Abstract Abstract
Recent inferences of phylogeny from molecular characters, as well as a reexamination of morphological and biological characters, reject the monophyly of the nematode genus Koerneria Meyl, 1960 (Diplogastridae). Here, Koerneria sensu lato is revised. The genus, which previously consisted of 40 species, is separated into three genera. Almost all of the transferred species are moved to the resurrected genus Allodiplogaster Paramonov & Sobolev in Skrjabin et al. (1954). Koerneria and Allodiplogaster are distinguished from each other by a weakly vs. clearly striated body surface, an undivided vs. divided stomatal cheilostom, and arrangement of the terminal ventral triplet of male genital papillae, namely in that v5 and v6 are paired and separated from v7 vs. v5–v7 being close to each other. Allodiplogaster is further divided into two groups of species, herein called the henrichae and striata groups, based on both morphological and life-history traits. The henrichae group is characterized by papilliform labial sensilla and male genital papillae, a conical tail in both males and females, and an association with terrestrial habitats and insects, whereas the striata group is characterized by setiform labial sensilla and male genital papillae, an elongated conical tail in both sexes, and an association with aquatic habitats. A second genus, Anchidiplogaster Paramonov, 1952, is resurrected to include a single species that is characterized by its miniscule stoma and teeth, unreflexed testis, and a distinct lack of male genital papillae or stomatal apodemes. Lastly, one further species that was previously included in Koerneria sensu lato is transferred to the genus Pristionchus Kreis, 1932. The revision of Koerneria sensu lato is necessitated by the great variability in its subordinate taxa, which occupy a variety of habitats, in addition to the increased attention to Diplogastridae as a model system for comparative mechanistic biology.
Keywords: Allodiplogaster, Anchidiplogaster, Koerneria, Pristionchus, revision, phylogeny, taxonomy
Introduction
Koerneria Meyl, 1960 heretofore consisted of 40 nominal species, following the revision by Sudhaus and Fürst von Lieven (2003) and including species described since then (Suppl. material 1). Several unidentified or undescribed species with molecular vouchers have also been reported (Mayer et al. 2007). Biological characters are variable for the genus, which contains terrestrial species isolated from rich soil environments, associates of several different groups of insects, and aquatic species. Such a diversity of ecologies raises the question of whether distinct subgroups of the genus can be identified and corroborated by independent characters.
In their revision of Diplogastridae, Sudhaus and Fürst von Lieven (2003) circumscribed Koerneria by the following characters: (1) presence of stomatal dimorphism, (2) cheilostom often separated into six per- and interradial figs or small stick-like figs (= rugae), (3) vertical striation, (4) stegostom with a dorsal claw-like tooth, a right subventral tooth, and left subventral serrated figs, (5) postdental region of stegostom with two subventrad directed apodemes, (6) female gonad amphidelphic or seldom prodelphic, and (7) no bursa. Later, Fürst von Lieven (2008) transferred Koerneria colobocerca (Andrássy, 1964) Fürst von Lieven, 2008 from Mononchoides Rahm, 1928 and revised the generic definition by adding two characters, (8) intestine sometimes with a prerectum, and (9) tail filiform or conical. Given the breadth of the most recent morphological definition of the genus, the only absolute generic character is the presence of articulated apodemes in the stoma. However, phylogenetic studies of molecular characters strongly indicate that the genus, as currently interpreted, is paraphyletic and thus separable into two or more clades (Fig. 1). Therefore, a taxonomic reorganization of Koerneria sensu lato (= Koerneria sensu Fürst von Lieven 2008) is needed. Such a revision is of particular importance for ongoing studies of the natural history of the genus and its relatives (e.g., Giblin-Davis et al. 2006, Kanzaki et al. 2009) as well as comparative research more generally in Diplogastridae (e.g., Mayer et al. 2009, Ragsdale et al. 2013), as Koerneria sensu lato includes taxa that are sister groups to most other known species in the family.
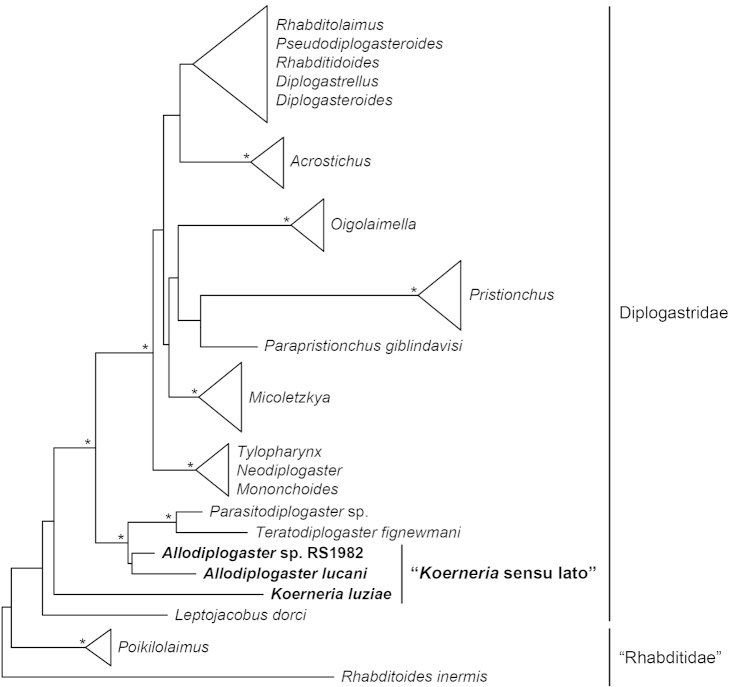
Figure 1.
