | 0.79 | 0.05 | 0.6 | 0.07 | 0.02 |
CTR : Controls; CH: Chronic hepatitis; C: Cirrhosis (Child A,B,C).
Figure 3.
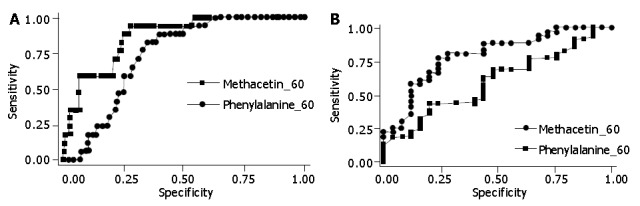
Area under the receiver operating characteristic (ROC) curves compared to the methacetin and phenylalanine breath tests between control subjects and liver disease patients as well as between chronic hepatitis and Child-Pugh A cirrhosis patients. A: Area under the receiver operating characteristic (ROC) curves compared to methacetin and phenylalanine breath tests between control subjects and liver disease patients. --M60 = cumulative percent oxidation of methacetin at 60 mins; -P60 = cumulative percent oxidation of phenylalanine at 60 min; B: Area under the receiver operating characteristic (ROC) curves compared to methacetin and phenylalanine breath tests between chronic hepatitis and Child-Pugh A liver cirrhosis patients. -M60 = cumulative percent oxidation of methacetin at 60 mins; --P60 = cumulative percent of oxidation of phenylalanine at 60 mins.
The results of the multiple linear regression analysis performed to evaluate whether MBT and PBT, serum CCA and CDCA levels and standard liver tests could predict the Child-Pugh scores are shown in Tables 5 and 6. CCDA and CCA, together with MBT, showed the highest regression coefficient value (Table 5). Similar results, but with lower regression coefficients, were obtained when PBT was considered (Table 6).
Table 5.
Multiple regression analysis of 13C-methacetin breath test, serum levels of cholic acid ( CCA) and chenodeoxycholic acid (CDCA) conjugates.
| Variable | Beta | P |
| 13C-methacetin (60 min % oxidation) | -0.544 | 0 |
| CCA | 0.576 | 0.0016 |
| CDCA | 0.855 | 0.001 |
r2 = 0.660.
Table 6.
Multiple regression analysis of 13C-phenylalanine breath test, serum levels of cholic acid (CCA) and chenodeoxycholic acid (CDCA) conjugates.
| Variable | Beta | P |
| 13C-phenylalanine (60 min % oxidation) | -0.252 | 0.057 |
| CCA | 0.487 | 0.07 |
| CDCA | 1.01 | 0 |
r2 = 0.545.
DISCUSSION
Results from the present study show that MBT and to a lesser extent PBT, could discriminate hepatic functional capacity both between healthy subjects and liver disease patients, and between the different categories of chronic liver disease patients. The diagnostic role of MBT was further confirmed by the correlation between MBT and liver function tests and, in particular between serum CCA and CDCA levels. The most useful expression of MBT kinetic parameters is the cumulative percent of oxidation at 60 min, since other modalities of expressed data (peak, AUC of the max percent of oxidation) are less accurate from a diagnostic point of view. Furthermore, MBT as well as serum bile acids, are highly predictive of Child-Pugh scores, as shown by the results of logistic regression. In the present study, the diagnostic power of PBT was always less than that of MBT.
In patients with liver diseases, various laboratory tests and indicators are used to grade liver damage. Conventionally, the degree of injury is assessed using tests which reflect hepatic structure (biopsy), hepatocyte permeability (transaminases) and synthetic activity (albumin, bilirubin and prothrombin time)[1].
These tests are static measurements based on the evaluation of the serum concentration of a particular substance at a given time[3] They do not quantitate functional hepatic reserve, but only hepatocellular damage[2]. In fact, evaluation of enzyme activity has not been considered adequate for the evaluation of hepatocyte function and reserve[8], which can be more accurately measured by dynamic tests.
Several quantitative tests have been proposed to evaluate the functional hepatic mass and numerous substrates have been used in the assessment of liver function, such as sulfobromo-phthalein dyes, indocyanine green, sorbitol. However, these tests, although accurate in evaluating hepatic functional mass, are found to be unpractical in the clinical setting for several reasons: need of repeated blood samples, prolonged catheterization, risk for anaphylactic reactions, elevated costs, etc.
