Abstract
Endoscopic retrograde cholangiopancreatography (ERCP) is a useful tool in the evaluation and management of acute pancreatitis. This review will focus on the role of ERCP in specific causes of acute pancreatitis, including microlithiasis and gallstone disease, pancreas divisum, Sphincter of Oddi dysfunction, tumors of the pancreaticobiliary tract, pancreatic pseudocysts, and pancreatic duct injury. Indications for endoscopic techniques such as biliary and pancreatic sphincterotomy, stenting, stricture dilation, treatment of duct leaks, drainage of fluid collections and stone extraction will also be discussed in this review. With the advent of less invasive and safer diagnostic modalities including endoscopic ultrasound (EUS) and magnetic retrograde cholangiopancreatography (MRCP), ERCP is appropriately becoming a therapeutic rather than diagnostic tool in the management of acute pancreatitis and its complications.
Keywords: Endoscopic retrograde cholangiopancreatography, Acute pancreatitis
INTRODUCTION
Most pancreatologists accept the 1992 Atlanta Symposium definition of acute pancreatitis as an acute inflammatory process of the pancreas with variable involvement of other regional tissues or remote organ systems[1]. It is recognized that the underlying process is reversible and the gland returns to normal once the injury resolves. The annual incidence is estimated to be 17 per 100000 in the United States[2]. Over 333000 hospital admissions and 911000 physician visits in the United States each year are due to acute pancreatitis[2]. The most common causes in US adults are gallstone disease and excessive alcohol use, although clinically detectable pancreatitis never develops in most persons with these risk factors[3].
The pathogenesis of acute pancreatitis involves the inappropriate activation of trypsinogen to trypsin in excessive quantities to overwhelm the mechanisms of elimination within the pancreas. Activation of these digestive enzymes causes pancreatic injury and an intense inflammatory response which results in microcirculatory injury, leukocyte chemoattraction, release of cytokines and oxidative stress. The release of pancreatic enzymes damages the vascular endothelium, the interstitium and acinar cells. Microcirculatory changes and progressive ischemia occur, which increase vascular permeability and leads to edema of the gland, commonly referred to as interstitial pancreatitis. Translocation of bacteria from the gut into the systemic circulation may occur as a consequence of gut ischemia secondary to hypovolemia and arteriovenous shunting. Severe pancreatitis may ensue, leading to life-threatening complications such as acute respiratory distress syndrome, renal failure, shock, metabolic derangements, and multi-organ failure. Approximately 20% of patients with acute pancreatitis will have a severe course with a 10% to 30% mortality rate[3]. Mortality related to acute pancreatitis decreased substantially from as high as 25%-30% in the 1970s to current rates since the early 1990s; however, since then, the mortality has remained relatively constant[3,4].Initial improvement appears to have been related to improvements in intensive care treatment rather than a better understanding of the natural history of disease or improved intervention.
Determining the etiology of acute pancreatitis can be challenging in those who do not give a significant history of alcohol use and in those who do not exhibit obvious gallstone disease. In about 20% to 40% of cases, no defined cause may be found and patients are subsequently labeled as having idiopathic acute pancreatitis[5]. Several causes for pancreatitis may be missed in the initial workup using the conventional imaging techniques of trans-abdominal ultrasound and/or CT scan and routine laboratory tests. Other modalities, including more advanced imaging techniques and endoscopic procedures, are often considered when working up the cause of unexplained acute pancreatitis. Although used primarily for diagnostic and therapeutic purposes in biliary disorders, endoscopic retrograde cholangiopancreatography (ERCP) has evolved as a diagnostic and therapeutic option in evaluating several pancreatic diseases[6].
CLINICAL USE OF ERCP IN ACUTE PANCREATITIS
When clear clinical, laboratory and imaging evidence for persistent biliary obstruction is present, patients should directly proceed to ERCP. As with all invasive procedures, the risk of procedure-related complications must be weighed against the potential benefit of the procedure. ERCP has a reported complication rate of 5% to 7%, with the main complications including pancreatitis, hemorrhage, perforation, cholangitis, cardiopulmonary complications and, rarely, death. Patient-related factors, such as underlying co-morbidities, age, and need for invasive evaluation, are considerable determinants of complication risk in any endoscopic procedures, especially those that carry a higher possibility for complications such as ERCP. Because of the potential morbidity and mortality associated with ERCP and the improvements in other less-invasive imaging modalities, the role of ERCP has become more well-defined in the diagnosis and treatment acute pancreatitis.
In the 30% of patients who may have no identifiable cause for acute pancreatitis with traditional non-invasive methods, ERCP with empiric biliary sphincterotomy is often performed without a more thorough evaluation for cause[7]. Freeman et al evaluated this concern and determined the risk of post-ERCP pancreatitis to be as high as 20% with a 3%-4% risk for severe pancreatitis. Though tempting, empiric sphincterotomy appears to have about an equal chance of causing complications as treating the underlying cause of the acute pancreatitis and, therefore, is not advocated[6,8]. Determining the etiology of acute pancreatitis is important, as it helps direct therapy, limits further unnecessary evaluation, and may improve a patient’s long term prognosis. Advanced endoscopic procedures, including ERCP, are emerging as valuable tools in the evaluation of this challenging group. The role of ERCP in the diagnostic and therapeutic evaluation of acute pancreatitis will be discussed in this review.
