Abstract
Recent advances in understanding of pancreatitis and advances in technology have uncovered the veils of idiopathic pancreatitis to a point where a thorough history and judicious use of diagnostic techniques elucidate the cause in over 80% of cases. This review examines the multitude of etiologies of what were once labeled idiopathic pancreatitis and provides the current evidence on each. This review begins with a background review of the current epidemiology of idiopathic pancreatitis prior to discussion of various etiologies. Etiologies of medications, infections, toxins, autoimmune disorders, vascular causes, and anatomic and functional causes are explored in detail. We conclude with management of true idiopathic pancreatitis and a summary of the various etiologic agents. Throughout this review, areas of controversies are highlighted.
Keywords: Idiopathic pancreatitis, Recurrence, Etiology, Endoscopic retrograde cholangiopancreatography, Magnetic resonance cholangiopancreatography, Sphincter of Oddi dysfunction
INTRODUCTION
Pancreatitis is a relatively common disorder with a myriad of etiologies all resulting in a common end result of inflammation within the pancreas. Acute pancreatitis (AP) results in acute inflammation typically presenting as abdominal pain with elevated levels of pancreatic enzymes[1]. Chronic pancreatitis (CP) is defined as a clinical syndrome of progressive inflammatory changes in the pancreas leading to permanent structural damage with subsequent impairment of both exocrine and endocrine function[2]. Chronic pancreatitis is often preceded by recurrent bouts of acute pancreatitis, however, occasionally it can present in a ‘silent’ fashion.
The label of “idiopathic pancreatitis” (IP) was originally designated to cases of pancreatitis wherein a diagnosis could not be made through a thorough history, physical examination, laboratory studies, and noninvasive imaging modalities such as abdominal ultrasonography/computerized tomography. Previously, this nomenclature had accounted for 8%-44% of cases being termed “idiopathic”[3-9]. Recent new laboratory and technological advances have been able to shred the enigmatic veils of IP to a point wherein with extensive evaluation it is possible to reveal the etiology in 79%-80% of patients previously labeled as having “idiopathic pancreatitis”[10,11]. More modern imaging investigations include, but are not limited to fine cut computerized tomography, endoscopic retrograde pancreatography, magnetic resonance pancreatography as well as endoscopic ultrasound.
EPIDEMIOLOGY
Longitudinal data on incidence trends report an increase in AP[12] which may be attributable to increases in all causes of pancreatitis as well as improved detection methods. The fatality rate for pancreatitis has continued to range between 3%-10%[13-18] despite a marked decrease in pancreatitis case fatality presumably secondary to early recognition of severity and complications as well as improved intensive care management[14,15]. Thomson[6] and others[7,17] have noted that in AP mortality rates are greater when the etiology is IP (14.1%)[6], compared with gallstone pancreatitis (7.2%)[6]. In addition, the clinical impact of IP is further highlighted by the fact that 40 percent (the largest subgroup) of all AP fatalities are attributable to IP[19,20]. The incidence of IP ranges from 4.21 per 100000[21] to as high as 45.33 per 100000[9] depending on the population studied and the time in which data was collected with a trend toward high incidence in populations studied more recently[12,13,22]. The incidence appears to be equal between the two sexes and tends to increase with age in both before starting to plateau around 70 years[18].
Elucidating the etiology of pancreatitis, if possible is paramount as it guides therapy and may theoretically subsequently improve patient outcomes, thereby preventing relapses. Some studies have shown that over 50% of untreated patients with acute IP experience recurrent episodes[23-25]. This contrasts with other studies where only 1 of 31 patients with a first episode of unexplained AP suffered another attack during a median follow up of 36 mo[26]. These conflicting results may reflect different patient populations, perhaps even within the same category known as IP.
Aside from the initial objective to decrease acute patient mortality and morbidity, repeated insults to the pancreas may progress to chronic pancreatitis with irreversible morphologic and functional changes[2,24,27]. This concern typically results in aggressive investigation for those patients with more than one episode of AP. This approach is supported by a study by Kaw and Brodmerkel in a study that included 126 patients with two or more episodes of IP. They demonstrated that investigations including bile for microlithiasis, a secretin stimulation test, and sphincter of Oddi manometry (SOM) was able to clarify the etiologies in 79 percent of patients[11]. In this study they were able to offer 75% of these patients treatment that resulted in the absence of AP in over 60% of cases over a 30 mo follow-up period[11].
However there is ongoing debate against aggressive evaluation. The Kaw and Brodmerkel study confirmed previous morbidity data demonstrating that SOM is not a benign procedure and that complications occur[11]. Additionally, many aggressive techniques for diagnosing the etiology of pancreatitis, including SOM, may not be routinely available. Therefore the generalizability of this study has been brought into question.
This article will examine some of the etiologies that must be excluded prior to a diagnosis of IP. An attempt will be made to highlight the areas of controversy, and make suggestions based on the best available evidence. For the purpose of this IP article, gallstones and alcohol related pancreatic disease will not be discussed as their presentation is usually easily determined. For a more comprehensive list of all etiologies of pancreatitis please refer to Table 1.
Table 1.
Etiologies of acute or acute recurrent pancreatitis
| Category | Agent/Diagnosis |
| Vascular | Atheroembolism |
| Intraoperative hypotension | |
| Hemorrhagic shock | |
| Vasculitis (systemic lupus erythematosus and polyarteritis nodosa) | |
| Infectious | Viral |
| Mumps | |
| Coxsackievirus type B | |
| Hepatitis B | |
| Cytomegalovirus | |
| Herpes simplex | |
| Varicella-zoster | |
| HIV | |
| Rubella (probable) | |
| Bacterial | |
| Legionella | |
| Leptospira | |
| Salmonella | |
| Mycoplasma | |
| Brucella | |
| Mycoplasma | |
| Salmonella typhi | |
| Fungal | |
| Aspergillus | |
| Parasites | |
| Toxoplasma | |
| Cryptosporidium | |
| Ascaris lumbricoides | |
| Trauma | Blunt or penetrating abdominal injury |
| Post-ERCP pancreatitis | |
| ERCP sphincterotomy | |
| Manometry of sphincter of Oddi | |
| Iatrogenic operative complication | |
| Metabolic | Hypertriglyceridemia (typesI, IV, V) |
| Hypercalcemia | |
| Hyperparathyroidism | |
| Toxins | Ethyl alcohol |
| Scorpion venom | |
| Methyl alcohol | |
| Organophosphorous insecticides | |
| Medications The following drugs were definitely associated with pancreatitis | Antimicrobial agents |
| Metronidazole, Stibogluconate, Sulfonamides, Tetracycline, Nitrofurantoin, Erythromycin, Isoniazid | |
| HIV Therapy | |
| Didanosine, Pentamidine | |
| Diuretics | |
| Furosemide, Thiazides | |
| Commonly used Gastroenterology Medications | |
| 5-ASA, Sulphasalazine, Cimetidine, Ranitidine, Mercaptopurine, Proton pump inhibitors | |
| Cardiac Agents | |
| Procainamide | |
| Immunosuppressives or Chemotherapeutics | |
| L-asparaginase, Azathioprine, Cytosine arabinoside, Dexamethasone | |
| Neuropsychiatric Agents | |
| Mechanical | Valproic Acid, αMethyl Dopa |
| Other Commonly Used | |
| Acetaminophen, Salicylates, Sulindac, Calcium, Ethinylestradiol, Norethindrone | |
| Gallstones | |
| Microlithiasis and Biliary Sludge. | |
| Sphincter of Oddi dysfunction | |
| Pancreas divisum | |
| Annular pancreas | |
| Autoimmune pancreatitis | |
| Pancreatobiliary Tumours | |
| Cholodochocele | |
| Duodenal stricture or obstruction | |
| Miscellaneous | Ascariasis |
| Post ERCP | |
| Renal transplant | |
| Genetic | Hyper IgG4 disease |
| CFTR | |
| Serine protease inhibitor Kazal type 1 mutation | |
| Cationic trypsinogen gene PRSS1 mutation | |
| Autoimmune | Sjogren’s syndrome |
| Primary biliary cirrhosis | |
| Renal tubular acidosis |
PANCREATITIS ETIOLOGIES OFTEN LABELLED AS IDIOPATHIC PANCREATITIS
Microlithiasis and biliary sludge
Biliary sludge refers to the viscous suspension in gallbladder bile formed by modification of hepatic bile by the gallbladder mucosa that may contain small stones (< 5 mm in diameter)[28]. Microlithiasis refers to stones of < 3 mm in diameter and is often used interchangeably at times with “microcrystals” and sometimes with biliary sludge[28-30]. Microscopy of bile in patients with sludge often shows cholesterol monohydrate crystals, calcium carbonate microspheroliths, or calcium bilirubinate granules[31]. A known risk factor for the development of sludge typically includes prolonged fasting states as well as some antibiotics (ceftriaxone)[32]. Microlithiasis has been suggested to be the most common causes of IP[31,33] with a prevalence ranging from 6%-73%[11,25,31,33,34].
It has been demonstrated that treatment with chole-cystectomy, endoscopic sphincterotomy, or ursodiol significantly reduces further attacks of IP[31,33]. Therefore it was inferred that in the absence of other identifiable risk factors, the presence of microlithiasis was enough to cause IP[11,26,35]. The caveat to interpreting these studies is that combined, they involved only 74 patients who had IP and treatments were clearly not double blinded[31,33].
An Indian study published earlier this year evaluating 51 patients with recurrent IP found that microlithiasis accounted for a mere 13 percent of these patients (using duodenal bile samples)[36]. In the setting of suspected biliary pancreatitis up to 88% of patients will have microlithiasis demonstrating the difference between these two patient groups[37]. It is speculated that there may be some gender, age, racial, and procedural differences in bile sample collection and analysis as these are not well standardized.
The pathogenesis of AP via microlithiasis remains unclear however it is thought that the microlithiasis may transiently impact the papilla, cause pancreatic duct obstruction and thereby pancreatitis[29,37,38]. The diagnostic workup for microlithiasis includes routine abdominal ultrasound (US) which has limited sensitivity when looking for stones less than 3 mm in diameter[28,39]. Endoscopic US (EUS) may be considered as well as it carries a lower risk of complications than endoscopic retrograde cholangiopancreatography (ERCP)[34,40-45]. On average, EUS is able to identify gallbladder sludge in up to 75 percent of IP cases[40-45]. All patients with recurrent IP should have microlithiasis excluded and there is even some evidence that it should be excluded for patients who have their first IP[41-43].
It remains to be clarified which method of bile collection for microscopic analysis is clinically the best and there are no standardized methods or recommendations at this time[46-48].
Cholecystectomy is recommended for patients with biliary sludge/microlithiasis once they recover from their episode of pancreatitis as it reduces the relapse rates[31,33]. In patients who are poor surgical candidates, ERCP with biliary sphincterotomy or ursodeoxycholic acid may be alternative forms of treatment[31,33].
Sphincter of Oddi dysfunction
At times referred to as hypertensive or fibrotic SOD, SOD causes diminished transphincteric flow of bile and/or pancreatic juice due to either organic obstruction (stenosis) or functional obstruction (dysmotility)[3,35,49]. SOD often causes recurrent pain with or without abnormalities of either hepatic/pancreatic profiles as well as duct dilation. It is thought that SOD causes pancreatitis as a result of bile reflux into the pancreatic duct or from pancreatic duct outflow obstruction[25,50]. SOD is considered to cause up to one third of all cases of IP[25,30,51,52].
In order to diagnose SOD, one often needs to perform sphincter of Oddi manometry (SOM)[53,54]. The diagnostic gold standard is a water-perfused catheter system that can be inserted into the common bile duct or pancreatic duct with the positive finding being a hypertensive sphincter of Oddi pressure greater than 40 mmHg[53]. Unfortunately whether ERCP in patients with suspected SOD is done for diagnostic SOM or therapeutic purposes it is undeniable that there have been high complication rates, particularly pancreatitis[51,55-59]. Additionally “severe” pancreatitis that is associated with death has even been quoted in 1-3% in SOM[15,25,51]. Despite this, ERCP with SOM has been advocated for the evaluation of recurrent IP[25,60]. In patients who have normal biliary manometry, pancreatic sphincter manometry may be reasonable to perform[61,62] which does carry a higher risk of pancreatitis[54] that may be reduced by aspirating through the middle port of the triple lumen manometry catheter[63].
Once the diagnosis of SOD is made, endoscopic sphincterotomy is the treatment of choice as it is believed to decrease the risk of recurrent pancreatitis[25,60,64-66].
The Geenen-Hogan (Milwaukee) criteria was devised to predict the overall probability of response to biliary sphincterotomy taking into account the presence of abnormal liver chemistries and dilated bile ducts[44]. On the basis of this, patients with pain and both abnormalities (typeI) are definitely recommended to have sphincterotomy as they have high response rates ranging from 90%-100% regardless of SOM[67,68]. Along this stratification, type II patients have either abnormal liver chemistry or dilated bile ducts. If SOM is abnormal in type II patients they will have a response rate to sphincterotomy of 60%-91%[64,67,69] and thus this would be a reasonable course of action. On the contrary, type III patients do not have objective biliary abnormalities and even if they have abnormal SOM they have poor response rates of 6-58 percent[69-71], making sphincterotomy of questionable benefit (Table 2) .
Table 2.
Sphincter of Oddi dysfunction (Geenen and Hogan Classification)
| Biliary type | Pancreatic type |
| TypeI | TypeI |
| Biliary-type pain | Pancreatic-type pain |
| LFT elevation | Amylase/lipase elevation |
| CBD dilation | PD dilation |
| Delayed drainage | Delayed drainage |
| Type II | Type II |
| Biliary-type pain | Pancreatic-type pain |
| One or two of above criteria | One or two of above criteria |
| Type III | Type III |
| Biliary-type pain only | Pancreatic-type pain only |
CBD: Common bile duct; LFT: Liver function tests; PD: Pancreatic duct.
If a diagnosis of pancreatic sphincter dysfunction is made in patients who have had biliary sphincterotomy the answer to who best benefits from pancreatic sphincterotomy is less clear. A study by Freeman et al[72] this year treated suspected SOD with biliary sphincterotomy with additional pancreatic sphincterotomy at initial or subsequent ERCP if there was abnormal pancreatic manometry in conjunction with pain refractory to biliary sphincterotomy, continuous pain, or a history of amylase elevation. In this study of 121 predominantly female (92%) and post cholecystectomy (87%) patients, all patients underwent biliary sphincterotomy while 40 percent also underwent pancreatic sphincterotomy regardless of the modified Milwaukee biliary classification[72]. The result from this study was that a positive response at final follow up was reported in 69 percent of patients and that this response was not significantly different between biliary typesI, II, and III[72]. Freeman et al[72] concluded that patient characteristics of pancreatic manometry, delayed gastric emptying, daily opioid use, and age < 40 were significant as predictors of outcomes as opposed to the Milwaukee classification. Indeed it is becoming more accepted that a lack of improvement after biliary sphincterotomy may be representative of a failure to relieve pancreatic sphincter pressure[61,62,73-77].
Noninvasive strategies such as a low-fat diet, analgesics, anticholinergics, calcium-channel blockers, nitrates, and proton pump inhibitors are unfortunately seldom effective[78,79].
Pancreas divisum
In Pancreas divisum it is thought that 80%-95% of pancreatic juice volume flows via the dorsal duct through the smaller minor papillary orifice via the dorsal duct of Santorini as opposed to the more common route through the major papilla via the ventral duct of Wirsung[66]. It is postulated that the mechanism is that of relative minor papilla outflow obstruction leading to pancreatitis[23,80-82].
Pancreas divisum occurs as an anatomic variant in 5%-7.5% of patients[23,82]. There are those who are skeptical of the obstructive theory as fewer than 5% of patients with pancreas divisum develop pancreatitis[83-86]. It also appears that the incidence of pancreas divisum is the same in patients with and without pancreatitis[86]. Nonetheless there is consensus that while most individuals with pancreas divisum live normally, a few unfortunate patients are predisposed to develop recurrent AP[23-25,35] and that it accounts for 20 percent of the IP cases[23-25].
One explanation of why some patients with pancreas divisum are more likely to be affected was the smaller than usual minor papillary orifice causing a disproportionately high intrapancreatic dorsal ductal pressure especially during times of active secretion. In this situation a cascade of inadequate drainage leading to ductal distension and eventually pancreatitis could occur[23,87-89]. This theory is supported by at least one surgical study that demonstrated relief of pain as well as diminished attacks of AP with sphincteroplasty[87].
Recently, Choudari et al[90] found that prevalence of the cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations was similar in patients with pancreas divisum with recurrent acute pancreatitis and true idiopathic recurrent acute pancreatitis. Interesting, this study also found that the prevalence of CFTR gene mutations in patients with pancreas divisum but without recurrent AP was similar to controls without pancreatitis[90]. The significance of CFTR will be discussed in more detail under “Genetic Causes and Contributions” below, however, these genetic studies call into question the true role of pancreas divisum in IP.
At present, it is accepted that patients who present with severe pancreatobiliary type symptoms or recurrent IP should however be evaluated for pancreas divisum[35,48]. The diagnosis of divisum can be made either by ERCP or magnetic resonance cholangiopancreatography (MRCP)[91,92]. ERCP may be preferred to conventional MRCP as it has a higher sensitivity and specificity[91,92] and can offer therapy albeit with a complication rate that is higher than in MRCP. Secretin may be given intravenously to aid in finding the minor papillary orifice at ERCP[93], finding functional outflow obstructions[94-96], and assessing the relative obstruction of the minor papilla[94-96]. In settings where secretin is either contraindicated or does not produce a visible increase in pancreatic juice flow, a dilute (1:10) methylene blue solution can assist in the location of the minor papilla[97]. Confirmation is recommended to rule out pseudodivisum caused by a tumor.