Breath tests with carbon-labeled compounds have been proposed as sensitive and accurate dynamic tests, being useful for the non-invasive measurement of hepatic function[6] . However, although several studies have demonstrated the usefulness of breath tests as hepatic function tests, there is no general agreement regarding their application in the clinical setting[5,17,18].
Thus, in patients with liver diseases, Child-Pugh classification still represents the most widely used marker of liver function. This classification, however, does not strictly reflect the quantitative functional hepatic reserve, and measurement thereof could be influenced by the subjectivity of some parameters (i.e., degree of ascites or hepatic encephalopathy) and by modifications induced by concomitant treatments (i.e., albumin infusion).
The diagnostic reliability of MBT and /or PBT in chronic liver diseases has been proposed and evaluated by various authors, but with controversial results.
Using MBT, Klatt et al[32] revealed significant differences between chronic hepatitis and cirrhotic patients, but not between controls and chronic hepatitis patients. Burke et al[14] on the other hand, showed more encouraging results using PBT, and in particular, a good correlation with the Child-Pugh scores, but their findings have not been confirmed by others. Perri et al[21] in a comparative evaluation of MBT, PBT and aminopyrine breath tests, in a small group of chronic liver disease patients, failed to show any differences between the results obtained with the different substrates.
In the present study, we demonstrated that evaluation using MBT but not PBT, percent of oxidation could discriminate between different groups of chronic liver disease patients, and in particular, between chronic hepatitis and Child-Pugh A cirrhotic patients, and was related to Child-Pugh score status. The correlation between MBT and serum levels of primary bile acids considered as a sensitive index of liver function[25,33,34] further supports the diagnostic role of MBT.
Different results have been recently obtained by Lara Baruque et al[22] who demonstrated a high sensibility of both MBT and PBT for the diagnosis of hepatic dysfunction. The specificity, however, was very low. In addition, these authors did not find significant differences in the test results, between chronic hepatitis and Child-Pugh A patients.
This is an important aspect since the evolution from chronic hepatitis to cirrhosis represents a crucial moment in the natural history of chronic liver diseases. Furthermore, the differentiation between chronic hepatitis patients and cirrhotic patients, as we obtained using both MBT and serum bile acid levels, is also important to define the diagnostic strategy to be adopted (i.e., to proceed with liver biopsy or use a clinical-biochemical score).
We showed that MBT had a greater diagnostic capacity than PBT. We could not explain this observation and in particular the lower diagnostic power of PBT than expected. A possible explanation concerning the lower solubility and the slower metabolism of phenylalanine compared to methacetin, was reported also by Lara Baruque et al[22].
Other important results emerging from the present study were related to the timing of breath collection and the expression of the isotope breath kinetics. The best discrimination capacity was obtained on the basis of the areas under the ROC curves, for both MBT and PBT 60 min after substrate ingestion. This finding, which is in agreement with that of some studies[14,22] , but not of others[21,32,35], is of practical importance, since it suggests that further (up to 60 min) breath samples are not necessary. In our experience, additional parameters, such as isotope excretion peak and maximal AUC, are not necessary, since they do not increase the diagnostic accuracy of the cumulative percent of oxidation. However, further studies are needed to confirm this observation.
To date, the important unsolved question is the usefulness and possible superiority of breath tests in predicting the prognosis of liver disease and the rate of disease progression compared with Child-Pugh classification[19,20]. According to several authors, the use of breath tests for the prognosis of liver disease patients should be considered only when their superior accuracy with respect to Child-Pugh scores is demonstrated. Merkel et al[36] published a study on 125 patients with chronic liver diseases, who were followed for 48 mo, demonstrating that aminopyrine breath test was superior to Child-Pugh scores in predicting fatal cirrhotic-correlated events. Similar results were obtained by Figg[37] and by Herold[38] , while other investig-ators[39,40] were unable to document a superiority of quantitative function tests over Child-Pugh classification. Recently Zipprich et al[41] have suggested that the possibility of overlapping values in cirrhotic patients could be due to the influence of anaemia and oxygen supply to the cirrhotic liver.
Although our study is not a prospective study, it shows that MBT and serum primary bile acids are the only parameters found to be predictive of Child-Pugh scores. Furthermore, in our previous prospective study[41] performed on cirrhotic patients awaiting liver transplantation, we showed that percent of oxidation of MBT strictly followed the clinical course of liver diseases. In fact MBT progressively decreased until liver transplantation was performed and then increased to reach normal values in patients whose liver transplantation was successful.