MICROLITHIASIS
Microlithiasis or biliary sludge as a causative etiology for acute pancreatitis remains controversial and not well understood. Several studies have demonstrated the presence of biliary sludge in as many as 75% of patients with unexplained acute pancreatitis[5]. Microlithiasis is a viscous precipitate containing mucin, cholesterol and calcium bilirubinate which can obstruct the pancreatic duct. Ultrasonography has a sensitivity of only about 55% in detecting microlithiasis and does not allow for analysis of the chemical composition of bile[9]. Bile analysis with microscopic examination is considered the gold standard for diagnosis. Bile can be obtained directly while cannulating the bile duct during ERCP or following CCK stimulation on EGD. ERCP with bile aspiration from the common bile duct (CBD) has a reported sensitivity of 83% in detecting microlithiasis[7]. It is recognized, however, that gallbladder bile is preferred over ductal bile for examination, as transit through the hepatic and common ducts can be too rapid to allow formation of crystals large enough to detect on microscopy. Ko et al[9] propose the criteria of 2 or more crystals per 100X field or more than 4 crystals per sample as a positive result. It is also recommended to collect bile samples prior to contrast injection to avoid the formation of “pseudomicrolithiasis” from contrast precipitates. Using a guidewire under fluoroscopy with aspiration to clear the collection catheter prior to obtaining the bile sample can minimize contrast contamination and diminish this artifact. The specimen should be centrifuged immediately and examined under polarized microscopy to evaluate for crystals. If specimens are not examined immediately, crystals can precipitate and cause a false positive result. ERCP should be performed after complete recovery from acute pancreatitis, usually 4 to 6 wk after presentation. If microlithiasis is detected, patients should be considered for cholecystectomy or biliary sphincterotomy depending on surgical risk. In post- cholecystectomy patients, bile analysis need not be performed.
PANCREAS DIVISUM
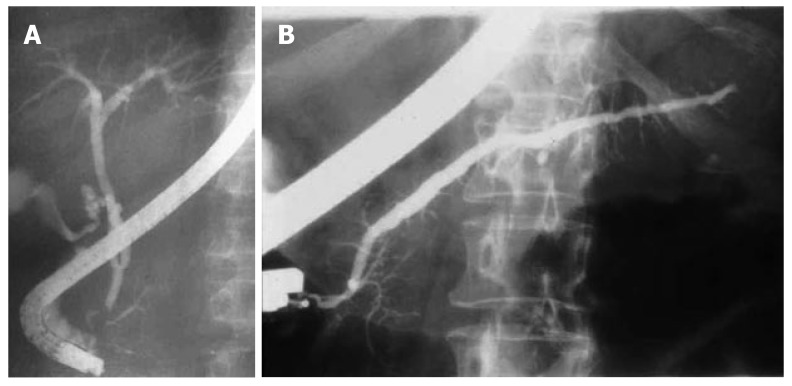
Pancreas divisum (PD) is the most common congenital anomaly of the pancreas and occurs in approximately 7%-10% of the population; however, less than 5% of those with PD are symptomatic[7]. PD is a small or absent ventral pancreatic duct that fails to fuse with the dorsal duct during embryologic development, resulting in a lack of communication between the ducts (Figure 1A and B). This lack of communication leads to a prominent dorsal duct that drains entirely through the minor papilla. Incomplete PD is the partial communication between the ventral and dorsal ducts; however, incomplete PD functions similar to that of complete PD. The divided drainage routes may result in a relative obstruction of flow through the minor papilla, leading to acute pancreatitis. Some controversy exists about recognizing PD as a causative etiology for pancreatitis. Regardless of the debate, several studies have demonstrated that dorsal duct outflow obstruction can lead to both acute and chronic pancreatitis[10,11]. It has been reported that patients with divisum have an increased prevalence of pancreatitis. Parenchymal changes consistent with chronic pancreatitis isolated to the dorsal pancreas have been observed on autopsy studies[6].
Figure 1.
A: Pancreas divisum with filling of the small ventral duct; B: Pancreas divisum with filling of dorsal duct through the minor orifice.
ERCP plays a limited role in diagnosis of PD given the less invasive and reasonably accurate modalities of MRCP and EUS. ERCP can, however, offer a therapeutic option in those who experience recurrent acute pancreatitis. Several studies have shown that endoscopic intervention, including sphincterotomy with or without stenting of the minor papilla, has resulted in decreased recurrence rates of acute pancreatitis and improved outcomes in patients with PD (Figure 2A-C)[12-17]. In the only randomized controlled trial evaluating endoscopic therapy in PD, Lans et al[18] observed an improvement in 90% of treated patients versus only 11% of controls over a mean of 12 mo follow-up. A retrospective study by Gerke et al[19] observed that 60% of patients who underwent minor papillotomy reported immediate improvement, but sustained results were seen in only half of those patients at a mean of 29 mo follow-up. Patients with well-defined bouts of pancreatitis appeared to benefit over those solely with chronic abdominal pain without objective evidence of pancreatitis. It is recognized that pain secondary to PD and chronic abdominal pain with incidentally found PD is difficult to differentiate. In order to distinguish between these two groups, objective criteria such as recurrent pain with ductal dilatation and elevations in pancreatic enzymes can be useful. Secretin stimulation imaging using either ultrasonography or MRI has proven promising as these modalities can give clues to the functionality of the pancreas. The study by Gerke et al underscores the importance of defining patients who may benefit from endoscopic therapy and reveals the difficulty in achieving long-term results in patients with PD.
