Abstract
Intermittent preventive treatment of malaria during pregnancy (IPTp) and insecticide-treated nets (ITN) are recommended malaria interventions during pregnancy; however, there is limited information on their efficacy in areas of low malaria transmission in sub-Saharan Africa.
An individually-randomised placebo-controlled trial involving 5775 women of all parities examined the effect of IPTp, ITNs alone or ITNs used in combination with IPTp on maternal anaemia and low birth weight (LBW) in a highland area of southwestern Uganda. The overall prevalence of malaria infection, maternal anaemia and LBW was 15.0%, 14.7% and 6.5%, respectively. Maternal and foetal outcomes were generally remarkably similar across all intervention groups (p>0.05 for all outcomes examined). A marginal difference in maternal Hb was observed in the dual intervention group (12.57 g/dL) compared with the IPTp and ITN alone groups (12.40 g/dL and 12.44 g/dL respectively; p=0.04), but this was too slight to be of clinical importance.
In conclusion, none of the preventive strategies was found to be superior to the others, and no substantial additional benefit to providing both IPTp and ITNs during routine antenatal services was observed. With ITNs offering a number of advantages over IPTp, yet showing comparable efficacy, we discuss why ITNs could be an appropriate preventive strategy for malaria control during pregnancy in areas of low and unstable transmission.
Trial Registration: www.ClinicalTrials.gov NCT0042207
Keywords: malaria prevention, pregnancy, intermittent preventive treatment, insecticide-treated nets, low transmission, highlands
Introduction
Malaria in pregnancy remains a major public health problem in sub-Saharan Africa. It is associated with low birth weight (LBW), intrauterine growth retardation and maternal anaemia.1-5 In addition to anaemia, malaria may contribute to maternal mortality by increasing the risk and severity of obstetric conditions such as pre-eclampsia/eclampsia and postpartum haemorrhage.6 In areas of low transmission, where women have little acquired immunity, malaria infections in pregnancy are more likely to give rise to clinical symptoms and may result directly in the loss of the foetus and/or maternal death.7,8 Malaria is also associated with an increased risk of stillbirth, with a seasonal pattern of higher incidence of stillbirths after peaks in rainfall.9 Dramatic increases in the risk of stillbirth have been seen during epidemics.9,10
Intermittent preventive treatment of malaria in pregnancy (IPTp) with sulfadoxine/pyrimethamine (SP) as well as insecticide-treated nets (ITN) are recommended methods of malaria control in pregnancy in areas of stable transmission in sub-Saharan Africa.11 LBW and maternal anaemia are key indicators for monitoring malaria intervention programmes in pregnancy,12 and have been shown to significantly improve with use of either IPTp3,13,14 or ITNs.15 Less information exists on the efficacy of both interventions together.16-18 Most trials have been conducted in areas of high malaria transmission, with very few studies undertaken in areas of low, unstable or highly seasonal transmission,8,19 and thus no specific guidance on malaria control during pregnancy is currently available for areas of low or unstable transmission in Africa.
A randomized trial was undertaken in the highlands of southwestern Uganda to evaluate the effect of IPTp and ITNs (used alone or in combination with IPTp) on maternal anaemia and LBW as well as to investigate the comparative cost-effectiveness of the three interventions in an area of low and unstable malaria transmission. This paper reports the effect of the interventions on maternal and foetal outcomes.
Materials and Methods
Study site and population
The study was conducted in Kabale district, a highland district, situated in southwestern Uganda, lying at an altitude between 1,219m and 2,347m above sea level. The majority of the district lies within the malaria epidemic-prone zone. According to the 2002 census, the population of Kabale district was 461,785 people, with a population density of 290 persons/km2. The temperature is fairly constant throughout the year, with maximum daily temperatures of 23.2 - 24.4 °C, dropping to a minimum of 9.8 - 12.6°C at night. Rainfall is seasonally bimodal, averaging between 1,000 and 1,480 mm per annum. Malaria transmission is low and unstable, with the entomological inoculation rate previously estimated at 0.41 infective bites/person/year.20 Malaria transmission occurs most years, usually following the seasonal pattern of rainfall which peaks during April-June and September-November. The intensity and extent of transmission varies from year to year resulting in periodic epidemics which can be associated with high morbidity and mortality rates, and an increased risk of stillbirths.10 Pregnant women were recruited at 10 rural health units, serving the 47 parishes included in the study area, covering approximately one-half the district. Each health unit was staffed by a qualified midwife.
Study design
An individually-randomized placebo-controlled three-arm intervention trial was conducted to examine the efficacy of three preventive approaches in improving pregnancy outcomes. The effect of using (i) ITNs + placebo; and (ii) ITNs in combination with IPTp (dual intervention) were compared to (iii) use of IPTp alone (current practice comparator). For ethical reasons, there was no placebo arm; all women received one or more preventive interventions against malaria. The primary trial endpoints were the prevalence of LBW and maternal anaemia. An independent data safety monitoring board oversaw implementation of the trial and approved the analytical plan prior to analysis. Analysis for all primary endpoints was completed before the data were unmasked.
Enrolment and administration of the interventions
Community sensitisation was carried out to inform local communities of the risks of malaria in pregnancy, the benefits of early attendance to antenatal care (ANC), and the research that would be taking place in local clinics. Women of all parities whose pregnancies were < 27 weeks gestation at first antenatal presentation were eligible for inclusion in the study. After written informed consent was obtained at first ANC attendance, women were individually assigned to one of the three study groups, using a computer-generated random number list with individual sealed envelopes (arranged in blocks of 12). Tablets of SP or placebo, identical in shape and colour, were obtained from Kampala Pharmaceutical Industries (Kampala, Uganda) and were stored in tins labeled only with a single identification letter corresponding to the study group. Solubility and drug content were confirmed using high performance liquid chromatography (HPLC). All study participants, health staff and researchers were blind to drug assignment (SP or placebo), but not to ITN use.
The first treatment dose of SP (or placebo) was given once gestational age was ≥ 16 weeks and women reported foetal movement, or at enrolment if later. Assessment of gestational age was made by bimanual palpation and use of a fundal height tape to estimate fundal height at each antenatal visit. A pregnancy test was administered when the fundus was not palpable. Women with a confirmed pregnancy of < 16 weeks gestation were enrolled into the study and were advised to return for their first treatment dose of SP as soon as they sensed any foetal movement. A second treatment dose was given in the third trimester of pregnancy (between gestational weeks 28 and 34). All doses of IPTp were given under conditions of directly observed treatment. Home visitors reminded women when to re-attend for their second dose of IPTp, as well as at week 36 for haemoglobin (Hb) assessment and delivery advice. Women were also encouraged to present to the health unit in the event of any fever or other illness during pregnancy. Study cards with a unique identification number facilitated identification at every contact throughout the study.
