Abstract
Cycloaddition is an essential tool in chemical synthesis. Instead of using light or heat as a driving force, marine sponges promote cycloaddition with a more versatile but poorly understood mechanism in producing pyrrole–imidazole alkaloids sceptrin, massadine, and ageliferin. Through de novo synthesis of sceptrin and massadine, we show that sponges may use single-electron oxidation as a central mechanism to promote three different types of cycloaddition. Additionally, we provide surprising evidence that, in contrast to previous reports, sceptrin, massadine, and ageliferin have mismatched chirality. Therefore, massadine cannot be an oxidative rearrangement product of sceptrin or ageliferin, as is commonly believed. Taken together, our results demonstrate unconventional chemical approaches to achieving cycloaddition reactions in synthesis and uncover enantiodivergence as a new biosynthetic paradigm for natural products.
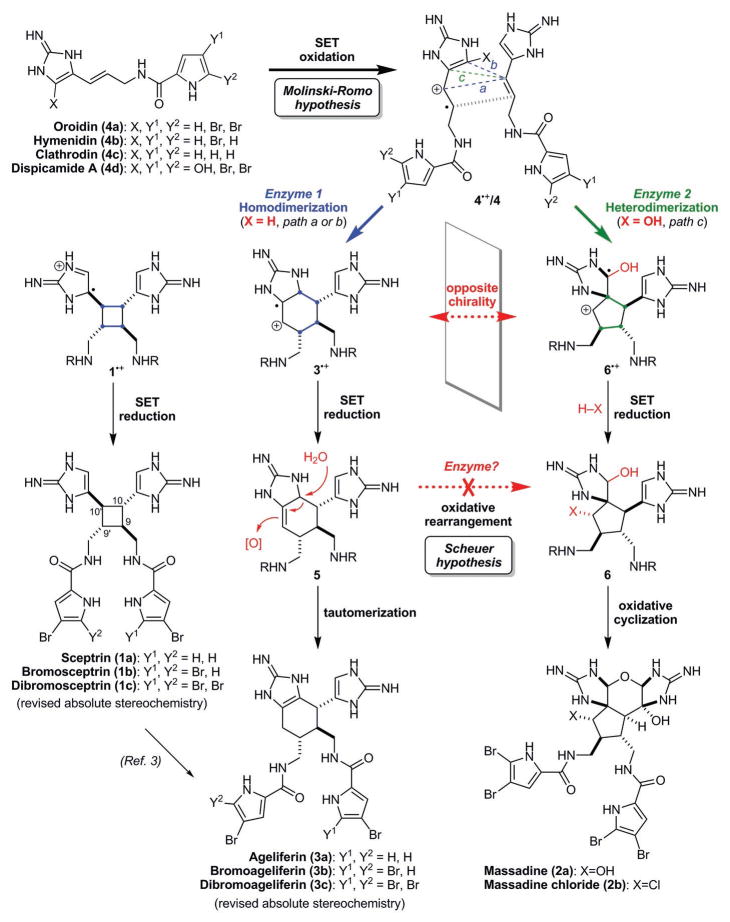
The dimeric pyrrole–imidazole alkaloids are structurally complex small molecules produced by marine sponges through reactions that are not well understood (1) (Fig. 1 and figs. S1 and S2). These natural products are highly polar, noncrystalline, redox labile, and pH sensitive because of their exceptionally high nitrogen content (~16% atomic composition or 15 to 30% by weight). The presence of multiple halogen atoms further contributes to their chemical instability and synthetic challenges (2–21). As depicted in Fig. 1, sceptrin (1a) (22), massadine (2a) (23, 24), and ageliferin (3a) (25, 26) are family members derived from formal [2+2], [3+2], and [4+2] cycloaddition reactions of oroidin (4a), hymenidin (4b), or their congeners. Our previous studies on the de novo synthesis of ageliferins (3) (4, 14, 15) suggest that the biogenic dimerization of 4 is likely a radical reaction. We now describe the de novo syntheses of sceptrin (1a) and massadine (2a), using oxidative reactions that are suspected to be involved in their biosyntheses. These asymmetric syntheses along with two new crystal structures allow us to unambiguously assign the absolute stereochemistries of the pyrrole–imidazole dimers. We also demonstrate that the [3+2] dimers cannot be derived from the [4+2] or [2+2] dimers through a skeletal rearrangement reaction. Instead, a surprising enantiodivergent pathway must be involved in the biosynthesis of these alkaloids.