Paraphyly of Koerneria sensu lato. Tree is simplified from Kanzaki et al. (2014b), which was inferred from nearly full-length small subunit ribosomal DNA sequences. A subsequent study that included several more species of Koerneria sensu lato likewise showed the well-supported exclusion (99% bootstrap support in likelihood analysis, 100% posterior probability in Bayesian analysis) of a clade of Koerneria luziae + Koerneria ruehmi from all other Diplogastridae, including a monophyletic clade of what is designated herein as Allodiplogaster (Atighi et al. 2013). Asterisks indicate nodes with very strong support as inferred in the former study (>95% bootstrap support, 100% posterior probability).
In this article, we revise the genus Koerneria by examining the original and subsequent descriptions of its nominal species. Based on morphological, biological, and molecular evidence, we separate Koerneria sensu lato into three genera. All renamed groups are hypothesized to be monophyletic and follow the precedent of previous classification systems (Andrássy 1984). Besides limiting the scope of Koerneria, we resurrect the genera Anchidiplogaster Paramonov, 1952 and Allodiplogaster Paramonov & Sobolev in Skrjabin et al. (1954). Furthermore, we distinguish two putative clades of Allodiplogaster species, each of which we hypothesize to be monophyletic on available information, although we leave formal revision of this genus to follow molecular studies of unsampled aquatic taxa.
Materials and methods
Species of Koerneria sensu lato are classified herein into four typological groups (three genera, with one genus further separated into two morphological and ecological groups) based on the following characters or traits, which were selected due to their high availability and reliability, being relatively accurate even in old descriptions:
(1) Stomatal morphology, specifically the separation of cheilostom and presence of apodemes
(2) Male tail morphology, including shape and arrangement of genital papillae
(3) Female tail morphology
(4) Life-history characters, particularly habitat preferences
In addition to published literature, several species available in culture were examined for typological characters: Koerneria luziae, isolated from stag beetles from Japan (Kanzaki et al. 2011); Koerneria sp., isolated from Dorcus rectus (Coleoptera: Lucanidae) from Japan; Allodiplogaster spp. RGD227 and RGD228, both isolated from soil-dwelling bees in the United States. Stomatal morphology and tail characters were examined by differential interference contrast (DIC) microscopy according to methods described by Kanzaki (2013). Several schematic illustrations were prepared based on original observations as well as published data. Following the reexamination of informative characters, the original descriptions and revisions were reviewed to determine the generic status of species following the International Code of Zoological Nomenclature (ICZN).
Systematics
Previous inferences of the phylogeny of Koerneria spp. indicate that two species, Koerneria luziae (Körner, 1954) Meyl, 1960 and Koerneria ruehmi Atighi, Pourjam, Kanzaki, Giblin-Davis, De Ley, Mundo-Ocampo & Pedram, 2013, form a well-supported clade that is the sister group to most or all other sequenced Diplogastridae (Atighi et al. 2013; Kanzaki et al. 2014b; Fig. 1). Separate from this group is a well-supported clade consisting of Koerneria lucani (Körner, 1954) Meyl, 1960 and some unidentified or undescribed species (Fig. 1). The former two species share two features that clearly distinguish them from other species of Koerneria sensu lato, namely (1) a tube- or ring-like (undivided) cheilostom and (2) male genital papillae arranged such that v5 and v6 (papillae nomenclature follows Sudhaus and Fürst von Lieven 2003) are close to each other and v7 is clearly apart from v6. These two characters diagnose six nominal species of Koerneria sensu lato, including the above two as well as the type species of the genus. We therefore revise the genus as follows:
