Abstract
AIM: Despite the presence of lymphocyte infiltration, human hepatocellular carcinoma (HCC) is typically a rapidly progressive disease. The mechanism of regulation of lymphocyte migration is poorly understood. In this study, we investigated various factors regulating T cell migration in HCC patients. We examined serum CXC chemokine levels in HCC patients and demonstrated the production of CXC chemokines by HCC cell lines. We determined the effect of both HCC patient serum and tumor cell conditioned supernatant upon lymphocyte expression of chemokine receptor CXCR3 as well as lymphocyte migration. Lastly, we examined the chemotactic responses of lymphocytes derived from HCC patients.
METHODS: The serum chemokines IP-10 (CXCL10) and Mig (CXCL9) levels were measured by cytometric bead array (CBA) and the tumor tissue IP-10 concentration was measured by ELISA. The surface expression of CXCR3 on lymphocytes was determined by flow cytometry. The migratory function of lymphocytes to the corresponding chemokines was assessed using an in vitro chemotactic assay. Phosphorylation of extracellular signal-regulated kinase (ERK) was determined by Western blot analysis.
RESULTS: Increased levels of IP-10 and Mig were detected in HCC patient serum and culture supernatants of HCC cell lines. The IP-10 concentration in the tumor was significantly higher than that in the non-involved adjacent liver tissues. HCC cell lines secreted functional chemokines that induced a CXCR3-specific chemotactic response of lymphocytes. Furthermore, tumor-cell-derived chemokines induced initial rapid phosphorylation of lymphocyte ERK followed by later inhibition of ERK phosphorylation. The culture of normal lymphocytes with HCC cell line supernatants or medium containing serum from HCC patients resulted in a significant reduction in the proportion of lymphocytes exhibiting surface expression of CXCR3. The reduction in T cell expression of CXCR3 resulted in reduced migration toward the ligand IP-10, and both CD4+ and CD8+ T cells from HCC patients exhibited diminished chemotactic responses to IP-10 in vitro compared to T cells from healthy control subjects.
CONCLUSION: This study demonstrates functional desensitization of the chemokine receptor CXCR3 in lymphocytes from HCC patients by CXCR3 ligands secreted by tumor cells. This may cause lymphocyte dysfunction and subsequently impaired immune defense against the tumor.
Keywords: Hepatocellular carcinoma, CXCR3 ligands
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common cancers in the world. The prognosis of patients with HCC is generally poor because the cancer is characterized by a rapid growth rate and vascular invasion[1]. T-lymphocyte-mediated cellular immunity plays an important role in host defense against tumors. Indeed, the presence of a heavy lymphocyte infiltration is associated with low recurrence and high survival rates in a variety of cancers including HCC[2-7] with most of the infiltrating lymphocytes in HCC being CD8+ or CD4+ T cells[6]. Activated CD8+ T cells constitute an essential arm of the immune system for controlling tumor growth[8], and stimulated CD4+ T cells are critical for successful tumor eradication[9-11]. However, marked lymphocyte infiltration was only observed in 10-20% cases of HCC, and the number of tumor-infiltrating lymphocytes (TIL) in HCC is generally lower than that evident in other cancers[6]. Although the mechanism underlying the regulation of lymphocyte traffic in patients with HCC is not well characterized, it is likely to involve a process of regulated adhesion and migration that requires tethering, triggering, integrin-mediated adhesion and chemotactic factors[12]. The most important molecules involved in triggering and directing lymphocyte migration are chemokines[13]. Lymphocyte migratory pathways are tightly regulated by chemokine receptor expression and exposure to chemokines. Lymphocytes could only be recruited if they express the corresponding chemokine receptors[13].
Chemokines are 8- to 12-kDa, heparin-binding cytokines distinguished by the specificity of their chemotactic properties for target cells[14]. They can be divided into four groups: CC, CXC, C and CX3C, based on the arrangement of conserved cysteine residues[15]. The importance of chemokines in regulating lymphocyte trafficking is suggested by the large number of chemokines in each of the four chemokine subfamilies that have been demonstrated to influence lymphocyte behaviors in vitro[16,17]. IP-10 and Mig, members of the CXC chemokine family, induce potent lymphocyte chemotaxis following interaction with their receptor, CXCR3[18,19], and play an important role in various inflammatory diseases[20-25]. CXCR3 exerts an important role in the recruitment of lymphocytes to both normal and diseased tissues[26-29]. However, the role of CXCR3 in the regulation of lymphocyte traffic in HCC remains unclear.
In this study, we examined the serum levels of chemokines IP-10 and Mig in HCC patients together with IP-10 levels in tumor and adjacent nontumor tissues. We also examined chemokine production by HCC cell lines and evaluated the interaction between these chemokines and their receptor CXCR3 in T lymphocytes. The role of these chemokines and their corresponding receptor in regulating T cell function in HCC were addressed in this study.