An emerging area is the role of EUS as it is a minimally invasive tool for the general workup of IP and recurrent IP[48]. It appears that EUS is able to detect pancreas divisum but more research in comparing ERCP to EUS specific to divisum is needed before recommendations are made.
In the setting of recurrent AP, once divisum is diagnosed, ERCP therapy to relieve minor papillary obstruction is recommended to decrease the rate of recurrent pancreatitis[98-101], particularly in patients with a dilated pancreatic duct who may be the most likely to benefit from therapy[98]. The rate of decrease of recurrent pancreatitis is quoted to range from 70%-90%[99,100] with a 40%-60% response rate in patients with CP also demonstrated[102]. The ERCP therapy involves a minor papillotomy usually with temporary stenting[98-101,103]. In one study, more favorable long-term results were achieved with minor papilla sphincterotomy than with repeated stenting in terms of both recurrence and fewer complications (25% vs 44% respectively)[98]. Some endoscopists are particularly cautious about endoscopic stenting as the procedure may cause permanent damage to the pancreatic ducts, possibly promoting the development of chronic pancreatic disease[104,105].
Anomalous union of pancreaticobiliary duct
Anomalous union of pancreaticobiliary duct (AUPBD) is defined as an anomalous junction of bile duct and pancreatic duct at an abnormal proximal site of these ducts[35]. The precise location of the junction is outside the duodenal musculature and independent of the sphincter of Oddi. This independence from the contractility of the sphincter of Oddi results in regurgitation of the pancreatic juice into the biliary tract and vice versa[106]. AUPBD is frequently associated with choledochal cysts which in itself has been linked to IP (see below)[107] and is more common in East Asia[35].
It is thought that in AUPBD reflux of bile into the pancreatic duct may cause recurrent AP[106,108] and that in cases with a dilated common channel the stasis of pancreatic secretion can occur in the common channel leading to pancreatitis[109,110]. Moreover it has also been found that SOD (see above) is sometimes associated with AUPBD[111].
The diagnosis of AUPBD is usually made during ERCP evaluation for IP when one notes a pancreatic duct and choledochus connecting with a long common channel of > 15 mm in adults[112]. Similarly AUPBD is also seen during MRCP[91]. The length of the common channel alone is not an absolute diagnostic criterion as other factors like the form of union, direction, and age and stature of patients need to be considered[108,113]. An alternate method of diagnosis, more importantly a ‘clue’ to the presence of AUPBD, may be the presence of high amylase levels in aspirated bile[114,115].
In AUPBD, endoscopic biliary sphincterotomy has been shown to prevent further attacks of AP[112] by a postulated mechanism of decreasing resistance at the major duodenal papilla[35]. Due to the fact that AUPBD has a propensity to increase gallbladder and biliary duct cancer[114,116-118], prophylactic cholecystectomy has been recommended in these patients[116]. As well, at this time there is some evidence that patients with AUPBD and choledochal cyst should have an extensive resection of the extrahepatic bile duct inclusive of the cyst in hopes of preventing development of biliary duct cancer[108] which may be relevant in 5% of patients[116]. It is reiterated that the understanding of AUPBD, its pathophysiology and therapy is very limited[35].
Choledochocele
Choledochocele is classified as a type III choledochal cyst that represents a prolapse or herniation of the intramural segment of the distal common bile duct into the duodenal lumen which may be either congenital or acquired[35,119]. Pancreatitis and recurrent AP is an important complication occurring in choledochoceles occurring in 12%-30% of patients[120,121]. Choledochoceles do not represent a common cause of IP, especially in adults[25,35,120].
It is thought that the choledochocele may create an obstruction to the pancreatic duct intermittently when the choledochocele becomes distended and that this may lead to reflux of bile into the pancreatic duct[121-123].
A characteristic “bulging” appearance of the papilla at ERCP and a soft “pillow” sign with pressure applied at the catheter tip suggests a choledochocele[120,124]. A CT or US in isolation may miss this diagnosis[120]. The role of MRCP in adults to diagnose this is still undefined[91,125,126].
Treatment can be initiated with endoscopic sphincterotomy combined with “unroofing” of the choledochocele with a papillotome with the goal of creating effective drainage of bile and pancreatic juice[120,127]. Patients that fail this treatment may be considered for a surgical sphincteroplasty[128]. Reports in the surgical literature about choledochal cysts in general recommend a single stage surgery usually comprising of complete cyst resection, cholecystectomy and Rou-en-Y hepatojejunostomy[129-131]. Malignancy is noted in 3-5 percent which may in some cases warrant prophylactic surgery[131,132].
Annular pancreas
Annular pancreas is a congenital condition that represents a band of pancreatic tissue partially or completely encircling the duodenum that is usually at the level of or immediately proximal to the major duodenal papilla[133]. It is thought that this defect occurs in utero due to the failure of the ventral bud to rotate with the duodenum[133]. This abnormality is rare and is detected in 1/7000-1/20000 autopsies[133,134] and in 1/1500 ERCPs[135-137].
Clinically it usually manifests in childhood with intractable vomiting[136,137] and is thought to result from descending duodenal narrowing leading to duodenal obstruction or recurrent AP[138]. In adults annular pancreas may present with abdominal pain, acute recurrent IP, CP, peptic ulcer disease, postprandial fullness, vomiting, or biliary obstruction[137,139]. Associated congenital anomalies such as Down’s syndrome, cardiac defects, tracheoesophageal fistula, Meckel’s diverticulum, and imperforate anus may be present[133,136]. If the diagnosis is made in adults it usually occurs between the ages of 20 to 50[140].
From a diagnostic view, barium studies, abdominal CT, or MRCP may suggest the diagnosis but an ERCP is recommended for confirmation[135,136,141]. A typical ERCP is that of the duct of the pancreatic annulus encircling the duodenum[24] and in one third of cases a pancreas divisum is also present[139]. The annular duct may communicate with the central duct but rarely drains into the dorsal duct, common bile duct, or independently into the duodenum[135,139]. In cases where ERCP may not be feasible, an EUS may offer an alternative means of diagnosis[142]. Recently some data has also become available on the role of MRCP which appears encouraging as a non-invasive method of diagnosis[143-145].
Surgery is the procedure of choice in patients in whom symptoms can be attributed to annular pancreas with the goal to relieve duodenal or gastric outlet obstruction[137,146].
Pancreatobiliary tumors
Any mass that obstructs the main pancreatic or biliary ducts, benign or malignant can result in acute pancreatitis. It has been estimated that 5%-14% of patients with pancreatobiliary tumors, benign or malignant, present with apparent IP[147-150]. Pancreatic cancer should be suspected in any patient older than 40 years with IP especially with a prolonged or recurrent course[3,5]. Neoplasia should be suspected in a patient with weight loss, steatorrhea, ductal dilation, new onset diabetes, or evidence of a solid or cystic pancreatic mass[24,149,150]. In younger patients lesions such as curable islet cell tumors should be in the differential whereas in the elderly they may have potentially curable lesions such as cystic neoplasms[24].
It is well known that CT, MRI, ERCP, and EUS have a role in identifying pancreatobiliary neoplasms[151-154]. It is recommended that if there is any clinical suspicion of malignancy that aggressive investigation including ERCP be performed even on the first attack of IP in older patients. In patients who are less than 40 years old, a CT may be sufficient in first attacks of AP[24]. If a malignancy is suspected, EUS is a favorable technique that can be used for diagnostic and staging purposes[155,156] especially when combined with fine needle biopsy[157,158].
Intraductal papillary mucinous neoplasm of the pancreas (IPMN) is a distinct pathologic entity formed of papillary proliferations of mucin-producing epithelial cells with or without excessive mucus production and or cystic dilation of the pancreatic duct. IPMN represents a precancerous lesion with a well described adenoma and carcinoma sequence that causes recurrent acute IP with symptoms suggestive of chronic obstructive pancreatitis due to intermittent obstruction of the pancreatic duct with mucus plugs[159]. It is worth noting this entity has an insidious nature and lack of awareness often delays diagnosis[160-163]. On ERCP, in cases of main duct involvement, the papilla is patulous and resembles a “fish-eye” frequently with mucus extruding from the orifice[161,164,165]. In terms of management, since the ten-year actuarial risk of high grade dysplasia and invasive cancer is significant[166] surgery is usually recommended, particularly for main duct disease[159,167-169]. It should also be noted that patients with IPMN may be at increased risk for extrapancreatic malignancies and because of this gastricadenocarcinoma and colorectal cancer should be screened for using endoscopy[170,171]. Patients with branch type disease may be at a lower risk of malignancy and theoretically can be monitored in regards to mural wall thickening, size of branch cystic lesion and tumor markers.
Cystic pancreatic tumors including IPMN, serous cystadenomas, mucinous cystadenomas, and mucinous cystadenocarcinomas, can be premalignant or malignant and surgery is generally indicated[172-174]. Tumor markers such as CA 19-9, CA 15-3, CA 72-4, and carcinoembryonic antigen in aspirated cystic fluid along with fluid viscosity and amylase level may be used to increase the diagnostic yield of cyst fluid cytology[175-179].
Ampullary adenomas are premalignant and in general, indicate the need for close monitoring and eventual removal[180,181]. In general, ampullary tumors have a more favorable prognosis than pancreatic tumors and pancreatoduodenectomy has historically been recommended[182-184]. An emerging development for ampullary tumors is the use of endoscopic techniques such as endoscopic ampullectomy for management of patients with small benign lesions or for carcinoma in situ[185-188] which appears to be safe and efficacious on long term follow up[188]. If endoscopic management is selected it has been recommended that surveillance and random biopsies be performed[24].
GENETIC ASSOCIATIONS AND CAUSES
An exciting development is the recognition of genetic mutations that are associated with pancreatitis.
CFTR
The cystic fibrosis transmembrane conductance regulator (CFTR) and its gene mutations cause cystic fibrosis in an autosomal recessive pattern. It is well established that CFTR mutations cause disease of the exocrine pancreas[189]. The CFTR gene encodes a chloride-channel protein that is regulated by cAMP. Bicarbonate ion is secreted into the duct lumen by the action of CFTR in the apical membranes and this sets up a gradient in which water follows[190]. In the absence of CFTR, the pancreatic duct cells cannot secrete fluid and bicarbonate and hence CF of the pancreas develops[191].
The actual mechanism that mutations in the CFTR gene causes recurrent AP or CP[90,192-194] is unknown. It may be that mutant CFTR channels are inefficient at flushing digestive enzymes out of the pancreatic duct and thereby limiting the major mechanism that prevents trypsin-associated injury within the pancreatic duct[195]. Along the same lines, it may be that mutant CFTR can limit bicarbonate secretion that can interfere with trypsin activation[195]. What is clear is that CFTR mutations have a demonstrated increased incidence in patients with CP, IP, and alcohol induced CP[194,196-201]. One recent study of 381 patients diagnosed with either CP or recurrent IP that used expansive CFTR genotyping showed mutant CFTR genes in 11% (43/381) of these patients[195]. This frequency is in keeping with previous reports of 28%-45% of IP patients being either heterozygous or homozygous for a CFTR gene mutation[202,203]. One study in Poland showed that the frequency of mutations in CFTR alleles was similar to controls (4.9% vs 5%, P = 0.587) but this study only looked at 3 CFTR defects[204].
Testing for this gene mutation may be considered in younger patients presenting with recurrent IP who have a positive family history of cystic fibrosis or IP[66,195,205]. If testing is to be done, a broad mutation panel to look for a multitude of mutations of CFTR is recommended[195].
SPINK1
The N34S mutation of the serine protease inhibitor Kazal type 1 (SPINK1) has been reported to be strongly associated with IP and hereditary/familial pancreatitis[206-208]. SPINK1 is a specific trypsin and trypsin activated trypsin like inhibitor expressed within the pancreas that provides a defense against prematurely activated trypsinogen[209,210]. It has been suggested that SPINK1 mutations are disease modifiers in that they lower the threshold for pancreatitis from other genetic or environmental factors[207]. For example this mutation was found in one study to be associated with CP and recurrent IP in 7.7% and 10% respectively[204]. Likewise, a larger study showed that 15.7% of patients with CP or recurrent IP carried at least one SPINK1 mutation[195].
A genetic test for SPINK1 should be done in any younger patient presenting with recurrent AP or CP and a family history of IP or CP-particularly if they have had prior negative workup with ERCP. When genetic testing is done, it is advised to perform a concomitant CFTR and PRSS1 mutation screen[195].
Cationic trypsinogen gene PRSS1
Two missense mutations R12211[211] and N291[212] in the human cationic trypsinogen gene [protease serine 1 (trypsin 1); PRSS1] were first detected in patients with hereditary pancreatitis and appear to manifest as autosomal dominant mutations[213]. Since the discovery of the first two genes many other PRSS1 mutations have been reported[214-219]. Pathophysiologically this mutation leads to impaired trypsin autolysis and degradation, impairment of SPINK1 defense mechanism, and promotion of auto-activation of trypsinogen all of which can cause AP and lead to CP[211,220].
Symptoms typically arise in childhood but may be delayed until the mid 30 s[221]. These usually include symptoms associated with CP[221]. The lifetime risk of pancreatic cancer is 40% and reportedly 75% with paternal inheritance[222]. Whereas in cases of CFTR and SPINK1 are associated with IP and CP, PRSS1 defects seem to be causative for AP[204].
As in CFTR and SPINK1, the diagnosis is usually suspected if younger patients with affected family members present with CP or recurrent IP. Complications of CP are usually dealt with endoscopically but given the high risk of malignancy surgical resection may be preferred[24].
Celiac disease
The frequent familial occurrence and the remarkably close association with the HLA-DQ2 and/or DQ8 gene locus suggests that celiac disease as an immune disorder that is triggered by an environmental agent, gliadin, in genetically predisposed individuals[223,224]. Intriguingly, recurrent pancreatitis can be caused by celiac disease[225,226]. The mechanism appears to be duodenal inflammation and associated papillary stenosis causing pancreatitis[225]. Endoscopic treatment is recommended at this time to relieve the obstruction.
There are consensus guidelines to the role of genetic testing in IP although they are evolving as our knowledge expands in the area[227].
MEDICATIONS
The list of medications that are associated with or that cause pancreatitis is increasing[228-230]. In one German study 1.4% of all acute pancreatitis was related to medications[231]. Another study reported an even lower association of 0.3%[232]. Drugs with the highest incidence of pancreatitis are azathioprine and mercaptopurine (incidence, 3 to 5%)[233] and didanosine (23%)[234].
Most medication related to pancreatitis is due to idiosyncratic response or a direct toxic effect. A high index of suspicion and astute drug history is crucial for making the diagnosis.
A few areas of medications causing pancreatitis will be highlighted. Firstly, physicians should be aware of a recent development showing an association of proton pump inhibitors and pancreatitis[235-237]. Secondly, some medications, such as pentamidine, valproic acid, and didanosine, appear to cause injury weeks to months after exposure, possibly through the accumulation of a toxic metabolite[238]. Hypersensitivity reactions have been implicated in other drugs, such as azathioprine, mercaptopurine, metronidazole, aminosalicylates, and sulfonamides, and these drugs characteristically lead to pancreatitis within one month after exposure[238]. Lastly, some medications like acetaminophen may cause pancreatitis with a single dose[239].
A complete list of medications that are classified as being definitely associated with pancreatitis derived from multiple references is shown in Table 1[228-230,240-242].
TOXINS
Toxins that have been linked to pancreatitis include the most common alcohol. The more deadly methanol can also cause AP[243]. Although exceedingly rare scorpion bites from Trinidad have been known to cause AP[244,245]. Another rare cause of IP is organophosphorous poi-soning[246].
INFECTIOUS CAUSES
A plethora of infectious agents have been associated with AP[247]. A full list of definite and probable etiologic agents is provided in Table 1 from data gathered from Parenti et al[247]. It is unclear how often an infectious etiology is responsible for IP but many appear to be associated with it[247].
A clinician must be suspicious of an infectious etiology in the characteristic syndrome caused by the particular infectious agent notwithstanding that this was evident in only 70% of patients in the prior review for definite cases[247]. A routine search for an infectious cause in IP is not recommended unless there is a strong clinical suspicion.
Ascariasis is an important cause of infectious obstructive AP in India where it is the second most common cause of AP[248]. At times this may also present with associated biliary tree infestation requiring endoscopic decompression[249].
An infectious group worth discussing in greater detail is HIV infections and their relation to AP[250]. One large series revealed that 4.7% of hospitalized patients with HIV had AP[251]. While primary HIV infection itself can be a cause of AP[252,253] it is more commonly attributable to a complication of medications taken as part of HIV treatment or medications for opportunistic infections such as Pneumocystis carinii and Mycobacterium avium-intracellulare[250].
METABOLIC
Hypertriglyceridemia
Serum triglyceride concentrations above 11.3 mmol/L are capable of causing AP although admittedly the pathogenesis of inflammation is unclear[254]. It is thought that hypertriglyceridemia represents 1.3%-3.8% of AP cases[255]. The incidence has been best defined in children with inherited disorders of lipoprotein metabolism that is associated with severe hypertriglyceridemia which are 35%, 15%, and up to 40% in hyperlipidemia typesI, II, and V respectively[256,257]. Other acquired causes of hypertriglyceridemia include obesity, diabetes mellitus, hypothyroidism, pregnancy, estrogen or tamoxifen therapy, glucocorticoid excess, nephritic syndrome, and beta blockers[258-260,255].
Drug-induced disease is more likely to occur in patients with underlying hypertriglyceridemia[256]. It is important to not neglect the lactescent serum as it is a vital clue to the diagnosis[255].
Controversy surrounds the contribution of hype-rlipidemia in causing AP in alcoholics[261] but it is thought that in most alcohol abusers the moderate elevations of triglyceride levels are transient and likely to be an epiphenomenon rather than a causative agent of pancreatitis[262].
Hypercalcemia
Occurring in uncommon frequency, hypercalcemia of any cause is a known cause of AP[263,264]. Postulated mechanisms include calcium deposition in the pancreatic duct and calcium activation of trypsinogen within the pancreatic parenchyma[265,266].