Women who were randomised to receive an ITN [(study arms (i) and (ii)] received a treated net, and instructions on its use at the time of enrolment. Nets were pre-treated with KO-tab® (deltamethrin) by study staff. Women were visited at home shortly after enrolment to advise them on use and care of the net and verify that it was being used. Women in the current practice arm (iii) received an ITN after delivery.
Exclusion criteria
Women presenting with gestational age >26 weeks at first antenatal booking, those who were not resident in the study area, visitors and temporary residents were not eligible for enrolment. Other exclusion criteria included history of adverse drug reaction, or severe disease (such as hepatitis, jaundice, tuberculosis, obvious AIDS symptoms). Women were screened for anaemia at first antenatal booking; women with severe anaemia (Hb<7.0g/dl) were treated with a full course of iron and folic acid, and excluded from enrolment. All women excluded from the trial received IPTp with SP, as per current practice.
Maternal and infant health outcomes
Primary maternal health outcomes were prevalence of maternal anaemia (Hb <11.0g/dl), and mean Hb concentration at 36-40 weeks gestation. Hb concentration was measured using a HemoCue photometer (HemoCue Corp., Angelholm, Sweden). Blood films were also prepared for malaria parasite examination at last ANC visit between 36-40 weeks gestation, as well as in any episode of suspected febrile illness presenting to the health unit. Peripheral and placental blood smears were taken at delivery. Blood slides were stained with Giemsa and all were read independently by two research technicians and were only declared negative after 200 high-power fields had been examined. In the case of discrepant slides, the results of microscopy performed by a senior technician and trainer with many years experience of reading malaria slides was considered definitive. A clinical episode of malaria was defined as a presence of malaria parasites in a peripheral blood film in a woman presenting with a history of fever and/or an axillary temperature ≥ 37.5°C. Evidence of any maternal malaria infection was defined as the presence of asexual parasites of any density detected in at least one maternal blood film taken at the end of pregnancy, including peripheral blood films taken at antenatal attendance between 36-40 weeks gestation, peripheral blood films taken at delivery, and blood films prepared from the placenta.
Primary infant health outcomes were prevalence of LBW (< 2500g) and mean birth weight. Other infant outcomes included numbers of abortions, stillbirths, premature births and neonatal deaths. Newborns were weighed on a digital scale, accurate to the nearest 10g. A blood sample was taken from the heel of infants at birth to measure neonatal Hb and prepare a blood film for malaria parasite examination. Midwives estimated gestational age at birth using the Ballard external criteria21,22 for all babies delivered at a health unit. Babies that were not delivered at a health unit were visited and weighed at home by a home visitor, and a blood film was prepared from mother, baby and placenta where possible. Mother and newborn were visited every week for the first 28 days to capture data on early and late neonatal mortality and/or maternal deaths.
Sample size calculations
Sample size was determined to detect a reduction in frequency of maternal anaemia at week 36, LBW and stillbirths. Data from an earlier study in Kenya,16 which showed an additive effect of ITNs on anaemia and an improvement in protective efficacy (PE) from 50% (IPTp alone) to 60% (IPTp+ITN), were used to inform sample size calculations. The additive effect was expected to be greater in a low transmission setting, and we further considered that an improvement in PE of <20% (increase in protective efficacy from 50% to 70%) to be of negligible public health importance to detect. Assuming the prevalence of maternal anaemia in the absence of intervention is 25%,19 the minimum sample size needed to detect a difference, assuming the prevalence of maternal anaemia after the intervention was 12.5% in the IPTp alone arm (PE=50%) and 7.5% in the dual intervention arm (IPTp+ITN; PE=70%), was 603 women in each arm (significance level of 0.05 and power of 0.80). The other study outcomes (frequency of LBW and stillbirths) are less common10 and required a larger sample size to detect a difference in PE between the intervention groups. A minimum sample size of 1604 women per arm was required to detect a difference if the prevalence of LBW after the intervention was 5% in the IPTp alone arm (PE=50%) and 3% in the dual intervention arm (PE=70%).
Data analysis
Data were analysed according to modified intention-to-treat, whereby study outcomes in all women were included regardless of whether they had received the intervention and the intended number of doses. Women who were found to have enrolled at more than one site, to be non-resident within the study area (enrollment errors) or to have attended ANC outside the study area were regarded as protocol violations and were removed from the dataset before analysis. Principal components analysis was used to generate an index of socioeconomic status based on ownership of household and agricultural assets ascertained during household surveys, and used to categorise women to wealth quintiles.23 The location of each household was mapped using a handheld Geko™ 201 global positioning system (Garmin, Olathe, Kansas, USA), which has an accuracy of ±15m, and used to estimate the elevation of residence using a digital elevation model for the study area.24 Birth weights, which were measured 1-7 days after birth, were adjusted to account for the expected changes in weight within a few days of birth25 using an estimate of daily mean weight change in a separate cohort of 100 non-study babies born in Kabale and weighed every day for the first 7 days after birth. Study babies weighed >7 days after birth were coded as missing data and were excluded from analysis of birth weight. Multiple births (twins) were excluded from all analyses of foetal outcomes, including LBW and preterm births.
Summary statistics were used initially to describe the study sample. These included number in each arm of the study and follow-up status, e.g. number of non-missing endpoint measurements, information on missing data and characteristics of women in each intervention arm at enrollment using information from antenatal booking record and household questionnaire. Data were examined to identify any imbalances in covariates and potential confounders between the three study arms (randomisation failure). Pre-specified covariates to consider in an adjusted analysis included recognised risk factors for LBW and maternal anaemia, such as maternal age, parity, education and nutritional status; as well as factors associated with exposure to malaria, including altitude of residence and use of an ITN outside the trial protocol.
The means of birth weight and Hb concentration in the three treatment arms were compared by Student’s t-test and one-way ANOVA. Proportions were compared using Chi square and P-values < 0.05 were considered significant in all tests. In highland area, altitudinal decrease in ambient temperature is the primary limiting factor for malaria transmission, such that the altitudinal zone between 1500m and 1750m probably represents the fringes of stable transmission in Uganda.26 Thus, in addition to the main analysis of pregnancy endpoints, pre-specified exploratory analyses were undertaken to include data only from women living below 1800m, at highest likelihood of malaria exposure during pregnancy. Data were analysed using Stata 10 (StataCorp LP, College Station, Texas, USA).