In conclusion, MBT and PBT represent a safe, simple and accurate test useful not only in diagnosing chronic liver disease in patients, but also in differentiating between different stages of chronic liver diseases. Furthermore, this study confirms the clinical usefulness of serum primary bile acid measurement in the diagnosis of chronic liver disease in patients.
If further longitudinal studies confirm the MBT diagnostic and prognostic values, this evaluation could represent an important tool for the overall diagnostic and therapeutic management of liver disease patients.
Footnotes
Edited by Wang XL
References
- 1.Tygstrup N. Assessment of liver function: principles and practice. J Gastroenterol Hepatol. 1990;5:468–482. doi: 10.1111/j.1440-1746.1990.tb01426.x. [DOI] [PubMed] [Google Scholar]
- 2.Friedman LS, Martin P, Munoz SJ. Liver function tests and the objective evaluation of the patient with liver disease. In: D , editor. Zakim, TD Boyer, editors, Hepatology, A textbook of liver disease. Philadelphia: Saunders; 1999. pp. 1134–1145. [Google Scholar]
- 3.Johnson PJ. Role of the standard 'liver function tests' in current clinical practice. Ann Clin Biochem. 1989;26(Pt 6):463–471. doi: 10.1177/000456328902600601. [DOI] [PubMed] [Google Scholar]
- 4.Jalan R, Hayes PC. Review article: quantitative tests of liver function. Aliment Pharmacol Ther. 1995;9:263–270. doi: 10.1111/j.1365-2036.1995.tb00380.x. [DOI] [PubMed] [Google Scholar]
- 5.Armuzzi A, Candelli M, Zocco MA, Andreoli A, De Lorenzo A, Nista EC, Miele L, Cremonini F, Cazzato IA, Grieco A, et al. Review article: breath testing for human liver function assessment. Aliment Pharmacol Ther. 2002;16:1977–1996. doi: 10.1046/j.1365-2036.2002.01374.x. [DOI] [PubMed] [Google Scholar]
- 6.Klein PD. 13C breath tests: visions and realities. J Nutr. 2001;131:1637S–1642S. doi: 10.1093/jn/131.5.1637S. [DOI] [PubMed] [Google Scholar]
- 7.Hepner GW, Vesell ES. Assessment of aminopyrine metabolism in man by breath analysis after oral administration of 14C-aminopyrine. Effects of phenobarbital, disulfiram and portal cirrhosis. N Engl J Med. 1974;291:1384–1388. doi: 10.1056/NEJM197412262912605. [DOI] [PubMed] [Google Scholar]
- 8.Perri F, Pastore M, Annese V, Andriulli A. The aminopyrine breath test. Ital J Gastroenterol. 1994;26:306–317. [PubMed] [Google Scholar]
- 9.Breen KJ, Bury RW, Calder IV, Desmond PV, Peters M, Mashford ML. A [14C]phenacetin breath test to measure hepatic function in man. Hepatology. 1984;4:47–52. doi: 10.1002/hep.1840040108. [DOI] [PubMed] [Google Scholar]
- 10.Kalow W, Tang BK. The use of caffeine for enzyme assays: a critical appraisal. Clin Pharmacol Ther. 1993;53:503–514. doi: 10.1038/clpt.1993.63. [DOI] [PubMed] [Google Scholar]
- 11.Oellerich M, Raude E, Burdelski M, Schulz M, Schmidt FW, Ringe B, Lamesch P, Pichlmayr R, Raith H, Scheruhn M. Monoethylglycinexylidide formation kinetics: a novel approach to assessment of liver function. J Clin Chem Clin Biochem. 1987;25:845–853. doi: 10.1515/cclm.1987.25.12.845. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto K, Suehiro M, Iio M, Kawabe T, Shiratori Y, Okano K, Sugimoto T. [13C] methacetin breath test for evaluation of liver damage. Dig Dis Sci. 1987;32:344–348. doi: 10.1007/BF01296285. [DOI] [PubMed] [Google Scholar]
- 13.Watkins PB, Murray SA, Winkelman LG, Heuman DM, Wrighton SA, Guzelian PS. Erythromycin breath test as an assay of glucocorticoid-inducible liver cytochromes P-450. Studies in rats and patients. J Clin Invest. 1989;83:688–697. doi: 10.1172/JCI113933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burke PA, Stack JA, Wagner D, Lewis DW, Jenkins RL, Forse RA. L-[1-(13)C] Phenylalanine oxidation as a measure of hepatocyte functional capacity in end-stage liver disease. Am J Surg. 1997;173:270–273, discussion 273-274. doi: 10.1016/S0002-9610(96)00392-3. [DOI] [PubMed] [Google Scholar]