MATERIALS AND METHODS
Patients and samples
Fresh whole blood and serum samples were obtained from 35 patients (31 men, 4 women; median age 54 years, range 36-84 years) with a diagnosis of HCC. Fresh tumor and peritumor liver specimens were obtained from 32 patients who underwent liver resection for HCC. Fresh whole blood and serum samples were also obtained from 12 healthy controls (10 men, 2 women; median age 46 years, range 27-58 years). None of the patients had received immunosuppressive drugs or chemotherapy. This study was approved by the Ethics Committee of our institution and informed consent was given by the patients.
Preparation of lymphocytes
Peripheral blood lymphocytes (PBLs) were isolated by Ficoll-Hypaque density gradient separation and washed twice in PBS. Mononuclear cells were suspended in RPMI-1640 medium and incubated at 37 °C for 90 min in a 6-well plate. Non-adherent lymphocytes were then collected for flow cytometric analysis, chemotaxis assays and Western blot analysis.
Cell lines
The HCC cell line Hep3B was obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) and HuH 7 was provided by Dr H. Nakabayashi (Hokkaido University School of Medicine, Hokkaido, Japan). Immortalized human hepatocyte MIHA was provided by Dr Roy Chowdhury (Albert Einstein College of Medicine, New York, USA). Hep3B and HuH 7 were cultured in DMEM medium containing penicillin/streptomycin and L-glutamine supplemented with 10% FCS. MIHA was cultured in Waymouth’s MB 752/1 medium (Gibco/BRL) supplemented with 10% FCS, penicillin/streptomycin and L-glutamine. Culture supernatants were collected from 3-d-old tumor cell cultures for further experiments.
Cytometric bead array (CBA) for chemokines
The concentrations of IP-10 and Mig in serum and cell culture supernatants were measured using the human chemokine CBA kit I (BD PharMingen, San Diego, CA, USA). In brief, 20 μL of mixed chemokine IP-10 and Mig capture beads was mixed with 20 μL of serum or culture supernatants and 20 μL of PE detection reagent and incubated for 3 h at room temperature. The beads were then washed with wash buffer and analyzed by FACS caliber (Becton Dickinson, San Jose, CA, USA).
Measurement of tumor and non-tumor IP-10 protein concentration
IP-10 of both tumor and adjacent nontumor tissue cytosolic samples was measured by the Quantikine human IP-10 ELISA kit (R&D Systems, Minneapolis, MN, USA). Protein fraction was obtained by homogenization of tissues in lysis buffer (1% Nonidet, 0.5% sodium deoxycholate, 0.1% SDS, 1 mmol/L PMSF and protease inhibitor cocktail at 1 tablet per 50 mL extraction solution). Homogenates were centrifuged at 20000 g at 4 °C for 10 min and the supernatants were collected for assay of IP-10 levels.
Flow cytometry analysis
The following antibodies were used in this study: APC-conjugated anti-CD4, PerCP-conjugated anti-CD8, phycoerythrin-conjugated anti-CXCR3 and IgG isotype control (BD PharMingen, San Diego, CA, USA). Lymphocytes were incubated with the antibodies for 30 min at 4 °C, and two-color or three-color flow cytometry was performed on an FACS caliber analyzer (Becton Dickinson, San Jose, CA, USA).
Chemotaxis assay
Lymphocyte chemotaxis assay was performed using 5.0-μm pore size Transwell inserts (Corning Costar, Cambridge, MA, USA). Six hundred mL of tumor culture supernatant, chemokine IP-10 (100 ng/mL) (PeproTech, London, UK) or medium alone were added to the lower Transwell chamber. A total of 5×105 PBL in 100 μL were added to the upper Transwell chamber containing 50 mL/L CO2 and incubated for 3 h at 37 °C. In some experiments, PBLs were incubated with blocking anti-CXCR3 monoclonal antibodies or isotype control antibody (R&D Systems, Minneapolis, MN, USA) for 1 h at room temperature before they were added to the upper chamber of the Transwell. Migrated cells were collected from the lower chamber after the lowerside of the filter was carefully washed. Cells were washed in flow cytometry buffer and then incubated for 30 min at 4 °C with APC-conjugated anti-CD4 and PerCP-conjugated anti-CD8. Cells were then washed and analyzed by flow cytometry with counting of viable cells for 1 min. Chemotaxis was quantified by dividing the number of CD4 or CD8 positive cells migrating in the presence of chemokines by the number of cells migrating toward the medium alone.
Assessment of chemokine receptor expression on normal PBLs pretreated with tumor conditioned medium or medium supplemented by serum derived from HCC patients
Hep3B and HuH-7 were seeded in 24-well plates and incubated in DMEM medium containing L-glutamine supplemented with 10% FCS for 3 d. The culture supernatants were aspirated and centrifuged. Freshly isolated normal PBLs from eight healthy control subjects were incubated with the conditioned supernatant for 20 h with 3-d MIHA culture medium serving as control. To determine the effect of the cell supernatants on the expression of CXCR3 by PBLs, surface CXCR3 expression was measured by flow cytometry. For the effect of the serum from HCC patients on lymphocyte CXCR3 expression, normal PBLs were incubated with either medium with 10% serum from HCC patients or 10% serum from healthy control subjects for 24 h. Normal PBLs cultured in the full medium with 10% FCS served as medium control. Surface expression of CXCR3 was measured by flow cytometry.