Results
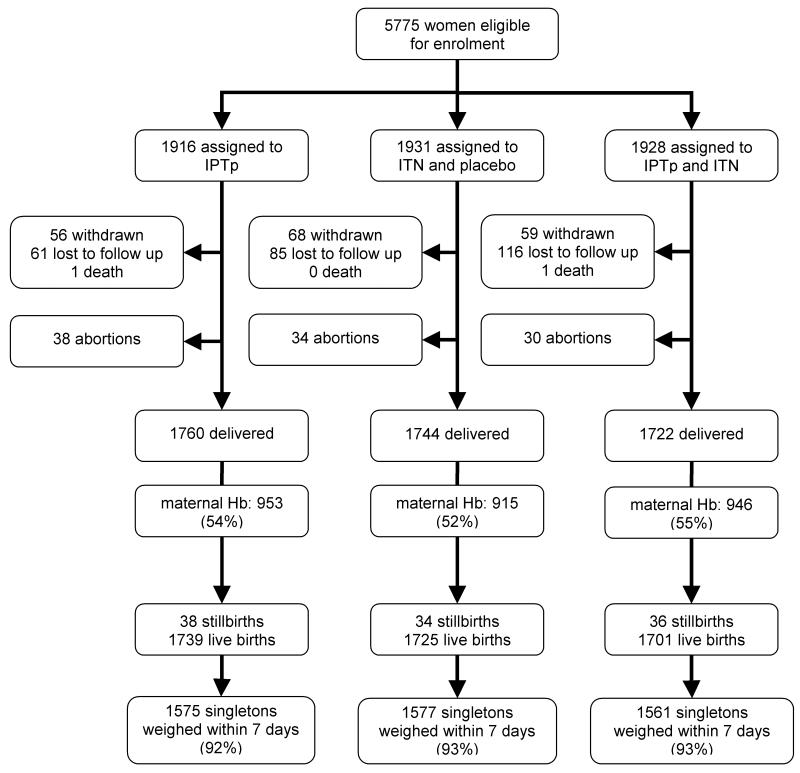
Overall, 5775 pregnant women were recruited into the study at their first ANC visit between 2004 and 2007, with 1916, 1931 and 1928 randomised to the IPTp, ITN and dual intervention (IPTp+ITN) groups, respectively (Figure 1). Retention rates were similar across the three intervention groups, with >90% of women evaluated at delivery. The main reasons for loss to follow-up were outmigration (51%); non-residence (19%) or ANC attendance outside the study area (10%). Twelve women withdrew consent and one woman experienced an adverse event after IPTp and was withdrawn from the study. Two women died before delivery, after 28 weeks and 36 weeks gestation in the IPTp and dual intervention groups, respectively. Verbal autopsy interviews revealed a history of general weakness and history of weight loss. Clinical reviews were inconclusive.
Figure 1.
Trial profile. IPTp: intermittent preventive treatment of malaria during pregnancy; ITN: insecticide-treated nets; Hb: haemoglobin
Baseline characteristics of the study women were comparable with respect to age, parity, Hb concentration at enrolment, nutritional and socioeconomic parameters, and altitude of residence (Table 1). The mean age of women enrolled in the study was 25.7 years (range 15-47 years), with 20% of women being primigravidae. Mean Hb concentration at enrolment was 12.16 g/dl. Altitude of residence ranged between 1367m and 2352m above sea level, with approximately one-half the study population living below 1800m in the altitudinal zone classified as ‘highland-fringe’ where the risk of malaria transmission is highest.26
Table 1.
Characteristics at enrollment of the 5328 women followed to terma
| Characteristic | IPTp (n = 1798) | ITN (n = 1778) | IPTp+ITN (n = 1752) | P-value |
|---|---|---|---|---|
| Age (years) (mean ± SD) | 25.55 ± 5.72 | 25.78 ± 5.56 | 25.82 ± 5.72 | 0.313 |
| Gestational age (weeks) (mean ± SD) | 18.21 ± 5.45 | 18.27 ± 5.70 | 18.10 ± 5.74 | 0.666 |
| Haemoglobin (g/dl) (mean ± SD) | 12.120 ± 1.522 | 12.157 ± 1.636 | 12.232 ± 1.567 | 0.104 |
| MUAC (cm) (mean ± SD) | 26.27 ± 2.41 | 26.28 ± 2.52 | 26.33 ± 2.57 | 0.710 |
| Gestational age (weeks) [n (%)] | ||||
| <20 | 942 (53) | 887 (50) | 926 (53) | 0.169 |
| 20 – 27 | 849 (47) | 883 (50) | 819 (47) | |
| Gravidity [n (%)] | ||||
| Primigravidae | 385 (21) | 347 (20) | 358 (20) | |
| Secundigravidae | 319 (18) | 321 (18) | 311 (18) | 0.829 |
| 2 -4 prior pregnancies | 691 (39) | 722 (41) | 696 (40) | |
| ≥ 5 prior pregnancies | 400 (22) | 386 (22) | 386 (22) | |
| Education [n (%)] | ||||
| None | 263 (15) | 228 (13) | 228 (13) | |
| Primary | 1353 (76) | 1366 (77) | 1301 (75) | 0.048 |
| Secondary | 172 (10) | 180 (10) | 213 (12) | |
| Socioeconomic quintile [n (%)] | ||||
| 1 (least poor) | 319 (18) | 372 (21) | 354 (20) | |
| 2 | 337 (19) | 362 (20) | 370 (21) | |
| 3 | 379 (21) | 354 (20) | 338 (19) | 0.067 |
| 4 | 382 (21) | 322 (18) | 345 (20) | |
| 5 (most poor) | 364 (20) | 361 (20) | 332 (19) | |
| MUAC index [n (%)] | ||||
| Normal (> 22cm) | 1644 (98) | 1603 (97) | 1572 (97) | 0.627 |
| Underweight (≤ 22cm) | 39 (2) | 47 (3) | 42 (3) | |
| Elevation of residence | ||||
| Mean ± SD (m) | 1788.28 ± 175.76 | 1782.06 ± 171.21 | 1780.80 ± 171.43 | 0.429 |
| <1500 m [n (%)] | 141 (9) | 142 (9) | 137 (9) | |
| 1500-1799 m [n (%)] | 547 (34) | 556 (35) | 566 (37) | 0.764 |
| ≥ 1800 m [n (%)] | 904 (57) | 883 (56) | 844 (55) |
IPTp: intermittent preventive treatment of malaria during pregnancy; ITN: insecticide-treated net; MUAC: mid-upper arm circumference.
The women who died before term (2), were withdrawn from the study (183) or were lost to follow-up (262) are excluded.
The three groups were also comparable with respect to mean gestational age at enrolment, and number of treatments received. The mean gestational age at recruitment was 18 weeks in all study groups (P=0.666), with over one-half the women presenting for antenatal booking before 20 weeks gestation. As a result, over 75% of the women received two or more doses of SP during pregnancy: (79.1%, 76.3% and 78.0% in the IPTp, ITN and dual intervention groups respectively; χ2 =4.36, df 2, P=0.113), amongst whom 16.2%, 15.2% and 14.4% of women, respectively, received three doses. Over 97% of women randomised to receive an ITN at enrolment used the net during pregnancy. In contrast, bed net use in the IPTp group was only 6%.
Hb was measured during ANC re-attendance visits at 36-40 weeks gestation and/or immediately prior to delivery. Owing to low antenatal re-attendance rates and high numbers of home births, Hb at 36-40 weeks gestation was only recorded for approximately 50% of the women (Figure 1). Completeness of maternal Hb recording was similar in all intervention groups (χ2 =2.31, df 2, P=0.315). However, recording of Hb differed between socioeconomic groups, ranging between 49 and 58%, with data being more complete for the least poor quintiles (χ2 =20.42, df 4, P<0.001). Birth weights were recorded within 7 days for over 92% of live singleton births in all three intervention groups (χ2 =1.70, df 2, P=0.427).