- 15.Saadeh S, Behrens PW, Parsi MA, Carey WD, Connor JT, Grealis M, Barnes DS. The utility of the 13C-galactose breath test as a measure of liver function. Aliment Pharmacol Ther. 2003;18:995–1002. doi: 10.1046/j.1365-2036.2003.01753.x. [DOI] [PubMed] [Google Scholar]
- 16.Armuzzi A, Marcoccia S, Zocco MA, De Lorenzo A, Grieco A, Tondi P, Pola P, Gasbarrini G, Gasbarrini A. Non-Invasive assessment of human hepatic mitochondrial function through the 13C-methionine breath test. Scand J Gastroenterol. 2000;35:650–653. doi: 10.1080/003655200750023633. [DOI] [PubMed] [Google Scholar]
- 17.Rating D, Langhans CD. Breath tests: concepts, applications and limitations. Eur J Pediatr. 1997;156 Suppl 1:S18–S23. doi: 10.1007/pl00014264. [DOI] [PubMed] [Google Scholar]
- 18.Romagnuolo J, Schiller D, Bailey RJ. Using breath tests wisely in a gastroenterology practice: an evidence-based review of indications and pitfalls in interpretation. Am J Gastroenterol. 2002;97:1113–1126. doi: 10.1111/j.1572-0241.2002.05664.x. [DOI] [PubMed] [Google Scholar]
- 19.Child CG, Turcotte JG. Surgery and portal hypertension. In: Child CG, editor , The liver and portal hypertension, editors. Philadelphia: W.B. Saunders; 1964. p. 50. [Google Scholar]
- 20.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 21.Galmiche JP, Delbende B, Perri F, Andriulli A. 13C octanoic acid breath test. Gut. 1998;43 Suppl 3:S28–S30. doi: 10.1136/gut.43.2008.s28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lara Baruque S, Razquin M, Jimenez I, Vazquez A, Gisbert JP, Pajares JM. 13C-phenylalanine and 13C-methacetin breath test to evaluate functional capacity of hepatocyte in chronic liver disease. Dig Liver Dis. 2000;32:226–232. doi: 10.1016/s1590-8658(00)80825-7. [DOI] [PubMed] [Google Scholar]
- 23.Schneider JF, Schoeller DA, Schreider BD. Use of 13C-phenacetin and 13C-methacetin for the detection of alterations in hepatic drug metabolism. In: Klein ER, Klein PD, editors , Proceedings of the 3rd International Conference on Stable Isotopes, May 1978, et al., editors. New York: Academic Press; 1979. pp. 507–516. [Google Scholar]
- 24.Monroe PS, Baker AL, Schneider JF, Krager PS, Klein PD, Schoeller D. The aminopyrine breath test and serum bile acids reflect histologic severity in chronic hepatitis. Hepatology. 1982;2:317–322. doi: 10.1002/hep.1840020305. [DOI] [PubMed] [Google Scholar]
- 25.Festi D, Morselli Labate AM, Roda A, Bazzoli F, Frabboni R, Rucci P, Taroni F, Aldini R, Roda E, Barbara L. Diagnostic effectiveness of serum bile acids in liver diseases as evaluated by multivariate statistical methods. Hepatology. 1983;3:707–713. doi: 10.1002/hep.1840030514. [DOI] [PubMed] [Google Scholar]
- 26.Stellard F, Elzinga H, Vonk RJ. Standardization and accuracy of breath tests. In: Perri F, Andriulli A, editors, Clinical application of breath tests in gastroenterology and hepatology, Rome: International University Press; 1998. pp. 13–16. [Google Scholar]
- 27.Bertz RJ, Granneman GR. Use of in vitro and in vivo data to stimulate the likelihood of metabolic pharmacokinetic interactions. Drug interactions. Clin Pharmacokinetic. 1997;3:32–40. doi: 10.2165/00003088-199732030-00004. [DOI] [PubMed] [Google Scholar]
- 28.Roda A, Girotti S, Lodi S, Preti S. Development of a sensitive enzyme immunoassay for plasma and salivary steroids. Talanta. 1984;31:895–900. doi: 10.1016/0039-9140(84)80218-0. [DOI] [PubMed] [Google Scholar]