Western blot analysis
Normal PBLs were incubated for 10 min or overnight with cell culture supernatants or IP-10 at 37 °C. The cells were collected, washed twice with PBS, and then lysed in lysis buffer (Cell signaling, Beverly, MA, USA) for 15 min at 4 °C. The lysates were centrifuged at 12000 g for 15 min at 4 °C and equal amounts of solubilized proteins were separated by SDS-PAGE, and then transferred to nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ, USA). The membranes were blocked with TBST (20 mmol/L Tris, pH 7.6, 135 mmol/L NaCl, 0.1% Tween 20) containing 5% non-fat milk and immunoblotted with anti-phosphorylated ERK (1:000) (Cell ignaling, Beverly, MA, USA) for 18 h at 4 °C, followed by detection using HRP-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 h at room temperature. Immunoreactive protein bands were visualized by the ECL system (Amersham Biosciences, Piscataway, NJ, USA).
Statistical analysis
Comparison of continuous variables between unpaired groups was performed using the Mann-Whitney U test. Wilcoxon’s rank sum test was used for the analysis of difference between paired groups. Correlation between continuous variables was performed using the Spearmann’s correlation coefficient (r). A P value less than 0.05 was defined as statistically significant. All statistical analyses were performed using the SPSS statistical software package (SPSS 10.0 for Windows, SPSS, Inc, Chicago, IL, USA).
RESULTS
Serum IP-10, Mig and tumor IP-10 levels in HCC patients
Serum IP-10 and Mig levels were significantly elevated in HCC patients compared with normal healthy subjects (Figure 1). In HCC patients, serum IP-10 level was significantly correlated with Mig level (correlation coefficient = 0.631, P<0.0001). Furthermore, analysis of tissue IP-10 levels indicated that tumor IP-10 level was significantly higher than that of the adjacent non-tumor tissues (Figure 2).
Figure 1.
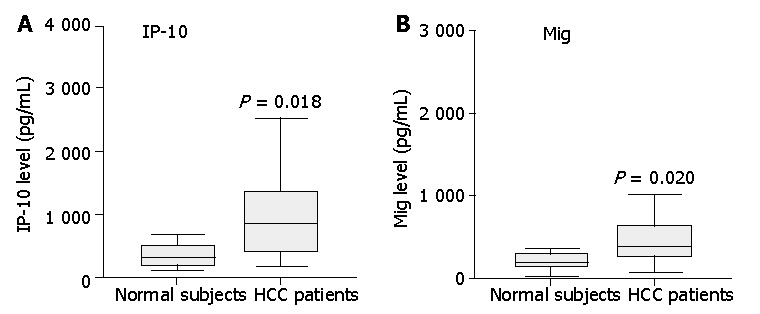
Assay of IP-10 and Mig in serum from 35 patients and 12 healthy controls by cytometric bead array analysis. Serum levels of IP-10 and Mig in patients with HCC were significantly higher than those of healthy controls. Data are presented as boxplots as medians and the 25th, 75th and 90th percentiles, P<0.05.
Figure 2.
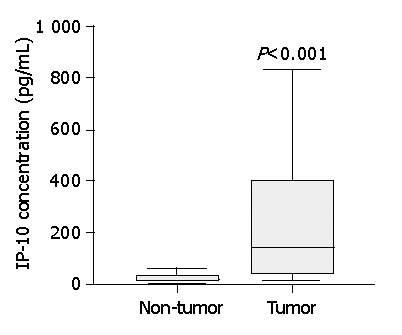
Boxplots show significant increase of tumor IP-10 level compared to that of non-tumor liver tissue. Data are presented as boxplots as medians and the 25th, 75th and 90th percentiles, n = 32, P<0.001.
HCC tumor cell lines secreted functional chemokines
IP-10 and Mig were also detected in the supernatants of tumor cells Hep3B and HuH 7 whilst immortalized hepatocytes produced negligible levels, thereby providing further evidence that HCC tumor tissue might be a likely source of the elevated serum levels of chemokines in HCC patients (Figure 3). We tested the chemotactic effect of tumor cell supernatants upon normal PBLs. Both CD4+ and CD8+ T cells exhibited a significant chemotactic response to Hep3B supernatants but not to supernatants of the control liver cell line MIHA. This chemotactic response was demonstrated to be CXCR3 specific since pre-incubation of T cells with a function blocking anti-CXCR3 monoclonal antibody (mAb) significantly reduced both the CD4+ and CD8+ T cell chemotactic activity of the Hep3B supernatants compared to isotype control mAb (Figure 4).
Figure 3.
