Abstract
AIM: To investigate the correlation between systemic hemodynamics and splanchnic circulation in recipients with cirrhosis undergoing living-donor liver transplantation (LDLT), and to clarify how systemic hemodynamics impact on local graft circulation after LDLT.
METHODS: Systemic hemodynamics, indocyanine green (ICG) elimination rate (KICG) and splanchnic circulation were simultaneously and non-invasively investigated by pulse dye densitometry (PDD) and ultrasound. Accurate estimators of optimal systemic hyperdynamics after LDLT [i.e., balance of cardiac output (CO) to blood volume (BV) and mean transit time (MTT), defined as the time required for half the administered ICG to pass through an attached PDD sensor in the first circulation] were also measured. Thirty recipients with cirrhosis were divided into two groups based on clinical outcomes corresponding to postoperative graft function.
RESULTS: Cirrhotic systemic hyperdynamics characterized by high CO, expanded BV and low total peripheral resistance (TPR) were observed before LDLT. TPR reflecting cirrhotic vascular alterations was slowly restored after LDLT in both groups. Although no significant temporal differences in TPR were detected between the two groups, CO/BV and MTT differed significantly. Recipients with good outcomes showed persistent cirrhotic systemic hyperdynamics after LDLT, whereas recipients with poor outcomes presented with unstable cirrhotic systemic hyperdynamics and severely decreased KICG. Systemic hyperdynamic disorders after LDLT impacted on portal venous flow but not hepatic arterial flow.
CONCLUSION: We conclude that subtle systemic hyperdynamics disorders impact on splanchnic circulation, and that an imbalance between CO and BV decreases portal venous flow, which results in critical outcomes.
Keywords: Cirrhosis, Hyperdynamic, Portal hypertension, Splanchnic, Indocyanine green
INTRODUCTION
We previously demonstrated that systemic hemodynamics affecting postoperative graft function are crucial for living-donor liver transplantation (LDLT)[1]. However, the relationship between systemic hemodynamic parameters and splanchnic circulation after LDLT remains to be fully elucidated. In particular, the influence of the systemic hemodynamic state on splanchnic circulation is unclear. Therefore, we carried out a detailed investigation of systemic and splanchnic hemodynamic behavior after LDLT in adult recipients with cirrhosis.
Prior to undergoing LDLT, recipients with cirrhosis generally develop peculiar systemic and splanchnic hemodynamics due to portal hypertension[2-4]. To ascertain correlations between systemic hemodynamics and splanchnic circulation, and to clarify how the systemic hemodynamic state impacts on the local graft circulation, we performed simultaneous assessments of systemic hemodynamics and directly measured splanchnic circulation by systemic dye distribution and ultrasound. We also determined the hemodynamic state required for an excellent clinical outcome corresponding to good graft function.
MATERIALS AND METHODS
Patients
From June 2003 to March 2006, indocyanine green (ICG) pharmacokinetics were analyzed using a non-invasive method in 30 adult recipients (average age 53.1 ± 9.3 years; 25 males, five females) who underwent orthotopic LDLT at Mie University Hospital. As well, splanchnic circulatory parameters were simultaneously assessed using Doppler ultrasound. All 30 patients received a right-lobe liver graft. Clinical diagnoses were 26 cases of liver cirrhosis with hepatitis B or C (18 complicated by hepatocellular carcinoma), two cases of biliary atresia (result of postoperative state of Kasai’s operation at childhood), and one case each of primary sclerosing cholangitis and alcoholic liver cirrhosis. All recipients were diagnosed with liver cirrhosis, based on histopathological examination of resected specimens. ABO blood group compatibility was identical in 24 recipients and compatible in six. The operative procedures and immunosuppression protocols used in our institute have been described in detail elsewhere[1,5-8]. All the protocols used in the present study were approved by the Ethics Review Committee for Human Studies of Mie University Graduate School of Medicine (Tsu, Mie, Japan), based on the Ethical Guidelines of the Helsinki Declaration of 1975. Informed consent was obtained from all patients before enrollment.
ICG, pulse dye densitometry (PDD) and analytical procedures
ICG is widely used for analysis of liver function[9,10]. Furthermore, the dye dilution curve of ICG can be used for measuring hemodynamic parameters[9,11]. A non-invasive method for measuring systemic hemodynamic parameters using ICG has been reported[12] and is relatively reliable compared with invasive ones[11,13-15]. It is also advantageous for clinical use because it is simple to use at the bedside, has quick real-time presentation of results and is cost-effective[16,17]. Hence, we used this non-invasive method in the present study.
ICG (Diagnogreen Inj., Daiichi Pharmaceutical, Tokyo, Japan), a non-toxic dye, has no known side effects other than a rare iodine allergy. Although a total of 630 ICG bolus injections were performed in the 30 recipients, no allergic responses or any other side effects were observed.
PDD, which measures the absorption of hemo-globin and ICG, is based on the principle of pulse spectrophotometry; the basic principles of which has been detailed elsewhere[11,12]. A PDD apparatus (DDG-2001; Nihon Kohden, Tokyo, Japan) was used to measure blood ICG concentrations and analyze dye densitography. A sensor was placed on the nose of each patient before ICG injection.
Twenty milligrams of ICG was injected through a peripheral cannula and immediately flushed with 20 mL normal saline[1,9,18]. PPD measurements were obtained before LDLT and from 1 to 14 d and at 21 d and 28 d postoperatively. In particular, measurements were performed every 12 h until 72 h postoperatively, because the hemodynamic parameters showed marked changes during the early postoperative period.
Systemic hemodynamic parameters and ICG elimination rate
The following parameters were measured and calculated using the PDD apparatus with the patients in a settled recumbent position: cardiac output (CO, L/min), cardiac index (CI, L/min per m2), mean transit time (MTT, s, blood volume (BV, L), heart rate (HR, beats/min) and ICG elimination rate constant (KICG). MTT was defined as the time required for half the administered ICG to pass through the attached nasal sensor in the first circulation. Details of the above calculations have been described elsewhere[11,12,17]. Measurement of mean arterial pressure (MAP) was performed simultaneously with the PDD. MAP, calculated as MAP (mmHg) = (pulse pressure/3) + diastolic pressure, was measured using a standard manual method[19]. Total peripheral resistance (TPR) was subsequently calculated according to the following formula: TPR (dyne/s5 per cm) = MAP × 80/CO[19].
