Abstract
AIM: To establish the extent to which contrast enhancement with SonoVue in combination with quantitative evaluation of contrast-medium dynamics facilitates the detection of hepatic tumors.
METHODS: One hundred patients with histologically confirmed malignant or benign hepatic tumor (maximum size 5 cm) were analyzed. Contrast-enhanced ultrasound (bolus injection 2.5 mL SonoVue) was carried out with intermittent breath-holding technique using a multifrequency transducer (2.5-4 MHz). Native vascularization was analyzed with power Doppler. The contrast-enhanced dynamic ultrasound investigation was carried out with contrast harmonic imaging in true detection mode during the arterial, portal venous and late phases. Mechanical index was set at 0.15. Perfusion analysis was performed by post-processing of the raw data [time intensity curve (TIC) analysis]. The cut-off of the gray value differences between tumor and normal liver tissue was established using Receiver Operating Characteristic (ROC) analysis 64-line multi-slice computed tomography served as reference method in all cases. Magnetic resonance tomography was used additionally in 19 cases.
RESULTS: One hundred patients with 59 malignant (43 colon, 5 breast, 2 endocrine metastases, 7 hepatocellular carcinomas and 2 kidney cancers) and 41 benign (15 hemangiomas, 7 focal nodular hyperplasias, 5 complicated cysts, 2 abscesses and 12 circumscribed fatty changes) tumors were included. The late venous phase proved to be the most sensitive for classification of the tumor type. Fifty-eight of the 59 malignant tumors were classified as true positive, and one as false negative. This resulted in a sensitivity of 98.3%. Of the 41 benign tumors, 37 were classified as true negative and 4 as false negative, which corresponds to a specificity of 90.2%. Altogether, 95.0% of the diagnoses were classified as correct on the basis of the histological classification. No investigator-dependency (P = 0.23) was noted.
CONCLUSION: The results show the possibility of accurate prediction of malignancy of hepatic tumors with a positive prognostic value of 93.5% using advanced contrast-enhanced ultrasound. Contrast enhancement with SonoVue in combination with quantitative evaluation of contrast-medium dynamics is a valuable tool to discriminate hepatic tumors.
Keywords: Liver tumors, Malignant tumor, Contrast harmonic imaging, Quantitative contrast enhancement
INTRODUCTION
Benign liver lesions have a prevalence of approximately 20% in the whole population, whereas tumor patients show hepatic metastases in 25%-50% at the time of diagnosis. The liver is the main region for the manifestation of distant metastases in oncological diseases. Therefore, reliable detection of hepatic metastases is a prerequisite for evaluating the prognosis and establishing a therapeutic procedure. To evaluate the prospects of resection or to plan interventional therapy, it is essential to establish the number and location of the lesions. Prior to this, the focal hepatic lesion must be accurately characterized. Since no single diagnostic tool has been able to clarify this question with sufficient reliability up to now, histological analysis remains the gold standard. Characterization of focal hepatic lesions with imaging techniques is an important part of radiological diagnosis and is still the focus of scientific research because of technical innovations of existing methods[1-15]. Special attention has been paid to the validation of new methods, especially the further development of contrast-enhanced ultrasound techniques because ultrasound is the most common and best available imaging technique[1-3,5-14].
In a patient with a known primary malignancy, any focal liver lesion seen on non-enhanced ultrasound must be regarded as suspicious of metastasis. However many lesions (25%-50% of lesions ≤ 2 cm) will eventually prove to be benign; next to contrast-enhanced ultrasound, other imaging modalities or biopsies are used to further characterize the lesion. For lesions < 1 cm, the false-negative rate of non-enhanced ultrasound is as high as 80%. Therefore contrast-enhanced ultrasound should be carried out in addition to conventional ultrasound in most cancer patients for definitive liver staging[6-9].
This prospective two-center study investigated the possibility of evaluating the malignancy of liver lesions on the basis of contrast-enhanced harmonic imaging (CHI) and SonoVue, by comparing the dynamics of the contrast agent in tumor lesions with those in the surrounding tissue. The objective of this study was to examine to what extent the detection of liver tumors will be simplified after contrast application with SonoVue, in combination with quantitative measurement of contrast agent dynamics. Special attention was paid to the feasibility of assessing the malignancy of the tumor by quantitative measurement of the tumor tissue contrast compared to the surrounding healthy tissue.
MATERIALS AND METHODS
Study design
This prospective study was performed at two centers. The equipment (Logic 9; GE Healthcare, Milwaukee, WI, USA), transducers (4C probe), data acquisition (PACS connection) and display, as well as the procedure of ultrasound examination were set identically at both centers.
Only patients with histologically confirmed malignant or benign hepatic lesions were included in the study. Tumor sizes were differentiated as follows: < 3 cm, and 3-5 cm. The maximum number of lesions considered was five.
Exclusion criteria were: tumor lesion > 5 cm, and more than five lesions, strong allergic reactions, diseases of liver and kidney with confirmed elevation of laboratory parameters, acute heart failure, acute myocardial infarction, subcutaneous emphysema, meteorism, tachypnea, and aerobilia.
Operational sequence and follow-up
A biopsy was taken from the malignant liver tumors to establish the histological result (if necessary, surgical resection or radiofrequency ablation was performed). In case of hemangioma, constant results of ultrasound examinations over 2 years and additional MRI or multiphase CT with constant results over 2 years was required. All contrast-enhanced ultrasound investigations were conducted with a multi-frequency linear transducer (2.5-4 MHz, Logic 9; GE Healthcare). Transmitted energy was reduced to a magnitude of < 30% with a mechanical index (MI) of 0.15. In fundamental B-scan, an optimal depth adjustment with three focus zones was made. After B-scan and analysis of vascularization with power Doppler ultrasound, an intravenous bolus injection of 2.5 mL SonoVue (Altana, Bracco, Konstanz, Germany) was administered through a 20-18 Ga peripheral cubital cannula, followed by a bolus injection of 10 mL NaCl.