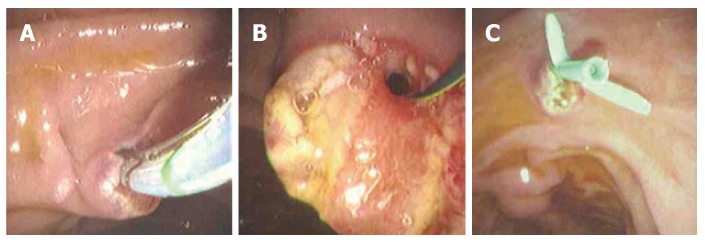
Figure 2.
A: Sphicterotome performing minor ampulla sphincter-otomy; B: Guidewire in place after minor sphincterotomy; C: Pancreatic stent placement post-sphincterotomy for pancreas divisum.
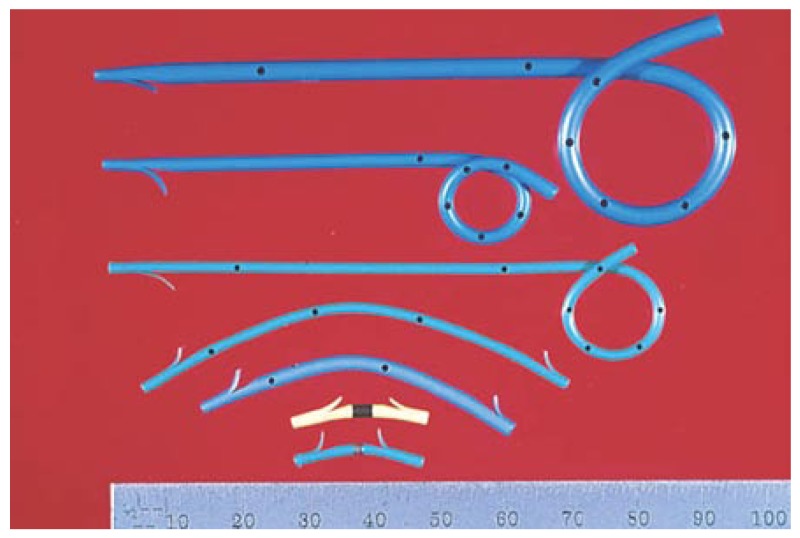
The role of using pancreatic stents in PD is limited to the prevention of post-ERCP pancreatitis and has demonstrated little efficacy in treating PD in comparison to sphincterotomy. The long-term use of pancreatic stents in PD may induce duct strictures or irregularities. Most experts recommend only the short-term use of pancreatic stents (less than 2 wk) in patients without pre-existing duct strictures. Size 3 or 4 French, non-phlanged stents, without an internal flap are recommended to decrease the risk of post-ERCP pancreatitis (Figure 3). These stents can pass spontaneously, therefore, obviating the need for a repeat procedure for stent removal. Note that the use of size 3 French stents requires a separate, smaller guidewire which may increase the overall cost of the procedure.
Figure 3.
Various designs of pancreatic stents (with permission from Cook Endoscopy).
SPHINCTER OF ODDI DYSFUNCTION
Sphincter of Oddi dysfunction (SOD) as an etiology for acute pancreatitis has been questioned by many due to the lack of concrete pathologic findings[20]. SOD refers to an abnormality in sphincter of Oddi contractility resulting in intermittent biliary and pancreatic duct obstruction. It has been estimated that SOD accounts for approximately 1/3rd of those with recurrent unexplained pancreatitis.
Management depends upon the classification of SOD. A classification system similar to the Milwaukee Biliary Group Classification has been developed for pancreatic SOD (Table 1): type I pancreatic SOD includes recurrent pancreatitis or pain suspected to be of pancreatic origin with elevated amylase and/or lipase up to 1.5 times upper limit of normal, a dilated pancreatic duct greater than 6 mm in the head and more than 5 mm in the body, and delayed emptying of greater than 9 min of the pancreatic duct. type II SOD includes the presence of presumed pancreatic pain plus at least one additional factor defining type I. type III is defined by pain alone. Some experts have suggested that due to the high incidence of stone disease, patients suspected to have SOD with an intact gallbladder should undergo cholecystectomy as initial therapy before evaluation for sphincter dysfunction due to less risk involved in surgery compared with manometry. Our approach in patients with intact gallbladder, but no stone disease on standard imaging, is to perform endoscopic ultrasound (EUS) looking for undetected stones or biliary sludge. If EUS is unremarkable, then ERCP with bile analysis is performed to evaluate for microlithiasis. If microlithiasis is not detected, we will proceed with sphincter of Oddi manometry during the same exam. In those who have previously had a cholecystectomy, dual sphincterotomy becomes the recommended treatment modality. A study by Gelrud et al[21] demonstrated significantly better outcomes when dual sphincterotomy was performed over a biliary sphincterotomy alone.
Table 1.
Sphincter of Oddi Dysfunction
| Type I |
| 1 Typical biliary-pancreatic pain |
| 2 Liver chemistries (total bilirubin, alkaline phosphatase, transaminases) ≥ 1.5-2X ULN and/or pancreatic chemistries (amylase and/or lipase) ≥ 1.5-2X ULN |
| 3 Dilated common bile duct (≥ 12 mm) or pancreatic duct (head ≥ 6 mm, body ≥ 5 mm) diameter |
| 4 Prolonged biliary drainage (> 45 min) with patient in supine position or pancreatic drainage (> 9 min) with patient in the prone position |
| Type II |
| 1 Typical biliary-pancreatic pain |
| 2 Positive findings for one or two items (2, 3, or 4) from type I |
| Type III |
| 1 Typical biliary-pancreatic pain and no other abnormalities |
Modified from Sherman et al[25].