Pregnancy outcomes
The majority of women (70%) delivered in the village and only 30% delivered at a health unit. Six women died during or shortly after delivery due to obstetric causes (3), complications following a spontaneous abortion (1) and suicide due to puerperal psychosis (1); the cause of death was undetermined in one woman with a history of fever. Most women delivered successfully (>95% live births), though 2% of the pregnancies ended in spontaneous abortion and 2% of babies were stillborn. Pregnancy outcomes were similar across the three intervention groups (Table 2).
Table 2.
Pregnancy outcomes (n=5375)a
| IPTp [n (%)] | ITN [n (%)] | IPTp+ITN [n (%)] | P-value | |
|---|---|---|---|---|
| Pregnancy outcomesa | ||||
| Spontaneous Abortions | 38 (2.1) | 34 (1.9) | 30 (1.7) | 0.686 |
| Stillbirths | 38 (2.1) | 34 (1.9) | 36 (2.0) | 0.910 |
| Live births | 1739 (95.8) | 1725 (96.2) | 1701 (96.3) | 0.748 |
| Total | 1815 | 1793 | 1767 | - |
| Neonatal Outcomesa | ||||
| Early neonatal deaths (within first 7 days of life) | 25 (1.4) | 24 (1.4) | 36 (2.1) | 0.175 |
| Late neonatal deaths (≥ 8 and <29 days of life) | 9 (0.5) | 11 (0.6) | 2 (0.1) | 0.050 |
| Perinatal deaths (stillbirths + early neonatal deaths) | 63 (3.5) | 58 (3.2) | 72 (4.1) | 0.382 |
| Maternal deaths (intra- and post-partum deaths) | 1 (<0.1) | 2 (0.1) | 3 (0.2) | 0.590 |
| Congenital abnormalities | 6 (0.3) | 3 (0.2) | 5 (0.3) | 0.612 |
IPTp: intermittent preventive treatment of malaria during pregnancy; ITN: insecticide-treated net.
Includes multiple births.
Effect of the interventions on birth weight and other foetal outcomes
Overall, 4713 singleton live births were weighed within 7 days of birth, with an overall prevalence of LBW of 6.54%. There was no difference between the three intervention groups in either the prevalence of LBW (P=0.802) or mean birth weight (P=0.261) (Table 3). Birth weight and other study outcomes were strongly associated with altitude of residence but not parity (Table 4). Nevertheless, when analysis was restricted to include only women living below 1800m, the mean birth weight and prevalence of LBW between the three intervention groups remained similar (P=0.501 and P=0.928, respectively) (Table 3).
Table 3.
Low birth weight and secondary foetal outcomes according to study group: overall and according to altitude of residence and gravidity.
| IPTp | ITN | IPTp+ITN | P-value | |
|---|---|---|---|---|
| Low birth weighta (<2500g) [n/N (%)] | ||||
| Overall | 102 / 1575 (6.48) | 99 / 1577 (6.28) | 107 / 1561 (6.85) | 0.802 |
| By elevation | ||||
| < 1800 m | 48 / 630 (7.62) | 46 / 626 (7.35) | 48 / 605 (7.93) | 0.928 |
| ≥ 1800 m | 40 / 739 (5.41) | 40 / 738 (5.42) | 47 / 730 (6.44) | 0.625 |
| By gravidity | ||||
| Primigravidae | 16 / 313 (5.11) | 18 / 329 (5.47) | 27 / 333 (8.11) | 0.224 |
| Secundigravidae | 22 / 287 (7.67) | 16 / 277 (5.78) | 14 / 276 (5.07) | 0.417 |
| 2-4 prior pregnancies | 40 / 610 (6.56) | 37 / 631 (5.86) | 44 / 614 (7.17) | 0.648 |
| ≥ 5 prior pregnancies | 21 / 347 (6.05) | 27 / 325 (8.31) | 21 / 315 (6.67) | 0.500 |
| Mean ± SD birth weighta (SD) | ||||
| Overall | 3135.62 ± 430.41 | 3161.06 ± 452.68 | 3144.37 ± 444.15 | 0.261 |
| By elevation | ||||
| < 1800 m | 3107.21 ± 416.84 | 3135.07 ± 455.19 | 3112.33 ± 465.70 | 0.501 |
| ≥ 1800 m | 3166.44 ± 443.12 | 3181.38 ± 445.73 | 3149.37 ± 426.31 | 0.376 |
| By gravidity | ||||
| Primigravidae | 3162.58 ± 466.00 | 3174.84 ± 462.62 | 3124.48 ± 471.38 | 0.352 |
| Secundigravidae | 3123.04 ± 414.05 | 3167.02 ± 417.62 | 3192.50 ± 456.05 | 0.152 |
| 2-4 prior pregnancies | 3134.68 ± 435.38 | 3149.76 ± 455.88 | 3130.44 ± 433.80 | 0.719 |
| ≥ 5 prior pregnancies | 3124.26 ± 401.30 | 3162.80 ± 467.60 | 3152.74 ± 425.18 | 0.485 |
| Secondary foetal outcomesb | ||||
| Pre-term birth (<37 weeks) [n/N (%)] | 42 / 489 (8.59) | 42 / 480 (8.75) | 45 / 495 (9.09) | 0.961 |
| Mean ± SD gestational age at birth (weeks) | 37.39 ± 0.66 | 37.37 ± 0.91 | 37.40 ± 0.73 | 0.803 |
| Total no. babies examined for Hb | 321 | 329 | 332 | |
| Infant Hb at birth (g/dl) (mean ± SD) | 19.227 ± 2.566 | 19.641 ± 2.932 | 19.532 ± 2.856 | 0.148 |
IPTp: intermittent preventive treatment of malaria during pregnancy; ITN: insecticide-treated net; Hb: haemoglobin
Singleton live births weighed within 7 days of birth, adjusted for number of days after birth and growth
Gestational age at birth and neonatal Hb was only available for singleton live births delivered at a health unit.
Table 4.