There are questions however on hyperparathyroidism, a cause of hypercalcemia, and the link to pancreatitis rose in one large study of 1153 patients that found that AP occurred in only 1.5 percent of patients that was of a statistically non significant difference from that of the general population[267]. This finding was mirrored in two other studies[268,269]. This is still an open controversy however as other studies have supported at least an association and a significantly increased relative risk of AP in patients with hyperparathyroidism[270,271]. Until the debate is resolved it is recommended that the parathyroid be checked in recurrent IP if hypercalcemia is present.
VASCULAR DISEASE
Pancreatic ischemia is an uncommon but an established cause of pancreatitis reported in: (1) vasculitis (systemic lupus erythematosus, polyarteritis nodosa, and microscopic polyangiitis)[272-274], (2) atheroembolism[275,276], (3) hypotension and shock[277-279].
While it is true that most patients have mild attacks of pancreatitis secondary to ischemia, fatal necrotizing pancreatitis is a rare occurrence[277].
TRAUMATIC CAUSES
Blunt or penetrating abdominal injuries can cause pancreatitis although it is incredibly rare given the retroperitoneal location of the gland[280]. Types of trauma that cause pancreatitis can range from a mild contusion, severe crush injury, or transection of the gland[280].
Post ERCP pancreatitis remains the commonest severe complication of ERCP found to occur in 5%-7% of patients in a recent review[281]. It may be possible that prophylactic stenting of the pancreatic duct in selective cases and minimally traumatic cannulation techniques may prevent some cases[281] and indeed this has been shown to be cost effective in high risk patients[282]. Octreotide infusion does not prevent ERCP-induced pancreatitis or affect serum amylase levels[283]. Likewise, transdermal glyceryl trinitrate did not improve the rate of success in ERCP cannulation or prevent post-ERCP pancreatitis in either average or high-risk patient groups[284]. Allopurinol at high doses has been shown in a prospective randomized trial to lower the risk of post-ERCP pancreatitis[285] but this study requires further verification as other studies have shown no benefit[285,286]. Risk factors for post-ERCP pancreatitis have been well described[287].
AUTOIMMUNE CAUSES
Autoimmune pancreatitis is a more recently described type of chronic pancreatitis characterized by an autoimmune inflammatory process in which prominent lymphoplasmacytic infiltration with associated fibrosis of the pancreas causes organ dysfunction[288-290]. The reported prevalence of autoimmune pancreatitis is between 5 and 6% of all patients with chronic pancreatitis[291]. A series from the United States shows that 11% of patients (27 of 254) with chronic pancreatitis received a diagnosis of autoimmune pancreatitis based on histological findings[292].
Immunologic abnormalities including hypergamma-globulinemia, elevated serum IgG4 levels, and the presence of autoantibodies against carbonic anhydrase and lactoferrin are important markers of the disease[288]. In particular, autoantibodies against lactoferrin and carbonic anhydrase II have been identified as potential serologic markers of autoimmune pancreatitis[293,294]. The finding of increased serum IgG levels or the presence of autoantibodies is supportive of the diagnosis, whereas an elevated serum IgG4 level is nearly diagnostic[288].
CP has also been found to be in association with Sjogren’s syndrome, primary biliary cirrhosis, and renal tubular acidosis[295,296]. The diagnosis is usually based on clinical suspicion and serum autoantibody to a pancreatic antigen previously discussed. Inflammatory bowel disease is also occasionally associated with autoimmune pancreatitis[297].
Differentiating the focal form of autoimmune pancreatitis rather than pancreatic carcinoma can be very difficult on the basis of CT imaging only[288]. Diffuse pancreatic-ductal narrowing is highly diagnostic of autoimmune pancreatitis[288,289,291]. There are also nonspecific endoscopic findings in the stomach or colon in patients with autoimmune pancreatitis, foci of slightly pale, thickened mucosa with loss of visible vascular pattern were observed in some cases[298]. The role for MRCP is at this time undefined[288].
EUS is a key tool in the diagnosis of autoimmune pancreatitis and its differentiation from other pancreatic diseases. The most common finding on endoscopic ultrasonography is diffuse or focal pancreatic enlargement along with a diffusely hypoechoic parenchyma, similar to findings on transabdominal ultrasonography[299,300].
The use of corticosteroid therapy is not mandatory in autoimmune pancreatitis, as there are reports of the spontaneous resolution of a pancreatic mass, stricture, and jaundice[301,302]. When steroids are given the response is often dramatic and can be monitored via clinical, laboratory and radiological parameters[303-306].
MISCELLANEOUS CAUSES
Recent reports of a new disease known as hyper IgG4 disease has been linked to IP[307] but more data is required before further comment. It is unclear if this is an entirely separate entity from autoimmune pancreatitis.
In renal transplant cases pancreatitis can occur as a result of the procedure itself[308], immunosuppressant medications (see above ‘Medications’), opportunistic infections[309] or through allograft pancreatitis[310].
CHRONIC PANCREATITIS
The aforementioned etiologies of IP can all theoretically lead to CP. The diagnosis is best made after considering the results of ERCP, pancreatic function tests, and EUS[24].
It has been suggested that pancreatic function testing may help establish the diagnosis of CP at an earlier stage[311]. It appears based on recent evidence that EUS may be the most sensitive test[311,312] for CP diagnosis with the caveat of false positives[313].
Mortality rates for CP are 3-4 times greater than in controls[314,315]. Pancreatitis in CP accounts for 20 percent of mortality but mortality is usually from non-pancreatic causes[314,315]. CP carries with it many complications such as pancreatic duct strictures, stones, pseudocyst, fistulas, pseudoaneurysm, or ascites[316-320].
TRUE IDIOPATHIC PANCREATITIS
In spite of extensive systematic investigations and exhaustive efforts, there will be patients with true IP (TIP). Recommendations are difficult to make given the heterogeneity of studies that have evaluated this problem. Generally there are 4 therapies that might be applicable in TIP taking into account that TIP most likely represents a group of heterogeneous disorders: antioxidants, ursodeoxycholic acid, pancreatic enzymes, and somatostatin or its analogue octreotide.
Antioxidants (e.g., vitamin C, and E, beta carotene) have been shown to reduce the pain involved in IP[321]. It is postulated that patients with AP or CP may have a deficiency in antioxidants either locally within the pancreatic parenchyma or systemically[322]. A cocktail of antioxidants including 600 μg of selenium, 9000 IU of β-carotene, 0.54 g of vitamin C, 270 IU of vitamin E, and 2 g of methionine daily[321] was shown to have a statistically significant benefit in reducing attacks of pancreatitis. It is again stressed that these trials have limited power since the above study evaluated only 28 patients[321].
In patients who continue to have attacks of pancreatitis despite having cholecystectomy or endoscopic sphincterotomy, or patients with contraindications to surgical and endoscopic treatment, maintenance therapy with ursodeoxycholic acid has also been suggested with some benefit[31,323].
Pancreatic enzyme therapy has recently been reviewed and the efficacy in IP or recurrent AP or CP is small[324]. However, given the low risk of enzyme therapy it has been suggested as a therapeutic trial in treating CP and IP[325,326].
Somatostatin or its analogue octreotide has been postulated to reduce pancreatic secretion and thereby be of benefit in the treatment of relapsing pancreatitis. This therapy entails either a continuous infusion or frequent injections in the past[327]. A 1 mo depot injection has recently become available and may make this therapy more attractive but data is limited with existing data on octreotide and somatostatin focused on altering outcomes during an index hospitalization of severe pancreatitis rather than preventing subsequent attacks[327].
APPROACH TO RECURRENT IP
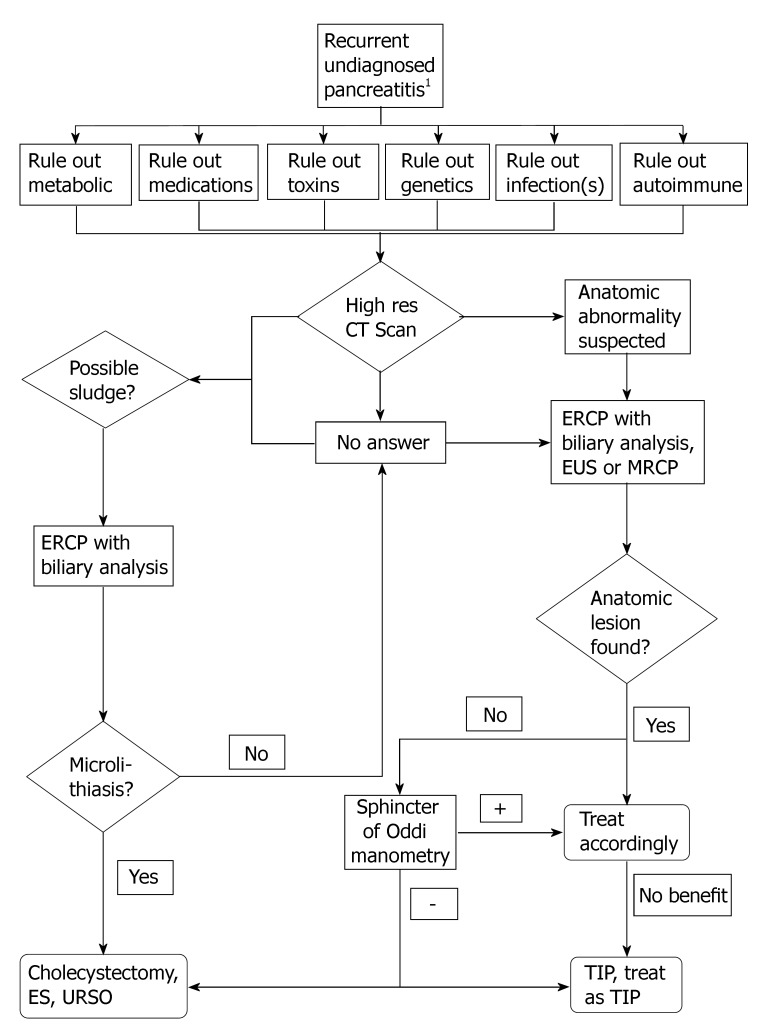
The authors of this review propose the following systematic approach for patients who have had more than one attack of IP. The following is not validated and is meant to be only used as a guide. For specific details please refer to the aforementioned sections (Figure 1).
Figure 1.
1To arrive at the label of recurrent undiagnosed pancreatitis, a history, physical exam, routine laboratory investigations, chest radiograph, abdominal ultrasound, and or computed tomography must fail to find the etiology of pancreatitis. Although sludge may be picked up on transabdominal ultrasound, the low sensitivity precludes exclusion of diagnosis of microlithiasis. ES: Endoscopic sphincterotomy; TIP: True idiopathic pancreatitis; EUS: Endoscopic ultrasound; CT: Computed tomography; URSO: Ursodeoxycholic acid.
CONCLUSION
True IP is declining as knowledge and technology advances. IP has been found now to represent a myriad of etiologies that have been elucidated with the advancement of laboratory and endoscopic studies. It is thought that a thorough workup of these cases should reveal an etiology in up to 80% of cases. If genetic screening is applied it is suspected the etiologies may be explained in a much higher percentage of cases. There are still many controversies surrounding some of the IP etiologies and full consensus agreement is lacking in many areas. This review comprehensively outlined the latest evidence in the etiology, pathogenesis, diagnosis, and treatment strategies. It is felt that establishing a diagnosis is key, for it has the potential to direct management. It is recognized, however, that for many of the disorders (particularly genetic abnormalities) there are limited therapies. Elucidating all the causes of IP is a challenge that needs to be faced so that patients can avoid unnecessary morbidity and mortality from subsequent invasive testing.
The treating physician should perform a detailed history to rule out medication, metabolic, and toxin related etiologies. Moreover a detailed history should raise the possibility of genetic associations and causes as well as infectious etiologies so that further testing can be best directed. Celiac disease, vascular disease, and autoimmune causes of pancreatitis should be considered in evaluating the optimal approach to IP.
The most common causes of IP include microlithiasis and biliary sludge, sphincter of Oddi Dysfunction, and anatomic abnormalities. When there is any risk or suggestion of malignancy either as a cause or as an association of the etiology, as in choledochoceles, appropriate management including the necessary diagnostic workup and consideration of surgical excision when the diagnosis is made needs to be considered. In the aforementioned causes it is clear that both imaging techniques such as MRCP, EUS, high resolution CT, and interventional techniques as ERCP play their respective roles.
Indeed with further research additional causes of IP and associations of IP will be revealed. Even with exhaustive efforts and considering all the etiologies in this article there will still be a few patients who will continue to have true IP. In these patients non specific therapy including pain control is often unfortunately the only option. Certainly further advances in pancreatitis will be welcome.