Management of types II and III disease has posed more of a treatment challenge to the clinician. Patients who meet type II classification criteria should undergo sphincter of Oddi manometry (SOM). A resting basal pressure of > 40 mm Hg is the best predictor of response to endoscopic sphincterotomy, with up to 90% demonstrating clinical benefit in 4 years follow-up[20]. Studies advocate the measurement of both biliary and pancreatic sphincter pressures during ERCP, as differences in pressure may be detected if either duct is measured alone[22-25]. There is no role for ERCP without manometry in the evaluation of type II SOD patients.
Traditional teaching has been that performing SOM increases the risk for pancreatitis[26]. However, more recently, it appears that performing any ERCP (with or without manometry) in the subset of patients at highest risk for SOD (typically young women with unexplained recurrent abdominal pain, normal anatomy, and normal serum bilirubin) increases the risk for pancreatitis[8,27,28]. Several randomized trials have proven a decreased risk of post-ERCP pancreatitis in this group of patients by performing prophylactic stent placement[29,30]. The NIH has recommended that diagnostic and therapeutic procedures in this select group of patients should be performed ONLY by endoscopist possessing expertise in this particular area because of the high rate of severe complications in this young, otherwise healthy population[20]. Because of this thought, the diagnosis and management of type III disease is the most difficult. Invasive procedures should be delayed or avoided if possible. Trials of anticholinergics, antidepressants, nonspecific pain relievers and/or calcium channel blockers should precede invasive approaches. Diagnostic ERCP without manometry has no role in the assessment of type III pancreatic SOD patients[20].
OCCULT TUMORS OF THE PANCREAS AND BILIARY TRACT
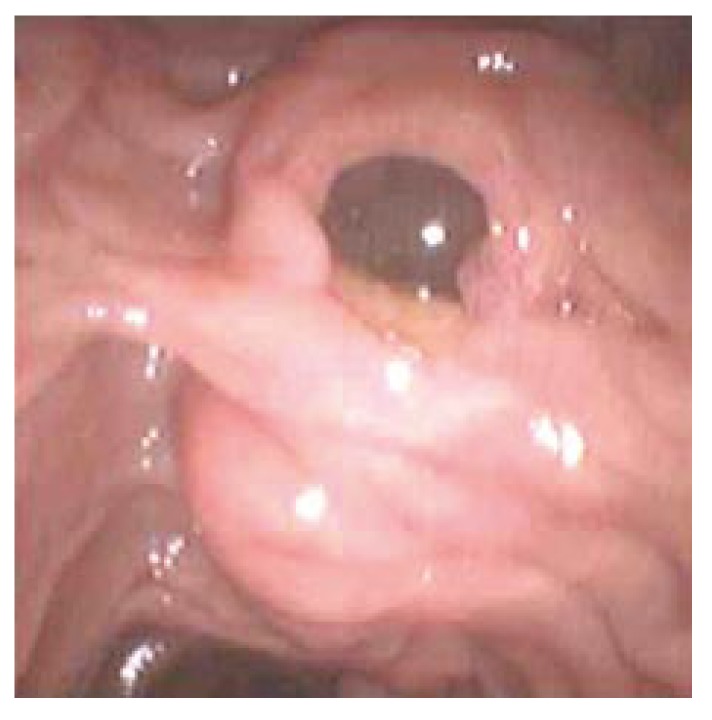
Approximately 30000 new cases of pancreatic cancer and 7000 biliary tract cancers are diagnosed annually in the United States. Prognosis remains dismal with less than 20% survival at 1 year and a 5 years mortality rate of > 95%[31]. Infrequently, tumors of the pancreas and biliary tree may present as acute pancreatitis. ERCP has proven to be valuable in the diagnosis and treatment of ampullary tumors and intraductal papillary mucinous tumors (IPMT) of the pancreas, both conditions which may present with acute pancreatitis due to obstruction from the pancreatic duct. ERCP may be the best means for direct visualization of the ampullary and periampullary region and offers the capability of sampling through biopsy. In non-operative patients with obstructing ampullary tumors, palliation or possibly cure can be achieved with endoscopic snare ampullectomy and ablative thermal therapy. Post-procedure stent placement in this clinical scenario has been demonstrated to reduce the risk of post-procedure pancreatitis[6]. IPMT can be diagnosed on ERCP with the classic “fish eye” appearance of the dilated ampulla and mucin extruding from the orifice (Figure 4). In non-operative patients with IPMT, ERCP with stent placement and/or sphincterotomy may offer palliation by decreasing the risk of acute recurrent pancreatitis by minimizing mucin impaction in the pancreatic duct.
Figure 4.
Classic “fish eye” appearance of IPMT with mucin draining from minor ampulla.
Peroral pancreatoscopy (PPS) is useful in cases of suspected IPMT by not only diagnosing but also localizing the tumor for planning surgical resection. PPS may also provide tissue sampling for histologic diagnosis[32].