Koerneria Meyl, 1960
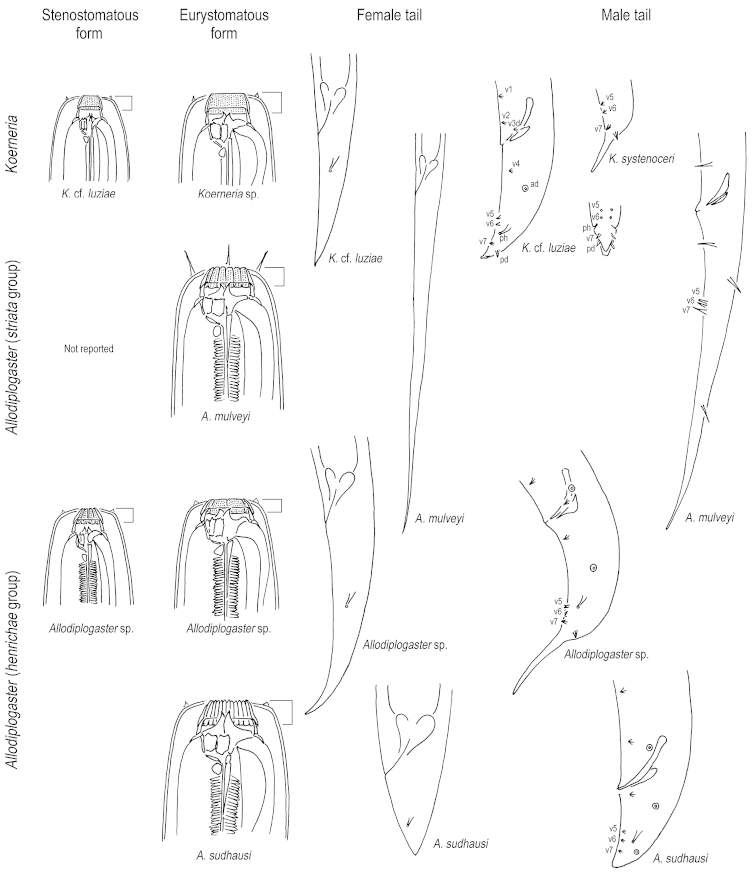
Fig. 2
Figure 2.
Schematic drawings of the generic characters of Koerneria and Allodiplogaster. From left to right: stenostomatous form, eurystomatous form, female tail, and male tail characters. From top to bottom: Koerneria, aquatic Allodiplogaster (“striata” species group) and two types of terrestrial Allodiplogaster (“henrichae” species group). For the stenostomatous form of Allodiplogaster sudhausi (not shown), see Fürst von Lieven (2008). Squared bracket indicates cheilostom, which as undivided separates Koerneria from Allodiplogaster. Further diagnosing Koerneria is the arrangement of male genital papillae v5-v7. Unique to the striata group of Allodiplogaster relative to the henrichae group and to Koerneria are a long tail in both sexes, setiform genital papillae, and in many cases setiform labial papillae. Genital papillae and phasmids are labeled following the terminology in Sudhaus and Fürst von Lieven (2003). The phasmids are not clearly described in species of the striata group.
Generic diagnosis
1) Stomatal dimorphism occasionally present1
2) Body-wall cuticle with weak vertical striations
3) Cheilostom usually forming short, undivided tube; rugae absent
4) Stegostom with dorsal claw-like tooth, right subventral tooth, and left subventral serrated figs or ridges
5) Postdental region of stegostom with left and right apodemes directed subventrad
6) Female gonad amphidelphic
7) Anterior two ventral and distal pairs of genital papillae (v5 and v6) close to each other, the posterior pair (v7) being clearly apart from v6
8) Short and conical male tail (c’ is usually ≤ 3) usually with short spike or with small, bursa-like or membranous appendage at tail tip
9) Known from terrestrial habitats, often in association with insects
Type species
Diplogaster goffarti Körner, 1954
comb. Koerneria goffarti Meyl, 1960
Other species
Koerneria erlangensis (Sachs, 1950) Sudhaus & Fürst von Lieven, 2003
Koerneria luziae (Körner, 1954) Meyl, 1960
Koerneria ruehmi Atighi, Pourjam, Kanzaki, Giblin-Davis, De Ley, Mundo-Ocampo & Pedram, 2013
Koerneria sinodendroni (Körner, 1954) Meyl, 1960
Koerneria systenoceri (Körner, 1954) Meyl, 1960
Following this restricted definition of Koerneria, most of the remaining species of Koerneria sensu lato are transferred to the resurrected genera Anchidiplogaster and Allodiplogaster. One species, which was previously combined as Koerneria dubia (Hnatewytsch, 1929) Sudhaus & Fürst von Lieven, 2003 is returned to Anchidiplogaster based on a suite of characters unique to this taxon as well as by the lack of stomatal apodemes, an absolute character of Koerneria as defined both previously and herein:
Anchidiplogaster Paramonov, 1952
Generic diagnosis
1) Miniscule, undivided stoma with two small, similarly sized pyramidal teeth (one dorsal and one right subventral)
2) Stomatal apodemes absent
3) Male genital papillae absent
4) Testis without flexure
Type and only species
=Diplogaster dubia Hnatewytsch, 1929
comb. Anchidiplogaster Paramonov, 1952
All but one of the remaining species of Koerneria sensu lato are transferred to the other resurrected genus, Allodiplogaster. This name has priority (ICZN 23.1) over other names that are available for this taxonomic grouping, which consist of Diplenteron Andrássy, 1964, Glauxinemella Gagarin, 1998, and Gobindonema Khera, 1970. Furthermore, Allodiplogaster is separated into two putatively monophyletic groups of species, which we designate as the “henrichae group” and “striata group” based on morphological and biological evidence.