Fig. 1. The enantiodivergent biosynthetic pathways for the dimeric pyrrole-imidazole alkaloids.
The biosyntheses of the [2+2] dimers sceptrins (1) and the [4+2] dimers ageliferins (3) involve a homo-dimerization of oroidin (4a) or hymenidin (4b). SET oxidation of 4 promotes a [2+2] cycloaddition reaction of 4•+/4 to give 1•+ (path a), or a [4+2] cycloaddition reaction to give 3•+ (path b). Subsequent SETreduction yields 1 and 5 that aromatize to 3. In contrast, the biosyntheses of the [3+2] dimers massadines (2) involve a heterodimerization of dispacamide A (4d) and oroidin (4a). SET oxidation of 4d promotes a [3+2] cycloaddition reaction of 4d•+/4a to give 6•+. Subsequent SET reduction, protonation, and trapping with water or chloride provide “pre-massadines” (6). Oxidative cyclization of 6 gives massadines (2). The homodimerization (path a and b) and heterodimerization (path c) of 4 are enantiomeric pathways.
Sceptrin (1a) is the first dimeric pyrrole–imidazole alkaloid discovered in nature (22). Because there is insufficient light where the sponges live (20 to 30 m below the ocean surface), it is unlikely for hymenidin (4b) to undergo a photocycloaddition reaction to give 1a biogenically (2, 22). In addition, a [2+2] photocycloaddition reaction of 4b would lead to the formation of racemic 1a, whereas the isolated 1a was optically active. Based on results of meta-biosynthetic experiments, Molinski and Romo proposed an enzyme-promoted single-electron transfer (SET) mechanism for the biogenic dimerization of 4 (27). We believe that a SET oxidation of 4 would give radical cation 4•+ that is highly reactive toward [2+2] (path a) and [4+2] (path b) cycloaddition reactions to afford 1•+ and 3•+, correspondingly (Fig. 1). Subsequent back-SET would provide sceptrins (1) and “iso-ageliferins” (5) that tautomerize to ageliferins (3).
Massadine (2a) is a densely functionalized polycyclic [3+2] dimer that contains 8 contiguous stereocenters, 10 nitrogen atoms, and multiple sensitive functional groups. These structural features preclude the use of many traditional tools for its synthesis. Because enzymes that promote [3+2] cycloaddition reactions remain elusive, it is commonly believed that 2a is derived from 5 through an oxidative skeletal rearrangement reaction (13, 28, 29). Oxidation of 5 would induce a ring-contraction reaction to give “pre-massadine” (6). Further oxidation of 6 would provide 2 and its congeners via different modes of cyclization (1, 30). This oxidative rearrangement hypothesis was first proposed by Scheuer for palau’amine (28) and recently modified by us (13), Al-Mourabit, and Romo (1) to account for its revised relative stereochemistry. The chemical viability of this skeletal rearrangement through two-electron oxidation has also been demonstrated by Romo, Lovely, and us (5, 12, 18, 19).
We were interested in using chemical synthesis to evaluate the Molinski-Romo and Scheuer biosynthetic hypotheses. The prior synthesis of sceptrin (1a) by Birman (20) and us (2, 4, 5) involved the construction of the cyclobutane ring by forming the C9/C10 and C9′/C10′ bonds using a [2+2] photocycloaddition reaction. The synthesis described herein involves the alternative formation of the C9/C9′ and C10/C10′ bonds (biogenic connection) via a SET-promoted [2+2] cycloaddition reaction (Molinski-Romo hypothesis). The synthesis of massadine (2a) has also been achieved by us, using an intramolecular aldol reaction (8) or a Pauson-Khand reaction (10) to construct the central cyclopentane ring. We now describe a complementary synthesis that involves an oxidative rearrangement (Scheuer hypothesis) of a dibromoageliferin derivative (14, 15), using an oxidative radical cyclization reaction as the key step.