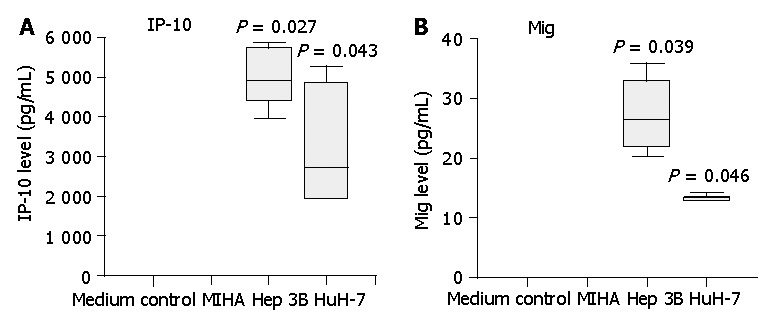
Secretion of IP-10 and Mig by HCC cell lines Hep3B and HuH-7, but not by control liver cell line MIHA. Three-day cell culture supernatants were collected and IP-10, Mig levels were quantified by cytometric bead array analysis. Significantly higher levels of IP-10 and Mig were detected in HCC cell line supernatants compared with those of control cell line MIHA. Data are presented as boxplots as medians and the 25th, 75th and 90th percentiles, aP<0.05.
Figure 4.
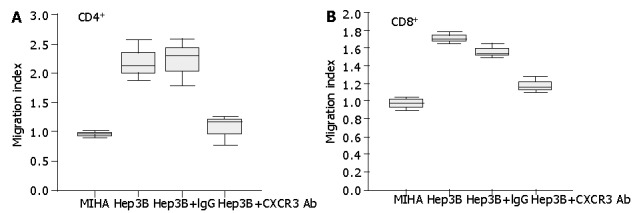
Secretion of chemotactic factors by Hep3B cells for lymphocytes from healthy subjects in vitro. The ability of culture supernatants from Hep3B cells to induce CD4+ (A) and CD8+ (B) T cell chemotaxis was tested by chemotactic experiments as described in Materials and Methods. To test the effect of blocking CXCR3 on the chemotactic response of lymphocytes to Hep3B culture supernatants, lymphocytes were pretreated with anti-CXCR3 monoclonal antibody or IgG control before the chemotaxis assay. Results are expressed as migration index, calculated as described in Materials and Methods.
We also studied the effect of tumor cell culture supernatants on the phosphorylation of ERK in normal PBLs. Stimulation of normal PBLs for 10 min with tumor cell supernatants but not immortalized liver cell supernatant induced the transient phosphorylation of ERK. However, prolonged overnight stimulation of normal PBLs with tumor cell culture supernatants inhibited ERK phosphorylation. Cells stimulated with IP-10 at 100 nmol/L for 10 min or overnight were included as controls (Figure 5).
Figure 5.

Early activation and later inhibition of ERK phosphorylation induced by tumor cell culture supernatants in lymphocytes from normal controls. A total of 5×106 freshly isolated lymphocytes were incubated with 500 μL of culture supernatants or IP-10 at 100 nmol/L in a 37 °C incubator for either 10 min or overnight. The cells were lysed and ERK phosphorylation was determined by Western blotting. The SDS-PAGE profile represents one experiment out of three.
Proportion of CXCR3-expressing T lymphocytes was downregulated by tumor-conditioned medium and tumor patient serum
Following incubation with medium conditioned by the tumor cell lines Hep3B and HuH-7 for 20 h, the proportion of CD4+ and CD8+ T cells from normal healthy subjects that expressed the chemokine receptor CXCR3 was significantly reduced (Figures 6A,B,P<0.05). However, such an effect was not observed following incubation with medium conditioned by the control cell line MIHA for 20 h (Figure 6). Furthermore, the proportion of CD4+ and CD8+ T cells from normal healthy subjects that expressed the chemokine receptor CXCR3 was significantly reduced following incubation with medium supplemented with 10% serum from HCC patients for 24 h (Figures 7A,B,P<0.05). Such an effect was not observed following incubation with medium containing the serum of normal subjects for 24 h (Figure 7).
Figure 6.
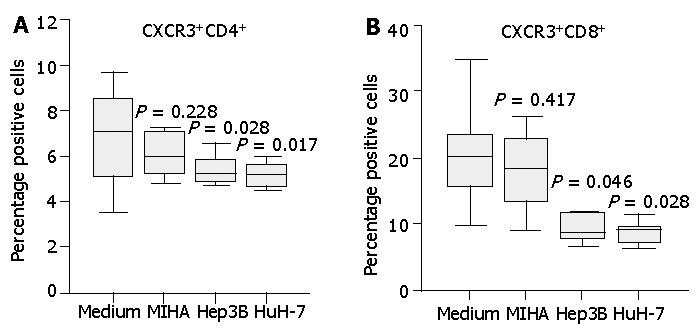
Expression of CXCR3 on normal CD4+ or CD8+ T cells incubated with unconditioned medium or medium conditioned with the tumor cell lines or a control cell line. Cell culture supernatants conditioned for 3 d by the HCC cell lines Hep3B, HuH-7 or the control cell line MIHA were collected. Freshly isolated normal PBLs were incubated in unconditioned or conditioned medium for 20 h and T cell expression of CXCR3 was assessed by flow cytometry. Diminished CXCR3 expression on CD4+ T cells (A) and CD8+ T cells (B) was observed following incubation in HCC cell line supernatants whilst the control cell line supernatant had no significant effect. Data represent the median and the 25th, 75th and 90th percentiles, n = 8, aP<0.05 vs medium control.