- 29.Schneider JF, Schoeller DA, Nemchausky B, Boyer JL, Klein P. Validation of 13CO2 breath analysis as a measurement of demethylation of stable isotope labeled aminopyrine in man. Clin Chim Acta. 1978;84:153–162. doi: 10.1016/0009-8981(78)90489-8. [DOI] [PubMed] [Google Scholar]
- 30.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 31.Weinstein MC, Fineberg HV. Clinical decision analysis. Philadelphia: WB Saunders; 1980. [Google Scholar]
- 32.Klatt S, Taut C, Mayer D, Adler G, Beckh K. Evaluation of the 13C-methacetin breath test for quantitative liver function testing. Z Gastroenterol. 1997;35:609–614. [PubMed] [Google Scholar]
- 33.Par P, Hoesf JC, Ashcavai M. Determinants of serum bile acids in chronic liver disease. Gastroenterology. 1981;81:959–964. [PubMed] [Google Scholar]
- 34.Poupon RY, Poupon RE, Lebrec D, Le Quernec L, Darnis F. Mechanisms for reduced hepatic clearance and elevated plasma levels of bile acids in cirrhosis. A study in patients with an end-to-side portacaval shunt. Gastroenterology. 1981;80:1438–1444. [PubMed] [Google Scholar]
- 35.Pfaffenbach B, Götze O, Szymanski C, Hagemann D, Adamek RJ. The 13C-methacetin breath test for quantitative noninvasive liver function analysis with an isotope-specific nondispersive infrared spectrometer in liver cirrhosis. Dtsch Med Wochenschr. 1998;123:1467–1471. doi: 10.1055/s-2007-1024202. [DOI] [PubMed] [Google Scholar]
- 36.Merkel C, Bolognesi M, Bellon S, Bianco S, Honisch B, Lampe H, Angeli P, Gatta A. Aminopyrine breath test in the prognostic evaluation of patients with cirrhosis. Gut. 1992;33:836–842. doi: 10.1136/gut.33.6.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Figg WD, Dukes GE, Lesesne HR, Carson SW, Songer SS, Pritchard JF, Hermann DJ, Powell JR, Hak LJ. Comparison of quantitative methods to assess hepatic function: Pugh's classification, indocyanine green, antipyrine, and dextromethorphan. Pharmacotherapy. 1995;15:693–700. [PubMed] [Google Scholar]
- 38.Herold C, Heinz R, Niedobitek G, Schneider T, Hahn EG, Schuppan D. Quantitative testing of liver function in relation to fibrosis in patients with chronic hepatitis B and C. Liver. 2001;21:260–265. doi: 10.1034/j.1600-0676.2001.021004260.x. [DOI] [PubMed] [Google Scholar]
- 39.Herold C, Berg P, Kupfal D, Becker D, Schuppan D, Hahn EG, Schneider HT. Parameters of microsomal and cytosolic liver function but not of liver perfusion predict portal vein velocity in noncirrhotic patients with chronic hepatitis C. Dig Dis Sci. 2000;45:2233–2237. doi: 10.1023/a:1026600921967. [DOI] [PubMed] [Google Scholar]
- 40.Albers I, Hartmann H, Bircher J, Creutzfeldt W. Superiority of the Child-Pugh classification to quantitative liver function tests for assessing prognosis of liver cirrhosis. Scand J Gastroenterol. 1989;24:269–276. doi: 10.3109/00365528909093045. [DOI] [PubMed] [Google Scholar]
- 41.Zipprich A, Meiss F, Steudel N, Sziegoleit U, Fleig WE, Kleber G. 13C-Methacetin metabolism in patients with cirrhosis: relation to disease severity, haemoglobin content and oxygen supply. Aliment Pharmacol Ther. 2003;17:1559–1562. doi: 10.1046/j.1365-2036.2003.01604.x. [DOI] [PubMed] [Google Scholar]
- 42.Petrolati A, Festi D, De Berardinis G, Colaiocco-Ferrante L, Di Paolo D, Tisone G, Angelico M. 13C-methacetin breath test for monitoring hepatic function in cirrhotic patients before and after liver transplantation. Aliment Pharmacol Ther. 2003;18:785–790. doi: 10.1046/j.1365-2036.2003.01752.x. [DOI] [PubMed] [Google Scholar]