Effect of gravidity and elevation of residence on study outcomes
| Any malaria infectiona [n/N (%)] | Maternal anaemia [n/N (%)] | Low birth weight [n/N (%)] | Mean ± SD birth weight | |
|---|---|---|---|---|
| Elevation | ||||
| <1700 m | 175 / 999 (17.52) | 172 / 746 (23.06) | 88 / 1028 (8.56) | 3085.14 ± 446.64 |
| 1700-1799 m | 151 / 861 (17.54) | 40 / 490 (8.16) | 54 / 833 (6.48) | 3159.10 ± 442.17 |
| 1800-1899 m | 205 / 1439 (14.25) | 88 / 793 (11.10) | 80 / 1421 (5.65) | 3180.73 ± 435.94 |
| ≥ 1900 m | 103 / 835 (12.34) | 59 / 443 (13.32) | 47 / 786 (5.98) | 3138.77 ± 442.11 |
| P-value | 0.003 | <0.001 | 0.028 | <0.001 |
| Gravidity | ||||
| Primigravidae | 141 / 982 (14.36) | 80 / 613 (13.05) | 61 / 975 (6.26) | 3153.71 ± 466.73 |
| Secundigravidae | 133 / 856 (15.54) | 74 / 501 (14.77) | 52 / 840 (6.19) | 3160.37 ± 429.90 |
| 2-4 prior pregnancies | 277 / 1858 (14.91) | 168 / 1074 (15.64) | 121 / 1855 (6.52) | 3138.41 ± 441.79 |
| ≥ 5 prior pregnancies | 159 / 1026 (15.50) | 91 / 618 (14.72) | 69 / 987 (6.99) | 3146.04 ± 431.52 |
| P-value | 0.870 | 0.554 | 0.892 | 0.639 |
Presence of malaria parasites in one or more blood films taken at the end of pregnancy, including peripheral blood films taken at antenatal attendance between 36-40 weeks gestation, peripheral blood films taken at delivery and blood films prepared from the placenta.
Likewise, there was no difference between the intervention groups in either the prevalence of prematurity (P=0.961) or mean gestational age at birth (P=0.803) in the subgroup of babies delivered at a health unit and assessed by a midwife (Table 3). Status at 28 days after birth was available for the majority of live births (99.6%), in whom the overall neonatal death rate was 20.80 per 1000 live births. The total number of neonatal deaths was similar in the IPTp, ITN and dual intervention groups, with 34, 35 and 38 neonatal deaths recorded, respectively (Table 2).
Effect of the interventions on maternal anaemia
Maternal Hb measured at 36 weeks of gestation or before delivery was available for 2814 of the 5328 women with delivery outcomes (53%, 51% and 54% of women in the IPTp, ITN and dual intervention groups, respectively; P=0.315). There was no difference in the prevalence of maternal anaemia (Hb < 11.0 g/dl) between the three intervention groups, which ranged from 14% to 16% across the three groups (P=0.234) (Table 5). Severe-to-moderate anaemia (Hb < 8.0 g/dl) was observed in 21 women (0.75%), comprising 7 in each group (χ2 =0.01, df 2, P=0.997). Mean Hb concentration was marginally higher in the dual intervention group, being recorded as 12.40, 12.44, and 12.57 g/dl in the IPTp, ITN and dual intervention groups, respectively (P=0.040) (Table 5). When analysis was restricted to include only women living below 1800m, the differences in mean Hb between the three intervention groups remained marginal and did not reach significance (P=0.125) (Table 5).
Table 5.
Maternal anaemia and parasitological outcomes by study groupa: Overall effect and categorized by altitude of residence.
| IPTp | ITN | IPTp+ITN | P-value | |
|---|---|---|---|---|
| Maternal anaemia (< 11.0 g/dl) [n/N (%)] | ||||
| Overall | 135 / 953 (14.17) | 149 / 915 (16.28) | 129 / 946 (13.64) | 0.234 |
| Elevation | ||||
| < 1800 m | 73 / 434 (16.82) | 80 / 402 (19.90) | 59 / 400 (14.75) | 0.150 |
| ≥ 1800 m | 48 / 424 (11.32) | 53 / 400 (13.25) | 46 / 412 (11.17) | 0.593 |
| Mean maternal haemoglobin (g/dl) (mean ± SD) | ||||
| Overall | 12.395 ± 1.471 | 12.441 ± 1.561 | 12.567 ± 1.557 | 0.040 |
| Elevation | ||||
| < 1800 m | 12.289 ± 1.403 | 12.252 ± 1.602 | 12.454 ± 1.492 | 0.125 |
| ≥ 1800 m | 12.501 ± 1.467 | 12.574 ± 1.487 | 12.725 ± 1.505 | 0.085 |
| Parasitological outcomes [n/N (%)] | ||||
| Peripheral parasitaemia at weeks 36-40 | 83 / 883 (9.40) | 60 / 841 (7.13) | 75 / 853 (8.79) | 0.219 |
| Peripheral parasitaemia at delivery | 157 / 1431 (10.97) | 148 / 1431 (10.34) | 160 / 1377 (11.62) | 0.557 |
| Placental parasitaemia | 28 / 651 (4.30) | 16 / 622 (2.57) | 19 / 613 (3.10) | 0.211 |
| No. of suspected malaria cases | 38 | 30 | 35 | |
| Slide-positive clinical malaria | 11 / 38 (28.95) | 7 / 30 (23.33) | 10 / 35 (28.57) | 0.853 |
| Any malaria infection at end of pregnancyb [n/N (%)] | ||||
| Overall | 254 / 1606 (15.82) | 212 / 1580 (13.42) | 246 / 1550 (15.87) | 0.088 |
| Elevation | ||||
| < 1800 m | 122 / 648 (18.83) | 95 / 602 (15.78) | 109 / 610 (17.87) | 0.354 |
| ≥ 1800 m | 105 / 779 (13.48) | 98 / 779 (12.58) | 105 / 716 (14.66) | 0.499 |
| Congenital parasitaemia | 46 / 1351 (3.40) | 45 / 1331 (3.38) | 32 / 1298 (2.47) | 0.284 |
IPTp: intermittent preventive treatment of malaria during pregnancy; ITN: insecticide-treated net.
Outcome data were not available for all womenowing to a lack of re-attendance late in pregnancy and delivery at home. Blood films were prepared from all women and babies delivering at a health unit and, where possible, those born at home.
Presence of malaria parasites in one or more blood films taken at the end of pregnancy, including peripheral blood films taken at antenatal attendance between 36-40 weeks gestation, peripheral blood films taken at delivery and blood films prepared from the placenta.
Effect of the interventions on parasitaemia
Overall, peripheral parasitaemia measured at 36-40 weeks of gestation or at delivery was available for 87% of the 5328 women with delivery outcomes (88%, 88% and 86% of women in the IPTp, ITN and dual intervention groups, respectively; χ2 =2.71, df 2, P=0.258). There was no difference in the prevalence of peripheral parasitaemia between the three intervention groups, which ranged between 7% and 9% across the three groups at weeks 36-40, and between 10% and 12% at delivery (P=0.219 and P=0.557 respectively) (Table 5). Similarly, there was no difference between the intervention groups in the prevalence of placental parasitaemia in the subgroup of women who delivered at a health unit (P=0.211). An additional 103 slides were taken from study women presenting with suspected malaria during pregnancy, of which 28 were found to be parasite-positive. Slide positivity in suspected malaria cases did not differ between the three intervention groups (P=0.853). The prevalence of congenital parasitaemia was also comparable between the three intervention groups (P=0.284).