Allodiplogaster Paramonov & Sobolev in Skrjabin, Shikobalova, Sobolev, Paramonov & Sudarikov, 1954
Fig. 2
=Diplenteron Andrássy, 1964: Diplenteron colobocercus Andrássy, 1964
=Gobindonema Khera, 1970: Gobindonema filicaudata Khera, 1970
nec Gobindonema Sood & Prashad, 1974 (Trichostrongylidae)
=Glauxinemella Gagarin, 1998: Glauxinemella striata Gagarin, 1998
Generic diagnosis
1) Stomatal dimorphism occasionally present
2) Body-wall cuticle with clear vertical striations
3) Cheilostom separated into six per- and interradial figs or rugae
4) Stegostom with dorsal claw-like tooth, right subventral tooth, and left subventral serrated figs or ridges
5) Postdental region of stegostom with left and right apodemes directed subventrad
6) Female gonad amphidelphic; rarely prodelphic
7) Distal triplet papillae of males (v5-7) close to each other
8) Tail of male and females highly variable in shape
9) Known from variable habitats including terrestrial insect associates and aquatic species
Type species
Diplogaster henrichae Sachs, 1950
comb. Allodiplogaster henrichae Paramonov & Sobolev in Skrjabin, Shikobalova, Sobolev, Paramonov & Sudarikov, 1954
Other species
henrichae group of Allodiplogaster
1) Labial sensilla usually papilliform
2) Female tail usually conical with or without filiform tip
3) Male tail usually conical with short spike
4) Male genital papillae short and papilliform
5) Known from terrestrial habitats, often in association with insects
Allodiplogaster colobocerca (Andrássy, 1964), comb. n.
=Mononchoides potohikus Yeates, 1969
Allodiplogaster hirschmannae (Sachs, 1950), comb. n.
Allodiplogaster histophora (Weingärtner, 1955), comb. n.
Allodiplogaster hylobii (Fuchs, 1915), comb. n.
Allodiplogaster incurva (Körner, 1954), comb. n.
Allodiplogaster labiomorpha (Kühne, 1995), comb. n.
Allodiplogaster lepida (Andrássy, 1958), comb. n.
Allodiplogaster lucani (Körner, 1954), comb. n.
Allodiplogaster pierci (Massey, 1967), comb. n.
Allodiplogaster pini (Fuchs, 1931), comb. n.
Allodiplogaster robinicola (Rühm, 1956), comb. n.
Allodiplogaster sudhausi (Fürst von Lieven, 2008), comb. n.
striata group of Allodiplogaster
1) Labial sensilla setiform
2) Male and female tail usually elongate-conical, with or without filiform tip
3) Male genital papillae setiform
4) Known from aquatic habitats
Allodiplogaster angarensis (Gagarin, 1983), comb. n.
Allodiplogaster aquatica (Dassonville & Heyns, 1984), comb. n.
Allodiplogaster baicalensis (Tsalolichin, 1972), comb. n.
Allodiplogaster carinata (Zullini, 1981), comb. n.
Allodiplogaster didentata (Hnatewytsch, 1929), comb. n.
=Diplogaster curvidentatus Altherr, 1938
=Diplogaster obscuricola Altherr, 1938
=Diplogaster quadridentatus Altherr, 1938
Allodiplogaster filicaudata (Khera, 1970), comb. n.
Allodiplogaster ivanegae (Gagarin, 1983), comb. n.
Allodiplogaster lupata (Shoshin, 1989), comb. n.
Allodiplogaster mordax (Shoshin, 1989), comb. n.
Allodiplogaster mulveyi (Ebsary, 1986), comb. n.
Allodiplogaster pantolaba (Shoshin, 1989), comb. n.
Allodiplogaster pararmata (Schneider, 1938), comb. n.
=Diplogaster armatus apud Filipjev, 1930, nec Hofmänner, 1913
Allodiplogaster regia (Shoshin, 1989), comb. n.
Allodiplogaster ruricula (Gagarin, 1983), comb. n.
Allodiplogaster sphagni (Soós, 1938), comb. n.
Allodiplogaster strenua (Gagarin, 1983), comb. n.
Allodiplogaster striata (Gagarin, 1998), comb. n.
Allodiplogaster tenuipunctata (Altherr, 1938), comb. n.
Allodiplogaster terranova (Ebsary, 1986), comb. n.
The single remaining species previously included in Koerneria sensu lato is transferred to Pristionchus Kreis, 1932. This transfer is supported by the absence of subventral apodemes, the key character diagnosing Koerneria sensu lato:
Pristionchus macrospiculum (Altherr, 1938), comb. n.