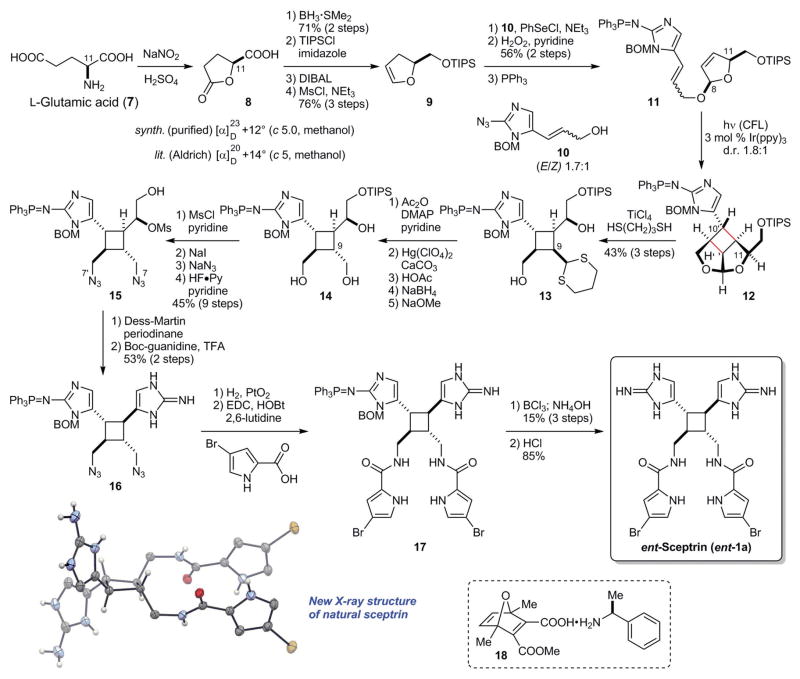
We first set out to develop an asymmetric synthesis of sceptrin (1a) using a SET-mediated [2+2] cycloaddition reaction to construct its core skeleton (Fig. 2). L-Glutamic acid (7) was used as the source of chirality to establish the absolute stereochemistry of our synthetic 1a. Diazotization of 7 induced an anchimeric lactonization to give (S)-(+)-5-oxo-2-tetrahydrofurancarboxylic acid (8), a commercially available chiral building block. Dihydrofuran 9 was then prepared from 8 through reduction, silylation, and dehydration. Electrophilic selenation of 9 proceeded from the less hindered face, allowing alcohol 10 to approach from the more hindered face of 9 with excellent stereoselectivity. Subsequent oxidative elimination of the phenylselenyl group and reduction of the azido group provided 11 with cis-C8/C11 stereochemistry, setting the stage for the key [2+2] cycloaddition reaction.
Fig. 2. Biomimetic synthesis of sceptrin and revision of its absolute stereochemistry.
The key step involves a SET-mediated [2+2] cycloaddition reaction that mechanistically mimics the biogenic dimerization of hymenidin (4b). ent-Sceptrin was obtained while targeting the originally reported absolute stereochemistry. A new crystal structure of natural sceptrin (shown in ORTEP representation) confirms that its absolute stereochemistry needs to be revised. TIPS, triisopropylsilyl; DIBAL, diisobutylaluminium hydride; Ms, methanesulfonyl; BOM, benzyloxymethyl; CFL, compact fluorescent lamp; DMAP, 4-(dimethylamino)pyridine; Boc, tert-butyloxycarbonyl; EDC, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide; HOBt, 1-hydroxybenzotriazole.