Doppler ultrasound and splanchnic hemodynamic measurements
Doppler ultrasound assessment of splanchnic hemodynamic parameters was conducted at the same time as PDD. Portal venous flow velocity (PVFVe), portal venous flow volume (PVFVo), hepatic arterial pulsatility index (HAPI), and hepatic arterial resistance index (HARI) were evaluated as splanchnic circulatory parameters. A Triplex Doppler ultrasound system (Prosound SSD-5000SV; ALOKA, Tokyo, Japan) and a convex probe (2-5 MHz; UST-9119; ALOKA) were used for the Doppler ultrasound assessment. The following parameters were measured at the extrahepatic but post-anastomosis area: (1) PVFVe (cm/s), representing the mean of the maximal flow velocity of the portal vein; (2) PVFVo (mL/min), calculated from a cross-sectional area, assuming a circular portal vein section, and the mean velocity; (3) HAPI, calculated from the Doppler trace over one cardiac cycle as: (peak systolic velocity-minimum velocity)/mean of maximal velocities; and (4) HARI, derived from the Doppler spectrum over one cardiac cycle according to: (peak systolic velocity-end diastolic velocity)/peak systolic velocity. The measurement methods for the above indices have been described in detail elsewhere[20-23].
Establishment of normal ranges of systemic hemodynamic parameters, KICG value and splanchnic circulatory parameters
To establish the normal ranges of the variables we investigated the variables using the above-described methods in seven donors before LDLT and in nine volunteers who agreed to the aims of this study. The data measured in these 16 healthy individuals represent the normal ranges of the parameters, and are shown in Table 1. The control population showed no significant differences in age or body surface area compared with the LDLT recipients (data not shown).
Table 1.
Systemic hemodynamic parameters, KICG values and splanchnic circulatory parameters before LDLT
| Parameters | Healthy individuals n = 16 | Group I n = 25 | Group II n = 5 |
| Systemic hemodynamics | |||
| CO (L/min) | 5.83 ± 1.52 | 6.87 ± 0.97a | 7.36 ± 1.07e |
| CI (L/min per m2) | 3.22 ± 0.71 | 4.10 ± 0.71b | 4.56 ± 0.58e |
| BV (L) | 3.40 ± 0.96 | 4.09 ± 0.51a | 4.40 ± 0.45e |
| CO/BV (/min) | 1.74 ± 0.28 | 1.69 ± 0.21 | 1.69 ± 0.28 |
| MTT (s) | 16.1 ± 2.3 | 16.5 ± 1.5 | 16.5 ± 1.2 |
| HR (beat/min) | 64.3 ± 9.9 | 77.9 ± 12.6b | 77.6 ± 9.8e |
| MAP (mmHg) | 89.3 ± 11.8 | 68.9 ± 6.5d | 70.8 ± 11.2e |
| TPR (dyne/s5 per cm) | 1275.1 ± 228.3 | 818.9 ± 166.7d | 785.3 ± 187.4e |
| ICG clearance test | |||
| KICG | 0.227 ± 0.076 | 0.037 ± 0.017d | 0.056 ± 0.038e |
| Splanchnic circulation | |||
| Portal vein | |||
| PVFVo (mL/min) | 1482.1 ± 335.6 | 327.3 ± 416.9d | 435.6 ± 592.6e |
| PVFVe (cm/s) | 45.1 ± 8.1 | 7.9 ± 12.8d | 10.5 ± 13.8f |
| Hepatic artery | |||
| HAPI | 0.95 ± 0.11 | 1.06 ± 0.28a | 1.16 ± 0.21e |
| HARI | 0.93 ± 0.26 | 1.04 ± 0.23a | 1.10 ± 0.10e |
There were no significant differences between Groups I and II in each parameter, respectively (P > 0.05, analyzed by Mann-Whitney’s U test ). Statistical differences between healthy individuals and Group I analyzed by Mann-Whitney’s U test (aP < 0.05,
P < 0.005,
P < 0.0005). Statistical differences between healthy individuals and Group II analyzed by Mann-Whitney’s U test (eP < 0.05,
P < 0.005). ICG: Indocyanine green; LDLT: Living-donor liver transplantation; CO: Cardiac output; CI: Cardiac index; BV: Blood volume; MTT: Mean transit time; HR: Heart rate; MAP: Mean arterial pressure; TPR: Total peripheral resistance; PVFVo: Portal veinous flow volume; PVFVe: Portal venous flow velocity; HAPI: Hepatic arterial pulsatility index; HARI: Hepatic arterial resistant index.
Computed tomographic (CT) volumetry of liver grafts and the standard liver volume (SLV)
In our institution, helical CT studies are routinely performed at 2 and 4 wk after LDLT. All 30 recipients underwent these studies after LDLT. The helical CT studies were conducted using a High Speed Advantage QX-1 (GE Medical Systems, Tokyo, Japan). The scanning parameters were 120 kV, 200 mA, collimation of 5 mm, and a table speed of 15 mm/rotation, with reconstruction increments of 5 mm. Graft volume was calculated by CT volumetry. SLV was calculated according to a previously described formula[24].
Technetium-99m-diethylenetriaminepenta-acetic acid-galactosyl-human serum albumin (99mTc-GSA) liver scintigraphy and ratio of liver to heart-plus-liver radioactivity at 15 min (LHL15)
Since asialoglycoprotein receptors on hepatocytes are characteristic of functional liver cells[25], 99mTc-GSA liver scintigraphy is used as a reliable assessment tool for functional hepatic volume[26]. A total of 60 measurements were performed in the 30 recipients at 2 and 4 wk after LDLT. After intravenous injection of 185 MBq of 99mTc-GSA (Nihon Medi-Physics, Nishinomiya, Japan), dynamic imaging was performed with the patient in the supine position using a large field-of-view gamma camera (GCA7200A; Toshiba, Tokyo, Japan). LHL15 was calculated by dividing the radioactivity of whole liver regions of interest (ROIs) by that of whole liver-plus-heart ROIs at 15 min after injection, as previously described[27].
Histopathological analysis and graft parenchymal damage score
In our institution, needle biopsies are performed after LDLT if necessary. Protocol biopsies are not performed because of the associated risks, such as hemorrhage[28]. In the present study, a total of 30 biopsy specimens from the 30 recipients were assessed within 4 wk after LDLT.