Discussion
The separation of Koerneria as circumscribed herein from Allodiplogaster, Anchidiplogaster, and Pristionchus is strongly supported by the structure of the cheilostom, arrangement of male genital papillae, and phylogeny as inferred independently from molecular sequence characters (Atighi et al. 2013; Kanzaki et al. 2011, 2013, 2014b). Although for the previous, wider definition of Koerneria the name Koerneria was itself a junior synonym of Allodiplogaster, the name Koerneria is retained for a group of six described species, all of which are unambiguously unified by morphological characters and which are represented by a clade not nested within any other valid genus. Furthermore, species of Koerneria in the revised sense are apparently the sister group to most or all species of Diplogastridae, with the possible one exception of Leptojacobus dorci (see Kanzaki et al. 2014b), and therefore the revision of this genus will be useful for ongoing research on the family as a comparative model system.
The distinct morphology of Anchidiplogaster dubia, especially its lack of genital papillae in several observed male specimens separates this species from all other Diplogastridae and supports its reestablishment in that genus. Further indicating the distinctness of this species from most other known Diplogastridae is its unreflexed testis flexure, as a flexure was previously considered a plesiomorphic character of the entire family (Sudhaus and Fürst von Lieven 2003, Andrássy 2005), although one other diplogastrid genus, Leptojacobus Kanzaki, Ragsdale, Susoy & Sommer, 2014, has since been described to have no flexure. In the original description of Anchidiplogaster dubia, the above missing features were explicitly given as diagnostic characters (Hnatewytsch 1929) and thus were unlikely to be simply missed in all specimens examined. An undivided stoma is also unusual among most diplogastrids with subventral teeth, although this type of stoma is present in Koerneria in the revised sense and is thus insufficient in itself to diagnose Anchidiplogaster. The presence of asymmetrical teeth and a glandular postcorpus clearly support its identity as a diplogastrid, the otherwise divergent stomatal morphology in this species, namely its diminutive, undivided stomatal cavity armed with triangular teeth, obscure its relationships to other taxa within the family. Because molecular characters are not available for this species, its position in Diplogastridae cannot be predicted with any certainty.
The split of Allodiplogaster into the henrichae and striata groups is also supported by morphology, principally by the tail of both sexes, which is usually much longer in striata group than in henrichae group, and by the male genital papillae and labial sensilla, which are distinctly setiform in striata group. The separation of the two proposed groups of Allodiplogaster is only confounded by overlapping morphological characters, namely the fish-bone-like swellings along the pharyngeal lumen that are present in henrichae group species, Allodiplogaster spp. RGD227 and RGD228, as well as in one species of the striata group, Allodiplogaster carinata, although not in another striata group species such as Allodiplogaster pararmata (Fürst von Lieven 2001, Fürst von Lieven and Sudhaus 2000, Kanzaki et al. unpubl. obs.). However, no species of the striata group have been molecularly characterized thus far, so the phylogenetic position of that group has yet to be tested by gene sequence data. Reisolation of species, particularly of the striata group, will be necessary for fully testing the system of intrageneric grouping presented here. Complete taxonomic revision of Allodiplogaster is therefore not performed here due to a lack of molecular evidence, although further studies may confirm the separation of the two species groups designated here into different genera.
Other morphological characters may further support to separate henrichae group from as a monophyletic clade distinct from the striata group and from Koerneria. Kühne (1995) examined three henrichae group species by scanning electron microscopy and reported important morphological features of the male tail: the modification in v5 and v6 subventral distal papillae, whereby v5 is rooted in a socket-like base and has split tip, and v6 having two anterior and posterior appendages and being likewise rooted in a socket-like base (Fig. 2). This morphology, which is similar to the trifurcate genital papillae of Diplogasteroides spp. (Kiontke et al. 2001, Kanzaki et al. 2013), is otherwise unique among Diplogastridae and Rhabditidae thus far characterized (e.g., Kiontke and Sudhaus 2000, Kanzaki et al. 2012a), and has additionally been found in two undescribed henrichae group species (Giblin-Davis et al. unpubl. obs.). The diagnostic utility of modified genital papillae has already been shown for another clade of Diplogastridae: in Pristionchus and the closely related genus Parapristionchus Kanzaki, Ragsdale, Herrmann, Mayer, Tanaka & Sommer, 2012, the v5 and v6 papillae are shrouded at the base by a socket-like structure, where the tip of v6 is split into two papilla-like projections (e.g., Kanzaki et al. 2012b, 2014a). Therefore, further examination of male tail morphology in other species of Koerneria sensu lato may confirm the importance of v5 and v6 as diagnostic of the henrichae group of Allodiplogaster. Yet another putative character for the henrichae group is the presence of a prerectum, as suggested to distinguish the “Diplenteron group” of Koerneria sensu lato (Fürst von Lieven 2008) and which was reported for Allodiplogaster colobocerca and Allodiplogaster sudhausi. The prerectum, which is a shallow but distinct constriction separating the posterior part of the intestine from the anterior part, can be found in three undescribed Allodiplogaster species (Kanzaki and Giblin-Davis unpubl. obs.), lending preliminary support to this idea. This character is not present in Koerneria nor in any species of striata group as interpreted from original descriptions, and therefore it may additionally support monophyly of the henrichae group.