Footnotes
S- Editor Liu Y L- Editor Alpini GD E- Editor Ma WH
References
- 1.Sarles H. Revised classification of pancreatitis--Marseille 1984. Dig Dis Sci. 1985;30:573–574. doi: 10.1007/BF01320265. [DOI] [PubMed] [Google Scholar]
- 2.Steer ML, Waxman I, Freedman S. Chronic pancreatitis. N Engl J Med. 1995;332:1482–1490. doi: 10.1056/NEJM199506013322206. [DOI] [PubMed] [Google Scholar]
- 3.Bank S, Indaram A. Causes of acute and recurrent pancreatitis. Clinical considerations and clues to diagnosis. Gastroenterol Clin North Am. 1999;28:571–589, viii. doi: 10.1016/s0889-8553(05)70074-1. [DOI] [PubMed] [Google Scholar]
- 4.Banks PA. Epidemiology, natural history, and predictors of disease outcome in acute and chronic pancreatitis. Gastrointest Endosc. 2002;56:S226–S230. doi: 10.1067/mge.2002.129022. [DOI] [PubMed] [Google Scholar]
- 5.Grendell JH. Idiopathic acute pancreatitis. Gastroenterol Clin North Am. 1990;19:843–848. [PubMed] [Google Scholar]
- 6.Thomson HJ. Acute pancreatitis in north and north-east Scotland. J R Coll Surg Edinb. 1985;30:104–111. [PubMed] [Google Scholar]
- 7.Thomson SR, Hendry WS, McFarlane GA, Davidson AI. Epidemiology and outcome of acute pancreatitis. Br J Surg. 1987;74:398–401. doi: 10.1002/bjs.1800740526. [DOI] [PubMed] [Google Scholar]
- 8.Giggs JA, Bourke JB, Katschinski B. The epidemiology of primary acute pancreatitis in Greater Nottingham: 1969-1983. Soc Sci Med. 1988;26:79–89. doi: 10.1016/0277-9536(88)90047-0. [DOI] [PubMed] [Google Scholar]
- 9.Svensson JO, Norbäck B, Bokey EL, Edlund Y. Changing pattern in aetiology of pancreatitis in an urban Swedish area. Br J Surg. 1979;66:159–161. doi: 10.1002/bjs.1800660306. [DOI] [PubMed] [Google Scholar]
- 10.Coyle WJ, Pineau BC, Tarnasky PR, Knapple WL, Aabakken L, Hoffman BJ, Cunningham JT, Hawes RH, Cotton PB. Evaluation of unexplained acute and acute recurrent pancreatitis using endoscopic retrograde cholangiopancreatography, sphincter of Oddi manometry and endoscopic ultrasound. Endoscopy. 2002;34:617–623. doi: 10.1055/s-2002-33245. [DOI] [PubMed] [Google Scholar]
- 11.Kaw M, Brodmerkel GJ. ERCP, biliary crystal analysis, and sphincter of Oddi manometry in idiopathic recurrent pancreatitis. Gastrointest Endosc. 2002;55:157–162. doi: 10.1067/mge.2002.118944. [DOI] [PubMed] [Google Scholar]
- 12.Yadav D, Lowenfels AB. Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas. 2006;33:323–330. doi: 10.1097/01.mpa.0000236733.31617.52. [DOI] [PubMed] [Google Scholar]
- 13.Gislason H, Horn A, Hoem D, Andrén-Sandberg A, Imsland AK, Søreide O, Viste A. Acute pancreatitis in Bergen, Norway. A study on incidence, etiology and severity. Scand J Surg. 2004;93:29–33. doi: 10.1177/145749690409300106. [DOI] [PubMed] [Google Scholar]
- 14.Goldacre MJ, Roberts SE. Hospital admission for acute pancreatitis in an English population, 1963-98: database study of incidence and mortality. BMJ. 2004;328:1466–1469. doi: 10.1136/bmj.328.7454.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Floyd A, Pedersen L, Nielsen GL, Thorladcius-Ussing O, Sorensen HT. Secular trends in incidence and 30-day case fatality of acute pancreatitis in North Jutland County, Denmark: a register-based study from 1981-2000. Scand J Gastroenterol. 2002;37:1461–1465. doi: 10.1080/003655202762671369. [DOI] [PubMed] [Google Scholar]
- 16.Mann DV, Hershman MJ, Hittinger R, Glazer G. Multicentre audit of death from acute pancreatitis. Br J Surg. 1994;81:890–893. doi: 10.1002/bjs.1800810632. [DOI] [PubMed] [Google Scholar]
- 17.Birgisson H, Möller PH, Birgisson S, Thoroddsen A, Asgeirsson KS, Sigurjónsson SV, Magnússon J. Acute pancreatitis: a prospective study of its incidence, aetiology, severity, and mortality in Iceland. Eur J Surg. 2002;168:278–282. doi: 10.1002/ejs.46. [DOI] [PubMed] [Google Scholar]
- 18.Lankisch PG, Assmus C, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic diseases in Lüneburg County. A study in a defined german population. Pancreatology. 2002;2:469–477. doi: 10.1159/000064713. [DOI] [PubMed] [Google Scholar]
- 19.Wilson C, Imrie CW, Carter DC. Fatal acute pancreatitis. Gut. 1988;29:782–788. doi: 10.1136/gut.29.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banerjee AK, Kaul A, Bache E, Parberry AC, Doran J, Nicholson ML. An audit of fatal acute pancreatitis. Postgrad Med J. 1995;71:472–475. doi: 10.1136/pgmj.71.838.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trapnell JE, Duncan EH. Patterns of incidence in acute pancreatitis. Br Med J. 1975;2:179–183. doi: 10.1136/bmj.2.5964.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindkvist B, Appelros S, Manjer J, Borgström A. Trends in incidence of acute pancreatitis in a Swedish population: is there really an increase? Clin Gastroenterol Hepatol. 2004;2:831–837. doi: 10.1016/s1542-3565(04)00355-6. [DOI] [PubMed] [Google Scholar]
- 23.Bernard JP, Sahel J, Giovannini M, Sarles H. Pancreas divisum is a probable cause of acute pancreatitis: a report of 137 cases. Pancreas. 1990;5:248–254. doi: 10.1097/00006676-199005000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Levy MJ, Geenen JE. Idiopathic acute recurrent pancreatitis. Am J Gastroenterol. 2001;96:2540–2555. doi: 10.1111/j.1572-0241.2001.04098.x. [DOI] [PubMed] [Google Scholar]
- 25.Venu RP, Geenen JE, Hogan W, Stone J, Johnson GK, Soergel K. Idiopathic recurrent pancreatitis. An approach to diagnosis and treatment. Dig Dis Sci. 1989;34:56–60. doi: 10.1007/BF01536154. [DOI] [PubMed] [Google Scholar]
- 26.Ballinger AB, Barnes E, Alstead EM, Fairclough PD. Is intervention necessary after a first episode of acute idiopathic pancreatitis? Gut. 1996;38:293–295. doi: 10.1136/gut.38.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682–707. doi: 10.1053/gast.2001.22586. [DOI] [PubMed] [Google Scholar]
- 28.Ko CW, Sekijima JH, Lee SP. Biliary sludge. Ann Intern Med. 1999;130:301–311. doi: 10.7326/0003-4819-130-4-199902160-00016. [DOI] [PubMed] [Google Scholar]
- 29.Farinon AM, Ricci GL, Sianesi M, Percudani M, Zanella E. Physiopathologic role of microlithiasis in gallstone pancreatitis. Surg Gynecol Obstet. 1987;164:252–256. [PubMed] [Google Scholar]
- 30.Levy MJ. The hunt for microlithiasis in idiopathic acute recurrent pancreatitis: should we abandon the search or intensify our efforts? Gastrointest Endosc. 2002;55:286–293. doi: 10.1067/mge.2002.121224. [DOI] [PubMed] [Google Scholar]
- 31.Ros E, Navarro S, Bru C, Garcia-Pugés A, Valderrama R. Occult microlithiasis in 'idiopathic' acute pancreatitis: prevention of relapses by cholecystectomy or ursodeoxycholic acid therapy. Gastroenterology. 1991;101:1701–1709. doi: 10.1016/0016-5085(91)90410-m. [DOI] [PubMed] [Google Scholar]
- 32.Lopez AJ, O'Keefe P, Morrissey M, Pickleman J. Ceftriaxone-induced cholelithiasis. Ann Intern Med. 1991;115:712–714. doi: 10.7326/0003-4819-115-9-712. [DOI] [PubMed] [Google Scholar]
- 33.Lee SP, Nicholls JF, Park HZ. Biliary sludge as a cause of acute pancreatitis. N Engl J Med. 1992;326:589–593. doi: 10.1056/NEJM199202273260902. [DOI] [PubMed] [Google Scholar]
- 34.Tandon M, Topazian M. Endoscopic ultrasound in idiopathic acute pancreatitis. Am J Gastroenterol. 2001;96:705–709. doi: 10.1111/j.1572-0241.2001.03609.x. [DOI] [PubMed] [Google Scholar]
- 35.Kim HJ, Kim MH, Bae JS, Lee SS, Seo DW, Lee SK. Idiopathic acute pancreatitis. J Clin Gastroenterol. 2003;37:238–250. doi: 10.1097/00004836-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Garg PK, Tandon RK, Madan K. Is biliary microlithiasis a significant cause of idiopathic recurrent acute pancreatitis? A long-term follow-up study. Clin Gastroenterol Hepatol. 2007;5:75–79. doi: 10.1016/j.cgh.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 37.Kohut M, Nowak A, Nowakowska-Duiawa E, Marek T. Presence and density of common bile duct microlithiasis in acute biliary pancreatitis. World J Gastroenterol. 2002;8:558–561. doi: 10.3748/wjg.v8.i3.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kohut M, Nowak A, Nowakowska-Duława E, Kaczor R, Marek T. The frequency of bile duct crystals in patients with presumed biliary pancreatitis. Gastrointest Endosc. 2001;54:37–41. doi: 10.1067/mge.2001.115475. [DOI] [PubMed] [Google Scholar]
- 39.Hernández CA, Lerch MM. Sphincter stenosis and gallstone migration through the biliary tract. Lancet. 1993;341:1371–1373. doi: 10.1016/0140-6736(93)90942-a. [DOI] [PubMed] [Google Scholar]
- 40.Grau F, Almela P, Aparisi L, Bautista D, Pascual I, Peña A, Rodrigo JM. Usefulness of alanine and aspartate aminotransferases in the diagnosis of microlithiasis in idiopathic acute pancreatitis. Int J Pancreatol. 1999;25:107–111. doi: 10.1385/IJGC:25:2:107. [DOI] [PubMed] [Google Scholar]
- 41.Queneau PE, Zeeh S, Lapeyre V, Thibault P, Heyd B, Carayon P, Miguet JP. Feasibility of and interest in combined endoscopic ultrasonography and biliary drainage in unexplained acute biliopancreatic disorders. Dig Dis Sci. 2002;47:2020–2024. doi: 10.1023/a:1019616711220. [DOI] [PubMed] [Google Scholar]
- 42.Yusoff IF, Raymond G, Sahai AV. A prospective comparison of the yield of EUS in primary vs. recurrent idiopathic acute pancreatitis. Gastrointest Endosc. 2004;60:673–678. doi: 10.1016/s0016-5107(04)02018-8. [DOI] [PubMed] [Google Scholar]
- 43.Norton SA, Alderson D. Endoscopic ultrasonography in the evaluation of idiopathic acute pancreatitis. Br J Surg. 2000;87:1650–1655. doi: 10.1046/j.1365-2168.2000.01587.x. [DOI] [PubMed] [Google Scholar]
- 44.Frossard JL, Sosa-Valencia L, Amouyal G, Marty O, Hadengue A, Amouyal P. Usefulness of endoscopic ultrasonography in patients with "idiopathic" acute pancreatitis. Am J Med. 2000;109:196–200. doi: 10.1016/s0002-9343(00)00478-2. [DOI] [PubMed] [Google Scholar]
- 45.Kurol M, Forsberg L. Ultrasonography in the diagnosis of acute cholecystitis. Acta Radiol Diagn (Stockh) 1984;25:379–383. doi: 10.1177/028418518402500506. [DOI] [PubMed] [Google Scholar]
- 46.Marotta PJ, Gregor JC, Taves DH. Biliary sludge: a risk factor for 'idiopathic' pancreatitis? Can J Gastroenterol. 1996;10:385–388. doi: 10.1155/1996/107353. [DOI] [PubMed] [Google Scholar]
- 47.Goodman AJ, Neoptolemos JP, Carr-Locke DL, Finlay DB, Fossard DP. Detection of gall stones after acute pancreatitis. Gut. 1985;26:125–132. doi: 10.1136/gut.26.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilcox CM, Varadarajulu S, Eloubeidi M. Role of endoscopic evaluation in idiopathic pancreatitis: a systematic review. Gastrointest Endosc. 2006;63:1037–1045. doi: 10.1016/j.gie.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 49.Lehman GA, Sherman S. Hypertensive pancreatic sphincter. Can J Gastroenterol. 1998;12:333–337. doi: 10.1155/1998/148150. [DOI] [PubMed] [Google Scholar]
- 50.Petersen BT. An evidence-based review of sphincter of Oddi dysfunction: part I, presentations with "objective" biliary findings (types I and II) Gastrointest Endosc. 2004;59:525–534. doi: 10.1016/s0016-5107(04)00012-4. [DOI] [PubMed] [Google Scholar]
- 51.Sherman S, Ruffolo TA, Hawes RH, Lehman GA. Complications of endoscopic sphincterotomy. A prospective series with emphasis on the increased risk associated with sphincter of Oddi dysfunction and nondilated bile ducts. Gastroenterology. 1991;101:1068–1075. [PubMed] [Google Scholar]
- 52.Tarnasky P, Cunningham J, Cotton P, Hoffman B, Palesch Y, Freeman J, Curry N, Hawes R. Pancreatic sphincter hypertension increases the risk of post-ERCP pancreatitis. Endoscopy. 1997;29:252–257. doi: 10.1055/s-2007-1004185. [DOI] [PubMed] [Google Scholar]
- 53.Hogan WJ, Geenen JE, Dodds WJ. Dysmotility disturbances of the biliary tract: classification, diagnosis, and treatment. Semin Liver Dis. 1987;7:302–310. doi: 10.1055/s-2008-1040585. [DOI] [PubMed] [Google Scholar]
- 54.Kuo WH, Pasricha PJ, Kalloo AN. The role of sphincter of Oddi manometry in the diagnosis and therapy of pancreatic disease. Gastrointest Endosc Clin N Am. 1998;8:79–85. [PubMed] [Google Scholar]
- 55.Freeman ML. Adverse outcomes of ERCP. Gastrointest Endosc. 2002;56:S273–S282. doi: 10.1067/mge.2002.129028. [DOI] [PubMed] [Google Scholar]
- 56.Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–393. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 57.Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909–918. doi: 10.1056/NEJM199609263351301. [DOI] [PubMed] [Google Scholar]
- 58.Freeman ML, Guda NM. Prevention of post-ERCP pancreatitis: a comprehensive review. Gastrointest Endosc. 2004;59:845–864. doi: 10.1016/s0016-5107(04)00353-0. [DOI] [PubMed] [Google Scholar]
- 59.Gottlieb K, Sherman S. ERCP and biliary endoscopic sphincterotomy-induced pancreatitis. Gastrointest Endosc Clin N Am. 1998;8:87–114. [PubMed] [Google Scholar]
- 60.Geenen JE, Nash JA. The role of sphincter of Oddi manometry and biliary microscopy in evaluating idiopathic recurrent pancreatitis. Endoscopy. 1998;30:A237–A241. doi: 10.1055/s-2007-1001447. [DOI] [PubMed] [Google Scholar]
- 61.Eversman D, Fogel EL, Rusche M, Sherman S, Lehman GA. Frequency of abnormal pancreatic and biliary sphincter manometry compared with clinical suspicion of sphincter of Oddi dysfunction. Gastrointest Endosc. 1999;50:637–641. doi: 10.1016/s0016-5107(99)80011-x. [DOI] [PubMed] [Google Scholar]
- 62.Aymerich RR, Prakash C, Aliperti G. Sphincter of oddi manometry: is it necessary to measure both biliary and pancreatic sphincter pressures? Gastrointest Endosc. 2000;52:183–186. doi: 10.1067/mge.2000.105985. [DOI] [PubMed] [Google Scholar]
- 63.Sherman S, Troiano FP, Hawes RH, Lehman GA. Sphincter of Oddi manometry: decreased risk of clinical pancreatitis with use of a modified aspirating catheter. Gastrointest Endosc. 1990;36:462–466. doi: 10.1016/s0016-5107(90)71115-7. [DOI] [PubMed] [Google Scholar]
- 64.Geenen JE, Hogan WJ, Dodds WJ, Toouli J, Venu RP. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter-of-Oddi dysfunction. N Engl J Med. 1989;320:82–87. doi: 10.1056/NEJM198901123200203. [DOI] [PubMed] [Google Scholar]
- 65.Toouli J, Roberts-Thomson IC, Kellow J, Dowsett J, Saccone GT, Evans P, Jeans P, Cox M, Anderson P, Worthley C, et al. Manometry based randomised trial of endoscopic sphincterotomy for sphincter of Oddi dysfunction. Gut. 2000;46:98–102. doi: 10.1136/gut.46.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Somogyi L, Martin SP, Venkatesan T, Ulrich CD. Recurrent acute pancreatitis: an algorithmic approach to identification and elimination of inciting factors. Gastroenterology. 2001;120:708–717. doi: 10.1053/gast.2001.22333. [DOI] [PubMed] [Google Scholar]
- 67.Rolny P, Geenen JE, Hogan WJ. Post-cholecystectomy patients with "objective signs" of partial bile outflow obstruction: clinical characteristics, sphincter of Oddi manometry findings, and results of therapy. Gastrointest Endosc. 1993;39:778–781. doi: 10.1016/s0016-5107(93)70264-3. [DOI] [PubMed] [Google Scholar]
- 68.Hogan WJ, Sherman S, Pasricha P, Carr-Locke D. Sphincter of Oddi manometry. Gastrointest Endosc. 1997;45:342–348. [PubMed] [Google Scholar]
- 69.Botoman VA, Kozarek RA, Novell LA, Patterson DJ, Ball TJ, Wechter DG, Neal LA. Long-term outcome after endoscopic sphincterotomy in patients with biliary colic and suspected sphincter of Oddi dysfunction. Gastrointest Endosc. 1994;40:165–170. doi: 10.1016/s0016-5107(94)70160-1. [DOI] [PubMed] [Google Scholar]
- 70.Wehrmann T, Wiemer K, Lembcke B, Caspary WF, Jung M. Do patients with sphincter of Oddi dysfunction benefit from endoscopic sphincterotomy? A 5-year prospective trial. Eur J Gastroenterol Hepatol. 1996;8:251–256. doi: 10.1097/00042737-199603000-00012. [DOI] [PubMed] [Google Scholar]