Tissue specimens were stained with hematoxylin-eosin using standard histopathological techniques, and reviewed by an experienced liver pathologist using a semi-quantitative scoring system for features of the graft parenchyma. The graft parenchymal damage score, representing liver damage, was calculated as the total of the following parenchymal feature scores: hepatocyte ballooning (0, no; 1, yes), hepatocyte necrosis (0, none; 1, small foci; 2, confluent areas; 3, bridging necrosis), congestion (0, no; 1, yes), the fraction of hepatocytes that contain microvesicular fat (0, none; 1, < 1/3 of hepatocytes, 2, between 1/3 and 2/3 of hepatocytes; 3, > 2/3 of hepatocytes), neutrophil aggregates (0, none; 1, minimal; 2, moderate; 3, extensive) and cholestasis (0, none; 1, mild; 2, moderate; 3, severe). The graft parenchymal damage score, which was modified from the score according to Neil et al[29], has been described in detail elsewhere[6].
Outcomes after LDLT
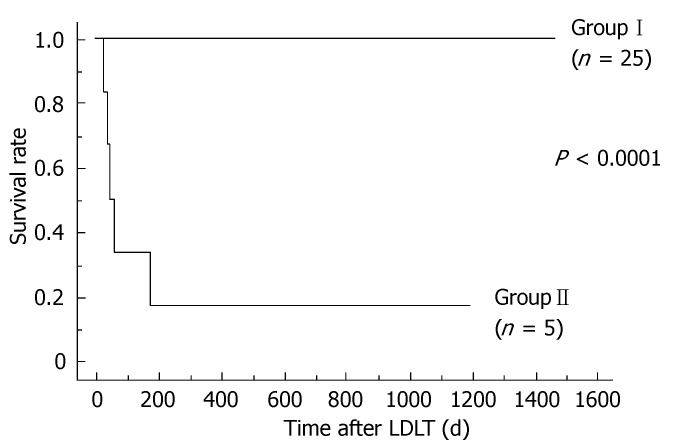
The clinical courses of all recipients were followed for 996.2 ± 436.5 d, ranging from 32 (patient died) to 1472 d after LDLT. The 30 recipients were retrospectively divided into two groups based on clinical outcomes corresponding to postoperative graft function. Although 25 recipients (Group I) presented with a good clinical course and excellent outcome, a subset of five recipients (Group II) required long-term intensive management, and finally died because of hepatic or extrahepatic reasons that originated from graft dysfunction with prolonged jaundice. Group II showed poor clinical outcome, and survival rate differed significantly between the two groups (P < 0.0001) (Figure 1).
Figure 1.
Survival rates after LDLT. The two lines represent the survival rates for Groups I and II. The P value analyzed by the log-rank test was < 0.0001.
Clinical profiles before, during and after LDLT
There were no significant differences in the clinical profiles before and during LDLT between the two groups. We considered that high portal venous pressure before removal of the native liver was due to portal hypertension. After LDLT, there was a significant difference in the length of stay in the intensive care unit between the two groups. Although there were no significant differences in SLV, LHL15 and graft parenchymal damage scores both differed significantly between the two groups (Table 2). Because LHL15 and graft parenchymal damage scores accurately reflect functional hepatocytes, these results clearly indicated graft dysfunction in Group II during the late postoperative period after LDLT.
Table 2.
Clinical profiles before, during and after LDLT
| Clinical profile | Group I(n = 25) | Group II(n = 5) | P valuea |
| Before LDLT | |||
| Age | 51.8 ± 9.8 | 58.6 ± 3.5 | NS |
| Body surface area (m2) | 1.69 ± 0.18 | 1.61 ± 0.12 | NS |
| Child-Pugh score (points) | 9.2 ± 2.3 | 10.8 ± 2.2 | NS |
| Model for end-stage liver disease score (points) | 17.6 ± 6.7 | 17.4 ± 7.1 | NS |
| During LDLT | |||
| Native liver weight (g) | 857.0 ± 227.5 | 946.0 ± 376.2 | NS |
| Portal venous pressure before removal of native liver (mmHg) | 21.5 ± 4.7 | 24.6 ± 7.1 | NS |
| Cold ischemic time (min) | 163.7 ± 79.0 | 139.6 ± 52.6 | NS |
| Warm ischemic time (min) | 55.1 ± 16.7 | 45.8 ± 12.7 | NS |
| Anhepatic phase (min) | 209.2 ± 104.9 | 184.4 ± 177.6 | NS |
| Operative time (min) | 899.4 ± 126.7 | 933.4 ± 131.0 | NS |
| Blood loss (mL) | 22515.7 ± 14200.5 | 22788.6 ± 19247.8 | NS |
| Graft weight (g) | 687.8 ± 124.6 | 632.0 ± 72.9 | NS |
| Graft-recipient weight ratio | 1.09 ± 0.21 | 1.23 ± 0.37 | NS |
| After LDLT | |||
| Intensive care unit stay (d) | 5.1 ± 1.9 | 35.6 ± 15.7 | < 0.005 |
| %SLV based on CT volumetry | |||
| 2 wk after LDLT | 1.14 ± 0.22 | 1.07 ± 0.09 | NS |
| 4 wk after LDLT | 1.05 ± 0.15 | 1.17 ± 0.16 | NS |
| LHL15 based on 99mTc-GSA liver scintigraphy | |||
| 2 wk after LDLT | 0.935 ± 0.026 | 0.846 ± 0.061 | < 0.005 |
| 4 wk after LDLT | 0.941 ± 0.017 | 0.751 ± 0.034 | < 0.005 |
| Histopathological fraft parenchymal damage score (points) | |||
| Within 4 wk after LDLT | 3.9 ± 1.4 | 10.6 ± 1.3 | < 0.005 |
Statistical differences between Groups I and II analyzed by Mann-Whitney’s U test (NS: P > 0.05). LDLT: Living-donor liver transplantation; SLV: Standard liver volume; CT: Computed tomographic; LHL15: The ratio of liver to heart-plus-liver radioactivity at 15 min; 99mTc-GSA: Technetium-99m-diethylenetriaminepenta-acetic acid-galactosyl-human serum albumin.
Statistical analysis
Results were expressed as means ± SD. For individually, temporally and repeatedly measured data, differences in the changes over time after LDLT between the two groups were analyzed by repeated-measures ANOVA[30,31]. Differences in unpaired discontinuous data between the two groups were analyzed by Mann-Whitney’s U test. Survival rates were calculated by the Kaplan-Meier method, and the log-rank test was used for between-group comparisons of recipient survival. All calculations were performed using Stat View-J 5.0 statistical software (SAS Institute, Cary, NC, USA) and values of P < 0.05 were considered significant.