When slide data were combined, such that the presence of at least one positive placental or peripheral blood film taken at the end of pregnancy was considered evidence of a maternal infection, the proportion of women with maternal parasitaemia increased to 15.0% overall, with 712 of the 4736 women examined having at least one positive slide. Maternal malaria infection was strongly associated with altitude of residence in this highland area, decreasing from 17.5% among women living below 1700m to 12.3% among women living above 1900m (P=0.003) (Table 4). Nonetheless, when analysis was restricted to include only women living at lower elevations, the proportion of women with any evidence of maternal parasitaemia in each of the three intervention groups remained comparable (P=0.354) (Table 5).
Discussion
This randomised placebo-controlled trial found no evidence of a difference in effect on LBW or maternal anaemia between three malaria preventive strategies during pregnancy using either IPTp alone (current practice), ITNs alone or a dual intervention with ITNs used in combination with IPTp in an area of low and unstable malaria transmission in Uganda. A marginal difference was observed in maternal Hb of 0.2 g/dl, which reached statistical significance (P=0.04) but is unlikely to be of clinical significance. No difference was observed in the prevalence of maternal anaemia or LBW. Indeed, across all the trial endpoints examined the prevalence of maternal and foetal outcomes was remarkably similar in all intervention groups. Thus, no evidence was found to indicate that any one preventive strategy was superior to prevent the deleterious impact of malaria during pregnancy in areas of low and unstable transmission. Moreover, no evidence was observed of any additional benefit to providing both IPTp and ITNs through routine antenatal services.
Preventive programmes delivered through ANC frequently face challenges due to late ANC attendance and non-compliance,27 which can limit the impact of interventions in pregnancy and give rise to a null result. However, in this study the lack of difference between the interventions cannot be explained by late or irregular attendance since most women attended ANC early due to community sensitisation carried out prior to the start of the study. Mean gestational age at enrollment was 18 weeks, with a substantial proportion presenting within the first 20 weeks of gestation, early enough for the interventions to have an impact on the study outcomes and to maximize the potential benefit of IPTp and ITNs delivered through ANC.8,28,29 Neither can poor compliance explain the lack of difference among the interventions on study outcomes, since more than 75% of women received two or more doses of IPTp in all the intervention groups, and ITN use among women randomised to receive a net was > 97%. In contrast, ITN use among women in the current practice group was low (< 10%). There are no contemporary data on SP resistance in the study area. The most recent study conducted in October 2001 reported 18.5% RI parasitological resistance to SP after 14 days of follow-up in malaria patients aged between 2-42 years (no RII or RIII resistance),30 but resistance may well have increased in the intervening period. Nevertheless, we do not believe that SP resistance compromised the efficacy of IPTp in this study since study outcomes in the group of women who only received IPTp with SP were no worse than among the two intervention groups in which women used ITNs. Although baseline characteristics of women enrolled into the three intervention groups were comparable and losses to follow-up were generally low, Hb at weeks 36-40 was only obtained for 50% of the enrolled women, with completeness of recording differing according to socioeconomic status and thus possibly subject to participation bias. In contrast, data on birth weight was recorded for > 90% of women enrolled into the study. Therefore, we can be confident that the lack of difference in LBW was not due to randomisation failure, non-compliance, suboptimal delivery, differential loss to follow-up or participation bias.
The entomological inoculation rate in the district is low, previously estimated at 0.41 infective bites/person per year between 1700 and 1960m elevation,20 though with marked geographical variation between villages31. A potential consideration is thus whether the level of malaria transmission was too low to impact on pregnancy outcomes. This is particularly problematic to evaluate since for ethical reasons there was no placebo arm, and all women received a preventive intervention against malaria. The probability of exposure will have differed between study subjects since the elevation at which women lived ranged between 1367 m and 2352 m above sea level, spanning the altitudinal limits of stable transmission in Uganda,26 and elevation of residence was strongly correlated with maternal parasitaemia, maternal anaemia and birth weight in this study population. Birth outcomes in women who are not exposed to malaria will not be affected by malaria interventions, and inclusion of data from these women in the analysis could dilute any observed effects of the interventions. Within the study population, the proportion of women with any evidence of malaria parasitaemia was found to be 15.0% overall, with the highest levels of infection seen in women living below 1700m in whom the proportion with parasitaemia increased to 17.5%. The difficulties of detecting malaria infection during pregnancy owing to placental sequestration are well recognised, and microscopy can significantly underestimate the true prevalence of infection in the study population.32 Owing to the high proportion of deliveries at home, we were rarely able to obtain placental samples, neither did we undertake placental histology. Nevertheless, the levels of parasitaemia observed in our study were not dissimilar to the 15% peripheral parasitaemia recorded in an area of moderate perennial transmission in Mozambique where the true level of maternal infection during pregnancy was shown to be 51% according to placental histology.18 Despite the limitations of light microscopy and peripheral blood films, we thus believe the parasitological findings from pregnant women in Kabale indicate that significant levels of malaria transmission were indeed occurring within this highland area. Furthermore, when pre-specified analyses were carried out by altitude, no evidence of a difference between the groups was seen even amongst women living at the lower altitudes below 1800m, where exposure is higher and any differences in PE between the interventions would be more likely to be apparent. Though doubtless not powered to detect statistical significance, in all sub-group analyses the prevalence of study endpoints were almost identical in all three intervention groups and it seems reasonable to conclude that the interventions do not differ in their effect on maternal and foetal outcomes, even in those women at greatest risk of malaria. The prevalence of parasitaemia observed was equal or greater than that previously reported in other highland areas33-35 and was comparable to the median prevalence of peripheral parasitaemia of 13.7% seen in low-transmission African settings.8 We thus consider that the background level of transmission in Kabale is probably representative of other low-transmission settings in Africa and these trial results are generalisable to other settings of low and unstable transmission. It should be noted, however, that no epidemic outbreaks occurred in the study area during the period of the trial (2004-2008), and the possibility that one strategy might prove to be more efficacious in protecting pregnant women from malaria, and the attendant risks of abortion and stillbirth, at times of increased transmission in the highlands cannot be ruled out.
These findings are broadly consistent with other published trials. In an individually-randomised trial conducted in an area of high malaria transmission in western Kenya, IPTp and ITNs were both found to be of comparable efficacy, although the combination of IPTp+ITNs showed a marginally better protective efficacy than either intervention given alone.16 All three interventions were found more effective in preventing maternal anaemia than case management alone. A second trial comparing the use of ITNs with and without the addition of IPTp in an area of moderate malaria transmission in Mozambique found no significant difference between the two interventions on the prevalence of LBW and maternal anaemia and concluded that ITNs in combination with case management was more appropriate than IPTp with SP alone.18 The current trial, conducted in an area of low and unstable transmission, similarly did not find any evidence of a difference in efficacy of IPTp and ITNs, whether given alone or in combination. We did not evaluate the impact of case management or of screening and treatment.