Whereas treating 11 with a variety of single-electron oxidants (31) resulted in complete decomposition, the photoredox method developed by Yoon (32) promoted the reversible SET reactions of 11 smoothly. Irradiation of a solution of 11 with a catalytic amount of Ir(ppy)3 induced the [2+2] cycloaddition to give 12 [diastereomeric ratio (d.r.) 1.8:1 at C10′]. Although this cycloaddition reaction could also proceed through an energy transfer mechanism (33), 9-fluorenone, which has the same triplet energy (ET = 55 kcal/mol) as Ir(ppy)3 (34, 35), could not catalyze this reaction. Therefore, we believe that the [2+2] cycloaddition of 11 was induced by reversible SET between Ir(ppy)3 and the aminoimidazole group of 11.
To complete the synthesis of sceptrin (1a), we used transthioketalization to reveal the cyclobutane core skeleton in 12. The requisite all-trans configuration of the central cyclobutane core was furnished by epimerization of the C9 stereogenic center after protecting the hydroxyl groups and hydrolyzing the dithiane group of 13. Subsequent reduction of the aldehyde group and deacetylation provided triol 14. With the sceptrin core skeleton in hand, we then functionalized the side chains to install the second aminoimidazole group and the two pyrrole groups. The N7 and N7′ groups were introduced by sequential mesylation, iodination, and azidation. Subsequent desilylation gave bisazide 15. The second aminoimidazole group was constructed by oxidizing the hydroxyl group of 15 and treating the resulting unstable mesyl aldehyde with Boc-guanidine. The azido groups of 16 were next reduced, and the pyrrole groups were introduced to give 17. Removal of the protecting groups of 17 afforded sceptrin (1a) (0.4% yield from L-glutamic acid).
Although we targeted the synthesis of sceptrin in its presumed natural enantiomeric form, the circular dichroism (CD) spectrum of our synthetic sceptrin (fig. S3) was the reverse of the natural samples’ spectrum (4, 36). In our synthesis, the absolute stereochemistry of sceptrin was established by the intramolecular [2+2] cycloaddition reaction of 11. The structure of the resulting pseudo-symmetric product 12 was determined by nuclear magnetic resonance (NMR) experiments. The rigid, cagelike structure of 12 rendered NMR a reliable tool for assigning its relative stereochemistry. We thus suspected that the original absolute stereochemistry of sceptrin was misassigned.
The absolute stereochemistry of sceptrin was determined in 1981 by Faulkner and Clardy through x-ray crystallographic analysis (fig. S1) (22). Although the Hamilton test favored ent-1a, the difference in the R factors was small (0.090 versus 0.094). In our previous synthetic study (4), a crystal of intermediate 18 was obtained from co-crystalization with (S)-α-methylbenzylamine. Although it supports the original assignment, the acid (18) used had only 75% enantiomeric excess. In light of our above-described findings, the ambiguity associated with enantio-impure sample of 18, and the unconvincing x-ray data by modern standards, we recrystallized a natural sample of sceptrin and used anomalous dispersion to determine its absolute stereochemistry. A suitable crystal was obtained with a slowly evaporating (4°C) biphasic mixture of ethyl acetate and water (1:10). With a Flack factor of −0.002(5) for 1a, we concluded that the previously reported absolute stereochemistry of sceptrin was incorrect. Because the absolute stereochemistry of ageliferin was determined based on that of sceptrin (4), this result suggests that a revision is also needed for ageliferin.