Using CHI, the microbubbles of the contrast medium were stimulated to vibrate and their energetic harmonic Doppler frequencies were employed for imaging. In the technique of pulse inversion harmonic imaging (PIHI) of CHI, emission frequencies are digitally encoded. Several pulses, each after a defined period of time, were produced. Then the received echos were subtracted from each other. The harmonic frequencies of contrast-enhanced echos in the flowing blood remained as image information. In the subtraction mode of the background information, the influx of contrast agent and its distribution were imaged, even with very low acoustic energy.
A low MI allowed the real-time evaluation of the contrast-agent enhancement. CHI suppressed the fundamental linear echoes from the liver tissue, whereas the non-linear echoes reflected from the microbubbles remained, which provided the ultrasound signal. The true agent detection mode was also used to process the fundamental non-linear signal generated by ultrasound contrast agents that were stronger than the harmonic signal, thereby increasing the specificity of the microbubble-to-tissue ratio. The combination of SonoVue with true agent detection mode using a low MI allowed dynamic enhancement of the blood supply of a liver nodule to be evaluated during the various phases of contrast-agent circulation. Perfusion curves described the ultrasound signal intensity over time after contrast bolus injection in a region of interest (ROI).
Modified ultrasound slices close to the tumor enabled a minimum depth of penetration to be maintained. One turn of the scanner head examined the whole liver during intermittent breath holding. Digital data from cine sequences allowed post-processing of the dynamic tumor blood flow and calculation of 3D data block images. Scanning was carried out during the arterial (< 30 s), portal venous (40-120 s) and late venous (> 120 s) phases in true agent detection mode. Parallel dynamic imaging in fundamental B-scan and recording of the dynamics of contrast agent in the subtraction mode of CHI were performed in the meantime, which greatly facilitated the localization of tumor lesions.
Color-coded Doppler sonography and power Doppler ultrasound were used to evaluate native vascularization. Color enhancement was adjusted to the lowest possible pulse repetition frequency (PRF, < 1000 Hz) and to the best possible, artifact-free color enhancement, in order to avoid artifacts. The complete data of the contrast-agent examination were recorded in up to 5 min. The length of the specific cine loops in the three stages was a minimum of 20 s each.
Measurements were done in the arterial phase (to quantify arterial vascularization), in the portal venous phase (to asses contrast-agent accumulation, pooling), and up to 5 min in the late phase (to assess the wash-out effect).
Altogether, five measurements were performed in each patient in the ROIs: one measurement within the lesion (T1) and four in the surrounding liver parenchyma at 12, 3, 6 and 9 o'clock positions (G1, G2, G3 and G4), with ROIs not larger than 5 mm. A careful adjustment of the position of the ROIs was effected manually depending on inspiration or expiration. Time intensity curve (TIC) analyses were made off-line and then transferred into an spreadsheet table (Excel 2003; Microsoft, Redmond, WA, USA) for off-line analysis. In addition, digital raw data were recorded and saved.
The different dynamics of the depletion of contrast agent - in comparison to the depletion within normal liver tissue - was used to assess the malignancy of the tumors. For this purpose, the recorded gray scale parameters of the ROIs were averaged over the time of recording, and then the following gray scale differences were calculated:
arterial phase: Diffart = T1art - (G1art + G2art + G3art + G4art)/4
portal venous phase: Diffpv = T1pv - (G1pv + G2pv + G3pv + G4pv)/4
late venous phase: Difflv = T1sv - (G1sv + G2sv + G3sv + G4sv)/4
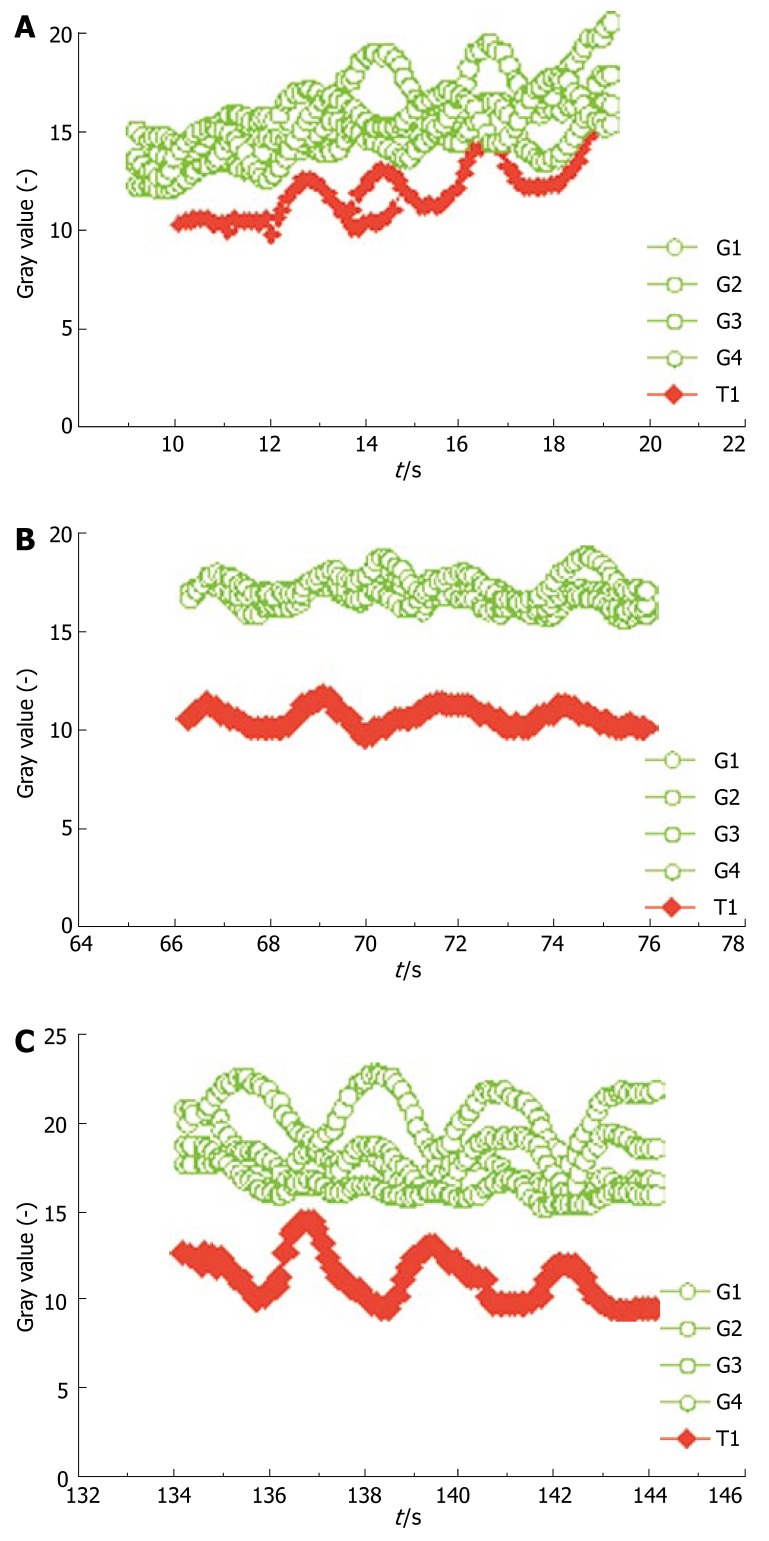
The malignancy of the tumor was then inferred from the absolute value of the differences. If the absolute value of Diffsv was smaller than -0.4 in the case of a malignant tumor, the assessment was rated as true positive, if the value was greater than -0.4, the assessment was rated as false negative. If the value was greater than -0.4 in the case of a benign tumor, the assessment was rated as true negative, otherwise as false positive. First, the critical value had been ascertained in a small preliminary study, which had shown characteristic contrast-agent dynamics in malignant liver tumors. The determination of the optimal scale value is shown in the section ROC analysis. Figure 1A-C presents an example of contrast-agent dynamics.
Figure 1.