- 71.Rosenblatt ML, Catalano MF, Alcocer E, Geenen JE. Comparison of sphincter of Oddi manometry, fatty meal sonography, and hepatobiliary scintigraphy in the diagnosis of sphincter of Oddi dysfunction. Gastrointest Endosc. 2001;54:697–704. doi: 10.1067/mge.2001.118946. [DOI] [PubMed] [Google Scholar]
- 72.Freeman ML, Gill M, Overby C, Cen YY. Predictors of outcomes after biliary and pancreatic sphincterotomy for sphincter of oddi dysfunction. J Clin Gastroenterol. 2007;41:94–102. doi: 10.1097/01.mcg.0000225584.40212.fb. [DOI] [PubMed] [Google Scholar]
- 73.Cohen S, Bacon BR, Berlin JA, Fleischer D, Hecht GA, Loehrer PJ, McNair AE, Mulholland M, Norton NJ, Rabeneck L, et al. National Institutes of Health State-of-the-Science Conference Statement: ERCP for diagnosis and therapy, January 14-16, 2002. Gastrointest Endosc. 2002;56:803–809. doi: 10.1067/mge.2002.129875. [DOI] [PubMed] [Google Scholar]
- 74.Sherman S, Troiano FP, Hawes RH, O'Connor KW, Lehman GA. Frequency of abnormal sphincter of Oddi manometry compared with the clinical suspicion of sphincter of Oddi dysfunction. Am J Gastroenterol. 1991;86:586–590. [PubMed] [Google Scholar]
- 75.Lans JL, Parikh NP, Geenen JE. Application of sphincter of Oddi manometry in routine clinical investigations. Endoscopy. 1991;23:139–143. doi: 10.1055/s-2007-1010641. [DOI] [PubMed] [Google Scholar]
- 76.Rolny P, Arlebäck A, Funch-Jensen P, Kruse A, Järnerot G. Clinical significance of manometric assessment of both pancreatic duct and bile duct sphincter in the same patient. Scand J Gastroenterol. 1989;24:751–754. doi: 10.3109/00365528909093117. [DOI] [PubMed] [Google Scholar]
- 77.Silverman WB, Slivka A, Rabinovitz M, Wilson J. Hybrid classification of sphincter of Oddi dysfunction based on simplified Milwaukee criteria: effect of marginal serum liver and pancreas test elevations. Dig Dis Sci. 2001;46:278–281. doi: 10.1023/a:1005692530034. [DOI] [PubMed] [Google Scholar]
- 78.Khuroo MS, Zargar SA, Yattoo GN. Efficacy of nifedipine therapy in patients with sphincter of Oddi dysfunction: a prospective, double-blind, randomized, placebo-controlled, cross over trial. Br J Clin Pharmacol. 1992;33:477–485. doi: 10.1111/j.1365-2125.1992.tb04074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kalloo AN, Pasricha PJ. Therapy of sphincter of Oddi dysfunction. Gastrointest Endosc Clin N Am. 1996;6:117–125. [PubMed] [Google Scholar]
- 80.Tarnasky PR, Hawes RH. Endoscopic diagnosis and therapy of unexplained (idiopathic) acute pancreatitis. Gastrointest Endosc Clin N Am. 1998;8:13–37. [PubMed] [Google Scholar]
- 81.Cotton PB. Pancreas divisum. Am J Gastroenterol. 1995;90:1898. [PubMed] [Google Scholar]
- 82.Cotton PB. Congenital anomaly of pancreas divisum as cause of obstructive pain and pancreatitis. Gut. 1980;21:105–114. doi: 10.1136/gut.21.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Delhaye M, Engelholm L, Cremer M. Pancreas divisum: controversial clinical significance. Dig Dis. 1988;6:30–39. doi: 10.1159/000171182. [DOI] [PubMed] [Google Scholar]
- 84.Burtin P, Person B, Charneau J, Boyer J. Pancreas divisum and pancreatitis: a coincidental association? Endoscopy. 1991;23:55–58. doi: 10.1055/s-2007-1010612. [DOI] [PubMed] [Google Scholar]
- 85.Carr-Locke DL. Pancreas divisum: the controversy goes on? Endoscopy. 1991;23:88–90. doi: 10.1055/s-2007-1010621. [DOI] [PubMed] [Google Scholar]
- 86.Delhaye M, Engelholm L, Cremer M. Pancreas divisum: congenital anatomic variant or anomaly? Contribution of endoscopic retrograde dorsal pancreatography. Gastroenterology. 1985;89:951–958. doi: 10.1016/0016-5085(85)90193-3. [DOI] [PubMed] [Google Scholar]
- 87.Lehman GA, Sherman S. Pancreas divisum. Diagnosis, clinical significance, and management alternatives. Gastrointest Endosc Clin N Am. 1995;5:145–170. [PubMed] [Google Scholar]
- 88.Krueger KJ, Wootton FT, Cunningham JT, Hoffman BJ. Unexpected anomalies of the common bile and pancreatic ducts. Am J Gastroenterol. 1992;87:1492–1495. [PubMed] [Google Scholar]
- 89.Cunningham JT. Pancreas divisum and acute pancreatitis: romancing the stone? Am J Gastroenterol. 1992;87:802–803. [PubMed] [Google Scholar]
- 90.Choudari CP, Imperiale TF, Sherman S, Fogel E, Lehman GA. Risk of pancreatitis with mutation of the cystic fibrosis gene. Am J Gastroenterol. 2004;99:1358–1363. doi: 10.1111/j.1572-0241.2004.30655.x. [DOI] [PubMed] [Google Scholar]
- 91.Kamisawa T, Tu Y, Egawa N, Tsuruta K, Okamoto A, Kamata N. MRCP of congenital pancreaticobiliary malformation. Abdom Imaging. 2007;32:129–133. doi: 10.1007/s00261-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 92.Bret PM, Reinhold C, Taourel P, Guibaud L, Atri M, Barkun AN. Pancreas divisum: evaluation with MR cholangiopancreatography. Radiology. 1996;199:99–103. doi: 10.1148/radiology.199.1.8633179. [DOI] [PubMed] [Google Scholar]
- 93.Devereaux BM, Fein S, Purich E, Trout JR, Lehman GA, Fogel EL, Phillips S, Etemad R, Jowell P, Toskes PP, et al. A new synthetic porcine secretin for facilitation of cannulation of the dorsal pancreatic duct at ERCP in patients with pancreas divisum: a multicenter, randomized, double-blind comparative study. Gastrointest Endosc. 2003;57:643–647. doi: 10.1067/mge.2003.195. [DOI] [PubMed] [Google Scholar]
- 94.Warshaw AL, Simeone J, Schapiro RH, Hedberg SE, Mueller PE, Ferrucci JT. Objective evaluation of ampullary stenosis with ultrasonography and pancreatic stimulation. Am J Surg. 1985;149:65–72. doi: 10.1016/s0002-9610(85)80011-8. [DOI] [PubMed] [Google Scholar]
- 95.Lowes JR, Lees WR, Cotton PB. Pancreatic duct dilatation after secretin stimulation in patients with pancreas divisum. Pancreas. 1989;4:371–374. doi: 10.1097/00006676-198906000-00015. [DOI] [PubMed] [Google Scholar]
- 96.Warshaw AL, Simeone JF, Schapiro RH, Flavin-Warshaw B. Evaluation and treatment of the dominant dorsal duct syndrome (pancreas divisum redefined) Am J Surg. 1990;159:59–64; discussion 64-66. doi: 10.1016/s0002-9610(05)80607-5. [DOI] [PubMed] [Google Scholar]
- 97.Park SH, de Bellis M, McHenry L, Fogel EL, Lazzell L, Bucksot L, Sherman S, Lehman GA. Use of methylene blue to identify the minor papilla or its orifice in patients with pancreas divisum. Gastrointest Endosc. 2003;57:358–363. doi: 10.1067/mge.2003.110. [DOI] [PubMed] [Google Scholar]
- 98.Heyries L, Barthet M, Delvasto C, Zamora C, Bernard JP, Sahel J. Long-term results of endoscopic management of pancreas divisum with recurrent acute pancreatitis. Gastrointest Endosc. 2002;55:376–381. doi: 10.1067/mge.2002.121602. [DOI] [PubMed] [Google Scholar]
- 99.Lans JI, Geenen JE, Johanson JF, Hogan WJ. Endoscopic therapy in patients with pancreas divisum and acute pancreatitis: a prospective, randomized, controlled clinical trial. Gastrointest Endosc. 1992;38:430–434. doi: 10.1016/s0016-5107(92)70471-4. [DOI] [PubMed] [Google Scholar]
- 100.Lehman GA, Sherman S, Nisi R, Hawes RH. Pancreas divisum: results of minor papilla sphincterotomy. Gastrointest Endosc. 1993;39:1–8. doi: 10.1016/s0016-5107(93)70001-2. [DOI] [PubMed] [Google Scholar]
- 101.Ertan A. Long-term results after endoscopic pancreatic stent placement without pancreatic papillotomy in acute recurrent pancreatitis due to pancreas divisum. Gastrointest Endosc. 2000;52:9–14. doi: 10.1067/mge.2000.106311. [DOI] [PubMed] [Google Scholar]
- 102.Kamisawa T. Clinical significance of the minor duodenal papilla and accessory pancreatic duct. J Gastroenterol. 2004;39:605–615. doi: 10.1007/s00535-004-1390-1. [DOI] [PubMed] [Google Scholar]
- 103.Lehman GA, Sherman S. Diagnosis and therapy of pancreas divisum. Gastrointest Endosc Clin N Am. 1998;8:55–77. [PubMed] [Google Scholar]
- 104.Smith MT, Sherman S, Ikenberry SO, Hawes RH, Lehman GA. Alterations in pancreatic ductal morphology following polyethylene pancreatic stent therapy. Gastrointest Endosc. 1996;44:268–275. doi: 10.1016/s0016-5107(96)70163-3. [DOI] [PubMed] [Google Scholar]
- 105.Sherman S, Hawes RH, Savides TJ, Gress FG, Ikenberry SO, Smith MT, Zaidi S, Lehman GA. Stent-induced pancreatic ductal and parenchymal changes: correlation of endoscopic ultrasound with ERCP. Gastrointest Endosc. 1996;44:276–282. doi: 10.1016/s0016-5107(96)70164-5. [DOI] [PubMed] [Google Scholar]
- 106.Guelrud M, Morera C, Rodriguez M, Prados JG, Jaén D. Normal and anomalous pancreaticobiliary union in children and adolescents. Gastrointest Endosc. 1999;50:189–193. doi: 10.1016/s0016-5107(99)70223-3. [DOI] [PubMed] [Google Scholar]
- 107.Okada A, Nakamura T, Higaki J, Okumura K, Kamata S, Oguchi Y. Congenital dilatation of the bile duct in 100 instances and its relationship with anomalous junction. Surg Gynecol Obstet. 1990;171:291–298. [PubMed] [Google Scholar]
- 108.Matsumoto Y, Fujii H, Itakura J, Matsuda M, Nobukawa B, Suda K. Recent advances in pancreaticobiliary maljunction. J Hepatobiliary Pancreat Surg. 2002;9:45–54. doi: 10.1007/s005340200004. [DOI] [PubMed] [Google Scholar]
- 109.Komi N, Takehara H, Kunitomo K, Miyoshi Y, Yagi T. Does the type of anomalous arrangement of pancreaticobiliary ducts influence the surgery and prognosis of choledochal cyst? J Pediatr Surg. 1992;27:728–731. doi: 10.1016/s0022-3468(05)80102-2. [DOI] [PubMed] [Google Scholar]
- 110.Tashiro S, Imaizumi T, Ohkawa H, Okada A, Katoh T, Kawaharada Y, Shimada H, Takamatsu H, Miyake H, Todani T. Pancreaticobiliary maljunction: retrospective and nationwide survey in Japan. J Hepatobiliary Pancreat Surg. 2003;10:345–351. doi: 10.1007/s00534-002-0741-7. [DOI] [PubMed] [Google Scholar]
- 111.Guelrud M, Morera C, Rodriguez M, Jaen D, Pierre R. Sphincter of Oddi dysfunction in children with recurrent pancreatitis and anomalous pancreaticobiliary union: an etiologic concept. Gastrointest Endosc. 1999;50:194–199. doi: 10.1016/s0016-5107(99)70224-5. [DOI] [PubMed] [Google Scholar]
- 112.Samavedy R, Sherman S, Lehman GA. Endoscopic therapy in anomalous pancreatobiliary duct junction. Gastrointest Endosc. 1999;50:623–627. doi: 10.1016/s0016-5107(99)80009-1. [DOI] [PubMed] [Google Scholar]
- 113.Nomura T, Shirai Y, Sandoh N, Nagakura S, Hatakeyama K. Cholangiographic criteria for anomalous union of the pancreatic and biliary ducts. Gastrointest Endosc. 2002;55:204–208. doi: 10.1067/mge.2002.121341. [DOI] [PubMed] [Google Scholar]
- 114.Kinoshita H, Nagata E, Hirohashi K, Sakai K, Kobayashi Y. Carcinoma of the gallbladder with an anomalous connection between the choledochus and the pancreatic duct. Report of 10 cases and review of the literature in Japan. Cancer. 1984;54:762–769. doi: 10.1002/1097-0142(1984)54:4<762::aid-cncr2820540429>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 115.Davenport M, Stringer MD, Howard ER. Biliary amylase and congenital choledochal dilatation. J Pediatr Surg. 1995;30:474–477. doi: 10.1016/0022-3468(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 116.Chijiiwa K, Kimura H, Tanaka M. Malignant potential of the gallbladder in patients with anomalous pancreaticobiliary ductal junction. The difference in risk between patients with and without choledochal cyst. Int Surg. 1995;80:61–64. [PubMed] [Google Scholar]
- 117.Sandoh N, Shirai Y, Hatakeyama K. Incidence of anomalous union of the pancreaticobiliary ductal system in biliary cancer. Hepatogastroenterology. 1997;44:1580–1583. [PubMed] [Google Scholar]
- 118.Yamauchi S, Koga A, Matsumoto S, Tanaka M, Nakayama F. Anomalous junction of pancreaticobiliary duct without congenital choledochal cyst: a possible risk factor for gallbladder cancer. Am J Gastroenterol. 1987;82:20–24. [PubMed] [Google Scholar]
- 119.Todani T, Watanabe Y, Narusue M, Tabuchi K, Okajima K. Congenital bile duct cysts: Classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1977;134:263–269. doi: 10.1016/0002-9610(77)90359-2. [DOI] [PubMed] [Google Scholar]
- 120.Kim MH, Myung SJ, Lee SK, Yoo BM, Seo DW, Lee MH, Jung SA, Kim YS, Min YI. Ballooning of the papilla during contrast injection: the semaphore of a choledochocele. Gastrointest Endosc. 1998;48:258–262. doi: 10.1016/s0016-5107(98)70187-7. [DOI] [PubMed] [Google Scholar]
- 121.Sarris GE, Tsang D. Choledochocele: case report, literature review, and a proposed classification. Surgery. 1989;105:408–414. [PubMed] [Google Scholar]
- 122.Goldberg PB, Long WB, Oleaga JA, Mackie JA. Choledochocele as a cause of recurrent pancreatitis. Gastroenterology. 1980;78:1041–1045. [PubMed] [Google Scholar]
- 123.Ochiai K, Kaneko K, Kitagawa M, Ando H, Hayakawa T. Activated pancreatic enzyme and pancreatic stone protein (PSP/reg) in bile of patients with pancreaticobiliary maljunction/ choledochal cysts. Dig Dis Sci. 2004;49:1953–1956. doi: 10.1007/s10620-004-9599-7. [DOI] [PubMed] [Google Scholar]
- 124.Venu RP, Geenen JE, Hogan WJ, Dodds WJ, Wilson SW, Stewart ET, Soergel KH. Role of endoscopic retrograde cholangiopancreatography in the diagnosis and treatment of choledochocele. Gastroenterology. 1984;87:1144–1149. [PubMed] [Google Scholar]
- 125.Shimizu T, Suzuki R, Yamashiro Y, Segawa O, Yamataka A, Kuwatsuru R. Magnetic resonance cholangiopancreatography in assessing the cause of acute pancreatitis in children. Pancreas. 2001;22:196–199. doi: 10.1097/00006676-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 126.Schaefer JF, Kirschner HJ, Lichy M, Schlemmer HP, Schick F, Claussen CD, Fuchs J. Highly resolved free-breathing magnetic resonance cholangiopancreatography in the diagnostic workup of pancreaticobiliary diseases in infants and young children--initial experiences. J Pediatr Surg. 2006;41:1645–1651. doi: 10.1016/j.jpedsurg.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 127.Gerritsen JJ, Janssens AR, Kroon HM. Choledochocele: treatment by endoscopic sphincterotomy. Br J Surg. 1988;75:495–496. doi: 10.1002/bjs.1800750533. [DOI] [PubMed] [Google Scholar]
- 128.Lopez RR, Pinson CW, Campbell JR, Harrison M, Katon RM. Variation in management based on type of choledochal cyst. Am J Surg. 1991;161:612–615. doi: 10.1016/0002-9610(91)90911-v. [DOI] [PubMed] [Google Scholar]
- 129.Jordan PH, Goss JA, Rosenberg WR, Woods KL. Some considerations for management of choledochal cysts. Am J Surg. 2004;187:790–795. doi: 10.1016/j.amjsurg.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 130.Lipsett PA, Pitt HA. Surgical treatment of choledochal cysts. J Hepatobiliary Pancreat Surg. 2003;10:352–359. doi: 10.1007/s00534-002-0797-4. [DOI] [PubMed] [Google Scholar]
- 131.Woon CY, Tan YM, Oei CL, Chung AY, Chow PK, Ooi LL. Adult choledochal cysts: an audit of surgical management. ANZ J Surg. 2006;76:981–986. doi: 10.1111/j.1445-2197.2006.03915.x. [DOI] [PubMed] [Google Scholar]
- 132.Ozawa K, Yamada T, Matumoto Y, Tobe R. Carcinoma arising in a choledochocele. Cancer. 1980;45:195–197. doi: 10.1002/1097-0142(19800101)45:1<195::aid-cncr2820450131>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 133.Schulte S. Embryology, and congenital anomalies of the bile and pancreatic ducts. In: Silvis S, Rohrmann CA, Ansel HJ, editors. Text and Atlas of Endoscopic Retrograde Cholangiopancreatography. New York: Igaku-Shoin; 1995. pp. 1–480. [Google Scholar]
- 134.Ravitch MM. The pancreas in infants and children. Surg Clin North Am. 1975;55:377–385. doi: 10.1016/s0039-6109(16)40587-6. [DOI] [PubMed] [Google Scholar]