RESULTS
Systemic hemodynamic states before LDLT and temporal differences in systemic hemodynamic parameters after LDLT
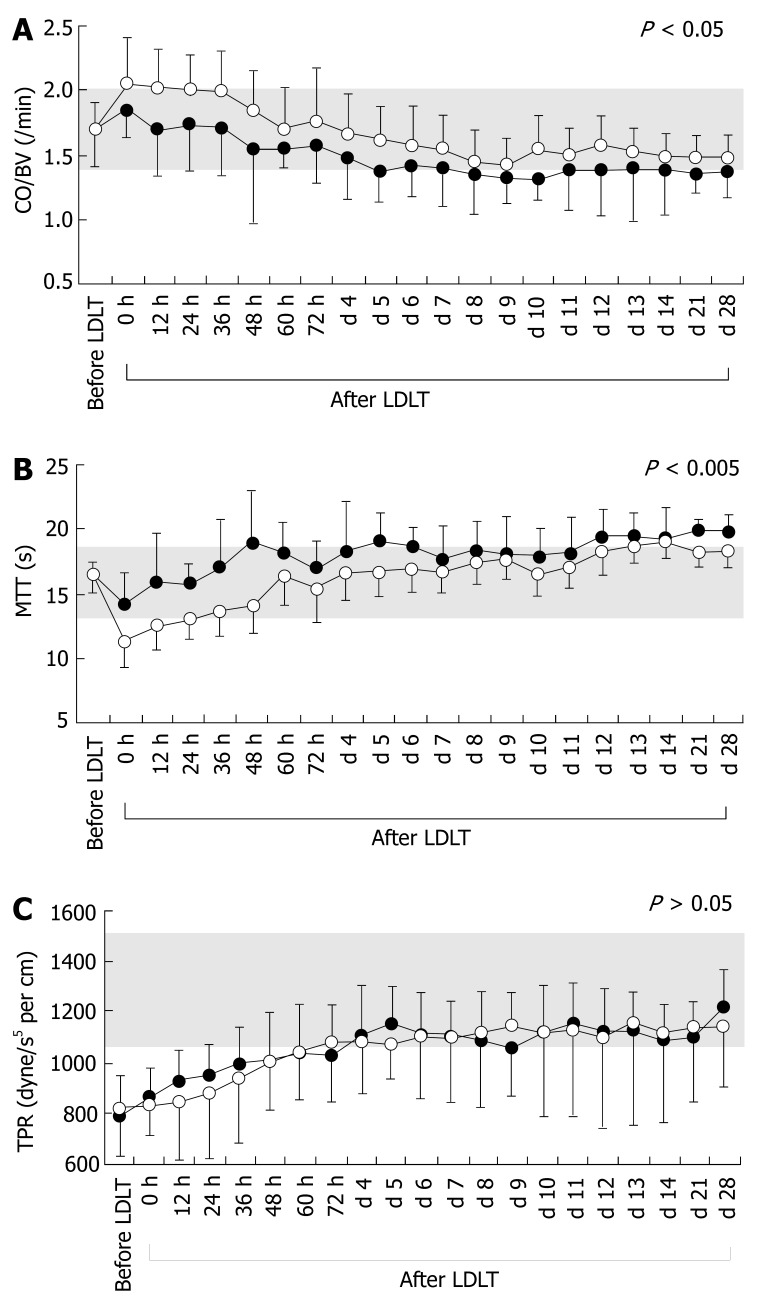
Cirrhotic systemic hemodynamics have been symbolized as hyperdynamic[1-4,32], and the hyperdynamic state characterized by high CO or CI, large BV, low TPR, mild tachycardia, and low or normal MAP[2,19,32-35]. Although hyperdynamic states were recognized in both groups, there were no significant differences between the two groups before LDLT. Interestingly, CO/BV and MTT were both constant before LDLT (Table 1). There were significant temporal differences after LDLT between the groups for CO/BV and MTT, but no significant differences in CO, CI, BV, HR, MAP or TPR (Table 3). The actual temporal changes in CO/BV, MTT and TPR are presented in Figure 2. When the absolute values of CO and BV in the recipients were compared with those of healthy individuals, recipients in Group I persisted in a hyperdynamic state after LDLT, while those in Group II showed a tendency to remain in a hyperdynamic state (actual temporal changes not shown). Thus, regardless of the outcome and graft function, the temporal changes in the absolute values of CO and BV between groups did not reach statistical significance. Therefore, as we have previously determined, detecting subtle disorders of optimal systemic hemodynamics in recipients with cirrhosis by comparing absolute values is not necessarily satisfactory (unpublished data). Indicators for peripheral resistance are thought to precisely reflect cirrhotic vascular alterations and the presence of collateral vessels and shunts[19,36,37]. It should be noted that the changes in TPR in the two groups exhibited similar patterns with no prompt restoration, despite normalization of the portal pressure after LDLT, and showed quite slow improvement (Figure 2C).
Table 3.
Statistical differences in post-operative temporal changes of systemic hemodynamic parameters, KICG values and splanchnic circulatory parameters
| Parameters | Statistical temporal differences after LDLT between Groups I and II P value1 |
| Systemic hemodynamics | |
| CO (L/min) | 0.2321 |
| CI (L/min per m2) | 0.5037 |
| BV (L) | 0.3420 |
| CO/BV (/min) | 0.0426a |
| MTT (s) | 0.0023b |
| HR (beat/min) | 0.0701 |
| MAP (mmHg) | 0.2453 |
| TPR (dyne/s5 per cm) | 0.8859 |
| ICG clearance test | |
| KICG | 0.0001d |
| Splanchnic circulation | |
| Portal vein | |
| PVFVo (mL/min) | 0.0113a |
| PVFVe (cm/s) | 0.0171a |
| Hepatic artery | |
| HAPI | 0.2504 |
| HARI | 0.4261 |
Statistical temporal differences between Groups I and II analyzed by repeated measures ANOVA (aP < 0.05,
P < 0.005,
P < 0.0005). ICG: Indocyanine green; LDLT: Living-donor liver transplantation; CO: Cardiac output; CI: Cardiac index; BV: Blood volume; MTT: Mean transit time; HR: Heart rate; MAP: Mean arterial pressure; TPR: Total peripheral resistance; PVFVo: Portal veinous flow volume; PVFVe: Portal venous flow velocity; HAPI: Hepatic arterial pulsatility index; HARI: Hepatic arterial resistant index.
Figure 2.
Temporal changes in systemic hemodynamic parameters before and after LDLT. A: Temporal changes in the ratio of CO to BV before and after LDLT; B: Temporal changes in MTT before and after LDLT; C: Temporal changes in TPR before and after LDLT. Open and closed circles represent systemic hemodynamic parameters for Groups I and II, respectively. Shaded areas show normal ranges measured in healthy individuals.