Gray value progression of the five ROIs over 10 s. A: For a patient with liver metastasis in the arterial phase; B: For the same patient in the portal-venous phase; C: For the same patient in the late venous phase. (red, gray-value progression in the tumor; green, gray-value progression in the surrounding healthy tissue).
An increased wash-out of contrast agent molecules appeared to occur in the malignant tumor vessels rather than in normal hepatic vessels. This was probably caused by arteriovenous shunts within the lesion and additional tumor necrosis, which prevented accumulation of microbubbles in the malignant tumor. Therefore, the tumor was more hypoechoic, especially in the late phase, i.e., the gray value imaging was less distinct. This characteristic behavior could then be used for quantitative evaluation of gray value differences in the diagnosis of liver tumor malignancy.
Ethical concerns
The study data were collected within the framework of an external quality control as a registry complying with the principles of the Helsinki/Edinburgh Declaration of 2002. Before each contrast agent application, patients were informed in detail about possible risks such as allergic reactions. The consent of all patients was obtained prior to the study.
Statistical analysis
All results were presented as the mean ± SD. Specificity, sensitivity, positive and negative prognostic value, as well as diagnostic accuracy were calculated to evaluate their diagnostic significance. Since there were, as a rule, no clinical findings or the history of the patient was not available, the examiner could not establish a pretest probability. In this situation, the resulting sensitivity and specificity of the three diagnostic tests were calculated on the basis of Table 1[17]:
Table 1.
The sensitivity and specificity of results analysis
| Disease existent | Disease non-existent | ||
| Test positive | a | b | a + b |
| Test negative | c | d | c + d |
| a + c | b + d |
Where a is the number of patients with existing disease and a positive test result (true positive); b is the number of patients with no disease, but a positive test result (false positive); c is the number of patients with existing disease and a negative test result (false negative); d is the number of patients with no disease and a negative test result (true negative); a/(a + c): sensitivity: true positive results/number of affected patients; d/(b + d): specificity: true negative results / number of healthy patients.
Using the Bayes formula it was possible to calculate the probability[17] that disease was present, on the basis of a positive result of the diagnostic test (positive prognostic value: pV). pV = (true positive results)/(true positive results + false positive results)
It was also possible to calculate the probability of no disease on the basis of a negative test result (negative prognostic value: nV). pV = (true negative results)/(true negative results + false negative results)
RESULTS
Patient characteristics
Altogether, 100 consecutive patients (43 women, 57 men, aged 25-83 years; mean 57 years) with suspicion of hepatic tumor were included in the study. Fifty-nine of these suffered from a malignant tumor (43 metastases of colon carcinoma, 5 metastases of breast cancer, 2 endocrine metastases, 7 HCC metastases, and 2 renal cell carcinoma), 41 patients had a benign tumor (9 hemangioma, 6 high-flow hemangioma, 7 nodular hyperplasia, 5 complicated cysts, 2 abscess, and 12 circumscribed fatty degeneration of the liver (focal hyposteatosis) with constant results over a minimum of 2 years. Maximum tumor size was 5 cm.
Analysis of contrast-agent dynamics
Arterial phase: Of the 59 patients with histologically confirmed malignant tumors, 40 were classified as true positive and 19 as false negative in the arterial phase. Of the 41 patients with histologically classified benign tumor, 22 were classified as true negative and 19 as false positive (Table 2, Figure 1A). In the arterial phase, a sensitivity of 67.8% with a specificity of 53.7 % (pV 67.8%, nV 53.7%) was achieved. Therefore, 62% of the test results were correct.
Table 2.
Results based on contrast-enhanced CHI during the arterial phase
| Tumor present | Tumor not present | ||
| Test positive | 40 | 19 | 59 |
| Test negative | 19 | 22 | 41 |
| 59 | 41 | 100 |
Portal venous phase: Of the 59 patients with histologically confirmed malignant tumor, 54 were classified as true positive and 5 as false negative in the portal venous phase. Of the 41 patients with histologically classified benign tumor, 31 were classified as true negative and 10 as false positive (Table 3, Figure 1B). This resulted in a sensitivity of 91.5% with a specificity of 75.6%, which gave a pV of 84.4% and nV of 86.1%. The correctly interpreted test evidence therefore amounted to 85.0%.