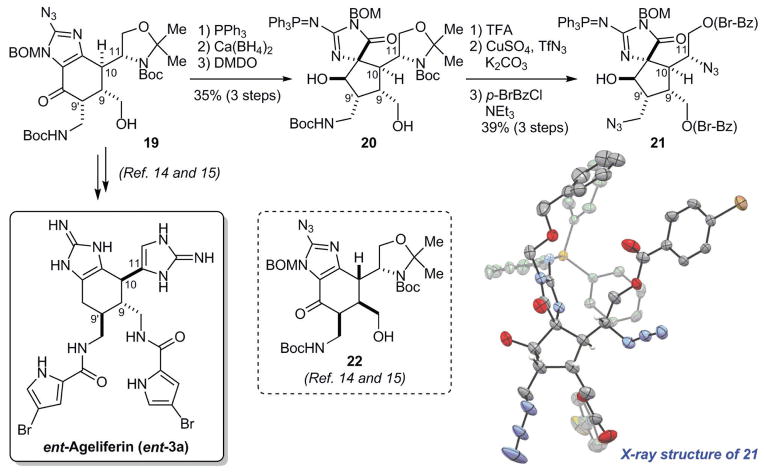
Previously, we targeted the synthesis of ageliferin via 19 using L-serine as the source of chirality (14, 15). However, we obtained ent-ageliferin instead of the expected nat-ageliferin (figs. S4 and S5). In light of the revision of the absolute stereochemistry of sceptrin (1a), we reinvestigated the structural assignment of 19 (Fig. 3). Reduction of the azido and carbonyl groups of 19 followed by oxidation with dimethyldioxirane (DMDO) induced the Scheuer-type rearrangement (5, 12, 18, 19) to give 20. The absolute and relative stereochemistry of 20 was determined by x-ray crystallographic analysis of its crystalline derivative 21. The C9/C10/C11 relative stereochemistry of 21 is consistent with that of 19 instead of 22, a structure that would be consistent with the original ent-ageliferin (fig. S5). The absolute stereochemistry of 21 determined by anomalous x-ray scattering is also consistent with the chirality of L-serine. These experiments provide independent evidence that the absolute stereochemistry of ageliferin also needs to be revised.
Fig. 3. Revision of the absolute stereochemistry of ageliferin (3a).
Oxidative ring-contraction of 19, the key intermediate in our ageliferin synthesis, gave 20 that bears a massadine core skeleton. The crystal structure of its crystalline derivative 21 (shown in ORTEP representation) confirms that the absolute stereochemistry of ageliferin needs to be revised. DMDO, dimethyldioxirane; TFA, trifluoroacetic acid; Br-Bz, 4-bromobenzoyl.
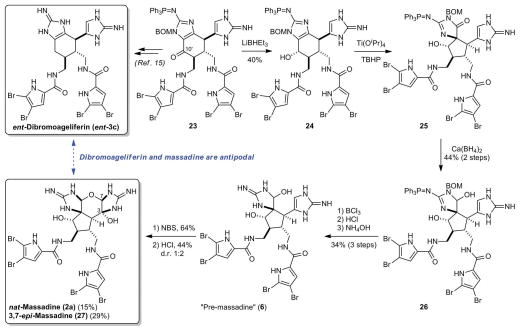
After completing the biomimetic synthesis of sceptrin (1a), we turned our attention to the asymmetric synthesis of massadine (2a). We chose to carry out the Scheuer-type oxidation on 23, a protected 10′-oxo-dibromoageliferin, to best mimic the putative Scheuer biosynthetic pathway (Fig. 4). Although we could induce the ring-contraction reaction with various oxidative methods, the undesired spiro-configuration was always obtained (15). We envisioned that the C10′ oxygen functionality of 23 could be used as a directing group (37) to reverse this stereo-selectivity. Indeed, reduction of 23 with LiBHEt3 gave 24 bearing the desired hydroxyl configuration. Oxidation of 24 with Ti(OiPr)4/TBHP induced the Scheuer-type ring contraction to give iminohydantoin 25 with the desired spiro configuration. This Ti(IV)-promoted oxidation was markedly chemo- and stereoselective. It only oxidized the more electron-rich aminoimidazole group in the presence of an unprotected aminoimidazole and two dibromopyrroles. It was also the only method that could provide the correct spiro configuration. Although alcohol 24 was only stable as an acid salt, the Ti(OiPr)4/TBHP-oxidation reaction failed completely under even slightly acidic conditions. Therefore, we washed 24•TFA with base and used it immediately to give 25 with good reproducibility. Without purification, 25 was reduced with Ca(BH4)2 to afford hemiaminal 26. Subsequent removal of the protecting groups gave “pre-massadine” (6).