KICG before LDLT and differences in temporal changes in KICG after LDLT
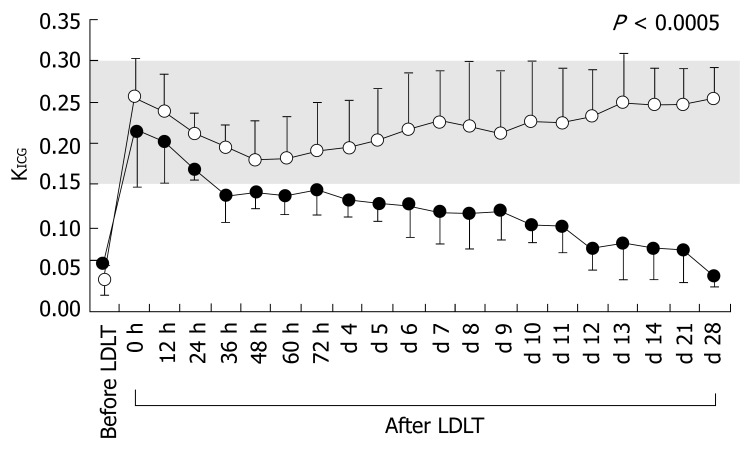
Recipients in both groups showed large decreases in KICG before LDLT (Table 1). Although there were no significant differences in KICG between the groups before LDLT, KICG changed significantly after LDLT (Figure 3, Table 3). The KICG value is dualistic, since it reflects functional hepatocytes and splanchnic blood flow[9,38-40]. However, splanchnic blood flow is a major determinant of KICG in normal liver[9,41,42]. We have previously demonstrated that KICG accurately evaluates functional hepatocytes during the late postoperative period, and sharply reflects splanchnic circulation during the early postoperative period, since LDLT restores functional hepatocyte volume drastically and immediately[1]. Extraordinary decreases in KICG from the early postoperative period were observed in Group II, in contrast to the findings for Group I. Therefore, in the present study we verified the detailed splanchnic circulatory parameters measured by Doppler ultrasound.
Figure 3.
Temporal changes in KICG before and after LDLT. Open and closed circles represent KICG values for Groups I and II, respectively. The shaded area shows the normal range measured in healthy individuals.
Splanchnic hemodynamics before LDLT and temporal differences in splanchnic circulatory parameters after LDLT
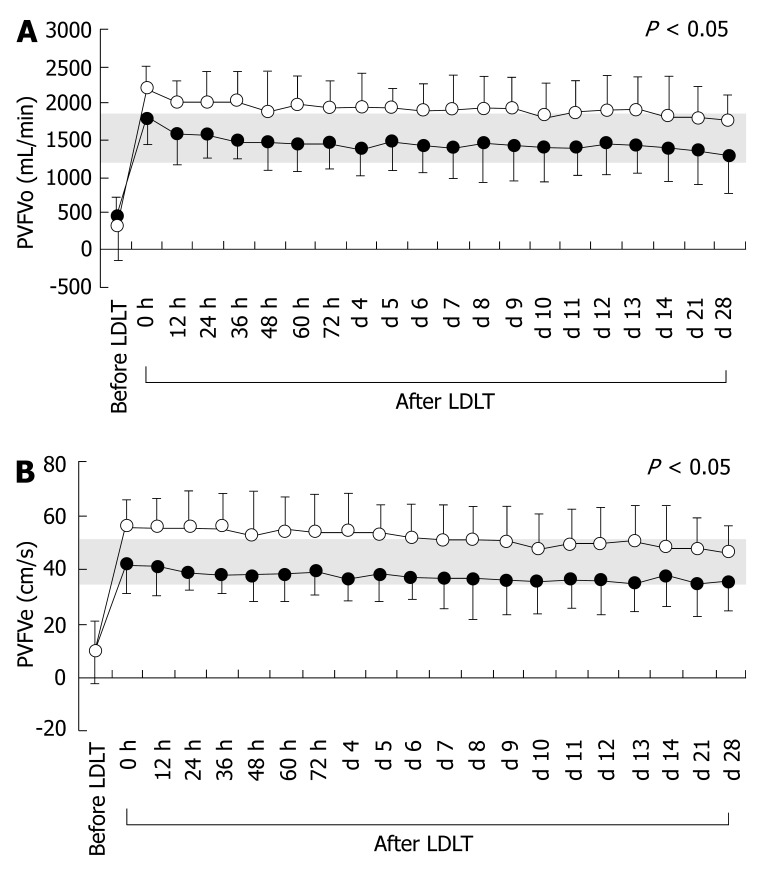
Cirrhotic splanchnic circulation is symbolized by decreased portal venous flow because of portal hypertension, despite a systemic hyperdynamic state. Although all splanchnic circulatory parameters in both groups before LDLT differed significantly from those in healthy individuals, there were no significant differences between the two groups (Table 1). However, after LDLT, there were significant temporal differences in PVFVo and PVFVe, but no significant differences in HAPI and HARI, between the two groups (Table 3). The actual temporal changes in PVFVo and PVFVe are shown in Figure 4. Interestingly, differences in portal venous parameters, but not hepatic arterial parameters, were observed.
Figure 4.
Temporal changes in splanchnic circulatory parameters before and after LDLT. A: Temporal changes in PVFVo before and after LDLT; B: Temporal changes in PVFVe before and after LDLT. Open and closed circles represent splanchnic circulatory parameters for Groups I and II, respectively. Shaded areas show normal ranges measured in healthy individuals.
DISCUSSION
Almost all adult recipients who undergo LDLT develop liver cirrhosis with long-term portal hypertension. Portal hypertension results in vascular dilatation and collateral pathways. Thus, various alterations in systemic hemodynamics and splanchnic circulation occur, and adult recipients often present characteristic hemodynamics before LDLT. Cirrhotic hemodynamic abnormalities were obviously present before LDLT in the present study.
Several investigators have demonstrated that the systemic hyperdynamic state remains despite normalization of liver function and restoration of portal pressure after LDLT[19,33,36,43-45], and have suggested that most systemic parameters are slowly restored to the normal range after LDLT[19,36]. In agreement with these suggestions, our results demonstrated that vascular alterations do not disappear within 4 wk after LDLT, regardless of the outcome. Thus, we have suggested that optimal persistence of a systemic hyperdynamic state after LDLT is necessary for successful outcomes in recipients with cirrhosis (unpublished data).