- 135.Itoh Y, Hada T, Terano A, Itai Y, Harada T. Pancreatitis in the annulus of annular pancreas demonstrated by the combined use of computed tomography and endoscopic retrograde cholangiopancreatography. Am J Gastroenterol. 1989;84:961–964. [PubMed] [Google Scholar]
- 136.Kiernan PD, ReMine SG, Kiernan PC, ReMine WH. Annular pancreas: May Clinic experience from 1957 to 1976 with review of the literature. Arch Surg. 1980;115:46–50. doi: 10.1001/archsurg.1980.01380010038007. [DOI] [PubMed] [Google Scholar]
- 137.Lloyd-Jones W, Mountain JC, Warren KW. Annular pancreas in the adult. Ann Surg. 1972;176:163–170. doi: 10.1097/00000658-197208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rizzo RJ, Szucs RA, Turner MA. Congenital abnormalities of the pancreas and biliary tree in adults. Radiographics. 1995;15:49–68; quiz 147-8. doi: 10.1148/radiographics.15.1.7899613. [DOI] [PubMed] [Google Scholar]
- 139.Dowsett JF, Rode J, Russell RC. Annular pancreas: a clinical, endoscopic, and immunohistochemical study. Gut. 1989;30:130–135. doi: 10.1136/gut.30.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Urayama S, Kozarek R, Ball T, Brandabur J, Traverso L, Ryan J, Wechter D. Presentation and treatment of annular pancreas in an adult population. Am J Gastroenterol. 1995;90:995–999. [PubMed] [Google Scholar]
- 141.Bret PM, Reinhold C. Magnetic resonance cholangiopancreatography. Endoscopy. 1997;29:472–486. doi: 10.1055/s-2007-1004252. [DOI] [PubMed] [Google Scholar]
- 142.Gress F, Yiengpruksawan A, Sherman S, Ikenberry S, Kaster S, Ng RY, Cerulli MA, Lehman GA. Diagnosis of annular pancreas by endoscopic ultrasound. Gastrointest Endosc. 1996;44:485–489. doi: 10.1016/s0016-5107(96)70108-6. [DOI] [PubMed] [Google Scholar]
- 143.Choi JY, Kim MJ, Kim JH, Lim JS, Oh YT, Chung JJ, Song SY, Chung JB, Yoo HS, Lee JT, et al. Annular pancreas: emphasis on magnetic resonance cholangiopancreatography findings. J Comput Assist Tomogr. 2004;28:528–532. doi: 10.1097/00004728-200407000-00015. [DOI] [PubMed] [Google Scholar]
- 144.Chevallier P, Souci J, Buckley MJ, Oddo F, Hastier P, Diaine B. Annular pancreas: MR imaging including MR cholangiopancreatography (MRCP) Pancreas. 1999;18:216–218. [PubMed] [Google Scholar]
- 145.Hidaka T, Hirohashi S, Uchida H, Koh M, Itoh T, Matsuo Y, Matsuo N, Ohishi H. Annular pancreas diagnosed by single-shot MR cholangiopancreatography. Magn Reson Imaging. 1998;16:441–444. doi: 10.1016/s0730-725x(97)00306-8. [DOI] [PubMed] [Google Scholar]
- 146.Maker V, Gerzenshtein J, Lerner T. Annular pancreas in the adult: two case reports and review of more than a century of literature. Am Surg. 2003;69:404–410. [PubMed] [Google Scholar]
- 147.Simpson WF, Adams DB, Metcalf JS, Anderson MC. Nonfunctioning pancreatic neuroendocrine tumors presenting as pancreatitis: report of four cases. Pancreas. 1988;3:223–231. doi: 10.1097/00006676-198804000-00019. [DOI] [PubMed] [Google Scholar]
- 148.Köhler H, Lankisch PG. Acute pancreatitis and hyperamylasaemia in pancreatic carcinoma. Pancreas. 1987;2:117–119. doi: 10.1097/00006676-198701000-00018. [DOI] [PubMed] [Google Scholar]
- 149.Kahrilas PJ, Hogan WJ, Geenen JE, Stewart ET, Dodds WJ, Arndorfer RC. Chronic recurrent pancreatitis secondary to a submucosal ampullary tumor in a patient with neurofibromatosis. Dig Dis Sci. 1987;32:102–107. doi: 10.1007/BF01296695. [DOI] [PubMed] [Google Scholar]
- 150.Robertson JF, Imrie CW. Acute pancreatitis associated with carcinoma of the ampulla of Vater. Br J Surg. 1987;74:395–397. doi: 10.1002/bjs.1800740524. [DOI] [PubMed] [Google Scholar]
- 151.Rösch T, Braig C, Gain T, Feuerbach S, Siewert JR, Schusdziarra V, Classen M. Staging of pancreatic and ampullary carcinoma by endoscopic ultrasonography. Comparison with conventional sonography, computed tomography, and angiography. Gastroenterology. 1992;102:188–199. doi: 10.1016/0016-5085(92)91800-j. [DOI] [PubMed] [Google Scholar]
- 152.Legmann P, Vignaux O, Dousset B, Baraza AJ, Palazzo L, Dumontier I, Coste J, Louvel A, Roseau G, Couturier D, et al. Pancreatic tumors: comparison of dual-phase helical CT and endoscopic sonography. AJR Am J Roentgenol. 1998;170:1315–1322. doi: 10.2214/ajr.170.5.9574609. [DOI] [PubMed] [Google Scholar]
- 153.Müller MF, Meyenberger C, Bertschinger P, Schaer R, Marincek B. Pancreatic tumors: evaluation with endoscopic US, CT, and MR imaging. Radiology. 1994;190:745–751. doi: 10.1148/radiology.190.3.8115622. [DOI] [PubMed] [Google Scholar]
- 154.Rösch T. Staging of pancreatic cancer. Analysis of literature results. Gastrointest Endosc Clin N Am. 1995;5:735–739. [PubMed] [Google Scholar]
- 155.Mujica VR, Barkin JS, Go VL. Acute pancreatitis secondary to pancreatic carcinoma. Study Group Participants. Pancreas. 2000;21:329–332. doi: 10.1097/00006676-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 156.Rösch T, Lightdale CJ, Botet JF, Boyce GA, Sivak MV, Yasuda K, Heyder N, Palazzo L, Dancygier H, Schusdziarra V. Localization of pancreatic endocrine tumors by endoscopic ultrasonography. N Engl J Med. 1992;326:1721–1726. doi: 10.1056/NEJM199206253262601. [DOI] [PubMed] [Google Scholar]
- 157.Jhala NC, Jhala D, Eltoum I, Vickers SM, Wilcox CM, Chhieng DC, Eloubeidi MA. Endoscopic ultrasound-guided fine-needle aspiration biopsy: a powerful tool to obtain samples from small lesions. Cancer. 2004;102:239–246. doi: 10.1002/cncr.20451. [DOI] [PubMed] [Google Scholar]
- 158.Volmar KE, Vollmer RT, Jowell PS, Nelson RC, Xie HB. Pancreatic FNA in 1000 cases: a comparison of imaging modalities. Gastrointest Endosc. 2005;61:854–861. doi: 10.1016/s0016-5107(05)00364-0. [DOI] [PubMed] [Google Scholar]
- 159.Bassi C, Procacci C, Zamboni G, Scarpa A, Cavallini G, Pederzoli P. Intraductal papillary mucinous tumors of the pancreas. Verona University Pancreatic Team. Int J Pancreatol. 2000;27:181–193. doi: 10.1385/ijgc:27:3:181. [DOI] [PubMed] [Google Scholar]
- 160.Bastid C, Bernard JP, Sarles H, Payan MJ, Sahel J. Mucinous ductal ectasia of the pancreas: a premalignant disease and a cause of obstructive pancreatitis. Pancreas. 1991;6:15–22. doi: 10.1097/00006676-199101000-00003. [DOI] [PubMed] [Google Scholar]
- 161.Cellier C, Cuillerier E, Palazzo L, Rickaert F, Flejou JF, Napoleon B, Van Gansbeke D, Bely N, Ponsot P, Partensky C, et al. Intraductal papillary and mucinous tumors of the pancreas: accuracy of preoperative computed tomography, endoscopic retrograde pancreatography and endoscopic ultrasonography, and long-term outcome in a large surgical series. Gastrointest Endosc. 1998;47:42–49. doi: 10.1016/s0016-5107(98)70297-4. [DOI] [PubMed] [Google Scholar]
- 162.Obara T, Saitoh Y, Maguchi H, Ura H, Yokota K, Okamura K, Namiki M. Papillary adenoma of the pancreas with excessive mucin secretion. Pancreas. 1992;7:114–117. doi: 10.1097/00006676-199201000-00016. [DOI] [PubMed] [Google Scholar]
- 163.Yamaguchi K, Chijiiwa K, Shimizu S, Yokohata K, Morisaki T, Yonemasu H, Tanaka M. Intraductal papillary neoplasm of the pancreas: a clinical review of 13 benign and four malignant tumours. Eur J Surg. 1999;165:223–229. doi: 10.1080/110241599750007081. [DOI] [PubMed] [Google Scholar]
- 164.Nickl NJ, Lawson JM, Cotton PB. Mucinous pancreatic tumors: ERCP findings. Gastrointest Endosc. 1991;37:133–138. doi: 10.1016/s0016-5107(91)70670-6. [DOI] [PubMed] [Google Scholar]
- 165.Raijman I, Kortan P, Walden D, Kandel G, Marcon NE, Haber GB. Mucinous ductal ectasia: cholangiopancreatographic and endoscopic findings. Endoscopy. 1994;26:303–307. doi: 10.1055/s-2007-1008972. [DOI] [PubMed] [Google Scholar]
- 166.Lévy P, Jouannaud V, O'Toole D, Couvelard A, Vullierme MP, Palazzo L, Aubert A, Ponsot P, Sauvanet A, Maire F, et al. Natural history of intraductal papillary mucinous tumors of the pancreas: actuarial risk of malignancy. Clin Gastroenterol Hepatol. 2006;4:460–468. doi: 10.1016/j.cgh.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 167.Traverso LW, Peralta EA, Ryan JA, Kozarek RA. Intraductal neoplasms of the pancreas. Am J Surg. 1998;175:426–432. doi: 10.1016/s0002-9610(98)00039-7. [DOI] [PubMed] [Google Scholar]
- 168.Sohn TA, Yeo CJ, Cameron JL, Iacobuzio-Donahue CA, Hruban RH, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an increasingly recognized clinicopathologic entity. Ann Surg. 2001;234:313–321; discussion 321-322. doi: 10.1097/00000658-200109000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–797; discussion 797-799. doi: 10.1097/01.sla.0000128306.90650.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sugiyama M, Atomi Y. Extrapancreatic neoplasms occur with unusual frequency in patients with intraductal papillary mucinous tumors of the pancreas. Am J Gastroenterol. 1999;94:470–473. doi: 10.1111/j.1572-0241.1999.879_h.x. [DOI] [PubMed] [Google Scholar]
- 171.Choi MG, Kim SW, Han SS, Jang JY, Park YH. High incidence of extrapancreatic neoplasms in patients with intraductal papillary mucinous neoplasms. Arch Surg. 2006;141:51–56; discussion 56. doi: 10.1001/archsurg.141.1.51. [DOI] [PubMed] [Google Scholar]
- 172.Sarr MG, Carpenter HA, Prabhakar LP, Orchard TF, Hughes S, van Heerden JA, DiMagno EP. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann Surg. 2000;231:205–212. doi: 10.1097/00000658-200002000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wilentz RE, Albores-Saavedra J, Zahurak M, Talamini MA, Yeo CJ, Cameron JL, Hruban RH. Pathologic examination accurately predicts prognosis in mucinous cystic neoplasms of the pancreas. Am J Surg Pathol. 1999;23:1320–1327. doi: 10.1097/00000478-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 174.Siech M, Tripp K, Schmidt-Rohlfing B, Mattfeldt T, Widmaier U, Gansauge F, Görich J, Beger HG. Cystic tumours of the pancreas: diagnostic accuracy, pathologic observations and surgical consequences. Langenbecks Arch Surg. 1998;383:56–61. doi: 10.1007/s004230050092. [DOI] [PubMed] [Google Scholar]
- 175.Anderson JH, Morran CG, Anderson JR, Carter DC. Acute pancreatitis and non-Hodgkin's lymphoma. Postgrad Med J. 1987;63:137–139. doi: 10.1136/pgmj.63.736.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.McLatchie GR, Imrie CW. Acute pancreatitis associated with tumour metastases in the pancreas. Digestion. 1981;21:13–17. doi: 10.1159/000198513. [DOI] [PubMed] [Google Scholar]
- 177.Sand JA, Hyoty MK, Mattila J, Dagorn JC, Nordback IH. Clinical assessment compared with cyst fluid analysis in the differential diagnosis of cystic lesions in the pancreas. Surgery. 1996;119:275–280. doi: 10.1016/s0039-6060(96)80113-9. [DOI] [PubMed] [Google Scholar]
- 178.Hammel P, Voitot H, Vilgrain V, Lévy P, Ruszniewski P, Bernades P. Diagnostic value of CA 72-4 and carcinoembryonic antigen determination in the fluid of pancreatic cystic lesions. Eur J Gastroenterol Hepatol. 1998;10:345–348. doi: 10.1097/00042737-199804000-00012. [DOI] [PubMed] [Google Scholar]
- 179.Kim HJ, Kim MH, Myung SJ, Lim BC, Park ET, Yoo KS, Seo DW, Lee SK, Min YI. A new strategy for the application of CA19-9 in the differentiation of pancreaticobiliary cancer: analysis using a receiver operating characteristic curve. Am J Gastroenterol. 1999;94:1941–1946. doi: 10.1111/j.1572-0241.1999.01234.x. [DOI] [PubMed] [Google Scholar]
- 180.Stolte M, Pscherer C. Adenoma-carcinoma sequence in the papilla of Vater. Scand J Gastroenterol. 1996;31:376–382. doi: 10.3109/00365529609006414. [DOI] [PubMed] [Google Scholar]
- 181.Yamaguchi K, Enjoji M. Carcinoma of the ampulla of vater. A clinicopathologic study and pathologic staging of 109 cases of carcinoma and 5 cases of adenoma. Cancer. 1987;59:506–515. doi: 10.1002/1097-0142(19870201)59:3<506::aid-cncr2820590326>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 182.Tarazi RY, Hermann RE, Vogt DP, Hoerr SO, Esselstyn CB, Cooperman AM, Steiger E, Grundfest S. Results of surgical treatment of periampullary tumors: a thirty-five-year experience. Surgery. 1986;100:716–723. [PubMed] [Google Scholar]
- 183.Sperti C, Pasquali C, Piccoli A, Sernagiotto C, Pedrazzoli S. Radical resection for ampullary carcinoma: long-term results. Br J Surg. 1994;81:668–671. doi: 10.1002/bjs.1800810512. [DOI] [PubMed] [Google Scholar]
- 184.Chijiiwa K, Yamashita H, Kuroki S. Wide ampullectomy for patients with villous adenoma of duodenal papilla and follow-up results of pancreaticobiliary tract. Int Surg. 1994;79:178–182. [PubMed] [Google Scholar]
- 185.Binmoeller KF, Boaventura S, Ramsperger K, Soehendra N. Endoscopic snare excision of benign adenomas of the papilla of Vater. Gastrointest Endosc. 1993;39:127–131. doi: 10.1016/s0016-5107(93)70051-6. [DOI] [PubMed] [Google Scholar]
- 186.Ponchon T, Berger F, Chavaillon A, Bory R, Lambert R. Contribution of endoscopy to diagnosis and treatment of tumors of the ampulla of Vater. Cancer. 1989;64:161–167. doi: 10.1002/1097-0142(19890701)64:1<161::aid-cncr2820640127>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 187.Dittrick GW, Mallat DB, Lamont JP. Management of ampullary lesions. Curr Treat Options Gastroenterol. 2006;9:371–376. doi: 10.1007/BF02738525. [DOI] [PubMed] [Google Scholar]
- 188.Katsinelos P, Paroutoglou G, Kountouras J, Beltsis A, Papaziogas B, Mimidis K, Zavos C, Dimiropoulos S. Safety and long-term follow-up of endoscopic snare excision of ampullary adenomas. Surg Endosc. 2006;20:608–613. doi: 10.1007/s00464-004-2278-0. [DOI] [PubMed] [Google Scholar]
- 189.Davis PB, Drumm M, Konstan MW. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1229–1256. doi: 10.1164/ajrccm.154.5.8912731. [DOI] [PubMed] [Google Scholar]
- 190.Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol. 2005;67:377–409. doi: 10.1146/annurev.physiol.67.031103.153247. [DOI] [PubMed] [Google Scholar]
- 191.Naruse S, Fujiki K, Ishiguro H. Is genetic analysis helpful for diagnosing chronic pancreatitis in its early stage? J Gastroenterol. 2007;42 Suppl 17:60–65. doi: 10.1007/s00535-006-1934-7. [DOI] [PubMed] [Google Scholar]
- 192.Shwachman H, Lebenthal E, Khaw KT. Recurrent acute pancreatitis in patients with cystic fibrosis with normal pancreatic enzymes. Pediatrics. 1975;55:86–95. [PubMed] [Google Scholar]
- 193.Atlas AB, Orenstein SR, Orenstein DM. Pancreatitis in young children with cystic fibrosis. J Pediatr. 1992;120:756–759. doi: 10.1016/s0022-3476(05)80242-2. [DOI] [PubMed] [Google Scholar]
- 194.Cohn JA, Friedman KJ, Noone PG, Knowles MR, Silverman LM, Jowell PS. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N Engl J Med. 1998;339:653–658. doi: 10.1056/NEJM199809033391002. [DOI] [PubMed] [Google Scholar]
- 195.Keiles S, Kammesheidt A. Identification of CFTR, PRSS1, and SPINK1 mutations in 381 patients with pancreatitis. Pancreas. 2006;33:221–227. doi: 10.1097/01.mpa.0000232014.94974.75. [DOI] [PubMed] [Google Scholar]
- 196.Audrézet MP, Chen JM, Le Maréchal C, Ruszniewski P, Robaszkiewicz M, Raguénès O, Quéré I, Scotet V, Férec C. Determination of the relative contribution of three genes-the cystic fibrosis transmembrane conductance regulator gene, the cationic trypsinogen gene, and the pancreatic secretory trypsin inhibitor gene-to the etiology of idiopathic chronic pancreatitis. Eur J Hum Genet. 2002;10:100–106. doi: 10.1038/sj.ejhg.5200786. [DOI] [PubMed] [Google Scholar]
- 197.Noone PG, Zhou Z, Silverman LM, Jowell PS, Knowles MR, Cohn JA. Cystic fibrosis gene mutations and pancreatitis risk: relation to epithelial ion transport and trypsin inhibitor gene mutations. Gastroenterology. 2001;121:1310–1319. doi: 10.1053/gast.2001.29673. [DOI] [PubMed] [Google Scholar]