Table 3.
Results based on contrast-enhanced CHI during the portal-venous phase
| Tumor present | Tumor not present | ||
| Test positive | 54 | 10 | 64 |
| Test negative | 5 | 31 | 36 |
| 59 | 41 | 100 |
Late venous phase: Of 59 patients with histologically confirmed malignant tumor, 58 were classified as true positive and 1 as false negative in the late phase. Of 41 patients with histologically confirmed benign tumor, 37 were classified as true negative and 4 as false positive (Table 4, Figure 1C). This resulted in a sensitivity of 98.3% with a specificity of 90.2%, which gave a pV of 93.5% and nV of 97.4%. In the late venous phase, the correctly interpreted test evidence amounted to 95.0%.
Table 4.
Results based on contrast-enhanced CHI during the late phase
| Tumor present | Tumor not present | ||
| Test positive | 58 | 4 | 62 |
| Test negative | 1 | 37 | 38 |
| 59 | 41 | 100 |
A comparison of the diagnostic values of the three phases shows that the best accuracy was achieved in the late venous phase (Table 5).
Table 5.
Diagnostic value of quantitative CHI for detection of malignant hepatic tumors in different phases after intravenous application of ultrasound contrast agent
| Arterial phase | Portal-venous phase | Late venous phase | |
| Specificity (%) | 53.7 | 75.6 | 90.2 |
| Sensitivity (%) | 67.8 | 91.5 | 98.3 |
| Positive prognostic value | 67.8 | 84.4 | 93.5 |
| Negative prognostic value | 53.7 | 86.1 | 97.4 |
Stability of diagnostic criteria
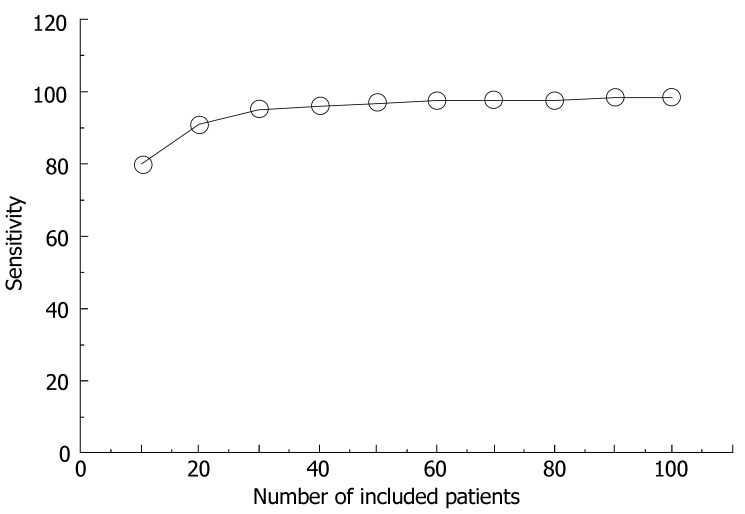
The sensitivity over the course of the study provided a sensitive indicator of the diagnostic value of this method in identifying malignant liver tumors in 100 patients (Figure 2). Only the late venous phase was analyzed since it showed the most sensitive results. Sensitivity over the course of the trial was cumulatively calculated after every 10 patients. This showed that sensitivity very clearly approached a stable final value. This meant that sensitivity would not have changed significantly if more patients were included. Therefore, a valid evaluation could be made. The other diagnostic parameters showed similar results.
Figure 2.
Sensitivity of the diagnostic test in relation to the number of patients examined. The sensitivity over the course of the study provides a sensitive indicator to the diagnostic value of this method in identifying malignant liver tumors in 100 patients.
ROC analysis
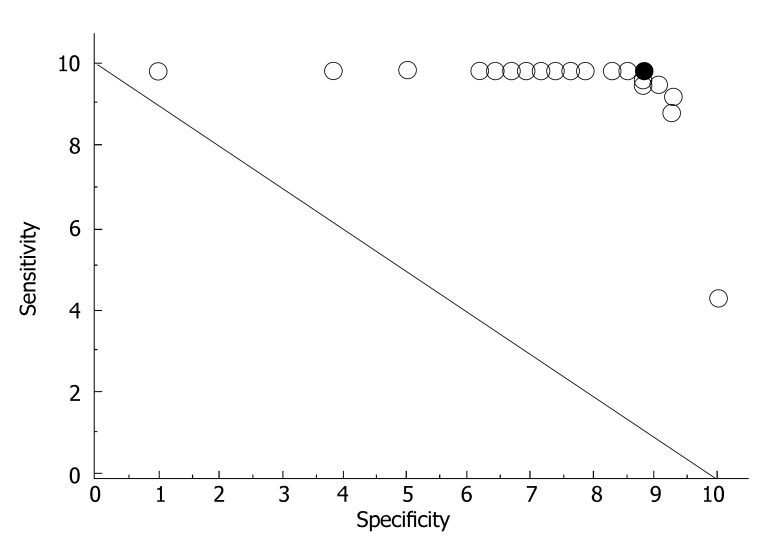
The test results depended on the cut-off value of the gray value differences that were chosen. By modifying the cut-off value by means of an ROC analysis, it was possible to attain the optimum selectivity by improving sensitivity and specificity[18]. Figure 5 shows the respective specificity and sensitivity for the late venous phase for different limiting values (varying the cut-off values from +5 to -5). Usually, the greater the distance from the points to the diagonal, the better the diagnostic test. It is evident that the point marked in red in the ROC diagram was the one with the most sensitivity and specificity. This optimum cut-off value was easy to calculate [cut-off valueopt = 0.5 × (specificity + sensitivity)/2] (Figure 3). The optimal cut-off value between the two groups of patients was readily established. The best selectivity for the two groups was at a cut-off value of -0.4 (Figure 4).