In conclusion, the three preventive strategies evaluated were found to be comparable in efficacy in an area of low and unstable transmission. Delivery of ITNs and IPTp through ANC was well accepted and compliance with their use was high. Thus, for policy the comparative cost of delivering each intervention and the wider public health benefits of ITN use may be more relevant considerations than efficacy. Cultural factors such as local acceptability of SP or ITNs, which can reduce the effectiveness of an intervention under operational conditions, may also be pertinent. The cost of delivery of IPTp with SP was found to be lower than the cost of distribution of ITNs through ANC.36 Nevertheless, the risk of waning efficacy of SP and current lack of alternative drugs could favour the distribution of long-lasting insecticidal nets (LLINs) over the use of IPTp as a preventive strategy in areas of low and unstable transmission. This would also reduce the risk of adverse effects of co-administration of SP with anti-retroviral drugs or co-trimoxazole in pregnant women with HIV/AIDS, and obviate concerns over the exposure to drugs in the first trimester.37 In contrast, little or no risk is associated with use of an ITN and they can be used within the first trimester, reducing the risk of infection throughout pregnancy. Continued use of a LLIN after pregnancy brings additional public health benefits, protecting the growing infant through the crucial first year of life and potentially serving to also protect the mother from the very first day of a subsequent pregnancy. Antenatal distribution, coupled with community-based initiatives, will also help to accelerate progress towards the goal of universal ITN coverage. On balance, ITNs provide a number of distinct advantages over IPTp and thus may be a more appropriate preventive strategy for malaria control during pregnancy in areas of low and unstable transmission.
Acknowledgments
The authors’ immense gratitude goes to the pregnant women who voluntarily agreed to participate in the study, the staff of the health facilities where the study was undertaken for their active involvement in the study, and the District Health Officials for their keen interest and logistical support to the research team. Special thanks to James Kirunda at the School of Entomology at Vector Control Division, Ministry of Health (Kampala, Uganda) for re-examining all the slides read initially by the laboratory technicians employed by the project. The authors also thank the Data Safety Monitoring Board (David Ross, Christine Watera, Moses Bateganya and Neal Alexander) for their advice and guidance during the conduct of the trial; Brian Beard for data management advice and technical support; Jon Cox for assistance with the digital elevation model; and Harparkash Kaur for undertaking HPLC analysis. They are also grateful to Brian Greenwood, Geoff Targett and Daniel Chandromohan for their advice throughout the study and comments on the manuscript. Sulfadoxine/pyrimethamine and placebo tablets were supplied by Kampala Pharmaceutical Industries (Kampala, Uganda).
Funding: The trial was funded by the Gates Malaria Partnership (London, UK), which was supported by a grant from the Bill and Melinda Gates Foundation (Seattle, WA, USA). SEC is supported by the Wellcome Trust (London, UK) through a Career Development Fellowship [084933]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest
There are no conflicts of interest to declare.
Ethical approval
The study protocol was approved by the Uganda National Council of Science and Technology, the Ethical Committee of the London School of Hygiene and Tropical Medicine, and the Danish National Committee on Biomedical Research Ethics, and was registered on a clinical trials database (www.clinicaltrials.gov: NCT00142207).
References
- 1.McGregor IA. Epidemiology, malaria and pregnancy. American Journal of Tropical Medicine and Hygiene. 1984;33:517–25. doi: 10.4269/ajtmh.1984.33.517. [DOI] [PubMed] [Google Scholar]
- 2.Brabin B. The risks and severity of malaria in pregnant women. Applied field research in malaria report 1. Special Programme for Research and Training in Tropical Diseases. World Health Organization; Geneva: 1991. TDR/FIELDMAL/1. [Google Scholar]
- 3.Shulman CE, Dorman EK, Cutts F, Kawuondo K, Bulmer JN, Peshu N, et al. Intermittent sulfadoxine-pyrimethamine to prevent severe anaemia secondary to malaria in pregnancy: a randomized placebo-controlled trial. Lancet. 1999;353(9153):632–6. doi: 10.1016/s0140-6736(98)07318-8. [DOI] [PubMed] [Google Scholar]
- 4.Ndyomugyenyi R, Magnussen P. Anaemia in pregnancy: Plasmodium falciparum infection is an important cause in primigravidae in Hoima district, western Uganda. Annals of Tropical Medicine & Parasitology. 1999;5:457–65. doi: 10.1080/00034989958195. [DOI] [PubMed] [Google Scholar]
- 5.Menendez C, Fleming AF, Alonso PL. Malaria-related anaemia. Parasitology Today. 2000;16:469–76. doi: 10.1016/s0169-4758(00)01774-9. [DOI] [PubMed] [Google Scholar]
- 6.Brabin BJ, Johnson PM. Placental malaria and pre-eclampsia through the looking glass backwards? Journal of Reproductive Immunology. 2005;65:1–15. doi: 10.1016/j.jri.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Hammerich A, Campbell OMR, Chandramohan D. Unstable malaria transmission and maternal mortality – experiences from Rwanda. Tropical Medicine and International Health. 2002;7:573–6. doi: 10.1046/j.1365-3156.2002.00898.x. [DOI] [PubMed] [Google Scholar]
- 8.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infectious Diseases. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 9.Uddenfeldt Wort U, Hastings I, Mutabingwa TK, Brabin BJ. The impact of endemic and epidemic malaria on the risk of stillbirth in two areas of Tanzania with different malaria transmission patterns. Malaria Journal. 2006;5:89. doi: 10.1186/1475-2875-5-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ndyomugyenyi R, Magnussen P. Malaria morbidity, mortality and pregnancy outcome in areas with different levels of malaria transmission in Uganda: a hospital record-based study. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2001;95:463–8. doi: 10.1016/s0035-9203(01)90003-3. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . A strategic framework for malaria prevention and control during pregnancy in the African region. World Health Organization Regional Office for Africa; Brazzaville: 2004. AFR/MAL/04/01. [Google Scholar]
- 12.Brabin BJ, Wasame M, Uddenfeldt-Wort U, Dellicour S, Hill J, Gies S. Monitoring and evaluation of malaria in pregnancy-developing national basis for control. Malaria Journal. 2008;7(Suppl 1):S6. doi: 10.1186/1475-2875-7-S1-S6. DOI: 10.1186/1475-2875-7-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sirima SB, Cotte AH, Konate A, Moran AC, Asamoa K, Bougouma EC, et al. Malaria prevention during pregnancy: assessing the disease burden one year after implementing a program of intermittent preventive treatment in Koupela District, Burkina Faso. American Journal of Tropical Medicine and Hygiene. 2006;75:205–11. [PubMed] [Google Scholar]