GALLSTONES
Gallstones account for nearly half of the cases of acute pancreatitis in the Western world. More than 70% of patients will spontaneously pass the culprit stone into the duodenum, however, approximately 3% to 7% may go on to develop acute pancreatitis[33]. Because differentiating patients who may have an uncomplicated course due to a transiently impacted gallstone from those who may progress on to severe acute pancreatitis with necrosis and sepsis is difficult, several studies have evaluated scoring systems and variables to predict severity[34-36]. Urgent endoscopy is generally reserved for patients who fail to demonstrate liver enzyme improvement within 24 to 48 h of admission, especially in total bilirubin by hospital d 2[34]; those demonstrating persistent choledocholithiasis on imaging; and those with clinical cholangitis. Fan et al[37] evaluated the role of early ERCP in patients with acute biliary pancreatitis prior to the onset of complications, regardless of mild or severe presentation. Within 24 h of presentation, patients were randomized to early ERCP versus conservative treatment with selective ERCP. A significant reduction in progression to biliary sepsis was seen in the early ERCP patients. Interestingly, however, the incidence of local and systemic complications was not significantly different, which suggests that removal of the impacted stone may not reverse the damage already occurring in the pancreas during the first hours or days of the illness. Other studies have advocated early intervention within 72 h after admission if persistent CBD stones were suspected[38,39].
Patients who recover from gallstone pancreatitis carry a 29% to 67% risk of recurrent pancreatitis if subsequent cholecystectomy and/or sphincterotomy are not performed[6,40]. In mild to moderate gallstone pancreatitis, ERCP is rarely required before cholecyste-ctomy unless cholangitis or clear evidence of persistent choledocholithiasis by imaging and laboratory data is observed. Chang et al[34] evaluated patients with acute gallstone pancreatitis who were suspected of persistent choledocholithiasis. Patients were randomized to either pre-operative ERCP or selective post-operative ERCP if choledocholithiasis was found intraoperatively. Hospital stay was significantly longer in the routine pre-operative ERCP group (11.7 d vs 9.0 d). In the post-operative group, ERCP was necessary in only 24% of patients, suggesting that the diagnostic and therapeutic yield of pre-operative ERCP is low. These findings are consistent with the NIH consensus statement recommending that patients suspected of having choledocholithiasis should undergo an operative cholangiogram at the time of cholecystectomy. Operative cholangiogram is efficient and preferable when surgical proficiency in this technique is available. Otherwise, post-operative ERCP is indicated for patients who demonstrate retained stones. In patients who have had a prior cholecystectomy and have a low probability of common bile duct stones, diagnostic evaluation for choledocholithiasis should be performed with less-invasive modalities including MRCP or EUS. In the clinical scenario where the potential for retained common bile duct stones is substantial, ERCP and, when indicated, sphincterotomy with stone removal is the preferred diagnostic and therapeutic option[20]. ERCP with sphincterotomy is the preferred therapeutic modality if cholangitis from retained common bile duct stones is present. Patients should receive close medical care and treatment with IV fluid resuscitation, hemodynamic monitoring, and intravenous antibiotic therapy. Patients who fail to improve should undergo ERCP with sphincterotomy as soon as possible. Those who do improve still require urgent ERCP with sphincterotomy, usually within 24 h, to relieve the obstruction[20].
Recurrence of pancreatitis after ERCP with sphinctero-tomy for gallstone pancreatitis is rare. Cholecystectomy versus endoscopic sphincterotomy for the treatment of recurrent gallstone pancreatitis remains a controversial topic. Several experts advocate that cholecystectomy should only be considered if there are overt manifestations of gallbladder disease (e.g., biliary pain, cholecystitis, cystic duct obstruction), but not for prevention of recurrent gallstone pancreatitis. Studies have demonstrated that ERCP can be an effective therapeutic option for prevention of recurrent gallstone pancreatitis[41]. Siegel et al[42] demonstrate that ERCP with sphincterotomy can be performed safely in both the elective and urgent setting in patients who are otherwise not ideal operative candidates, such as the aged or younger patients at risk for surgical complications. Our approach is to proceed with cholecystectomy if the patient is a good surgical candidate.
PANCREATIC PSEUDOCYSTS AND FLUID COLLECTIONS
Pancreatic pseudocysts occur mainly as a result of acute pancreatitis, pancreatic trauma or chronic pancreatitis. Fluid usually contains a high concentration of pancreatic enzymes and variable amount of tissue debris. Most pseudocysts are sterile. ERCP has a reported success rate of 65%-95% in treatment of pancreatic pseudocysts, with a complication rate of 10%-35%. Drainage of fluid collections is generally reserved for a later date, usually 4-6 wk after the acute pancreatitis episode resolves.
PANCREATIC DUCT INJURY
Pancreatic duct disruptions may result from acute and chronic pancreatitis, or they may be the primary cause for pancreatitis in cases of trauma or surgical injury. ERCP can be successful in detecting the presence of contrast extravasation from the duct, localizing the suspected site of injury, and treating the leak or fistula with stent or drain placement.
Approximately 37%-67% of patients with acute pancreatitis have pancreatic duct injury, suggesting that acute duct injury can be a relatively common finding[43,44]. Lau et al[43] observed that the presence of a leak was associated with a higher incidence of necrosis and prolonged length of stay. ERCP in this patient population was determined to be safe and not associated with increased mortality, prolonged hospital stay or need for necrosectomy provided that pancreatic duct leaks were detected and immediately treated.
ERCP to evaluate for pancreatic duct disruption in acute pancreatitis is controversial and should be reserved for investigational studies. A multidisciplinary approach is advocated when considering pancreatic stenting in the setting of acute necrosis, as the procedure carries a risk of introducing infection into an otherwise sterile environment. In patients with evidence of pancreatic duct injury or leak who are not responding to conservative treatment, ERCP should be considered.