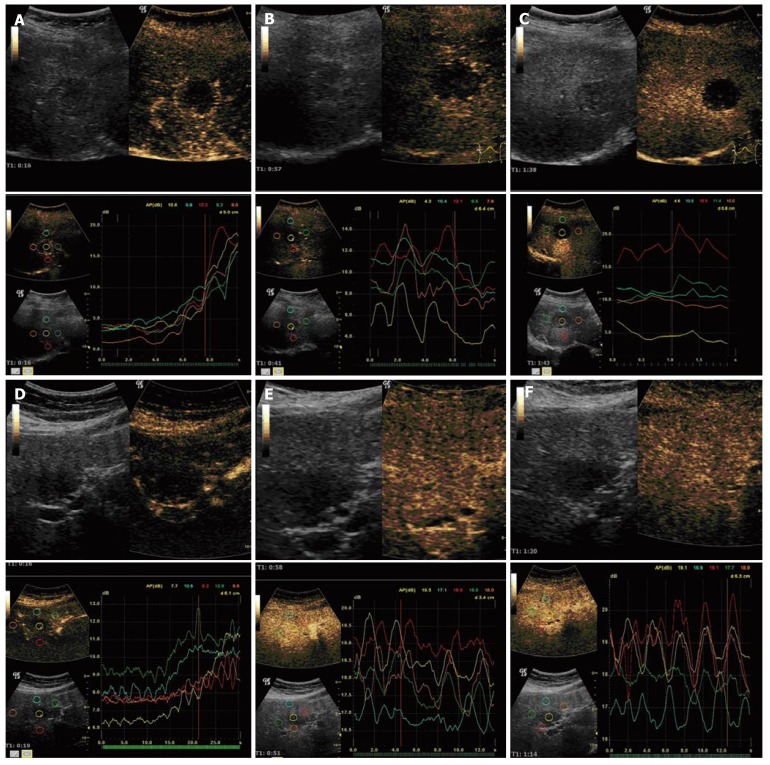
Figure 5.
True agent detection mode of CHI with TIC analysis. A: Malignant lesion in the arterial phase. Arterial enhancement of the tumor margin - metastasis of beast cancer; B: Malignant lesion in the portal-venous phase. Lower enhancement of the tumor - metastasis of beast cancer; C: Malignant lesion in the late venous phase (> 100 s). Lower enhancement of the tumor - metastasis of beast cancer; D: Benign lesion in the arterial phase. Lower enhancement of the tumor-adenoma histological confirmed in the early arterial phase; E: Benign lesion in the portal-venous phase. Enhancement of the tumor - adenoma histological confirmed-similar to normal liver tissue; F: Benign lesion in the late venous phase. Enhancement of the tumor-adenoma histological confirmed - similar to normal liver tissue.
Figure 3.
ROC diagram showing the specificity and sensitivity in the late venous phase for different limiting values (varying the cut-off values from +5 to -5). A greater distance from the diagonal line indicates that a diagnostic test had a higher reliability. The black point in the ROC diagram corresponds to the best sensitivity and specificity.
Figure 4.
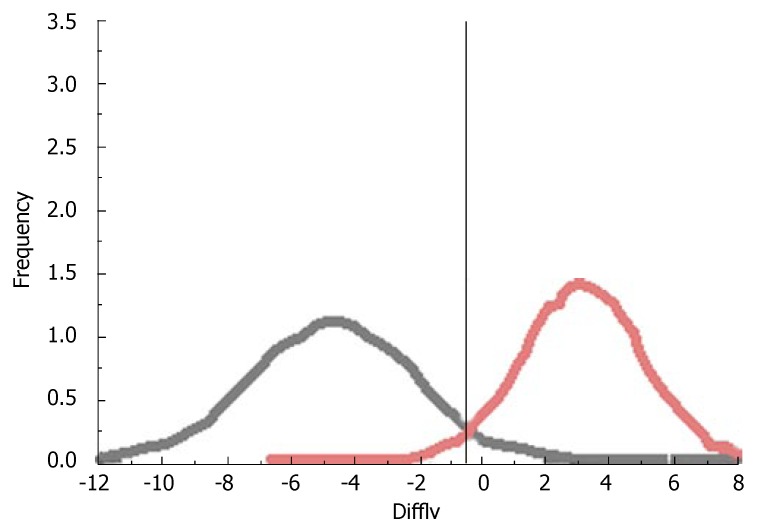
Distribution of the characteristic gray-value differences in the late venous phase for patients with benign (black) or malignant (red) hepatic tumor.
Center dependence
The diagnostic parameters were calculated and compared for the two centers (Table 6). Variations in the parameters were based on one single case in the whole study with a false-positive value in only one center, and a single case was still too small for a separate description. The quality of testing did not differ between the two centers: 47 out of 50 patients were classified correctly in center 1, and 48 out of 50 in center 2. There was therefore no center-dependence (P = 0.229) (Figure 5A-F).
Table 6.
Specificity and sensitivity for both testing centers
| Center 1 n = 50 | Center 2 n = 50 | Total n = 100 | |
| Specificity (%) | 85.7 | 95.0 | 90.2 |
| Sensitivity (%) | 100 | 96.7 | 98.3 |
| Negative prognostic value | 100 | 95.0 | 97.4 |
| Positive prognostic value | 90.6 | 96.7 | 93.5 |
DISCUSSION
All patients with suspected hepatic lesions, who were sent for further diagnostic workup to the two different centers, were consecutively included in the study. First, an ultrasound examination of the liver was undertaken using B-scan with a high-resolution multifrequency probe, resulting in a complete digital data set of the whole liver. Then, vascular ultrasound with power Doppler was performed to assess the vascularization of tumor lesions detected by the B-scan. After a bolus injection of 2.5 mL SonoVue, quantitative dynamic contrast-enhanced ultrasound was performed in low MI Technique for the arterial, portal-venous and late venous phase. As for the Contrast Harmonic Imaging (CHI) technique, Pulse Inversion Harmonic Imaging (PIHI) with simultaneous data acquisition of the B-image and the contrast-enhanced perfusion image in True Agent Detection mode was used. Data were stored with dynamic and cine sequences up to a maximum of 60 s. Contrast -agent dynamics were represented in terms of the digital raw data of the gray values, exported into a spreadsheet table, and evaluated by an external institution without any knowledge of imaging and the clinical data. The histological result was only communicated after the end of the study, so that diagnostic criteria were analyzed without any previous knowledge. An information bias was therefore precluded (which is especially important for radiological imaging), which enabled the prognostic values to be calculated according to Bayes[18]. Diagnostic tests were therefore evaluated in the same way as in the specific clinical situation.