A cirrhotic systemic hyperdynamic state is symbolized by expanded BV, high CO and low TPR[3,9,32], and the preload focuses on the balance between CO and BV[46,47]. Thus, we suggest that the balance of CO to BV is an accurate estimator of the optimal stability of the characteristic systemic hyperdynamic state (unpublished data). On the other hand, to determine the systemic hemodynamic parameters related to liver transplantation, the MTT is a rigorous indicator of kinetic behavior circuits[1,9]. MTT values precisely reflect systemic hemodynamics, which are especially influenced by preload factors. That is, a greater CO is proportional to a shorter MTT, and a large BV is proportional to a prolonged MTT. Accordingly, CO/BV and MTT represent mirror images. The results presented here showed significant temporal differences between the two groups in these precise systemic hemodynamic parameters. Thus, we suggest that the recipients in Group II showed subtle disorders of the systemic hyperdynamic state after LDLT, in contrast to the recipients in Group I.
Other studies have focused on systemic hemody-namics or splanchnic circulation after LDLT, and some investigators have demonstrated that systemic hemodynamics are well correlated with the splanchnic circulation[41,44,48]. Interestingly, the results for the splanchnic circulatory parameters in the current study reveal that subtle disorders of the optimal systemic hyperdynamic state easily influence portal venous flow, rather than hepatic arterial flow. Vascular alterations because of portal hypertension develop in vessels that originally flow into the portal vein under normal portal pressure, and represent one of the causes of a large BV. Hence, we suggest that the imbalance between the greater CO and larger BV after LDLT in Group II caused stagnation of the tributary blood flow in the dilated vein and collateral pathways, which resulted in a decrease in portal venous flow. It was also of interest that recipients with cirrhosis with good outcomes (i.e., Group I) showed a clear tendency toward postoperative portal venous overflow compared with that in healthy individuals. We have previously demonstrated that the persistence of a systemic hyperdynamic state is indispensable for recipients with cirrhosis after LDLT (unpublished data), and therefore consider that excessive portal flow after LDLT seems to be correlated with a postoperative systemic hyperdynamic state. Since portal venous flow has been shown to have a large influence on liver regeneration after LDLT[49], we conclude that successful clinical outcomes in cirrhotic LDLT recipients can be attributed to optimal stability of the systemic hyperdynamic state, which yields sufficient portal venous flow. Based on our results for Group I as compared with Group II, we suggest that continuous sufficient portal venous flow, with even a slight surplus, supported by the optimal systemic hyperdynamic state, is necessary for good outcomes after LDLT in recipients with cirrhosis. Since reversible graft damage might begin slowly from the early postoperative period, we suggest that appropriate intensive clinical management of hemodynamics will greatly impact on further improvements in LDLT outcomes.
COMMENTS
Background
Prior to undergoing LDLT, recipients with cirrhosis generally develop peculiar systemic and splanchnic hemodynamics due to portal hypertension. To ascertain correlations between systemic hemodynamics and splanchnic circulation, we performed simultaneous assessment of systemic hemodynamics and directly measured splanchnic circulation by systemic dye distribution and ultrasound.
Research frontiers
We clarify how the systemic hemodynamic state impacts on the local graft circulation in recipients with cirrhosis who underwent LDLT. Vascular alterations due to portal hypertension develop in vessels that originally flow into the portal vein under normal portal pressure, and represent one of the causes of a large BV. Hence, we suggest that the imbalance between the greater CO and larger BV after LDLT caused stagnation of the tributary blood flow in the dilated veins and collateral pathways, which resulted in a decrease in portal venous flow.
Innovations and breakthroughs
We also identified the hemodynamic state required for an excellent clinical outcome after LDLT. Since portal venous flow has been shown to have a large influence on liver regeneration after LDLT, we suggest that successful clinical outcomes in LDLT recipients with cirrhosis can be attributed to optimal stability of the systemic hyperdynamic state, which yields sufficient portal venous flow.
Applications
The methods in this study (PDD and ultrasound) are advantageous for clinical applications because of their simplicity of bedside use, rapid real-time presentation of results, and cost-effectiveness. Hence, we suggest that appropriate intensive clinical management of hemodynamics based on real-time and reliable results measured by non-invasive methods will have a large impact on further improvements in LDLT outcomes.
Terminology
Splanchnic blood flow in this study refers to that in cirrhotic recipients after living-donor liver transplantation.
Peer review
This study builds on previous observations by the same group that hyperdynamic systemic circulation persists following transplantation in patients who previously had cirrhosis, and that this is important for sustaining portal venous flow. The current manuscript focuses on the changes with respect to splanchnic hemodynamics. The authors have demonstrated significant differences in portal venous flow dynamics between a group of 25 individuals that had a good clinical outcome post-transplantation compared with five that had a poor postoperative course. This article has sufficient originality regarding the understanding of post-liver transplant hemodynamics.