Fig. 4. Asymmetric synthesis of massadine and evidence against the Scheuer biosynthetic hypothesis.
Deoxygenation and deprotection of 10′-oxo-dibromoageliferin (23) gave ent-dibromoageliferin (ent-3c) (the unnatural enantiomer), whereas skeletal rearrangement and oxidation of 23 provided nat-massadine (2a) (the natural enantiomer). TBHP, tert-butyl hydroperoxide; NBS, N-bromosuccinimide.
To complete the synthesis of massadine (2a), we would need to selectively oxidize the aminoimidazole group of 6 and promote the intra-molecular cyclization to form the oxygen bridge between the two cyclic guanidine groups of 2a. Both the oxidation and cyclization steps were pH-sensitive. Oxidation of 6 under acidic conditions resulted in considerable decomposition because the reactivity of aminoimidazole toward oxidation was suppressed when protonated and competing oxidation of other functionalities occurred. Although oxidation of 6 under neutral or slightly basic conditions proceeded more smoothly, the undesired N-cyclization occurred spontaneously to give the axinellamine skeleton (8). Eventually, we found that oxidation of 6 with N-bromosuccinimide (NBS) proceeded smoothly and cleanly in methanol to give a diastereomeric mixture of massadine-methanol adducts (11). Upon treatment with aqueous HCl, this mixture of oxidation products slowly converted into massadine (2a) (0.014% yield from L-serine) and 3,7-epi-massadine (27) (8).
The CD spectrum of our synthetic 2a suggested that we had obtained the natural enantiomer of massadine (fig. S6), confirming the correct assignment of its absolute stereochemistry by Fusetani (23) and us (11) previously. Because of our revision of the absolute stereochemistry of ageliferins (3), 2 and 3 have mismatched absolute stereochemistries. To verify this surprising result, we repeated the syntheses of 2a and 3c, using the same batch of common intermediate 23. We confirmed that oxidation and deprotection of 23 gave nat-2a, whereas deoxygenation and deprotection gave ent-3c. These experiments provided direct evidence that natural massadine (2a) and natural dibromoageliferin (3c) are antipodal, and an enantio-divergent pathway must be involved in their biosynthesis.
Sceptrins (1), massadines (2), and ageliferins (3) were previously thought to be derived from a common biosynthetic intermediate and have matched absolute stereochemistries (11). We have now shown that massadine (2a) and dibromoageliferin (3c) are antipodal. Therefore, 2a cannot be produced from a biogenic oxidation of 3c or 5. Considering that both enantiomeric forms of phakellin, a monomeric pyrrole–imidazole alkaloid, have been found in nature (38), it is possible that sponges also produce both enantiomers of 1–3. However, literature reports suggest that all the cyclic pyrrole–imidazole dimers exist in only one enantiomeric form. Because both the sign and magnitude of the optical rotation of 1–3 change with the salt form (4, 26), CD spectroscopy is currently the only reliable method to study their absolute stereochemistries. Ageliferins isolated from different species of sponges (two Agelas, one Astrosclera, and one Stylissa sponge) in the Caribbean and Pacific oceans all have matched CD spectra (table S1). Similarly, massadines, stylissadines, axinellamines, and donnazoles isolated from different species of sponges (one Agelas, two Axinella, and two Stylissa sponges) in the Caribbean, Pacific, Indian, and Southern oceans all show a positive exciton splitting centered at ~275 nm (table S1). The only two reported CD spectra of sceptrin are also consistent with one another (4, 36). Currently, there is no evidence that both enantiomers of cyclic pyrrole–imidazole dimers exist in nature, although it has also been shown that enantiomeric natural products can arise from a single species or from different genera and/or species (39). Because massadine (2a) and dibromoageliferin (3c) are antipodal, they have to be produced by independent bio-synthetic pathways.