Conventional ultrasound methods are limited for depicting and characterizing focal liver lesions by a low contrast between the lesion and normal parenchyma. The use of specific contrast agents for ultrasound improves the diagnostic value of conventional ultrasound and enables a complete diagnosis of liver lesions. Solutions for a complete ultrasound diagnosis have been proposed with the introduction of second-generation contrast agents and the use of low MI techniques, such as pulse inversion, which allows a continuous sweep with the same bubbles[1-3,5-10]. The support of gray-scale images is essential to delineate the lesions better and to check the perfect matching of contrast-enhanced and B-mode images. The availability of the true agent detection mode is able to overcome these limitations by higher signal sensitivity and simultaneous acquisition of gray scale and contrast-enhanced images.
SonoVue provides strong and persistent harmonic resonance at low MI (≤ 0.2), with which minimal or no bubble destruction occurs. This allows for continuous real-time imaging of a lesion during its vascular phase. With real-time low-MI imaging, the dynamic enhancement pattern and the vascular morphology of a lesion is assessed during the arterial phase (10-20 s until 25-50 s after bolus injection) and portal-venous phase (30-45 s until 120 s after bolus injection). The delayed phase (> 120 s after bolus injection) is particularly useful for the detection of, as they show as non-enhancing defects. Characterization is also improved by the late phase, as the vast majority of benign lesions show contrast uptake in this phase[5,7,8]. Our results show that by means of CHI and subsequent quantitative gray-value analysis of contrast-agent dynamics in the late venous phase, it is possible to assess malignant liver tumors with a sensitivity of 98.3% with an nV of 97.4%.
High-resolution digital contrast-enhanced ultrasound techniques have considerably improved the assessment of malignant and benign tumor lesions in the liver[1-12]. Under optimal examination conditions that enable imaging of the entire liver, it is possible to attain a diagnostic reliability comparable to that of contrast-enhanced MRI with a liver-specific contrast agent, and an even higher diagnostic certainty of > 90%, compared with contrast-enhanced multi-detector-spiral CT[1,4,15]. Using ultrasound contrast agents, a signal amplification of up to 10 dB is feasible. This allows a better differentiation between regular liver tissue and malignant tissue within the liver, which has an increased contrast wash-out in the portal-venous and, especially, in the late phase. The perfusion curves in healthy liver tissue show a slow increase, which reaches a plateau at the portal-venous phase, followed by a very slow decrease. In most benign tumors, enhancement can also be detected in the late phase after contrast agent injection. In cases of malignancy, the decrease begins in the portal-venous phase in most cases after slow marginal arterial enhancement. Tumor lesions of HCC, liver adenoma, high-flow hemangioma and focal nodular hyperplasia can show an early arterial enhancement in the first 30 s in perfusion curves[4,6,8,12,13]. These lesions may be masked in the portal-venous phase. Therefore, the most important phase for tissue differentiation is the late phase, because malignant tissue shows a contrast-agent wash-out in the late phase, whereas regular tissue still has a slowly descending plateau. This is the explanation for the significant differences of the gray-value analysis of the contrast-enhanced TIC analysis in the late phase.
Using a continuously acquired contrast-enhanced dynamic CHI, the diagnostic accuracy in characterization of benign lesions, such as partially thrombosed or high flow hemangioma, focal nodular hyperplasia or local fatty and regenerative changes, is superior to that of B-scan or contrast-enhanced spiral CT[1,2,5,8,10].
The general problem for liver ultrasound still remains unchanged. The subdiaphragmatic liver segments IVa and VIII are difficult to visualize, which makes it very difficult to assess a lesion within these segments. Investigation of only part of the liver was possible owing to the high attenuation of ultrasound especially in the cranial and dorsal parts of the liver where the position of the liver was very high under the diaphragm or when patients had difficulty holding their breath. Secondly, fatty areas or cirrhosis of the liver, as well as lesions location deeper than 10 cm, diminish the penetration of contrast-specific imaging modes, which results in a decreased signal noise[1,5,8-10]. Another major problem for all imaging modalities such as contrast-enhanced CT or MRI remains the detection and characterization of lesions smaller than 5 mm[7].
SonoVue is a pure intravascular blood-pool contrast agent without a specific liver phase. The microbubbles help to visualize small parenchymal vessels. Malignant liver lesions such as metastases or HCC lesions are characterized by demarcation during the late phase. SonoVue late phase is based on visualization of parenchymal vessels. It is possible for very small HCC lesions, hemangiona or adenoma with vascular architecture comparable to that of the liver parenchyma to be missed in the detection study. Hypervascularization is a feature of HCC with a diameter > 2 cm. This may explain why the early phase of enhancement might not be effective for some very small HCC lesions. Early arterial enhancement can be found in 76%-96% of HCC lesions, and homogeneous enhancement in the late phase in 3%-30% of the patients[4,6,8,12,13].
The detection rate still remains examiner-dependent, even with low-MI contrast-agent techniques. In our study, we were able to demonstrate the feasibility of objective digital raw data analysis, which was not influenced by different examiners in our two-center approach. Therefore, dedicated software for computer-aided diagnosis can be developed, which allows the user an objective assessment of benign or malignant liver lesions based on digital raw data.
Contrast-enhanced ultrasound (CEUS) reveals typical patterns of contrast enhancement in the different lesion histotypes, and provides equivalent accuracy to CT and MRI in focal liver lesion characterization. CEUS should be considered in every patient with a known malignancy with proven or suspected liver metastases at baseline ultrasound during preoperative staging or postoperative follow-up.