The use of ERCP in the treatment of acute pancreatitis from traumatic pancreatic duct injury has also been evaluated in both the adult and pediatric patient population. Several studies have demonstrated that ERCP with transpapillary stent placement is an effective technique in closing pancreatic duct disruption[45-47]. Successful therapy, however, appears to be associated with positioning of the stent to bridge the disruption and leak, not simply across the papilla as in biliary leaks[48,49]. Several studies in children have also reported successful results in treating traumatic duct injury, however the authors call attention to the risk of iatrogenic ductitis when stenting smaller pancreatic ducts, especially those in children[50,51].
UNUSUAL CAUSES OF ACUTE PANCREATITIS
Type III choledochal cysts are dilations of the joined portion of the pancreaticobiliary ducts. These cysts can be large enough to obstruct the pancreatic duct, which may result in recurrent acute pancreatitis[52,53]. Biliary sphincterotomy has been suggested as treatment; however, some patients may require a dual sphincterotomy for long term benefit.
Annular pancreas, anomalous pancreaticobiliary junction, and pancreatic intraductal parasites have all been reported as causes for acute and recurrent acute pancreatitis. ERCP can occasionally offer benefit in treatment of these rare conditions[6].
CONCLUSION
ERCP is a useful tool in the evaluation and management of acute pancreatitis. The main role of ERCP in acute pancreatitis is the diagnosis and treatment of biliary tract stone disease and other potential causes of pancreatic duct obstruction including sphincter dysfunction or anomalies such as pancreas divisum. With the advent of less-invasive and safer diagnostic modalities, ERCP is appropriately becoming a therapeutic tool in the management of acute pancreatitis and its complications.
Footnotes
S- Editor Liu Y L- Editor Rippe RA E- Editor Lu W
References
- 1.Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 2.National Digestive Disease Information Clearinghouse. National Institute of Health 1976-2002 [Google Scholar]
- 3.Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354:2142–2150. doi: 10.1056/NEJMcp054958. [DOI] [PubMed] [Google Scholar]
- 4.Neoptolemos JP, Raraty M, Finch M, Sutton R. Acute pancreatitis: the substantial human and financial costs. Gut. 1998;42:886–891. doi: 10.1136/gut.42.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SP, Nicholls JF, Park HZ. Biliary sludge as a cause of acute pancreatitis. N Engl J Med. 1992;326:589–593. doi: 10.1056/NEJM199202273260902. [DOI] [PubMed] [Google Scholar]
- 6.Kinney TP, Lai R, Freeman ML. Endoscopic approach to acute pancreatitis. Rev Gastroenterol Disord. 2006;6:119–135. [PubMed] [Google Scholar]
- 7.Hernandez L, Catalano M. Endoscopic Techniques (ERCP, EUS) for the Evaluation of Unexplained Acute Pancreatitis. Techn in Gastrointestinal Endoscopy. 2004;6:84–90. [Google Scholar]
- 8.Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909–918. doi: 10.1056/NEJM199609263351301. [DOI] [PubMed] [Google Scholar]
- 9.Ko CW, Sekijima JH, Lee SP. Biliary sludge. Ann Intern Med. 1999;130:301–311. doi: 10.7326/0003-4819-130-4-199902160-00016. [DOI] [PubMed] [Google Scholar]
- 10.Bank S, Indaram A. Causes of acute and recurrent pancreatitis. Clinical considerations and clues to diagnosis. Gastroenterol Clin North Am. 1999;28:571–589, viii. doi: 10.1016/s0889-8553(05)70074-1. [DOI] [PubMed] [Google Scholar]
- 11.Bernard JP, Sahel J, Giovannini M, Sarles H. Pancreas divisum is a probable cause of acute pancreatitis: a report of 137 cases. Pancreas. 1990;5:248–254. doi: 10.1097/00006676-199005000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Bradley EL, Stephan RN. Accessory duct sphincteroplasty is preferred for long-term prevention of recurrent acute pancreatitis in patients with pancreas divisum. J Am Coll Surg. 1996;183:65–70. [PubMed] [Google Scholar]
- 13.Keith RG, Shapero TF, Saibil FG, Moore TL. Dorsal duct sphincterotomy is effective long-term treatment of acute pancreatitis associated with pancreas divisum. Surgery. 1989;106:660–666; discussion 666-667. [PubMed] [Google Scholar]
- 14.Coleman SD, Eisen GM, Troughton AB, Cotton PB. Endoscopic treatment in pancreas divisum. Am J Gastroenterol. 1994;89:1152–1155. [PubMed] [Google Scholar]
- 15.Ertan A. Long-term results after endoscopic pancreatic stent placement without pancreatic papillotomy in acute recurrent pancreatitis due to pancreas divisum. Gastrointest Endosc. 2000;52:9–14. doi: 10.1067/mge.2000.106311. [DOI] [PubMed] [Google Scholar]