Figure 7.
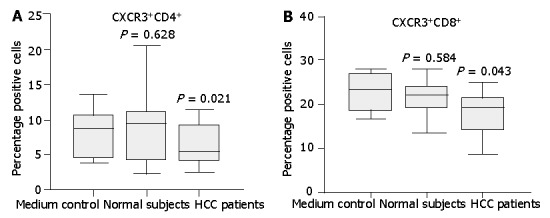
Expression of CXCR3 on normal CD4+ or CD8+ T cells incubated with medium supplemented with 10% serum from either HCC patients or normal subjects. Normal T cells cultured in the full medium with 10% FCS served as medium control. After 24 h, surface expression of CXCR3 on T cells was assessed by flow cytometry. Diminished CXCR3 expression on CD4+ T cells (A) and CD8+ T (B) cells was observed after incubation with HCC patient serum compared with normal serum. Data represent the median and the 25th, 75th and 90th percentiles, n = 9, aP<0.05.
Migration of T lymphocytes toward chemokine IP-10 was reduced in HCC patients
To determine whether the reduced CXCR3 expression on lymphocytes was biologically significant, we examined the migration of T cells toward the corresponding chemokine ligands. As shown in Figure 8A, CD4+ T cells from patients with HCC failed to display a significant migratory response to the CXCR3 ligand IP-10. In contrast, CD4+ T cells from healthy controls exhibited a significant chemotactic response to IP-10 (P<0.01). Similar results were obtained when CD8+ T cells derived from HCC cases were studied, but no significant migratory activity toward IP-10 was observed (Figure 8B). In contrast to HCC cases, CD8+ T cells from normal controls displayed a significant chemotactic response to IP-10 (Figure 8B,P<0.01).
Figure 8.
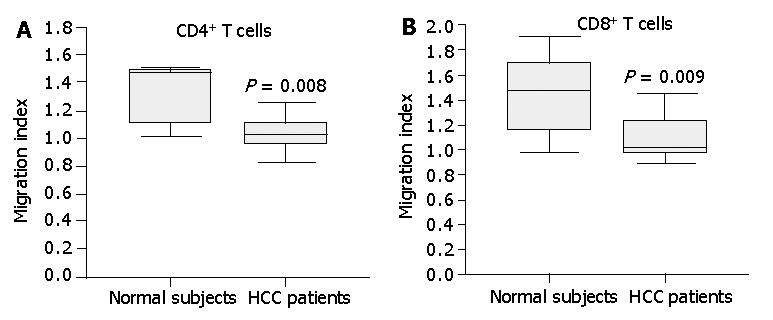
Chemotactic responsiveness of CD4+ T cells and CD8+T cells to IP-10. CD4+ T cells and CD8+T cells from HCC patients did not exhibit a significant chemotactic response toward IP-10 compared to CD4+ T cells and CD8+T cells from normal subjects. Results are expressed as migration index, calculated as described in Materials and Methods. Data are the median and the 25th, 75th and 90th percentiles, n = 7, aP<0.05.
DISCUSSION
The low proportion of HCC exhibiting a significant lymphocyte infiltration compared with some other cancers is an intriguing phenomenon, and might well be related to the generally poor prognosis of HCC patients[6,7]. In this study, we attempted to elucidate the role of chemokines and chemokine receptors in the regulation of lymphocyte migration in HCC. We measured the serum levels of chemokines in HCC patients and compared them to normal control subjects. The serum levels of IP-10 and Mig were significantly elevated in HCC patients. Furthermore, serum IP-10 level was significantly positively correlated with serum Mig level in HCC cases. In addition, the IP-10 level in tumor tissues was significantly higher than that in noninvolved adjacent liver tissues. Lastly, analysis of cell culture supernatants indicated that HCC tumor cell lines secreted a significant amount of IP-10 and Mig that was functionally active in chemotactic assays.