Footnotes
S- Editor Liu Y L- Editor Kerr C E- Editor Li HY
References
- 1.Hori T, Iida T, Yagi S, Taniguchi K, Yamamoto C, Mizuno S, Yamagiwa K, Isaji S, Uemoto S. K(ICG) value, a reliable real-time estimator of graft function, accurately predicts outcomes in adult living-donor liver transplantation. Liver Transpl. 2006;12:605–613. doi: 10.1002/lt.20713. [DOI] [PubMed] [Google Scholar]
- 2.Kowalski HJ, Abwlmann WH. The cardiac output at rest in Laennec's cirrhosis. J Clin Invest. 1953;32:1025–1033. doi: 10.1172/JCI102813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vorobioff J, Bredfeldt JE, Groszmann RJ. Increased blood flow through the portal system in cirrhotic rats. Gastroenterology. 1984;87:1120–1126. [PubMed] [Google Scholar]
- 4.Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1998;8:1151–1157. doi: 10.1002/hep.1840080532. [DOI] [PubMed] [Google Scholar]
- 5.Yagi S, Iida T, Taniguchi K, Hori T, Hamada T, Fujii K, Mizuno S, Uemoto S. Impact of portal venous pressure on regeneration and graft damage after living-donor liver transplantation. Liver Transpl. 2005;11:68–75. doi: 10.1002/lt.20317. [DOI] [PubMed] [Google Scholar]
- 6.Iida T, Yagi S, Taniguchi K, Hori T, Uemoto S, Yamakado K, Shiraishi T. Significance of CT attenuation value in liver grafts following right lobe living-donor liver transplantation. Am J Transplant. 2005;5:1076–1084. doi: 10.1111/j.1600-6143.2005.00799.x. [DOI] [PubMed] [Google Scholar]
- 7.Yagi S, Iida T, Hori T, Taniguchi K, Yamamoto C, Yamagiwa K, Uemoto S. Optimal portal venous circulation for liver graft function after living-donor liver transplantation. Transplantation. 2006;81:373–378. doi: 10.1097/01.tp.0000198122.15235.a7. [DOI] [PubMed] [Google Scholar]
- 8.Yamagiwa K, Yokoi H, Isaji S, Tabata M, Mizuno S, Hori T, Yamakado K, Uemoto S, Takeda K. Intrahepatic hepatic vein stenosis after living-related liver transplantation treated by insertion of an expandable metallic stent. Am J Transplant. 2004;4:1006–1009. doi: 10.1111/j.1600-6143.2004.00440.x. [DOI] [PubMed] [Google Scholar]
- 9.Niemann CU, Yost CS, Mandell S, Henthorn TK. Evaluation of the splanchnic circulation with indocyanine green pharmacokinetics in liver transplant patients. Liver Transpl. 2002;8:476–481. doi: 10.1053/jlts.2002.33218. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler HO, Cranston WI, Meltzer JI. Hepatic uptake and biliary excretion of indocyanine green in the dog. Proc Soc Exp Biol Med. 1958;99:11–14. doi: 10.3181/00379727-99-24229. [DOI] [PubMed] [Google Scholar]
- 11.Haruna M, Kumon K, Yahagi N, Watanabe Y, Ishida Y, Kobayashi N, Aoyagi T. Blood volume measurement at the bedside using ICG pulse spectrophotometry. Anesthesiology. 1998;89:1322–1328. doi: 10.1097/00000542-199812000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Iijima T, Aoyagi T, Iwao Y, Masuda J, Fuse M, Kobayashi N, Sankawa H. Cardiac output and circulating blood volume analysis by pulse dye-densitometry. J Clin Monit. 1997;13:81–89. doi: 10.1023/a:1007339924083. [DOI] [PubMed] [Google Scholar]
- 13.Iijima T, Iwao Y, Sankawa H. Circulating blood volume measured by pulse dye-densitometry: comparison with (131)I-HSA analysis. Anesthesiology. 1998;89:1329–1335. doi: 10.1097/00000542-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Imai T, Mitaka C, Nosaka T, Koike A, Ohki S, Isa Y, Kunimoto F. Accuracy and repeatability of blood volume measurement by pulse dye densitometry compared to the conventional method using 51Cr-labeled red blood cells. Intensive Care Med. 2000;26:1343–1349. doi: 10.1007/s001340000618. [DOI] [PubMed] [Google Scholar]
- 15.Bremer F, Schiele A, Tschaikowsky K. Cardiac output measurement by pulse dye densitometry: a comparison with the Fick's principle and thermodilution method. Intensive Care Med. 2002;28:399–405. doi: 10.1007/s00134-002-1252-3. [DOI] [PubMed] [Google Scholar]
- 16.Ishigami Y, Masuzawa M, Miyoshi E, Kato M, Tamura K, Kanda M, Awazu K, Taniguchi K, Kurita M, Hayashi N. Clinical applications of ICG Finger Monitor in patients with liver disease. J Hepatol. 1993;19:232–240. doi: 10.1016/s0168-8278(05)80577-x. [DOI] [PubMed] [Google Scholar]
- 17.Nishioka M, Ishikawa M, Hanaki N, Kashiwagi Y, Miki H, Miyake H, Tashiro S. Perioperative hemodynamic study of patients undergoing abdominal surgery using pulse dye densitometry. Hepatogastroenterology. 2006;53:874–878. [PubMed] [Google Scholar]
- 18.Niemann CU, Roberts JP, Ascher NL, Yost CS. Intraoperative hemodynamics and liver function in adult-to-adult living liver donors. Liver Transpl. 2002;8:1126–1132. doi: 10.1053/jlts.2002.36493. [DOI] [PubMed] [Google Scholar]
- 19.Piscaglia F, Zironi G, Gaiani S, Mazziotti A, Cavallari A, Gramantieri L, Valgimigli M, Bolondi L. Systemic and splanchnic hemodynamic changes after liver transplantation for cirrhosis: a long-term prospective study. Hepatology. 1999;30:58–64. doi: 10.1002/hep.510300112. [DOI] [PubMed] [Google Scholar]
- 20.Sabbà C, Merkel C, Zoli M, Ferraioli G, Gaiani S, Sacerdoti D, Bolondi L. Interobserver and interequipment variability of echo-Doppler examination of the portal vein: effect of a cooperative training program. Hepatology. 1995;21:428–433. doi: 10.1002/hep.1840210225. [DOI] [PubMed] [Google Scholar]
- 21.Piscaglia F, Gaiani S, Zironi G, Gramantieri L, Casali A, Siringo S, Serra C, Bolondi L. Intra- and extrahepatic arterial resistances in chronic hepatitis and liver cirrhosis. Ultrasound Med Biol. 1997;23:675–682. doi: 10.1016/s0301-5629(97)00012-4. [DOI] [PubMed] [Google Scholar]
- 22.Zironi G, Gaiani S, Fenyves D, Rigamonti A, Bolondi L, Barbara L. Value of measurement of mean portal flow velocity by Doppler flowmetry in the diagnosis of portal hypertension. J Hepatol. 1992;16:298–303. doi: 10.1016/s0168-8278(05)80660-9. [DOI] [PubMed] [Google Scholar]
- 23.Nakanishi S, Shiraki K, Yamamoto K, Saitou Y, Ohmori S, Nakano T, Mizuno S, Tabata M, Yamagiwa K, Yokoi H, et al. Early graft hemodynamics in living related liver transplantation evaluated by Doppler ultrasonography. Int J Mol Med. 2004;14:265–269. [PubMed] [Google Scholar]