- 198.Weiss FU, Simon P, Bogdanova N, Mayerle J, Dworniczak B, Horst J, Lerch MM. Complete cystic fibrosis transmembrane conductance regulator gene sequencing in patients with idiopathic chronic pancreatitis and controls. Gut. 2005;54:1456–1460. doi: 10.1136/gut.2005.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cohn JA. Reduced CFTR function and the pathobiology of idiopathic pancreatitis. J Clin Gastroenterol. 2005;39:S70–S77. doi: 10.1097/01.mcg.0000155522.89005.bf. [DOI] [PubMed] [Google Scholar]
- 200.Sharer N, Schwarz M, Malone G, Howarth A, Painter J, Super M, Braganza J. Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. N Engl J Med. 1998;339:645–652. doi: 10.1056/NEJM199809033391001. [DOI] [PubMed] [Google Scholar]
- 201.Pezzilli R, Morselli-Labate AM, Mantovani V, Romboli E, Selva P, Migliori M, Corinaldesi R, Gullo L. Mutations of the CFTR gene in pancreatic disease. Pancreas. 2003;27:332–336. doi: 10.1097/00006676-200311000-00011. [DOI] [PubMed] [Google Scholar]
- 202.Cohn JA, Neoptolemos JP, Feng J, Yan J, Jiang Z, Greenhalf W, McFaul C, Mountford R, Sommer SS. Increased risk of idiopathic chronic pancreatitis in cystic fibrosis carriers. Hum Mutat. 2005;26:303–307. doi: 10.1002/humu.20232. [DOI] [PubMed] [Google Scholar]
- 203.Bishop MD, Freedman SD, Zielenski J, Ahmed N, Dupuis A, Martin S, Ellis L, Shea J, Hopper I, Corey M, et al. The cystic fibrosis transmembrane conductance regulator gene and ion channel function in patients with idiopathic pancreatitis. Hum Genet. 2005;118:372–381. doi: 10.1007/s00439-005-0059-z. [DOI] [PubMed] [Google Scholar]
- 204.Sobczyńska-Tomaszewska A, Bak D, Oralewska B, Oracz G, Norek A, Czerska K, Mazurczak T, Teisseyre M, Socha J, Zagulski M, et al. Analysis of CFTR, SPINK1, PRSS1 and AAT mutations in children with acute or chronic pancreatitis. J Pediatr Gastroenterol Nutr. 2006;43:299–306. doi: 10.1097/01.mpg.0000232570.48773.df. [DOI] [PubMed] [Google Scholar]
- 205.Teich N, Ockenga J, Keim V, Mössner J. Genetic risk factors in chronic pancreatitis. J Gastroenterol. 2002;37:1–9. doi: 10.1007/s535-002-8125-1. [DOI] [PubMed] [Google Scholar]
- 206.Witt H, Luck W, Hennies HC, Classen M, Kage A, Lass U, Landt O, Becker M. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet. 2000;25:213–216. doi: 10.1038/76088. [DOI] [PubMed] [Google Scholar]
- 207.Pfützer RH, Barmada MM, Brunskill AP, Finch R, Hart PS, Neoptolemos J, Furey WF, Whitcomb DC. SPINK1/PSTI polymorphisms act as disease modifiers in familial and idiopathic chronic pancreatitis. Gastroenterology. 2000;119:615–623. doi: 10.1053/gast.2000.18017. [DOI] [PubMed] [Google Scholar]
- 208.Threadgold J, Greenhalf W, Ellis I, Howes N, Lerch MM, Simon P, Jansen J, Charnley R, Laugier R, Frulloni L, et al. The N34S mutation of SPINK1 (PSTI) is associated with a familial pattern of idiopathic chronic pancreatitis but does not cause the disease. Gut. 2002;50:675–681. doi: 10.1136/gut.50.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Khalid A, Finkelstein S, Thompson B, Kelly L, Hanck C, Godfrey TE, Whitcomb DC. A 93 year old man with the PRSS1 R122H mutation, low SPINK1 expression, and no pancreatitis: insights into phenotypic non-penetrance. Gut. 2006;55:728–731. doi: 10.1136/gut.2005.067959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mitchell RM, Byrne MF, Baillie J. Pancreatitis. Lancet. 2003;361:1447–1455. doi: 10.1016/s0140-6736(03)13139-x. [DOI] [PubMed] [Google Scholar]
- 211.Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK, Amann ST, Toskes PP, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–145. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 212.Gorry MC, Gabbaizedeh D, Furey W, Gates LK, Preston RA, Aston CE, Zhang Y, Ulrich C, Ehrlich GD, Whitcomb DC. Mutations in the cationic trypsinogen gene are associated with recurrent acute and chronic pancreatitis. Gastroenterology. 1997;113:1063–1068. doi: 10.1053/gast.1997.v113.pm9322498. [DOI] [PubMed] [Google Scholar]
- 213.Whitcomb DC, Preston RA, Aston CE, Sossenheimer MJ, Barua PS, Zhang Y, Wong-Chong A, White GJ, Wood PG, Gates LK, et al. A gene for hereditary pancreatitis maps to chromosome 7q35. Gastroenterology. 1996;110:1975–1980. doi: 10.1053/gast.1996.v110.pm8964426. [DOI] [PubMed] [Google Scholar]
- 214.Férec C, Raguénès O, Salomon R, Roche C, Bernard JP, Guillot M, Quéré I, Faure C, Mercier B, Audrézet MP, et al. Mutations in the cationic trypsinogen gene and evidence for genetic heterogeneity in hereditary pancreatitis. J Med Genet. 1999;36:228–232. [PMC free article] [PubMed] [Google Scholar]
- 215.Teich N, Ockenga J, Hoffmeister A, Manns M, Mössner J, Keim V. Chronic pancreatitis associated with an activation peptide mutation that facilitates trypsin activation. Gastroenterology. 2000;119:461–465. doi: 10.1053/gast.2000.9312. [DOI] [PubMed] [Google Scholar]
- 216.Chen JM, Piepoli Bis A, Le Bodic L, Ruszniewski P, Robaszkiewicz M, Deprez PH, Raguenes O, Quere I, Andriulli A, Ferec C. Mutational screening of the cationic trypsinogen gene in a large cohort of subjects with idiopathic chronic pancreatitis. Clin Genet. 2001;59:189–193. doi: 10.1034/j.1399-0004.2001.590308.x. [DOI] [PubMed] [Google Scholar]
- 217.Le Maréchal C, Chen JM, Quéré I, Raguénès O, Férec C, Auroux J. Discrimination of three mutational events that result in a disruption of the R122 primary autolysis site of the human cationic trypsinogen (PRSS1) by denaturing high performance liquid chromatography. BMC Genet. 2001;2:19. doi: 10.1186/1471-2156-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Simon P, Weiss FU, Sahin-Toth M, Parry M, Nayler O, Lenfers B, Schnekenburger J, Mayerle J, Domschke W, Lerch MM. Hereditary pancreatitis caused by a novel PRSS1 mutation (Arg-122 --& gt; Cys) that alters autoactivation and autodegradation of cationic trypsinogen. J Biol Chem. 2002;277:5404–5410. doi: 10.1074/jbc.M108073200. [DOI] [PubMed] [Google Scholar]
- 219.Pfützer R, Myers E, Applebaum-Shapiro S, Finch R, Ellis I, Neoptolemos J, Kant JA, Whitcomb DC. Novel cationic trypsinogen (PRSS1) N29T and R122C mutations cause autosomal dominant hereditary pancreatitis. Gut. 2002;50:271–272. doi: 10.1136/gut.50.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sahin-Tóth M, Gráf L, Tóth M. Trypsinogen stabilization by mutation Arg117--& gt; His: a unifying pathomechanism for hereditary pancreatitis? Biochem Biophys Res Commun. 1999;264:505–508. doi: 10.1006/bbrc.1999.1565. [DOI] [PubMed] [Google Scholar]
- 221.Perrault J. Hereditary pancreatitis. Gastroenterol Clin North Am. 1994;23:743–752. [PubMed] [Google Scholar]
- 222.Lowenfels AB, Maisonneuve P, DiMagno EP, Elitsur Y, Gates LK, Perrault J, Whitcomb DC. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;89:442–446. doi: 10.1093/jnci/89.6.442. [DOI] [PubMed] [Google Scholar]
- 223.Kagnoff MF. Celiac disease. A gastrointestinal disease with environmental, genetic, and immunologic components. Gastroenterol Clin North Am. 1992;21:405–425. [PubMed] [Google Scholar]
- 224.Schuppan D. Current concepts of celiac disease pathogenesis. Gastroenterology. 2000;119:234–242. doi: 10.1053/gast.2000.8521. [DOI] [PubMed] [Google Scholar]
- 225.Patel RS, Johlin FC, Murray JA. Celiac disease and recurrent pancreatitis. Gastrointest Endosc. 1999;50:823–827. doi: 10.1016/s0016-5107(99)70166-5. [DOI] [PubMed] [Google Scholar]
- 226.Thomson A. Celiac disease as a cause of pancreatitis. Gastroenterology. 2005;129:1137. doi: 10.1053/j.gastro.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 227.Ulrich CD. Pancreatic cancer in hereditary pancreatitis: consensus guidelines for prevention, screening and treatment. Pancreatology. 2001;1:416–422. doi: 10.1159/000055841. [DOI] [PubMed] [Google Scholar]
- 228.Rünzi M, Layer P. Drug-associated pancreatitis: facts and fiction. Pancreas. 1996;13:100–109. doi: 10.1097/00006676-199607000-00014. [DOI] [PubMed] [Google Scholar]
- 229.Wilmink T, Frick TW. Drug-induced pancreatitis. Drug Saf. 1996;14:406–423. doi: 10.2165/00002018-199614060-00006. [DOI] [PubMed] [Google Scholar]
- 230.McArthur KE. Review article: drug-induced pancreatitis. Aliment Pharmacol Ther. 1996;10:23–38. doi: 10.1111/j.1365-2036.1996.tb00174.x. [DOI] [PubMed] [Google Scholar]
- 231.Lankisch PG, Dröge M, Gottesleben F. Drug induced acute pancreatitis: incidence and severity. Gut. 1995;37:565–567. doi: 10.1136/gut.37.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Werth B, Kuhn M, Hartmann K, Reinhart WH. Drug-induced pancreatitis: experience of the Swiss Drug Adverse Effects Center (SANZ) 1981-1993. Schweiz Med Wochenschr. 1995;125:731–734. [PubMed] [Google Scholar]
- 233.Haber CJ, Meltzer SJ, Present DH, Korelitz BI. Nature and course of pancreatitis caused by 6-mercaptopurine in the treatment of inflammatory bowel disease. Gastroenterology. 1986;91:982–986. doi: 10.1016/0016-5085(86)90703-1. [DOI] [PubMed] [Google Scholar]
- 234.Maxson CJ, Greenfield SM, Turner JL. Acute pancreatitis as a common complication of 2',3'-dideoxyinosine therapy in the acquired immunodeficiency syndrome. Am J Gastroenterol. 1992;87:708–713. [PubMed] [Google Scholar]
- 235.Youssef SS, Iskandar SB, Scruggs J, Roy TM. Acute pancreatitis associated with omeprazole. Int J Clin Pharmacol Ther. 2005;43:558–561. doi: 10.5414/cpp43558. [DOI] [PubMed] [Google Scholar]
- 236.Eland IA, Alvarez CH, Stricker BH, Rodríguez LA. The risk of acute pancreatitis associated with acid-suppressing drugs. Br J Clin Pharmacol. 2000;49:473–478. doi: 10.1046/j.1365-2125.2000.00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sundström A, Blomgren K, Alfredsson L, Wiholm BE. Acid-suppressing drugs and gastroesophageal reflux disease as risk factors for acute pancreatitis--results from a Swedish Case-Control Study. Pharmacoepidemiol Drug Saf. 2006;15:141–149. doi: 10.1002/pds.1137. [DOI] [PubMed] [Google Scholar]
- 238.Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med. 1994;330:1198–1210. doi: 10.1056/NEJM199404283301706. [DOI] [PubMed] [Google Scholar]
- 239.Mofenson HC, Caraccio TR, Nawaz H, Steckler G. Acetaminophen induced pancreatitis. J Toxicol Clin Toxicol. 1991;29:223–230. doi: 10.3109/15563659109038615. [DOI] [PubMed] [Google Scholar]
- 240.Mallory A, Kern F. Drug-induced pancreatitis: a critical review. Gastroenterology. 1980;78:813–820. [PubMed] [Google Scholar]
- 241.Banerjee AK, Patel KJ, Grainger SL. Drug-induced acute pancreatitis. A critical review. Med Toxicol Adverse Drug Exp. 1989;4:186–198. doi: 10.1007/BF03259996. [DOI] [PubMed] [Google Scholar]
- 242.Underwood TW, Frye CB. Drug-induced pancreatitis. Clin Pharm. 1993;12:440–448. [PubMed] [Google Scholar]
- 243.Bennett IL, Cary FH, Mitchell GL Jr, Cooper MN. Acute methyl alcohol poisoning: a review based on experiences in an outbreak of 323 cases. Medicine (Baltimore) 1953;32:431–463. doi: 10.1097/00005792-195312000-00002. [DOI] [PubMed] [Google Scholar]
- 244.Bartholomew C. Acute scorpion pancreatitis in Trinidad. Br Med J. 1970;1:666–668. doi: 10.1136/bmj.1.5697.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Bartholomew C, McGeeney KF, Murphy JJ, Fitzgerald O, Sankaran H. Experimental studies on the aetiology of acute scorpion pancreatitis. Br J Surg. 1976;63:807–810. doi: 10.1002/bjs.1800631019. [DOI] [PubMed] [Google Scholar]
- 246.Lee HS. Acute pancreatitis and organophosphate poisoning--a case report and review. Singapore Med J. 1989;30:599–601. [PubMed] [Google Scholar]
- 247.Parenti DM, Steinberg W, Kang P. Infectious causes of acute pancreatitis. Pancreas. 1996;13:356–371. doi: 10.1097/00006676-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 248.Khuroo MS, Zargar SA, Mahajan R. Hepatobiliary and pancreatic ascariasis in India. Lancet. 1990;335:1503–1506. doi: 10.1016/0140-6736(90)93037-p. [DOI] [PubMed] [Google Scholar]
- 249.Khuroo MS, Zargar SA, Yattoo GN, Javid G, Dar MY, Boda MI, Khan BA. Worm extraction and biliary drainage in hepatobiliary and pancreatic ascariasis. Gastrointest Endosc. 1993;39:680–685. doi: 10.1016/s0016-5107(93)70222-9. [DOI] [PubMed] [Google Scholar]
- 250.Dassopoulos T, Ehrenpreis ED. Acute pancreatitis in human immunodeficiency virus-infected patients: a review. Am J Med. 1999;107:78–84. doi: 10.1016/s0002-9343(99)00169-2. [DOI] [PubMed] [Google Scholar]
- 251.Cappell MS, Marks M. Acute pancreatitis in HIV-seropositive patients: a case control study of 44 patients. Am J Med. 1995;98:243–248. doi: 10.1016/S0002-9343(99)80370-2. [DOI] [PubMed] [Google Scholar]
- 252.Rizzardi GP, Tambussi G, Lazzarin A. Acute pancreatitis during primary HIV-1 infection. N Engl J Med. 1997;336:1836–1837. doi: 10.1056/NEJM199706193362516. [DOI] [PubMed] [Google Scholar]
- 253.Cuenca Carvajal C, Ortiz Vega M, Gómez Antúnez M, Pérez Tamayo I, Filgueira Rubio JS. Acute pancreatitis complicating primary HIV-1 infection. An Med Interna. 1998;15:324–326. [PubMed] [Google Scholar]
- 254.Toskes PP. Hyperlipidemic pancreatitis. Gastroenterol Clin North Am. 1990;19:783–791. [PubMed] [Google Scholar]
- 255.Fortson MR, Freedman SN, Webster PD. Clinical assessment of hyperlipidemic pancreatitis. Am J Gastroenterol. 1995;90:2134–2139. [PubMed] [Google Scholar]
- 256.Krauss RM, Levy AG. Subclinical chronic pancreatitis in type I hyperlipoproteinemia. Am J Med. 1977;62:144–149. doi: 10.1016/0002-9343(77)90361-8. [DOI] [PubMed] [Google Scholar]
- 257.Salen S, Kessler JI, Janowitz HD. The development of pancreatic secretory insufficiency in a patient with recurrent pancreatitis and type V hyperlipoproteinemia. Mt Sinai J Med. 1970;37:103–107. [PubMed] [Google Scholar]
- 258.Nair S, Yadav D, Pitchumoni CS. Association of diabetic ketoacidosis and acute pancreatitis: observations in 100 consecutive episodes of DKA. Am J Gastroenterol. 2000;95:2795–2800. doi: 10.1111/j.1572-0241.2000.03188.x. [DOI] [PubMed] [Google Scholar]
- 259.Glueck CJ, Lang J, Hamer T, Tracy T. Severe hypertriglyceridemia and pancreatitis when estrogen replacement therapy is given to hypertriglyceridemic women. J Lab Clin Med. 1994;123:59–64. [PubMed] [Google Scholar]
- 260.Hozumi Y, Kawano M, Saito T, Miyata M. Effect of tamoxifen on serum lipid metabolism. J Clin Endocrinol Metab. 1998;83:1633–1635. doi: 10.1210/jcem.83.5.4753. [DOI] [PubMed] [Google Scholar]
- 261.Toskes PP. Is there a relationship between hypertriglyceridemia and development of alcohol- or gallstone-induced pancreatitis? Gastroenterology. 1994;106:810–812. doi: 10.1016/0016-5085(94)90720-x. [DOI] [PubMed] [Google Scholar]
- 262.Haber PS, Wilson JS, Apte MV, Hall W, Goumas K, Pirola RC. Lipid intolerance does not account for susceptibility to alcoholic and gallstone pancreatitis. Gastroenterology. 1994;106:742–748. doi: 10.1016/0016-5085(94)90710-2. [DOI] [PubMed] [Google Scholar]
- 263.Brandwein SL, Sigman KM. Case report: milk-alkali syndrome and pancreatitis. Am J Med Sci. 1994;308:173–176. doi: 10.1097/00000441-199409000-00010. [DOI] [PubMed] [Google Scholar]
- 264.Ullian ME, Linas SL. The milk-alkali syndrome in pregnancy. Case report. Miner Electrolyte Metab. 1988;14:208–210. [PubMed] [Google Scholar]
- 265.Mithöfer K, Fernández-del Castillo C, Frick TW, Lewandrowski KB, Rattner DW, Warshaw AL. Acute hypercalcemia causes acute pancreatitis and ectopic trypsinogen activation in the rat. Gastroenterology. 1995;109:239–246. doi: 10.1016/0016-5085(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 266.Ward JB, Petersen OH, Jenkins SA, Sutton R. Is an elevated concentration of acinar cytosolic free ionised calcium the trigger for acute pancreatitis? Lancet. 1995;346:1016–1019. doi: 10.1016/s0140-6736(95)91695-4. [DOI] [PubMed] [Google Scholar]