On the basis of our previous computational and experimental studies (12, 15), we believe that sceptrin (1a) and ageliferin (3a) are derived from two hymenidin (4b, X=H) molecules whereas massadine (2a) is assembled from one oroidin (4a, X=H) and one dispacamide A (4d, X=OH) molecule (Fig. 1). The homodimerization (path a and b) and heterodimerization (path c) of 4 are enantiomeric pathways. The pathway selectivity is determined by the stability of the radical intermediates. The redox-neutral, reversible SET-promoted heterodimerization of 4a and 4d would give “pre-massadines” (6) with a C9 aminal group that is present in all [3+2] pyrrole–imidazole dimers (fig. S2). Although enantiomeric biosynthesis that produces both enantiomers of a natural product has been reported (39), enantiodivergent biosynthesis that produces opposite enantiomers of natural products as congeners described herein is unknown. This discovery may serve as a new guiding principle for protein engineering and catalyst design.
Supplementary Material
Acknowledgments
We thank M. Köck (Alfred Wegener Institute) for providing the natural samples of sceptrin and massadine, and J. Muñoz (Alfred Wegener Institute) for providing a copy of the CD spectrum of natural sceptrin•TFA. We also thank V. Lynch (University of Texas at Austin), J. Clardy (Harvard University), and T. Molinski (University of California, San Diego) for helpful discussions. The CD experiments were performed by H. Shi [University of Texas (UT) Southwestern]. Financial support was provided by NIH (NIGMS R01-GM079554 and R01-GM073949), the Welch Foundation (I-1596), and UT Southwestern. Metrical parameters for the structures of natural sceptrin (1a) and 21 are available free of charge from the Cambridge Crystallographic Data Centre under reference numbers CCDC-995387 (1a), 1014159 (21), and 1016848 (21).
Footnotes
www.sciencemag.org/content/346/6206/219/suppl/DC1
Materials and Methods
References (40–52)
NMR spectra
REFERENCES AND NOTES
- 1.Al-Mourabit A, Zancanella MA, Tilvi S, Romo D. Nat Prod Rep. 2011;28:1229–1260. doi: 10.1039/c0np00013b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baran PS, Zografos AL, O’Malley DP. J Am Chem Soc. 2004;126:3726–3727. doi: 10.1021/ja049648s. [DOI] [PubMed] [Google Scholar]
- 3.Baran PS, O’Malley DP, Zografos AL. Angew Chem Int Ed. 2004;43:2674–2677. doi: 10.1002/anie.200453937. [DOI] [PubMed] [Google Scholar]
- 4.Baran PS, Li K, O’Malley DP, Mitsos C. Angew Chem Int Ed. 2005;45:249–252. doi: 10.1002/anie.200503374. [DOI] [PubMed] [Google Scholar]
- 5.O’Malley DP, Li K, Maue M, Zografos AL, Baran PS. J Am Chem Soc. 2007;129:4762–4775. doi: 10.1021/ja069035a. [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi J, et al. Angew Chem Int Ed. 2008;47:3578–3580. doi: 10.1002/anie.200705913. [DOI] [PubMed] [Google Scholar]
- 7.O’Malley DP, Yamaguchi J, Young IS, Seiple IB, Baran PS. Angew Chem Int Ed. 2008;47:3581–3583. doi: 10.1002/anie.200801138. [DOI] [PubMed] [Google Scholar]
- 8.Su S, Seiple IB, Young IS, Baran PS. J Am Chem Soc. 2008;130:16490–16491. doi: 10.1021/ja8074852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seiple IB, et al. Angew Chem Int Ed. 2010;49:1095–1098. doi: 10.1002/anie.200907112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su S, Rodriguez RA, Baran PS. J Am Chem Soc. 2011;133:13922–13925. doi: 10.1021/ja206191g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seiple IB, et al. J Am Chem Soc. 2011;133:14710–14726. doi: 10.1021/ja2047232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan X, Chen C. Angew Chem Int Ed. 2006;45:4345–4348. doi: 10.1002/anie.200601208. [DOI] [PubMed] [Google Scholar]