Citation
Kanzaki N, Ragsdale EJ, Giblin-Davis RM (2014) Revision of the paraphyletic genus Koerneria Meyl, 1960 and resurrection of two other genera of Diplogastridae (Nematoda). ZooKeys 442: 17–30. doi: 10.3897/zookeys.442.7459
Footnotes
Dimorphism has been confirmed in at least two species in this group, including one undescribed species (Kanzaki and Ragsdale et al. unpubl. obs.)
Supplementary materials
Species status and several key characters of Koerneria sensu lato in the revised genera.
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Natsumi Kanzaki, Erik J. Ragsdale, Robin M. Giblin-Davis
Data type: Summary of typological characters as a spread sheet.
Explanation note: Taxonomic history, morphological (typological) characters, and life-history traits of Koerneria sensu lato in the revised genera.
References
- Altherr E. (1938) La faune des mines de Bex, avec étude spécial des Nématodes. Revue Suisse de Zoologie 45: 567–720 [Google Scholar]
- Andrássy I. (1958) Diplogaster lepidus n. sp. und der Schlüssel der Diplogaster-Arten von unpaarigem Ovar. Nematologica 3: 295–300. doi: 10.1163/187529258X00058 [Google Scholar]
- Andrássy I. (1964) Neue Nematoden-Arten aus Ungarn, III. Fünf Arten. Opuscula Zoologica Budapest 5: 9–23 [Google Scholar]
- Andrássy I. (1984) Klasse Nematoda. Gustav Fischer Verlag, Stuttgart, 509 pp [Google Scholar]
- Andrássy I. (2005) Free-living nematodes of Hungary, I (Nematoda errantia). Hungarian National History Museum, Budapest, 518 pp [Google Scholar]
- Atighi MR, Pourjam E, Kanzaki N, Giblin-Davis RM, De Ley IT, Mundo-Ocampo M, Pedram M. (2013) Description of two new species of diplogastrid nematodes (Nematoda: Diplogastridae) from Iran. Journal of Nematode Morphology and Systematics 16: 113–129 [Google Scholar]
- Dassonville AF, Heyns J. (1984) Freshwater nematodes from South Africa. 7. New and known species collected in Skinnerspruit, Pretoria. Phytophylactica 16: 15–32 [Google Scholar]
- Ebsary BA. (1986) Mononchoides andersoni n. sp. and two new species of Koerneria (Nematoda: Diplogasteridae). Canadian Journal of Zoology 64: 2012–2020. doi: 10.1139/z86-304 [Google Scholar]
- Filipjev IN. (1930) Les nématodes libres de la baie de la Neva et de l’extrémité orientale du Golfe de Finlande. Archiv für Hydrobiologie 21: 1–64 [Google Scholar]
- Fuchs G. (1915) Die Naturgeschichte der Nematoden und einiger anderer Parasiten 1. des Ips typographus L. 2. des Hylobius abietis L. Zoologische Jahrbücher (Systematik) 38: 109–222 [Google Scholar]
- Fuchs G. (1931) Die Genera: 1. Rhabditolaimus Fuchs, 2. Neodiplogaster Cobb, 3. Tylenchodon Fuchs. Zentralblatt für das gesamte Forstwesen 57: 177–194 [Google Scholar]
- Fürst von Lieven A. (2001) Vergleichende und funktionelle Morphologie der Mundhöhle der Diplogastrina (Nematoda) mit einem ersten Entwurf der Phylogenie dieses Taxons. Ph.D. thesis, Freie Universität Berlin, Germany.