- 267.Bess MA, Edis AJ, van Heerden JA. Hyperparathyroidism and pancreatitis. Chance or a causal association? JAMA. 1980;243:246–247. [PubMed] [Google Scholar]
- 268.Prinz RA, Aranha GV. The association of primary hyperparathyroidism and pancreatitis. Am Surg. 1985;51:325–329. [PubMed] [Google Scholar]
- 269.Shearer MG, Imrie CW. Parathyroid hormone levels, hyperparathyroidism and acute pancreatitis. Br J Surg. 1986;73:282–284. doi: 10.1002/bjs.1800730412. [DOI] [PubMed] [Google Scholar]
- 270.Jacob JJ, John M, Thomas N, Chacko A, Cherian R, Selvan B, Nair A, Seshadri M. Does hyperparathyroidism cause pancreatitis? A South Indian experience and a review of published work. ANZ J Surg. 2006;76:740–744. doi: 10.1111/j.1445-2197.2006.03845.x. [DOI] [PubMed] [Google Scholar]
- 271.Abdullah M. Pancreatitis in primary hyperparathyroidism. Med J Malaysia. 2003;58:600–603. [PubMed] [Google Scholar]
- 272.Watts RA, Isenberg DA. Pancreatic disease in the autoimmune rheumatic disorders. Semin Arthritis Rheum. 1989;19:158–165. doi: 10.1016/0049-0172(89)90028-0. [DOI] [PubMed] [Google Scholar]
- 273.Haraguchi K, Gunji K, Ito Y, Yokomori N, Kawaguchi A, Ohomori M, Inoue H, Shimura H, Saito T, Kobayashi T. Extensive pancreatic necrosis in microscopic polyangiitis. Clin Exp Nephrol. 2005;9:326–331. doi: 10.1007/s10157-005-0378-3. [DOI] [PubMed] [Google Scholar]
- 274.Adsay NV, Basturk O, Thirabanjasak D. Diagnostic features and differential diagnosis of autoimmune pancreatitis. Semin Diagn Pathol. 2005;22:309–317. doi: 10.1053/j.semdp.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 275.Moolenaar W, Lamers CB. Cholesterol crystal embolization to liver, gallbladder, and pancreas. Dig Dis Sci. 1996;41:1819–1822. doi: 10.1007/BF02088752. [DOI] [PubMed] [Google Scholar]
- 276.Orvar K, Johlin FC. Atheromatous embolization resulting in acute pancreatitis after cardiac catheterization and angiographic studies. Arch Intern Med. 1994;154:1755–1761. [PubMed] [Google Scholar]
- 277.Fernández-del Castillo C, Harringer W, Warshaw AL, Vlahakes GJ, Koski G, Zaslavsky AM, Rattner DW. Risk factors for pancreatic cellular injury after cardiopulmonary bypass. N Engl J Med. 1991;325:382–387. doi: 10.1056/NEJM199108083250602. [DOI] [PubMed] [Google Scholar]
- 278.Sakorafas GH, Tsiotos GG, Sarr MG. Ischemia/Reperfusion-Induced pancreatitis. Dig Surg. 2000;17:3–14. doi: 10.1159/000018793. [DOI] [PubMed] [Google Scholar]
- 279.Warshaw AL, O'Hara PJ. Susceptibility of the pancreas to ischemic injury in shock. Ann Surg. 1978;188:197–201. doi: 10.1097/00000658-197808000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wilson RH, Moorehead RJ. Current management of trauma to the pancreas. Br J Surg. 1991;78:1196–1202. doi: 10.1002/bjs.1800781017. [DOI] [PubMed] [Google Scholar]
- 281.Frank CD, Adler DG. Post-ERCP pancreatitis and its prevention. Nat Clin Pract Gastroenterol Hepatol. 2006;3:680–688. doi: 10.1038/ncpgasthep0654. [DOI] [PubMed] [Google Scholar]
- 282.Das A, Singh P, Sivak MV, Chak A. Pancreatic-stent placement for prevention of post-ERCP pancreatitis: a cost-effectiveness analysis. Gastrointest Endosc. 2007;65:960–968. doi: 10.1016/j.gie.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 283.Kisli E, Baser M, Aydin M, Guler O. The role of octreotide versus placebo in the prevention of post-ERCP pancreatitis. Hepatogastroenterology. 2007;54:250–253. [PubMed] [Google Scholar]
- 284.Kaffes AJ, Bourke MJ, Ding S, Alrubaie A, Kwan V, Williams SJ. A prospective, randomized, placebo-controlled trial of transdermal glyceryl trinitrate in ERCP: effects on technical success and post-ERCP pancreatitis. Gastrointest Endosc. 2006;64:351–357. doi: 10.1016/j.gie.2005.11.060. [DOI] [PubMed] [Google Scholar]
- 285.Katsinelos P, Kountouras J, Chatzis J, Christodoulou K, Paroutoglou G, Mimidis K, Beltsis A, Zavos C. High-dose allopurinol for prevention of post-ERCP pancreatitis: a prospective randomized double-blind controlled trial. Gastrointest Endosc. 2005;61:407–415. doi: 10.1016/s0016-5107(04)02647-1. [DOI] [PubMed] [Google Scholar]
- 286.Mosler P, Sherman S, Marks J, Watkins JL, Geenen JE, Jamidar P, Fogel EL, Lazzell-Pannell L, Temkit M, Tarnasky P, et al. Oral allopurinol does not prevent the frequency or the severity of post-ERCP pancreatitis. Gastrointest Endosc. 2005;62:245–250. doi: 10.1016/s0016-5107(05)01572-5. [DOI] [PubMed] [Google Scholar]
- 287.Cheng CL, Sherman S, Watkins JL, Barnett J, Freeman M, Geenen J, Ryan M, Parker H, Frakes JT, Fogel EL, et al. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol. 2006;101:139–147. doi: 10.1111/j.1572-0241.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 288.Finkelberg DL, Sahani D, Deshpande V, Brugge WR. Autoimmune pancreatitis. N Engl J Med. 2006;355:2670–2676. doi: 10.1056/NEJMra061200. [DOI] [PubMed] [Google Scholar]
- 289.Sahani DV, Kalva SP, Farrell J, Maher MM, Saini S, Mueller PR, Lauwers GY, Fernandez CD, Warshaw AL, Simeone JF. Autoimmune pancreatitis: imaging features. Radiology. 2004;233:345–352. doi: 10.1148/radiol.2332031436. [DOI] [PubMed] [Google Scholar]
- 290.van Buuren HR, Vleggaar FP, Willemien Erkelens G, Zondervan PE, Lesterhuis W, Van Eijck CH, Puylaert JB, Van Der Werf SD. Autoimmune pancreatocholangitis: a series of ten patients. Scand J Gastroenterol Suppl. 2006:70–78. doi: 10.1080/00365520600664326. [DOI] [PubMed] [Google Scholar]
- 291.Kim KP, Kim MH, Song MH, Lee SS, Seo DW, Lee SK. Autoimmune chronic pancreatitis. Am J Gastroenterol. 2004;99:1605–1616. doi: 10.1111/j.1572-0241.2004.30336.x. [DOI] [PubMed] [Google Scholar]
- 292.Pearson RK, Longnecker DS, Chari ST, Smyrk TC, Okazaki K, Frulloni L, Cavallini G. Controversies in clinical pancreatology: autoimmune pancreatitis: does it exist? Pancreas. 2003;27:1–13. doi: 10.1097/00006676-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 293.Okazaki K, Uchida K, Ohana M, Nakase H, Uose S, Inai M, Matsushima Y, Katamura K, Ohmori K, Chiba T. Autoimmune-related pancreatitis is associated with autoantibodies and a Th1/Th2-type cellular immune response. Gastroenterology. 2000;118:573–581. doi: 10.1016/s0016-5085(00)70264-2. [DOI] [PubMed] [Google Scholar]
- 294.Aparisi L, Farre A, Gomez-Cambronero L, Martinez J, De Las Heras G, Corts J, Navarro S, Mora J, Lopez-Hoyos M, Sabater L, et al. Antibodies to carbonic anhydrase and IgG4 levels in idiopathic chronic pancreatitis: relevance for diagnosis of autoimmune pancreatitis. Gut. 2005;54:703–709. doi: 10.1136/gut.2004.047142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Epstein O, Chapman RW, Lake-Bakaar G, Foo AY, Rosalki SB, Sherlock S. The pancreas in primary biliary cirrhosis and primary sclerosing cholangitis. Gastroenterology. 1982;83:1177–1182. [PubMed] [Google Scholar]
- 296.Nishimori I, Yamamoto Y, Okazaki K, Morita M, Onodera M, Kino J, Tamura S, Yamamoto Y. Identification of autoantibodies to a pancreatic antigen in patients with idiopathic chronic pancreatitis and Sjögren's syndrome. Pancreas. 1994;9:374–381. doi: 10.1097/00006676-199405000-00015. [DOI] [PubMed] [Google Scholar]
- 297.Zamboni G, Lüttges J, Capelli P, Frulloni L, Cavallini G, Pederzoli P, Leins A, Longnecker D, Klöppel G. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch. 2004;445:552–563. doi: 10.1007/s00428-004-1140-z. [DOI] [PubMed] [Google Scholar]
- 298.Kamisawa T, Egawa N, Nakajima H, Tsuruta K, Okamoto A, Hayashi Y, Funata N. Gastrointestinal findings in patients with autoimmune pancreatitis. Endoscopy. 2005;37:1127–1130. doi: 10.1055/s-2005-870369. [DOI] [PubMed] [Google Scholar]
- 299.Farrell JJ, Garber J, Sahani D, Brugge WR. EUS findings in patients with autoimmune pancreatitis. Gastrointest Endosc. 2004;60:927–936. doi: 10.1016/s0016-5107(04)02230-8. [DOI] [PubMed] [Google Scholar]
- 300.Levy MJ, Reddy RP, Wiersema MJ, Smyrk TC, Clain JE, Harewood GC, Pearson RK, Rajan E, Topazian MD, Yusuf TE, et al. EUS-guided trucut biopsy in establishing autoimmune pancreatitis as the cause of obstructive jaundice. Gastrointest Endosc. 2005;61:467–472. doi: 10.1016/s0016-5107(04)02802-0. [DOI] [PubMed] [Google Scholar]
- 301.Wakabayashi T, Kawaura Y, Satomura Y, Fujii T, Motoo Y, Okai T, Sawabu N. Clinical study of chronic pancreatitis with focal irregular narrowing of the main pancreatic duct and mass formation: comparison with chronic pancreatitis showing diffuse irregular narrowing of the main pancreatic duct. Pancreas. 2002;25:283–289. doi: 10.1097/00006676-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 302.Ozden I, Dizdaroğlu F, Poyanli A, Emre A. Spontaneous regression of a pancreatic head mass and biliary obstruction due to autoimmune pancreatitis. Pancreatology. 2005;5:300–303. doi: 10.1159/000085287. [DOI] [PubMed] [Google Scholar]
- 303.Ito T, Nakano I, Koyanagi S, Miyahara T, Migita Y, Ogoshi K, Sakai H, Matsunaga S, Yasuda O, Sumii T, et al. Autoimmune pancreatitis as a new clinical entity. Three cases of autoimmune pancreatitis with effective steroid therapy. Dig Dis Sci. 1997;42:1458–1468. doi: 10.1023/a:1018862626221. [DOI] [PubMed] [Google Scholar]
- 304.Saito T, Tanaka S, Yoshida H, Imamura T, Ukegawa J, Seki T, Ikegami A, Yamamura F, Mikami T, Aoyagi Y, et al. A case of autoimmune pancreatitis responding to steroid therapy. Evidence of histologic recovery. Pancreatology. 2002;2:550–556. doi: 10.1159/000066092. [DOI] [PubMed] [Google Scholar]
- 305.Kojima E, Kimura K, Noda Y, Kobayashi G, Itoh K, Fujita N. Autoimmune pancreatitis and multiple bile duct strictures treated effectively with steroid. J Gastroenterol. 2003;38:603–607. [PubMed] [Google Scholar]
- 306.Kamisawa T, Yoshiike M, Egawa N, Nakajima H, Tsuruta K, Okamoto A. Treating patients with autoimmune pancreatitis: results from a long-term follow-up study. Pancreatology. 2005;5:234–238; discussion 238-240. doi: 10.1159/000085277. [DOI] [PubMed] [Google Scholar]
- 307.Neild GH, Rodriguez-Justo M, Wall C, Connolly JO. Hyper-IgG4 disease: report and characterisation of a new disease. BMC Med. 2006;4:23. doi: 10.1186/1741-7015-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Troppmann C, Gruessner AC, Dunn DL, Sutherland DE, Gruessner RW. Surgical complications requiring early relaparotomy after pancreas transplantation: a multivariate risk factor and economic impact analysis of the cyclosporine era. Ann Surg. 1998;227:255–268. doi: 10.1097/00000658-199802000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Sinha S, Jha R, Lakhtakia S, Narayan G. Acute pancreatitis following kidney transplantation - role of viral infections. Clin Transplant. 2003;17:32–36. doi: 10.1034/j.1399-0012.2003.02041.x. [DOI] [PubMed] [Google Scholar]
- 310.Pike JL, Rice JC, Sanchez RL, Kelly EB, Kelly BC. Pancreatic panniculitis associated with allograft pancreatitis and rejection in a simultaneous pancreas-kidney transplant recipient. Am J Transplant. 2006;6:2502–2505. doi: 10.1111/j.1600-6143.2006.01463.x. [DOI] [PubMed] [Google Scholar]
- 311.Catalano MF, Lahoti S, Geenen JE, Hogan WJ. Prospective evaluation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and secretin test in the diagnosis of chronic pancreatitis. Gastrointest Endosc. 1998;48:11–17. doi: 10.1016/s0016-5107(98)70122-1. [DOI] [PubMed] [Google Scholar]
- 312.Sahai AV, Zimmerman M, Aabakken L, Tarnasky PR, Cunningham JT, van Velse A, Hawes RH, Hoffman BJ. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc. 1998;48:18–25. doi: 10.1016/s0016-5107(98)70123-3. [DOI] [PubMed] [Google Scholar]
- 313.Wiersema MJ, Hawes RH, Lehman GA, Kochman ML, Sherman S, Kopecky KK. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy. 1993;25:555–564. doi: 10.1055/s-2007-1010405. [DOI] [PubMed] [Google Scholar]
- 314.Levy P, Milan C, Pignon JP, Baetz A, Bernades P. Mortality factors associated with chronic pancreatitis. Unidimensional and multidimensional analysis of a medical-surgical series of 240 patients. Gastroenterology. 1989;96:1165–1172. [PubMed] [Google Scholar]
- 315.Ammann RW, Akovbiantz A, Largiader F, Schueler G. Course and outcome of chronic pancreatitis. Longitudinal study of a mixed medical-surgical series of 245 patients. Gastroenterology. 1984;86:820–828. [PubMed] [Google Scholar]
- 316.Dumonceau JM, Devière J, Le Moine O, Delhaye M, Vandermeeren A, Baize M, Van Gansbeke D, Cremer M. Endoscopic pancreatic drainage in chronic pancreatitis associated with ductal stones: long-term results. Gastrointest Endosc. 1996;43:547–555. doi: 10.1016/s0016-5107(96)70189-x. [DOI] [PubMed] [Google Scholar]
- 317.DiMagno MJ, Dimagno EP. Chronic pancreatitis. Curr Opin Gastroenterol. 2006;22:487–497. doi: 10.1097/01.mog.0000239862.96833.89. [DOI] [PubMed] [Google Scholar]
- 318.Adler DG, Lichtenstein D, Baron TH, Davila R, Egan JV, Gan SL, Qureshi WA, Rajan E, Shen B, Zuckerman MJ, et al. The role of endoscopy in patients with chronic pancreatitis. Gastrointest Endosc. 2006;63:933–937. doi: 10.1016/j.gie.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 319.Catalano MF, Geenen JE, Schmalz MJ, Johnson GK, Dean RS, Hogan WJ. Treatment of pancreatic pseudocysts with ductal communication by transpapillary pancreatic duct endoprosthesis. Gastrointest Endosc. 1995;42:214–218. doi: 10.1016/s0016-5107(95)70094-3. [DOI] [PubMed] [Google Scholar]
- 320.Kozarek RA, Ball TJ, Patterson DJ. Endoscopic approach to pancreatic duct calculi and obstructive pancreatitis. Am J Gastroenterol. 1992;87:600–603. [PubMed] [Google Scholar]
- 321.Uden S, Bilton D, Nathan L, Hunt LP, Main C, Braganza JM. Antioxidant therapy for recurrent pancreatitis: placebo-controlled trial. Aliment Pharmacol Ther. 1990;4:357–371. doi: 10.1111/j.1365-2036.1990.tb00482.x. [DOI] [PubMed] [Google Scholar]
- 322.Uden S, Schofield D, Miller PF, Day JP, Bottiglier T, Braganza JM. Antioxidant therapy for recurrent pancreatitis: biochemical profiles in a placebo-controlled trial. Aliment Pharmacol Ther. 1992;6:229–240. doi: 10.1111/j.1365-2036.1992.tb00266.x. [DOI] [PubMed] [Google Scholar]
- 323.Testoni PA, Caporuscio S, Bagnolo F, Lella F. Idiopathic recurrent pancreatitis: long-term results after ERCP, endoscopic sphincterotomy, or ursodeoxycholic acid treatment. Am J Gastroenterol. 2000;95:1702–1707. doi: 10.1111/j.1572-0241.2000.02292.x. [DOI] [PubMed] [Google Scholar]
- 324.Brown A, Hughes M, Tenner S, Banks PA. Does pancreatic enzyme supplementation reduce pain in patients with chronic pancreatitis: a meta-analysis. Am J Gastroenterol. 1997;92:2032–2035. [PubMed] [Google Scholar]
- 325.Warshaw AL, Banks PA, Fernández-Del Castillo C. AGA technical review: treatment of pain in chronic pancreatitis. Gastroenterology. 1998;115:765–776. doi: 10.1016/s0016-5085(98)70157-x. [DOI] [PubMed] [Google Scholar]
- 326.Mossner J, Secknus R, Meyer J, Niederau C, Adler G. Treatment of pain with pancreatic extracts in chronic pancreatitis: results of a prospective placebo-controlled multicenter trial. Digestion. 1992;53:54–66. doi: 10.1159/000200971. [DOI] [PubMed] [Google Scholar]
- 327.Steinberg WM, Chari ST, Forsmark CE, Sherman S, Reber HA, Bradley EL, DiMagno E. Controversies in clinical pancreatology: management of acute idiopathic recurrent pancreatitis. Pancreas. 2003;27:103–117. doi: 10.1097/00006676-200308000-00001. [DOI] [PubMed] [Google Scholar]