Without dynamic evaluation of contrast enhancement, in approximately 10% of cases focal liver lesions remain indeterminate even after microbubble injection, and therefore Gadobenate-Dimeglumine (Gd-BOPTA) enhanced MRI should be employed, followed when necessary by ultrasound-guided biopsy. Standard cross-sectional imaging procedures including multislice CT or Gd-BOPTA-enhanced MRI should be referenced in every case not explorable by ultrasound, or in patients with no evidence of liver metastases at CEUS and with clinically suspected liver metastases, such as in cases with increased serum levels of tumor markers.
Using dynamic CHI, the malignancy of hepatic tumors can be predicted with a positive prognostic value of 93.5%. CHI with SonoVue in combination with dynamic quantitative evaluation of contrast-agent dynamics is a valuable tool for discrimination.
COMMENTS
Background
Liver lesions are a common diagnostic problem in medical imaging. Contrast-enhanced ultrasound is a recently introduced new modality in lesion assessment. In our study the quantitative evaluation of raw ultrasound data was assessed compared with histology as a gold standard.
Innovations and breakthroughs
Based on this raw data evaluation, new computer-assisted algorithms can be created, which allow automated liver lesion diagnosis.
Applications
For contrast-enhanced ultrasound-based lesion evaluation, three phases (arterial, portal-venous, and late phase) are mandatory. Computer-based dynamic analysis of raw digital ultrasound data facilitates lesion characterization.
Peer review
In this study, the authors review their experience of CHI and its diagnostic value in patients with space-occupying liver lesions. They conclude that using dynamic CHI, the malignancy of hepatic tumors can be predicted with a pV of 93.5%. CHI with SonoVue in combination with dynamic quantitative evaluation of contrast-agent dynamics is a valuable tool for discrimination.
Footnotes
S- Editor Zhu LH L- Editor Kerr C E- Editor Lu W
References
- 1.Hohmann J, Albrecht T, Oldenburg A, Skrok J, Wolf KJ. Liver metastases in cancer: detection with contrast-enhanced ultrasonography. Abdom Imaging. 2004;29:669–681. doi: 10.1007/s00261-004-0175-6. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht T, Blomley MJ, Burns PN, Wilson S, Harvey CJ, Leen E, Claudon M, Calliada F, Correas JM, LaFortune M, et al. Improved detection of hepatic metastases with pulse-inversion US during the liver-specific phase of SHU 508A: multicenter study. Radiology. 2003;227:361–370. doi: 10.1148/radiol.2272011833. [DOI] [PubMed] [Google Scholar]
- 3.Bartolozzi C, Donati F, Cioni D, Procacci C, Morana G, Chiesa A, Grazioli L, Cittadini G, Cittadini G, Giovagnoni A, et al. Detection of colorectal liver metastases: a prospective multicenter trial comparing unenhanced MRI, MnDPDP-enhanced MRI, and spiral CT. Eur Radiol. 2004;14:14–20. doi: 10.1007/s00330-003-1966-9. [DOI] [PubMed] [Google Scholar]
- 4.Boozari B, Lotz J, Galanski M, Gebel M. Diagnostic imaging of liver tumours. Current status. Internist (Berl) 2007;48:8, 10–2, 14-6, 18-20. doi: 10.1007/s00108-006-1773-x. [DOI] [PubMed] [Google Scholar]
- 5.Dietrich CF, Kratzer W, Strobe D, Danse E, Fessl R, Bunk A, Vossas U, Hauenstein K, Koch W, Blank W, et al. Assessment of metastatic liver disease in patients with primary extrahepatic tumors by contrast-enhanced sonography versus CT and MRI. World J Gastroenterol. 2006;12:1699–1705. doi: 10.3748/wjg.v12.i11.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 7.Bleuzen A, Huang C, Olar M, Tchuenbou J, Tranquart F. Diagnostic accuracy of contrast-enhanced ultrasound in focal lesions of the liver using cadence contrast pulse sequencing. Ultraschall Med. 2006;27:40–48. doi: 10.1055/s-2005-858944. [DOI] [PubMed] [Google Scholar]
- 8.von Herbay A, Haeussinger D, Gregor M, Vogt C. Characterization and detection of hepatocellular carcinoma (HCC): comparison of the ultrasound contrast agents SonoVue (BR 1) and Levovist (SH U 508A)--comparison of SonoVue and Levovist in HCC. Ultraschall Med. 2007;28:168–175. doi: 10.1055/s-2007-963070. [DOI] [PubMed] [Google Scholar]
- 9.Konopke R, Bunk A, Kersting S. The role of contrast-enhanced ultrasound for focal liver lesion detection: an overview. Ultrasound Med Biol. 2007;33:1515–1526. doi: 10.1016/j.ultrasmedbio.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Oldenburg A, Hohmann J, Foert E, Skrok J, Hoffmann CW, Frericks B, Wolf KJ, Albrecht T. Detection of hepatic metastases with low MI real time contrast enhanced sonography and SonoVue. Ultraschall Med. 2005;26:277–284. doi: 10.1055/s-2005-858526. [DOI] [PubMed] [Google Scholar]
- 11.D'Onofrio M, Rozzanigo U, Masinielli BM, Caffarri S, Zogno A, Malagò R, Procacci C. Hypoechoic focal liver lesions: characterization with contrast enhanced ultrasonography. J Clin Ultrasound. 2005;33:164–172. doi: 10.1002/jcu.20111. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa S, Kumada T, Toyoda H, Ichikawa H, Kawachi T, Otobe K, Hibi T, Takeshima K, Kiriyama S, Sone Y, et al. Evaluation of pathological features of hepatocellular carcinoma by contrast-enhanced ultrasonography: comparison with pathology on resected specimen. Eur J Radiol. 2006;59:74–81. doi: 10.1016/j.ejrad.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Wu W, Chen MH, Yin SS, Yan K, Fan ZH, Yang W, Dai Y, Huo L, Li JY. The role of contrast-enhanced sonography of focal liver lesions before percutaneous biopsy. AJR Am J Roentgenol. 2006;187:752–761. doi: 10.2214/AJR.05.0535. [DOI] [PubMed] [Google Scholar]