- Fürst von Lieven A. (2008) Koerneria sudhausi n. sp. (Nematoda: Diplogastridae); a hermaphroditic diplogastrid with an egg shell formed by zygote and uterine components. Nematology 10: 27–45. doi: 10.1163/156854108783360087 [Google Scholar]
- Fürst von Lieven A, Sudhaus W. (2000) Comparative and functional morphology of the buccal cavity of Diplogastrina (Nematoda) and a first outline of the phylogeny of this taxon. Journal of Zoological Systematics and Evolutionary Research 38: 37–63. doi: 10.1046/j.1439-0469.2000.381125.x [Google Scholar]
- Gagarin VG. (1983) New species of the genus Mononchoides, Nematoda, Diplogasterinae. Zoologicheskii Zhurnal 56: 1245–1248. [In Russian] [Google Scholar]
- Gagarin VG. (1998) Glauxinemella striata gen. et sp. n. from Europe (Nematoda: Diplogasterida. Zoosystematica Rossica 7: 239–241 [Google Scholar]
- Giblin-Davis RM, Ye W, Kanzaki N, Williams D, Morris K, Thomas WK. (2006) Stomatal ultrastructure, molecular phylogeny, and description of Parasitodiplogaster laevigata n. sp. (Nematoda: Diplogastridae), a parasite of fig wasps from Ficus laevigata from Florida. Journal of Nematology 38: 137–149 [PMC free article] [PubMed] [Google Scholar]
- Hnatewytsch B. (1929) Die Fauna der Erzgruben von Schneeberg im Erzgebirge. Zoologische Jahrbücher (Systematik) 56: 173–261 [Google Scholar]
- Hofmänner B. (1913) Contribution à l’étude des Nématodes libres de Lac Léman. Revue Suisse de Zoologie 21: 589–658 [Google Scholar]
- Kanzaki N. (2013) Simple methods for morphological observation of nematodes. Nematological Research 43: 9–13 [Google Scholar]
- Kanzaki N, Giblin-Davis RM, Davies K, Ye W, Center BJ, Thomas WK. (2009) Teratodiplogaster fignewmani gen. nov., sp. nov. (Nematoda: Diplogastridae) from the syconia of Ficus racemosa in Australia. Zoological Science 26: 569–578. doi: 10.2108/zsj.26.569 [DOI] [PubMed] [Google Scholar]
- Kanzaki N, Ragsdale EJ, Herrmann M, Mayer WE, Sommer RJ. (2012a) Description of three Pristionchus species (Nematoda: Diplogastridae) from Japan that form a cryptic species complex with the model organism P. pacificus. Zoological Science 29: 403–417. doi: 10.2108/zsj.29.403 [DOI] [PubMed] [Google Scholar]
- Kanzaki N, Ragsdale EJ, Herrmann M, Mayer WE, Tanaka R, Sommer RJ. (2012b) Parapristionchus giblindavisi n. gen, n. sp. (Rhabditida: Diplogastridae) isolated from stag beetles (Coleoptera: Lucanidae) in Japan. Nematology 14: 933–947. doi: 10.1163/156854112X635878 [Google Scholar]
- Kanzaki N, Ragsdale EJ, Herrmann M, Sommer RJ. (2014a) Two new and two recharacterized species from a radiation of Pristionchus (Nematoda: Diplogastridae) in Europe. Journal of Nematology 46: 60–74 [PMC free article] [PubMed] [Google Scholar]
- Kanzaki N, Ragsdale EJ, Susoy V, Sommer RJ. (2014b) Leptojacobus dorci n. gen., n. sp. (Nematoda: Diplogastridae), an associate of Dorcus stag beetles (Coleoptera: Lucanidae). Journal of Nematology 46: 50–59 [PMC free article] [PubMed] [Google Scholar]
- Kanzaki N, Taki H, Masuya H, Okabe K, Tanaka R, Abe F. (2011) Diversity of stag beetle-associated nematodes in Japan. Environmental Entomology 40: 281–288. doi: 10.1603/EN10182 [Google Scholar]
- Kanzaki N, Tanaka R, Hirooka Y, Maehara N. (2013) Description of Diplogasteroides andrassyi n. sp., associated with Monochamus grandis and Pinaceae trees in Japan. Journal of Nematode Morphology and Systematics 16: 35–47 [Google Scholar]
- Khera S. (1970) Nematodes from the banks of still and running waters. IX. Two new genera belonging to subfamily Diplogasterinae Micoletzky from India. Revista Brasileira de Biologia (Rio de Janeiro) 30: 405–409 [PubMed] [Google Scholar]
- Kiontke K, Manegold A, Sudhaus W. (2001) Redescription of Diplogasteroides nasuensis Takaki, 1941 and D. magnus Völk, 1950 (Nematoda: Diplogastrina) associated with Scarabaeidae (Coleoptera). Nematology 3: 817–832. doi: 10.1163/156854101753625317 [Google Scholar]
- Kiontke K, Sudhaus W. (2000) Phasmids in male Rhabditida and other secernentean nematodes. Journal of Nematode Morphology and Systematics 3: 1–37 [Google Scholar]
- Körner H. (1954) Die Nematodenfauna des vergehenden Holzes und ihre Beziehungen zu den Insekten. Zoologische Jahrbücher (Systematik) 82: 245–353 [Google Scholar]
- Kreis HA. (1932) Beiträge zur Kenntnis pflanzenparasitischer Nematoden. Zeitschrift für Parasitenkunde 5: 184–194. doi: 10.1007/BF02120641 [Google Scholar]