The interaction between chemokines and chemokine receptors is a key determinant for the selectivity of local immunity. We therefore investigated whether tumor-cell-derived chemokines acted to direct lymphocyte movements. Our data indicated that the tumor cell line Hep3B secreted functional chemotactic factors for normal lymphocytes. Furthermore, function blocking monoclonal antibody to CXCR3 could partially inhibit lymphocyte chemotaxis, indicating the involvement of ligands to CXCR3. Treatment of lymphocytes with IP-10 resulted in rapid ERK phosphorylation, but the activation was transient and rapidly desensitized[30]. Interestingly, we found that exposure of normal lymphocytes to tumor cell culture supernatants resulted in rapid phosphorylation of tyrosine residues on ERK but inhibition of ERK activation after prolonged stimulation, an effect that may well be secondary to the presence of IP-10 in the tumor cell supernatant. It was previously documented that phosphorylation of ERK was linked to cell migration, adhesion, proliferation and survival[31,32]. Therefore, it is possible that sustained exposure of lymphocytes to IP-10 secreted by tumors might inhibit lymphocyte function. To investigate this hypothesis, we analyzed the chemokine receptor expression following incubation of normal PBLs with tumor cell conditioned medium. This treatment led to the reduction in the proportion of both CD4+ and CD8+ T cells with surface expression of CXCR3, thereby suggesting that HCC might secret factors that reduce chemokine receptor expression on PBLs. Previous work indicated that the chemokine receptor CXCR3 on PBLs could be modulated by internalization and desensitization following interaction with their chemokine ligands[33], and that chemokine receptors could be desensitized by either high levels of chemokines or prolonged exposure to chemokines[34]. We therefore speculated that the reduced surface expression of CXCR3 on normal PBLs after exposure to tumor- cell-conditioned medium might partially be due to the desensitization of lymphocytes by tumor-cell-derived chemokines. To further test this hypothesis, we also examined the effect of serum from either HCC patients or normal control subjects upon lymphocyte CXCR3 expression. Our data indicated that normal lymphocytes exhibited a reduced lymphocyte expression of CXCR3 after treatment with HCC patient serum compared to normal serum. These data resonate with the work by Kurt et al[35] who pointed out that T cells from naive animals demonstrated a greater chemotactic response to tumor-derived chemokines compared to T cells from tumor-bearing mice, suggesting the presence of chemokine receptor desensitization in the tumor bearing mice.
Undoubtedly, there is an inadequate induction of tumor immunity in HCC patients. Indeed, the function of peripheral blood dendritic cells was also decreased in this patient group[36]. We demonstrated that CXCR3 expression on PBLs from HCC patients was reduced compared to that of healthy control subjects[37]. Furthermore, we demonstrated that the reduced CXCR3 expression in HCC correlated with decreased migratory responses of CD4+ and CD8+ T cells toward chemokine IP-10. Chemokine receptor expression level is critically important in lymphocyte function[29], and our results therefore indicated that impaired migratory function of peripheral lymphocytes in HCC patients was due to decreased chemokine receptor expression. Despite the presence of lymphocyte recruitment likely mediated by CCR5, CCR6 and CXCR3[37], the levels of infiltrating lymphocytes might still be inadequate. Indeed, elevated levels of circulating CXCR3 ligands can act to induce peripheral desensitization of circulating T cells and this could limit further on-going lymphocyte recruitment. This may well underlie the somewhat paradoxical combination of chemokine production by HCC tumor tissue in the absence of marked lymphocytic infiltration of HCC.
In conclusion, this study demonstrated elevated serum levels of IP-10 and Mig in HCC patients as well as increased IP-10 concentration in tumor tissues compared to adjacent non-tumor tissues. These findings are associated with the reduced migratory function of peripheral blood T cells in HCC patients and our data suggest that this is likely to be a consequence of desensitization of the chemokine receptor CXCR3 by prolonged exposure to a high level of circulating CXCR3 ligands. T-cell based immunotherapy provides a great future potential for treatment of cancer including HCC[38]. The insufficient anti-tumor response in HCC patients may in part lie in the deficient expression of appropriate chemokine receptors by T cells. Chemokines are expressed by a range of tumors including HCC and may serve as suitable targets for directing specific T cells toward the tumor[39]. Chemokine receptors such as CCR5 play a critical role in T cell migration to cancer and subsequent induction of tumor regression[40]. However, the reduced expression of specific chemokine receptors on peripheral lymphocytes in HCC patients and the resultant loss of lymphocyte responsiveness to tumor-derived chemokines may partially ‘blind’ them to the presence of life threatening tumors. The clinical challenge is therefore to find novel approaches to augment the specific chemokine receptor expression of lymphocytes in HCC patients.
ACKNOWLEDGEMENTS
The authors thank Professor David Adams (Liver Research Laboratories, MRC Centre for Immune Regulation, The University of Birmingham) for his suggestion.