- 13.Ma Z, Lu J, Wang X, Chen C. Chem Commun. 2011;47:427–429. doi: 10.1039/c0cc02214d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Ma Z, Lu J, Tan X, Chen C. J Am Chem Soc. 2011;133:15350–15353. doi: 10.1021/ja207386q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, et al. J Am Chem Soc. 2012;134:18834–18842. doi: 10.1021/ja309172t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Overman LE, Rogers BN, Tellew JE, Trenkle WC. J Am Chem Soc. 1997;119:7159–7160. [Google Scholar]
- 17.Starr JT, Koch G, Carreira EM. J Am Chem Soc. 2000;122:8793–8794. [Google Scholar]
- 18.Dilley AS, Romo D. Org Lett. 2001;3:1535–1538. doi: 10.1021/ol015864j. [DOI] [PubMed] [Google Scholar]
- 19.Lovely CJ, Du H, He Y, Rasika Dias HV. Org Lett. 2004;6:735–738. doi: 10.1021/ol036403w. [DOI] [PubMed] [Google Scholar]
- 20.Birman VB, Jiang XT. Org Lett. 2004;6:2369–2371. doi: 10.1021/ol049283g. [DOI] [PubMed] [Google Scholar]
- 21.Ding H, Roberts AG, Harran PG. Chem Sci. 2013;4:303–306. doi: 10.1039/C2SC21651E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker RP, Faulkner DJ, van Engen D, Clardy J. J Am Chem Soc. 1981;103:6772–6773. [Google Scholar]
- 23.Nishimura S, et al. Org Lett. 2003;5:2255–2257. doi: 10.1021/ol034564u. [DOI] [PubMed] [Google Scholar]
- 24.Grube A, Immel S, Baran PS, Köck M. Angew Chem Int Ed. 2007;46:6721–6724. 8107. doi: 10.1002/anie.200701935. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi J, et al. Tetrahedron. 1990;46:5579–5586. [Google Scholar]
- 26.Keifer PA, et al. J Org Chem. 1991;56:2965–2975. [Google Scholar]
- 27.Stout EP, Wang YG, Romo D, Molinski TF. Angew Chem Int Ed. 2012;51:4877–4881. doi: 10.1002/anie.201108119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinnel RB, Gehrken HP, Swali R, Skoropowski G, Scheuer PJ. J Org Chem. 1998;63:3281–3286. [Google Scholar]
- 29.Northrop BH, O’Malley DP, Zografos AL, Baran PS, Houk KN. Angew Chem Int Ed. 2006;45:4126–4130. doi: 10.1002/anie.200600514. [DOI] [PubMed] [Google Scholar]
- 30.Köck M, Grube A, Seiple IB, Baran PS. Angew Chem Int Ed. 2007;46:6586–6594. doi: 10.1002/anie.200701798. [DOI] [PubMed] [Google Scholar]
- 31.Bauld NL. Tetrahedron. 1989;45:5307–5363. [Google Scholar]
- 32.Yoon TP. ACS Catal. 2013;3:895–902. doi: 10.1021/cs400088e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Z, Yoon TP. Angew Chem Int Ed. 2012;51:10329–10332. doi: 10.1002/anie.201204835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valentine D, Jr, Hammond GS. J Am Chem Soc. 1972;94:3449–3454. [Google Scholar]
- 35.Chen FC, et al. J Polym Sci, B, Polym Phys. 2003;41:2681–2690. [Google Scholar]
- 36.Muñoz J, Moriou C, Gallard JF, Marie PD, Al-Mourabit A. Tetrahedron Lett. 2012;53:5828–5832. 6865. [Google Scholar]
- 37.Hoveyda AH, Evans DA, Fu GC. Chem Rev. 1993;93:1307–1370. [Google Scholar]
- 38.Gautschi JT, Whitman S, Holman TR, Crews P. J Nat Prod. 2004;67:1256–1261. doi: 10.1021/np0340495. [DOI] [PubMed] [Google Scholar]
- 39.Finefield JM, Sherman DH, Kreitman M, Williams RM. Angew Chem Int Ed. 2012;51:4802–4836. doi: 10.1002/anie.201107204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.