- Kühne R. (1995) Nematoden mit phoretischer Bindung an Dung vergrabende Geotrupes-Käfer: ein morphologischer Vergleich von Diplogaster henrichae Sachs, 1950, D. hirschmannae Sachs, 1950, und D. labiomorphus sp. n. (Nematoda: Diplogasteridae). Nematologica 41: 7–34. doi: 10.1163/003925995X00026 [Google Scholar]
- Massey CL. (1967) Nematodes associated with tree-infesting insects: Paurodontidae new family and Misticiinae new subfamily with a description of one new genus and four new species. Canadian Journal of Zoology 45: 779–786. doi: 10.1139/z67-089 [Google Scholar]
- Mayer WE, Herrmann M, Sommer RJ. (2007) Phylogeny of the nematode genus Pristionchus and implications for biodiversity, biogeography and the evolution of hermaphroditism. BMC Evolutinary Biology 7: . doi: 10.1186/1471-2148-7-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer WE, Herrmann M, Sommer RJ. (2009) Molecular phylogeny of beetle associated diplogastrid nematodes suggests host switching rather than nematode-beetle coevolution. BMC Evolutionary Biology 9: . doi: 10.1186/1471-2148-9-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyl AH. (1960) Die Freilebenden Erd- und Süßwassernematoden (Fadenwürmer). In: Brohmer P, Ehrmann P, Ulmer G. (Eds) Die Tierwelt Mitteleuropas, Vol.1 (5a). Germany, Quelle & Meyer, Leipzig, 1–164
- Paramonov AA. (1952) Opyt ekologicheskoi klassifikatsii fitonematod. Trudy Gelmintologicheskoi Laboratorii, Akademia Nauk SSSR (Moskva) 6: 338–369 [Google Scholar]
- Ragsdale EJ, Kanzaki N, Roeseler W, Herrmann M, Sommer RJ. (2013) Three new species of Pristionchus (Nematoda: Diplogastridae) show morphological divergence through evolutionary intermediates of a novel feeding polymorphism. Zoological Journal of Linnean Society 168: 671–698. doi: 10.1111/zoj.12041 [Google Scholar]
- Rahm G. (1928) Alguns nematodes parasitas e semi-parasitas das plantas culturães do Brasil. Achivos do Instituto de Biológico de Defesa Agricola e Animal (São Paulo) 1: 239–251 [Google Scholar]
- Rühm W. (1956) Die Nematoden der Ipiden. Parasitologische Schriften, Reihe 6: 1–435 [Google Scholar]
- Sachs H. (1950) Die Nematodenfauna der Rinderexkremente. Eine ökologisch-systematische Studie. Zoologische Jahrbücher (Systematik) 79: 209–272 [Google Scholar]
- Schneider W. (1938) Diplogaster pararmatus n. n. nebst Bemerkungen über einige andere Diplogaster-Arten. Zoologischer Anzeiger 121: 37–43 [Google Scholar]
- Shoshin AV. (1989) New nematode species of the families Diplogasteridae and Diplogasteroididae from Lake Baikal. In: Ryss AY. (Ed) Tylenchida and Rhabditida (Nematoda) – Parasites of plants and insects.Trudy Zoologicheskogo Instituta Akademii Nauk SSSR, Leningrad, 83–95. [In Russian]
- Skrjabin KI, Shikobalova NP, Sobolev AA, Paramonov AA, Sudarikov AA. (1954) Camellanata, Rhabditata, Tylenchata, Trichocephalata and Dioctophymata and the distribution of parasitic nematodes by hosts. Izdatel’stvo Akademii Nauk SSSR (Moskva) 4: 1–927. [In Russian] [Google Scholar]
- Sood ML, Parshad VR. (1974) Gobindonema boodugi gen.n., sp.n. Nematoda, Trichostrongylidae from rodents in Ludhiana Punjab, India. Acta Parasitologica Polonica 22: 93–96 [Google Scholar]
- Soós Á. (1938) Zwei neue tyrphobionte Nematoden-Arten. Zoologischer Anzeiger 124: 281–286 [Google Scholar]
- Sudhaus W, Fürst von Lieven A. (2003) A phylogenetic classification and catalogue of the Diplogastridae (Secernentea, Nematoda). Journal of Nematode Morphology and Systematics 6: 43–89 [Google Scholar]
- Tsalolichin SY. (1972) New species of free-living nematodes from Lake Baikal. Zoologicheskii Zhurnal 51: 1559–1653. [In Russian] [Google Scholar]
- Weingärtner I. (1955) Versuch einer Neuordnung der Gattung Diplogaster Schulze 1857 (Nematoda). Zoologische Jahrbücher (Systematik) 83: 248–317 [Google Scholar]
- Yeates GW. (1969) Three new Rhabditida (Nematoda) from New Zealand dine sands. Nematologica 15: 115–121. doi: 10.1163/187529269X00155 [Google Scholar]
- Zullini A. (1981) Three new species of Diplogastrids (Nematoda). Bollettino del Museo Civico di Storia Naturale di Verona 7: 395–404 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Species status and several key characters of Koerneria sensu lato in the revised genera.
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Natsumi Kanzaki, Erik J. Ragsdale, Robin M. Giblin-Davis
Data type: Summary of typological characters as a spread sheet.
Explanation note: Taxonomic history, morphological (typological) characters, and life-history traits of Koerneria sensu lato in the revised genera.