- 16.Lehman GA, Sherman S, Nisi R, Hawes RH. Pancreas divisum: results of minor papilla sphincterotomy. Gastrointest Endosc. 1993;39:1–8. doi: 10.1016/s0016-5107(93)70001-2. [DOI] [PubMed] [Google Scholar]
- 17.Soehendra N, Kempeneers I, Nam VC, Grimm H. Endoscopic dilatation and papillotomy of the accessory papilla and internal drainage in pancreas divisum. Endoscopy. 1986;18:129–132. doi: 10.1055/s-2007-1018352. [DOI] [PubMed] [Google Scholar]
- 18.Lans JI, Geenen JE, Johanson JF, Hogan WJ. Endoscopic therapy in patients with pancreas divisum and acute pancreatitis: a prospective, randomized, controlled clinical trial. Gastrointest Endosc. 1992;38:430–434. doi: 10.1016/s0016-5107(92)70471-4. [DOI] [PubMed] [Google Scholar]
- 19.Gerke H, Byrne MF, Stiffler HL, Obando JV, Mitchell RM, Jowell PS, Branch MS, Baillie J. Outcome of endoscopic minor papillotomy in patients with symptomatic pancreas divisum. JOP. 2004;5:122–131. [PubMed] [Google Scholar]
- 20.Cohen S, Bacon BR, Berlin JA, Fleischer D, Hecht GA, Loehrer PJ, McNair AE, Mulholland M, Norton NJ, Rabeneck L, et al. National Institutes of Health State-of-the-Science Conference Statement: ERCP for diagnosis and therapy, January 14-16, 2002. Gastrointest Endosc. 2002;56:803–809. doi: 10.1067/mge.2002.129875. [DOI] [PubMed] [Google Scholar]
- 21.Gelrud M, Plaz J, Mendoz S. Endoscopic treatment in type II pancreatic sphincter dysfunction. Gastrointest Endosc. 1995;41:398A. [Google Scholar]
- 22.Eversman D, Fogel EL, Rusche M, Sherman S, Lehman GA. Frequency of abnormal pancreatic and biliary sphincter manometry compared with clinical suspicion of sphincter of Oddi dysfunction. Gastrointest Endosc. 1999;50:637–641. doi: 10.1016/s0016-5107(99)80011-x. [DOI] [PubMed] [Google Scholar]
- 23.Silverman WB, Ruffolo TA, Sherman S, Hawes RH, Lehman GA. Correlation of basal sphincter pressures measured from the bile duct and the pancreatic duct in patients with suspected sphincter of Oddi dysfunction. Gastrointest Endosc. 1992;38:440–443. doi: 10.1016/s0016-5107(92)70473-8. [DOI] [PubMed] [Google Scholar]
- 24.Funch-Jensen P, Kruse A. Manometric activity of the pancreatic duct sphincter in patients with total bile duct sphincterotomy for sphincter of Oddi dyskinesia. Scand J Gastroenterol. 1987;22:1067–1070. doi: 10.3109/00365528708991959. [DOI] [PubMed] [Google Scholar]
- 25.Sherman S, Troiano FP, Hawes RH, O'Connor KW, Lehman GA. Frequency of abnormal sphincter of Oddi manometry compared with the clinical suspicion of sphincter of Oddi dysfunction. Am J Gastroenterol. 1991;86:586–590. [PubMed] [Google Scholar]
- 26.Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–393. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 27.Sherman S, Hawes RH, Troiano FP, Lehman GA. Pancreatitis following bile duct sphincter of Oddi manometry: utility of the aspirating catheter. Gastrointest Endosc. 1992;38:347–350. doi: 10.1016/s0016-5107(92)70430-1. [DOI] [PubMed] [Google Scholar]
- 28.Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Bjorkman DJ, Overby CS, Aas J, Ryan ME, Bochna GS, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54:425–434. doi: 10.1067/mge.2001.117550. [DOI] [PubMed] [Google Scholar]
- 29.Tarnasky PR, Palesch YY, Cunningham JT, Mauldin PD, Cotton PB, Hawes RH. Pancreatic stenting prevents pancreatitis after biliary sphincterotomy in patients with sphincter of Oddi dysfunction. Gastroenterology. 1998;115:1518–1524. doi: 10.1016/s0016-5085(98)70031-9. [DOI] [PubMed] [Google Scholar]
- 30.Fogel EL, Eversman D, Jamidar P, Sherman S, Lehman GA. Sphincter of Oddi dysfunction: pancreaticobiliary sphincterotomy with pancreatic stent placement has a lower rate of pancreatitis than biliary sphincterotomy alone. Endoscopy. 2002;34:280–285. doi: 10.1055/s-2002-23629. [DOI] [PubMed] [Google Scholar]
- 31.Ries L, Eisner M, Kosary C. Seer Cancer Statistics Review 1973-1996. National Cancer Institute. 2000 [Google Scholar]
- 32.Yasuda K, Sakata M, Ueda M, Uno K, Nakajima M. The use of pancreatoscopy in the diagnosis of intraductal papillary mucinous tumor lesions of the pancreas. Clin Gastroenterol Hepatol. 2005;3:S53–S57. doi: 10.1016/s1542-3565(05)00263-6. [DOI] [PubMed] [Google Scholar]
- 33.Moreau JA, Zinsmeister AR, Melton LJ, DiMagno EP. Gallstone pancreatitis and the effect of cholecystectomy: a population-based cohort study. Mayo Clin Proc. 1988;63:466–473. doi: 10.1016/s0025-6196(12)65644-4. [DOI] [PubMed] [Google Scholar]