Footnotes
Supported by the Sun C. Y. Research Foundation for Hepatobiliary and Pancreatic Surgery of the University of Hong Kong
Edited by Wang XL Proofread by Zhu LH
References
- 1.Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445–454. doi: 10.3748/wjg.v7.i4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watanabe H, Enjoji M, Imai T. Gastric carcinoma with lymphoid stroma. Its morphologic characteristics and prognostic correlations. Cancer. 1976;38:232–243. doi: 10.1002/1097-0142(197607)38:1<232::aid-cncr2820380135>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 3.Svennevig JL, Lunde OC, Holter J, Bjørgsvik D. Lymphoid infiltration and prognosis in colorectal carcinoma. Br J Cancer. 1984;49:375–377. doi: 10.1038/bjc.1984.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimokawara I, Imamura M, Yamanaka N, Ishii Y, Kikuchi K. Identification of lymphocyte subpopulations in human breast cancer tissue and its significance: an immunoperoxidase study with anti-human T- and B-cell sera. Cancer. 1982;49:1456–1464. doi: 10.1002/1097-0142(19820401)49:7<1456::aid-cncr2820490724>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Itoh K, Platsoucas CD, Balch CM. Autologous tumor-specific cytotoxic T lymphocytes in the infiltrate of human metastatic melanomas. Activation by interleukin 2 and autologous tumor cells, and involvement of the T cell receptor. J Exp Med. 1988;168:1419–1441. doi: 10.1084/jem.168.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998;27:407–414. doi: 10.1002/hep.510270214. [DOI] [PubMed] [Google Scholar]
- 7.Ng IO, Lai EC, Fan ST, Ng MM, So MK. Prognostic significance of pathologic features of hepatocellular carcinoma. A multivariate analysis of 278 patients. Cancer. 1995;76:2443–2448. doi: 10.1002/1097-0142(19951215)76:12<2443::aid-cncr2820761207>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 8.Shrikant P, Mescher MF. Control of syngeneic tumor growth by activation of CD8+ T cells: efficacy is limited by migration away from the site and induction of nonresponsiveness. J Immunol. 1999;162:2858–2866. [PubMed] [Google Scholar]
- 9.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Overwijk WW, Lee DS, Surman DR, Irvine KR, Touloukian CE, Chan CC, Carroll MW, Moss B, Rosenberg SA, Restifo NP. Vaccination with a recombinant vaccinia virus encoding a "self" antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4(+) T lymphocytes. Proc Natl Acad Sci USA. 1999;96:2982–2987. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ossendorp F, Toes RE, Offringa R, van der Burg SH, Melief CJ. Importance of CD4(+) T helper cell responses in tumor immunity. Immunol Lett. 2000;74:75–79. doi: 10.1016/s0165-2478(00)00252-2. [DOI] [PubMed] [Google Scholar]
- 12.Adams DH, Shaw S. Leucocyte-endothelial interactions and regulation of leucocyte migration. Lancet. 1994;343:831–836. doi: 10.1016/s0140-6736(94)92029-x. [DOI] [PubMed] [Google Scholar]
- 13.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 14.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 15.Gale LM, McColl SR. Chemokines: extracellular messengers for all occasions? Bioessays. 1999;21:17–28. doi: 10.1002/(SICI)1521-1878(199901)21:1<17::AID-BIES3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Hedrick JA, Zlotnik A. Chemokines and lymphocyte biology. Curr Opin Immunol. 1996;8:343–347. doi: 10.1016/s0952-7915(96)80123-3. [DOI] [PubMed] [Google Scholar]
- 17.Mackay CR. Chemokine receptors and T cell chemotaxis. J Exp Med. 1996;184:799–802. doi: 10.1084/jem.184.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao F, Rabin RL, Yannelli JR, Koniaris LG, Vanguri P, Farber JM. Human Mig chemokine: biochemical and functional characterization. J Exp Med. 1995;182:1301–1314. doi: 10.1084/jem.182.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taub DD, Longo DL, Murphy WJ. Human interferon-inducible protein-10 induces mononuclear cell infiltration in mice and promotes the migration of human T lymphocytes into the peripheral tissues and human peripheral blood lymphocytes-SCID mice. Blood. 1996;87:1423–1431. [PubMed] [Google Scholar]
- 20.Uguccioni M, Gionchetti P, Robbiani DF, Rizzello F, Peruzzo S, Campieri M, Baggiolini M. Increased expression of IP-10, IL-8, MCP-1, and MCP-3 in ulcerative colitis. Am J Pathol. 1999;155:331–336. doi: 10.1016/S0002-9440(10)65128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flier J, Boorsma DM, van Beek PJ, Nieboer C, Stoof TJ, Willemze R, Tensen CP. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398–405. doi: 10.1002/1096-9896(200108)194:4<397::aid-path899>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 22.Belperio JA, Keane MP, Burdick MD, Lynch JP, Xue YY, Li K, Ross DJ, Strieter RM. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J Immunol. 2002;169:1037–1049. doi: 10.4049/jimmunol.169.2.1037. [DOI] [PubMed] [Google Scholar]