- 14.Krix M, Plathow C, Essig M, Herfarth K, Debus J, Kauczor HU, Delorme S. Monitoring of liver metastases after stereotactic radiotherapy using low-MI contrast-enhanced ultrasound--initial results. Eur Radiol. 2005;15:677–684. doi: 10.1007/s00330-004-2620-x. [DOI] [PubMed] [Google Scholar]
- 15.Regge D, Campanella D, Anselmetti GC, Cirillo S, Gallo TM, Muratore A, Capussotti L, Galatola G, Floriani I, Aglietta M. Diagnostic accuracy of portal-phase CT and MRI with mangafodipir trisodium in detecting liver metastases from colorectal carcinoma. Clin Radiol. 2006;61:338–347. doi: 10.1016/j.crad.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Mamdani B. The Helsinki Declaration, 2000, and ethics of human research in developing countries. Indian J Med Ethics. 2004;1:94–95. doi: 10.20529/IJME.2004.046. [DOI] [PubMed] [Google Scholar]
- 17.Touitou Y, Smolensky MH, Portaluppi F. Ethics, standards, and procedures of animal and human chronobiology research. Chronobiol Int. 2006;23:1083–1096. doi: 10.1080/07420520601055308. [DOI] [PubMed] [Google Scholar]
- 18.Linnert K. A Review on the methodology for assessing diagnostic tests. Clin Chem. 1988;34:1379–1386. [PubMed] [Google Scholar]
- 19.Delorme S, Krix M, Albrecht T. Ultrasound contrast media--principles and clinical applications. Rofo. 2006;178:155–164. doi: 10.1055/s-2005-858648. [DOI] [PubMed] [Google Scholar]
- 20.Krix M, Plathow C, Kiessling F, Herth F, Karcher A, Essig M, Schmitteckert H, Kauczor HU, Delorme S. Quantification of perfusion of liver tissue and metastases using a multivessel model for replenishment kinetics of ultrasound contrast agents. Ultrasound Med Biol. 2004;30:1355–1363. doi: 10.1016/j.ultrasmedbio.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Quaia E. Microbubble ultrasound contrast agents: an update. Eur Radiol. 2007;17:1995–2008. doi: 10.1007/s00330-007-0623-0. [DOI] [PubMed] [Google Scholar]
- 22.Quaia E, Bartolotta TV, Midiri M, Cernic S, Belgrano M, Cova M. Analysis of different contrast enhancement patterns after microbubble-based contrast agent injection in liver hemangiomas with atypical appearance on baseline scan. Abdom Imaging. 2006;31:59–64. doi: 10.1007/s00261-005-0358-9. [DOI] [PubMed] [Google Scholar]
- 23.Kim SH, Lee JM, Lee JY, Han JK, An SK, Han CJ, Lee KH, Hwang SS, Choi BI. Value of contrast-enhanced sonography for the characterization of focal hepatic lesions in patients with diffuse liver disease: receiver operating characteristic analysis. AJR Am J Roentgenol. 2005;184:1077–1084. doi: 10.2214/ajr.184.4.01841077. [DOI] [PubMed] [Google Scholar]
- 24.Quaia E, Palumbo A, Rossi S, Degobbis F, Cernic S, Tona G, Cova M. Comparison of visual and quantitative analysis for characterization of insonated liver tumors after microbubble contrast injection. AJR Am J Roentgenol. 2006;186:1560–1570. doi: 10.2214/AJR.05.0527. [DOI] [PubMed] [Google Scholar]
- 25.Leen E, Ceccotti P, Kalogeropoulou C, Angerson WJ, Moug SJ, Horgan PG. Prospective multicenter trial evaluating a novel method of characterizing focal liver lesions using contrast-enhanced sonography. AJR Am J Roentgenol. 2006;186:1551–1559. doi: 10.2214/AJR.05.0138. [DOI] [PubMed] [Google Scholar]
- 26.Vilana R, Bianchi L, Varela M, Nicolau C, Sánchez M, Ayuso C, García M, Sala M, Llovet JM, Bruix J, et al. Is microbubble-enhanced ultrasonography sufficient for assessment of response to percutaneous treatment in patients with early hepatocellular carcinoma? Eur Radiol. 2006;16:2454–2462. doi: 10.1007/s00330-006-0264-8. [DOI] [PubMed] [Google Scholar]
- 27.Quaia E, D'Onofrio M, Palumbo A, Rossi S, Bruni S, Cova M. Comparison of contrast-enhanced ultrasonography versus baseline ultrasound and contrast-enhanced computed tomography in metastatic disease of the liver: diagnostic performance and confidence. Eur Radiol. 2006;16:1599–1609. doi: 10.1007/s00330-006-0192-7. [DOI] [PubMed] [Google Scholar]
- 28.Bryant TH, Blomley MJ, Albrecht T, Sidhu PS, Leen EL, Basilico R, Pilcher JM, Bushby LH, Hoffmann CW, Harvey CJ, et al. Improved characterization of liver lesions with liver-phase uptake of liver-specific microbubbles: prospective multicenter study. Radiology. 2004;232:799–809. doi: 10.1148/radiol.2323030596. [DOI] [PubMed] [Google Scholar]
- 29.Delorme S, Krix M, Albrecht T. Ultrasound contrast media--principles and clinical applications. Rofo. 2006;178:155–164. doi: 10.1055/s-2005-858648. [DOI] [PubMed] [Google Scholar]
- 30.Jung EM, Kubale R, Jungius KP, Jung W, Lenhart M, Clevert DA. Vascularization of liver tumors - preliminary results with Coded Harmonic Angio (CHA), phase inversion imaging, 3D power Doppler and contrast medium-enhanced B-flow with second generation contrast agent (Optison) Clin Hemorheol Microcirc. 2006;34:483–497. [PubMed] [Google Scholar]
- 31.Jung EM, Kubale R, Jungius KP. Vascularization and perfusion of hepatocellular carcinoma: assessment with contrast-enhanced ultrasound using perflutren protein-type A microspheres. Clin Hemorheol Microcirc. 2005;33:63–73. [PubMed] [Google Scholar]