- 14.Garner P, Gulmezoglu AM. Drugs for preventing malaria in pregnancy. Vol. 4. Cochrane Database Systematic Reviews; 2006. CD000169. [DOI] [PubMed] [Google Scholar]
- 15.Gamble C, Ekwaru JP, ter Kuile FO. Insecticide-treated nets for preventing malaria in pregnancy. Vol. 2. Cochrane Database Systematic Reviews; 2006. CD003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Njagi JK, Magnussen P, Estambale B, Ouma J, Mugo B. Prevention of anaemia in pregnancy using insecticide-treated bednets and sulphadoxine-pyrimethamine in a highly malarious area of Kenya: a randomized controlled trial. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2003;97(3):277–82. doi: 10.1016/s0035-9203(03)90141-6. [DOI] [PubMed] [Google Scholar]
- 17.Mbaye A, Richardson K, Balajo B, Dunyo S, Shulman C, Milligan P, et al. A randomized, placebo-controlled trial of intermittent preventive treatment with sulphadoxine-pyrimethamine in Gambian multigravidae. Tropical Medicine and International Health. 2006;11(7):992–1002. doi: 10.1111/j.1365-3156.2006.01649.x. [DOI] [PubMed] [Google Scholar]
- 18.Menendez C, Bardaji A, Sigauque B, Romagosa C, Sanz S, Serra-Casas E, et al. A randomized placebo-controlled trial of intermittent preventive treatment in pregnant women in the context of insecticide treated nets delivered through the antenatal care clinic. PloS ONE. 2008;3:e1934. doi: 10.1371/journal.pone.0001934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nosten F, ter Kuile F, Maelankiri L, Decludt B, White NJ. Malaria during pregnancy in an area of unstable endemicity. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1991;85:424–9. doi: 10.1016/0035-9203(91)90205-d. [DOI] [PubMed] [Google Scholar]
- 20.Lindblade KA, Walker ED, Onapa AW, Katungu J, Wilson ML. Highland malaria in Uganda: prospective analysis of an epidemic associated with El Ninõ. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1999;93:480–7. doi: 10.1016/s0035-9203(99)90344-9. [DOI] [PubMed] [Google Scholar]
- 21.Ballard JL, Novak KK, Driver M. A simplified score for assessment of fetal maturation of newly born infants. Journal of Paediatrics. 1979;95:769–74. doi: 10.1016/s0022-3476(79)80734-9. [DOI] [PubMed] [Google Scholar]
- 22.Verhoeff FH, Milligan P, Brabin BJ, Malanga S, Nakoma V. Gestational age assessment by nurses in a developing country, using the Ballard method, external criteria only. Annals of Tropical Paediatrics. 1997;17(4):333–42. doi: 10.1080/02724936.1997.11747907. [DOI] [PubMed] [Google Scholar]
- 23.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data - or tears: An application to educational enrollments in states of India. Demography. 2001;38(1):115–32. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 24.Jarvis A, Reuter HI, Nelson A, Guevara E. Hole-filled SRTM for the globe Version 4. 2008 available from the CGIAR-CSI SRTM 90m Database . http://srtm.csi.cgiar.org.
- 25.Greenwood BM, Greenwood AM, Snow RW, Byass P, Bennett S, Hatib-N’Jie AB. The effects of malaria chemoprophylaxis given by traditional birth attendants on the course and outcome of pregnancy. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1989;83:589–94. doi: 10.1016/0035-9203(89)90362-3. [DOI] [PubMed] [Google Scholar]
- 26.Cox J, Craig M, Le Sueur D, Sharp B. Mapping malaria risk in the highlands of Africa. WHO/TDR; Geneva: 1999. MARA/HIMAL Technical Report. [Google Scholar]
- 27.Crawley J, Hill J, Yartey J, Robalo M, Serufilira A, Ba-Nguz A, et al. From evidence to action? Challenges to policy change and programme delivery for malaria in pregnancy. Lancet Infectious Diseases. 2007;7:145–55. doi: 10.1016/S1473-3099(07)70026-9. [DOI] [PubMed] [Google Scholar]
- 28.Brabin BJ. An analysis of malaria in pregnancy in Africa. Bulletin of the World Health Organization. 1983;61:1005–16. [PMC free article] [PubMed] [Google Scholar]
- 29.Cottrell G, Mary JY, Barro D, Cot M. The importance of the period of malarial infection during pregnancy on birth weight in tropical Africa. American Journal of Tropical Medicine and Hygiene. 2007;76(5):849–54. [PubMed] [Google Scholar]
- 30.Ndyomugyenyi R, Magnussen P, Clarke SE. The efficacy of chloroquine, sulfadoxine-pyrimethamine and a combination of both for the treatment of uncomplicated Plasmodium falciparum malaria in an area of low transmission in western Uganda. Tropical Medicine and International Health. 2004;9:47–52. doi: 10.1046/j.1365-3156.2003.01167.x. [DOI] [PubMed] [Google Scholar]
- 31.Lindblade KA, Walker ED, Onapa AW, Katungu J, Wilson ML. Land use change alters malaria transmission parameters by modifying temperature in a highland area of Uganda. Tropical Medicine and International Health. 2000;5:263–74. doi: 10.1046/j.1365-3156.2000.00551.x. [DOI] [PubMed] [Google Scholar]
- 32.Shulman CE, Marshall T, Dorman EK, Bulmer JN, Cutts F, Peshu N, et al. Malaria in pregnancy: adverse effects on haemoglobin levels and birthweight in primigravidae and multigravidae. Tropical Medicine and International Health. 2001;6(10):770–8. doi: 10.1046/j.1365-3156.2001.00786.x. [DOI] [PubMed] [Google Scholar]
- 33.Cot M, Brutus L, Pinell V, Ramarosan H, Raveloson A, Rabeson D, et al. Malaria prevention during pregnancy in unstable transmission areas: the highlands of Madagascar. Tropical Medicine and International Health. 2002;7:565–72. doi: 10.1046/j.1365-3156.2002.00897.x. [DOI] [PubMed] [Google Scholar]
- 34.Newman RD, Hailemariam A, Jimma D, Degifie A, Kebede D, Rietveld AE, et al. Burden of malaria during pregnancy in areas of stable and unstable transmission in Ethiopia during a non-epidemic year. Journal of Infectious Disease. 2003;187:1765–72. doi: 10.1086/374878. [DOI] [PubMed] [Google Scholar]
- 35.Van Geertruyden JP, Ntakirutimana D, Erhart A, Rwagocondo C, Kabana A, D’Alessandro U. Malaria infection among pregnant women attending antenatal clinics in six Rwandan districts. Tropical Medicine and International Health. 2005;10(7):681–8. doi: 10.1111/j.1365-3156.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- 36.Ndyomugyenyi R, Schultz Hansen K, Clarke SE, Magnussen P. Cost-effectiveness analysis of three health interventions to prevent malaria in pregnancy in an area of low transmission in Uganda. International Health. 2012 doi: 10.1016/j.inhe.2011.10.001. forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menendez C, D’Alessandro U, ter Kuile FO. Reducing the burden of malaria in pregnancy by preventive strategies. Lancet Infectious Diseases. 2007;7:126–35. doi: 10.1016/S1473-3099(07)70024-5. [DOI] [PubMed] [Google Scholar]