- 34.Chang L, Lo SK, Stabile BE, Lewis RJ, de Virgilio C. Gallstone pancreatitis: a prospective study on the incidence of cholangitis and clinical predictors of retained common bile duct stones. Am J Gastroenterol. 1998;93:527–531. doi: 10.1111/j.1572-0241.1998.159_b.x. [DOI] [PubMed] [Google Scholar]
- 35.Chatzicostas C, Roussomoustakaki M, Vlachonikolis IG, Notas G, Mouzas I, Samonakis D, Kouroumalis EA. Comparison of Ranson, APACHE II and APACHE III scoring systems in acute pancreatitis. Pancreas. 2002;25:331–335. doi: 10.1097/00006676-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Leese T, Shaw D. Comparison of three Glasgow multifactor prognostic scoring systems in acute pancreatitis. Br J Surg. 1988;75:460–462. doi: 10.1002/bjs.1800750519. [DOI] [PubMed] [Google Scholar]
- 37.Fan ST, Lai EC, Mok FP, Lo CM, Zheng SS, Wong J. Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Engl J Med. 1993;328:228–232. doi: 10.1056/NEJM199301283280402. [DOI] [PubMed] [Google Scholar]
- 38.Neoptolemos JP, Carr-Locke DL, London NJ, Bailey IA, James D, Fossard DP. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet. 1988;2:979–983. doi: 10.1016/s0140-6736(88)90740-4. [DOI] [PubMed] [Google Scholar]
- 39.Fölsch UR, Nitsche R, Lüdtke R, Hilgers RA, Creutzfeldt W. Early ERCP and papillotomy compared with conservative treatment for acute biliary pancreatitis. The German Study Group on Acute Biliary Pancreatitis. N Engl J Med. 1997;336:237–242. doi: 10.1056/NEJM199701233360401. [DOI] [PubMed] [Google Scholar]
- 40.Frakes JT. Biliary pancreatitis: a review. Emphasizing appropriate endoscopic intervention. J Clin Gastroenterol. 1999;28:97–109. doi: 10.1097/00004836-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Kaw M, Al-Antably Y, Kaw P. Management of gallstone pancreatitis: cholecystectomy or ERCP and endoscopic sphincterotomy. Gastrointest Endosc. 2002;56:61–65. doi: 10.1067/mge.2002.125544. [DOI] [PubMed] [Google Scholar]
- 42.Siegel JH, Veerappan A, Cohen SA, Kasmin FE. Endoscopic sphincterotomy for biliary pancreatitis: an alternative to cholecystectomy in high-risk patients. Gastrointest Endosc. 1994;40:573–575. doi: 10.1016/s0016-5107(94)70255-1. [DOI] [PubMed] [Google Scholar]
- 43.Lau ST, Simchuk EJ, Kozarek RA, Traverso LW. A pancreatic ductal leak should be sought to direct treatment in patients with acute pancreatitis. Am J Surg. 2001;181:411–415. doi: 10.1016/s0002-9610(01)00606-7. [DOI] [PubMed] [Google Scholar]
- 44.Kozarek R, Traverso L, Bail T. Pancreatic duct leak in necrotizing pancreatitis: role of diagnostic and therapeutic ERCP as part of a multidisciplinary approach. Gastrointest Endosc. 2000;51:AB138. [Google Scholar]
- 45.Kim HS, Lee DK, Kim IW, Baik SK, Kwon SO, Park JW, Cho NC, Rhoe BS. The role of endoscopic retrograde pancreatography in the treatment of traumatic pancreatic duct injury. Gastrointest Endosc. 2001;54:49–55. doi: 10.1067/mge.2001.115733. [DOI] [PubMed] [Google Scholar]
- 46.Kozarek RA, Ball TJ, Patterson DJ, Freeny PC, Ryan JA, Traverso LW. Endoscopic transpapillary therapy for disrupted pancreatic duct and peripancreatic fluid collections. Gastroenterology. 1991;100:1362–1370. [PubMed] [Google Scholar]
- 47.Kozarek RA. Endoscopic therapy of complete and partial pancreatic duct disruptions. Gastrointest Endosc Clin N Am. 1998;8:39–53. [PubMed] [Google Scholar]
- 48.Varadarajulu S, Noone TC, Tutuian R, Hawes RH, Cotton PB. Predictors of outcome in pancreatic duct disruption managed by endoscopic transpapillary stent placement. Gastrointest Endosc. 2005;61:568–575. doi: 10.1016/s0016-5107(04)02832-9. [DOI] [PubMed] [Google Scholar]
- 49.Telford JJ, Farrell JJ, Saltzman JR, Shields SJ, Banks PA, Lichtenstein DR, Johannes RS, Kelsey PB, Carr-Locke DL. Pancreatic stent placement for duct disruption. Gastrointest Endosc. 2002;56:18–24. doi: 10.1067/mge.2002.125107. [DOI] [PubMed] [Google Scholar]
- 50.Canty TG, Weinman D. Treatment of pancreatic duct disruption in children by an endoscopically placed stent. J Pediatr Surg. 2001;36:345–348. doi: 10.1053/jpsu.2001.20712. [DOI] [PubMed] [Google Scholar]
- 51.Canty TG, Weinman D. Management of major pancreatic duct injuries in children. J Trauma. 2001;50:1001–1007. doi: 10.1097/00005373-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Goldberg PB, Long WB, Oleaga JA, Mackie JA. Choledochocele as a cause of recurrent pancreatitis. Gastroenterology. 1980;78:1041–1045. [PubMed] [Google Scholar]
- 53.Greene FL, Brown JJ, Rubinstein P, Anderson MC. Choledochocele and recurrent pancreatitis. Diagnosis and surgical management. Am J Surg. 1985;149:306–309. doi: 10.1016/s0002-9610(85)80092-1. [DOI] [PubMed] [Google Scholar]