- 24.Urata K, Kawasaki S, Matsunami H, Hashikura Y, Ikegami T, Ishizone S, Momose Y, Komiyama A, Makuuchi M. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317–1321. [PubMed] [Google Scholar]
- 25.Ashwell G, Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–554. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- 26.Jochum C, Beste M, Penndorf V, Farahani MS, Testa G, Nadalin S, Malago M, Broelsch CE, Gerken G. Quantitative liver function tests in donors and recipients of living donor liver transplantation. Liver Transpl. 2006;12:544–549. doi: 10.1002/lt.20627. [DOI] [PubMed] [Google Scholar]
- 27.Kudo M, Todo A, Ikekubo K, Yamamoto K, Vera DR, Stadalnik RC. Quantitative assessment of hepatocellular function through in vivo radioreceptor imaging with technetium 99m galactosyl human serum albumin. Hepatology. 1993;17:814–819. [PubMed] [Google Scholar]
- 28.Bubak ME, Porayko MK, Krom RA, Wiesner RH. Complications of liver biopsy in liver transplant patients: increased sepsis associated with choledochojejunostomy. Hepatology. 1991;14:1063–1065. [PubMed] [Google Scholar]
- 29.Neil DA, Hubscher SG. Histologic and biochemical changes during the evolution of chronic rejection of liver allografts. Hepatology. 2002;35:639–651. doi: 10.1053/jhep.2002.31726. [DOI] [PubMed] [Google Scholar]
- 30.Chekaluk E, Hutchinson TP, Cairns D. Repeated measures ANOVA for responses developing over time. Eur J Anaesthesiol. 1998;15:381–382. [PubMed] [Google Scholar]
- 31.Vickers AJ. Analysis of variance is easily misapplied in the analysis of randomized trials: a critique and discussion of alternative statistical approaches. Psychosom Med. 2005;67:652–655. doi: 10.1097/01.psy.0000172624.52957.a8. [DOI] [PubMed] [Google Scholar]
- 32.Henriksen JH, Kiszka-Kanowitz M, Bendtsen F. Review article: volume expansion in patients with cirrhosis. Aliment Pharmacol Ther. 2002;16 Suppl 5:12–23. doi: 10.1046/j.1365-2036.16.s5.3.x. [DOI] [PubMed] [Google Scholar]
- 33.Henderson JM, Mackay GJ, Hooks M, Chezmar JL, Galloway JR, Dodson TF, Kutner MH. High cardiac output of advanced liver disease persists after orthotopic liver transplantation. Hepatology. 1992;15:258–262. doi: 10.1002/hep.1840150214. [DOI] [PubMed] [Google Scholar]
- 34.Murray JF, Dawson AM, Sherlock S. Circulatory changes in chronic liver disease. Am J Med. 1958;24:358–367. doi: 10.1016/0002-9343(58)90322-x. [DOI] [PubMed] [Google Scholar]
- 35.Lee SS. Cardiac abnormalities in liver cirrhosis. West J Med. 1989;151:530–535. [PMC free article] [PubMed] [Google Scholar]
- 36.Gadano A, Hadengue A, Widmann JJ, Vachiery F, Moreau R, Yang S, Soupison T, Sogni P, Degott C, Durand F. Hemodynamics after orthotopic liver transplantation: study of associated factors and long-term effects. Hepatology. 1995;22:458–465. [PubMed] [Google Scholar]
- 37.Navasa M, Feu F, García-Pagán JC, Jiménez W, Llach J, Rimola A, Bosch J, Rodés J. Hemodynamic and humoral changes after liver transplantation in patients with cirrhosis. Hepatology. 1993;17:355–360. [PubMed] [Google Scholar]
- 38.Tsubono T, Todo S, Jabbour N, Mizoe A, Warty V, Demetris AJ, Starzl TE. Indocyanine green elimination test in orthotopic liver recipients. Hepatology. 1996;24:1165–1171. doi: 10.1002/hep.510240531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiao LR, El-Desoky AA, Seifalian AM, Habib N, Davidson BR. Effect of liver blood flow and function on hepatic indocyanine green clearance measured directly in a cirrhotic animal model. Br J Surg. 2000;87:568–574. doi: 10.1046/j.1365-2168.2000.01399.x. [DOI] [PubMed] [Google Scholar]
- 40.Groszmann RJ. The measurement of liver blood flow using clearance techniques. Hepatology. 1983;3:1039–1040. doi: 10.1002/hep.1840030625. [DOI] [PubMed] [Google Scholar]
- 41.Hashimoto M, Watanabe G. Simultaneous measurement of effective hepatic blood flow and systemic circulation. Hepatogastroenterology. 2000;47:1669–1674. [PubMed] [Google Scholar]
- 42.Huet PM, Villeneuve JP. Determinants of drug disposition in patients with cirrhosis. Hepatology. 1983;3:913–918. doi: 10.1002/hep.1840030604. [DOI] [PubMed] [Google Scholar]
- 43.Henderson JM, Mackay GJ, Kutner MH, Noe B. Volumetric and functional liver blood flow are both increased in the human transplanted liver. J Hepatol. 1993;17:204–207. doi: 10.1016/s0168-8278(05)80039-x. [DOI] [PubMed] [Google Scholar]
- 44.Hadengue A, Lebrec D, Moreau R, Sogni P, Durand F, Gaudin C, Bernuau J, Belghiti J, Gayet B, Erlinger S. Persistence of systemic and splanchnic hyperkinetic circulation in liver transplant patients. Hepatology. 1993;17:175–178. [PubMed] [Google Scholar]
- 45.Paulsen AW, Klintmalm GB. Direct measurement of hepatic blood flow in native and transplanted organs, with accompanying systemic hemodynamics. Hepatology. 1992;16:100–111. doi: 10.1002/hep.1840160118. [DOI] [PubMed] [Google Scholar]
- 46.Ueyama H, He YL, Tanigami H, Mashimo T, Yoshiya I. Effects of crystalloid and colloid preload on blood volume in the parturient undergoing spinal anesthesia for elective Cesarean section. Anesthesiology. 1999;91:1571–1576. doi: 10.1097/00000542-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Sakka SG, Reinhart K, Wegscheider K, Meier-Hellmann A. Comparison of cardiac output and circulatory blood volumes by transpulmonary thermo-dye dilution and transcutaneous indocyanine green measurement in critically ill patients. Chest. 2002;121:559–565. doi: 10.1378/chest.121.2.559. [DOI] [PubMed] [Google Scholar]
- 48.Mizushima Y, Tohira H, Mizobata Y, Matsuoka T, Yokota J. Assessment of effective hepatic blood flow in critically ill patients by noninvasive pulse dye-densitometry. Surg Today. 2003;33:101–105. doi: 10.1007/s005950300021. [DOI] [PubMed] [Google Scholar]
- 49.Eguchi S, Yanaga K, Sugiyama N, Okudaira S, Furui J, Kanematsu T. Relationship between portal venous flow and liver regeneration in patients after living donor right-lobe liver transplantation. Liver Transpl. 2003;9:547–551. doi: 10.1053/jlts.2003.50128. [DOI] [PubMed] [Google Scholar]