- 23.Narumi S, Tominaga Y, Tamaru M, Shimai S, Okumura H, Nishioji K, Itoh Y, Okanoue T. Expression of IFN-inducible protein-10 in chronic hepatitis. J Immunol. 1997;158:5536–5544. [PubMed] [Google Scholar]
- 24.Nishioji K, Okanoue T, Itoh Y, Narumi S, Sakamoto M, Nakamura H, Morita A, Kashima K. Increase of chemokine interferon-inducible protein-10 (IP-10) in the serum of patients with autoimmune liver diseases and increase of its mRNA expression in hepatocytes. Clin Exp Immunol. 2001;123:271–279. doi: 10.1046/j.1365-2249.2001.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apolinario A, Majano PL, Alvarez-Pérez E, Saez A, Lozano C, Vargas J, García-Monzón C. Increased expression of T cell chemokines and their receptors in chronic hepatitis C: relationship with the histological activity of liver disease. Am J Gastroenterol. 2002;97:2861–2870. doi: 10.1111/j.1572-0241.2002.07054.x. [DOI] [PubMed] [Google Scholar]
- 26.Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999;163:6236–6243. [PubMed] [Google Scholar]
- 27.Haydon G, Lalor PF, Hubscher SG, Adams DH. Lymphocyte recruitment to the liver in alcoholic liver disease. Alcohol. 2002;27:29–36. doi: 10.1016/s0741-8329(02)00208-2. [DOI] [PubMed] [Google Scholar]
- 28.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunkel EJ, Boisvert J, Murphy K, Vierra MA, Genovese MC, Wardlaw AJ, Greenberg HB, Hodge MR, Wu L, Butcher EC, et al. Expression of the chemokine receptors CCR4, CCR5, and CXCR3 by human tissue-infiltrating lymphocytes. Am J Pathol. 2002;160:347–355. doi: 10.1016/S0002-9440(10)64378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tilton B, Ho L, Oberlin E, Loetscher P, Baleux F, Clark-Lewis I, Thelen M. Signal transduction by CXC chemokine receptor 4. Stromal cell-derived factor 1 stimulates prolonged protein kinase B and extracellular signal-regulated kinase 2 activation in T lymphocytes. J Exp Med. 2000;192:313–324. doi: 10.1084/jem.192.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stupack DG, Cho SY, Klemke RL. Molecular signaling mechanisms of cell migration and invasion. Immunol Res. 2000;21:83–88. doi: 10.1385/IR:21:2-3:83. [DOI] [PubMed] [Google Scholar]
- 32.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 33.Sauty A, Colvin RA, Wagner L, Rochat S, Spertini F, Luster AD. CXCR3 internalization following T cell-endothelial cell contact: preferential role of IFN-inducible T cell alpha chemoattractant (CXCL11) J Immunol. 2001;167:7084–7093. doi: 10.4049/jimmunol.167.12.7084. [DOI] [PubMed] [Google Scholar]
- 34.Morris AJ, Malbon CC. Physiological regulation of G protein-linked signaling. Physiol Rev. 1999;79:1373–1430. doi: 10.1152/physrev.1999.79.4.1373. [DOI] [PubMed] [Google Scholar]
- 35.Kurt RA, Baher A, Wisner KP, Tackitt S, Urba WJ. Chemokine receptor desensitization in tumor-bearing mice. Cell Immunol. 2001;207:81–88. doi: 10.1006/cimm.2000.1754. [DOI] [PubMed] [Google Scholar]
- 36.Kakumu S, Ito S, Ishikawa T, Mita Y, Tagaya T, Fukuzawa Y, Yoshioka K. Decreased function of peripheral blood dendritic cells in patients with hepatocellular carcinoma with hepatitis B and C virus infection. J Gastroenterol Hepatol. 2000;15:431–436. doi: 10.1046/j.1440-1746.2000.02161.x. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Poon RT, Feng X, Yu WC, Luk JM, Fan ST. Reduced expression of chemokine receptors on peripheral blood lymphocytes in patients with hepatocellular carcinoma. Am J Gastroenterol. 2004;99:1111–1121. doi: 10.1111/j.1572-0241.2004.30265.x. [DOI] [PubMed] [Google Scholar]
- 38.Jones RL, Young LS, Adams DH. Immunotherapy in hepatocellular carcinoma. Lancet. 2000;356:784–785. doi: 10.1016/S0140-6736(00)02648-9. [DOI] [PubMed] [Google Scholar]
- 39.Kershaw MH, Wang G, Westwood JA, Pachynski RK, Tiffany HL, Marincola FM, Wang E, Young HA, Murphy PM, Hwu P. Redirecting migration of T cells to chemokine secreted from tumors by genetic modification with CXCR2. Hum Gene Ther. 2002;13:1971–1980. doi: 10.1089/10430340260355374. [DOI] [PubMed] [Google Scholar]
- 40.Uekusa Y, Yu WG, Mukai T, Gao P, Yamaguchi N, Murai M, Matsushima K, Obika S, Imanishi T, Higashibata Y, et al. A pivotal role for CC chemokine receptor 5 in T-cell migration to tumor sites induced by interleukin 12 treatment in tumor-bearing mice. Cancer Res. 2002;62:3751–3758. [PubMed] [Google Scholar]


