Abstract
Mycobacteria inhabit a wide range of intracellular and extracellular environments. Many of these environments are highly dynamic and therefore mycobacteria are faced with the constant challenge of redirecting their metabolic activity to be commensurate with either replicative growth or a non-replicative quiescence. A fundamental feature in this adaptation is the ability of mycobacteria to respire, regenerate reducing equivalents and generate ATP via oxidative phosphorylation. Mycobacteria harbor multiple primary dehydrogenases to fuel the electron transport chain and two terminal respiratory oxidases, an aa3-type cytochrome c oxidase and cytochrome bd-type menaquinol oxidase, are present for dioxygen reduction coupled to the generation of a protonmotive force. Hypoxia leads to the downregulation of key respiratory complexes, but the molecular mechanisms regulating this expression are unknown. Despite being obligate aerobes, mycobacteria have the ability to metabolize in the absence of oxygen and a number of reductases are present to facilitate the turnover of reducing equivalents under these conditions (e.g. nitrate reductase, succinate dehydrogenase/fumarate reductase). Hydrogenases and ferredoxins are also present in the genomes of mycobacteria suggesting the ability of these bacteria to adapt to an anaerobic-type of metabolism in the absence of oxygen. ATP synthesis by the membrane-bound F1FO-ATP synthase is essential for growing and non-growing mycobacteria and the enzyme is able to function over a wide range of protonmotive force values (aerobic to hypoxic). The discovery of lead compounds that target respiration and oxidative phosphorylation in Mycobacterium tuberculosis highlights the importance of this area for the generation of new front line drugs to combat tuberculosis.
Overview of mycobacterial respiration and oxidative phosphorylation
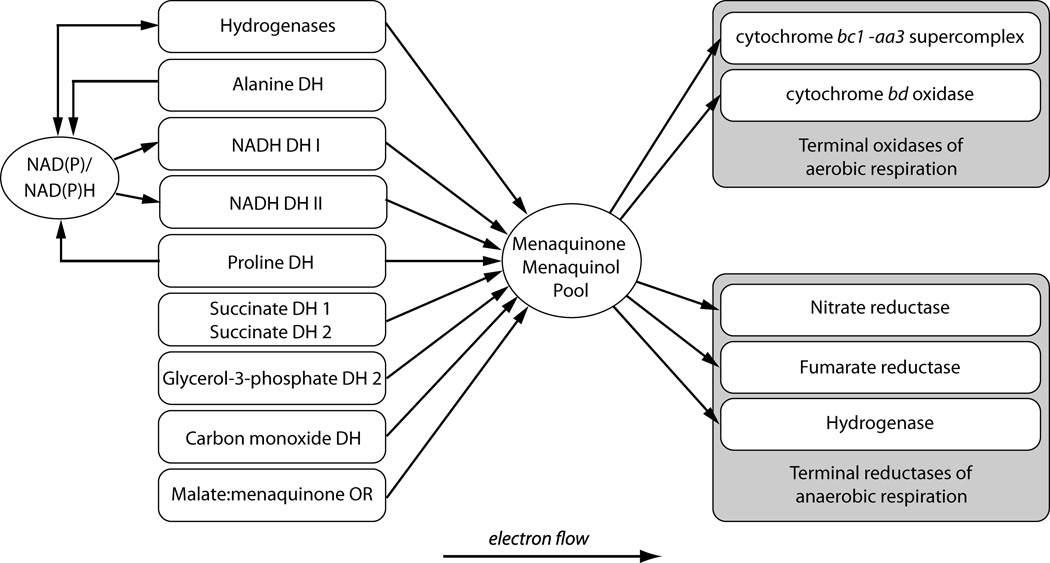
The genus Mycobacterium comprises a group of obligately aerobic bacteria that have adapted to inhabit a wide range of intracellular and extracellular environments. A fundamental feature in this adaptation is the ability to respire and generate energy from variable sources or to sustain metabolism in the absence of growth. Early studies on respiration demonstrated that Mycobacterium tuberculosis H37Rv, grown in the lungs of infected mice, had high rates of endogenous respiration that were not stimulated by exogenous substrates (e.g. acetate, pyruvate, glucose, glycerol, lactate) (1). In contrast, cells grown in vitro respired these substrates at high rates. Fatty acids, however, stimulated the respiration of in vivo grown M. tuberculosis, suggesting for the first time that M. tuberculosis switches to different energy sources in host tissues to fuel respiration. These early studies pointed to the fact that electron donor utilization and respiration are precisely controlled in M. tuberculosis, not only in response to growth rate, but also in response to the carbon and energy sources used for growth. The pioneering work of Brodie and colleagues on Mycobacterium phlei established much of the primary information on the electron transport chain and oxidative phosphorylation system in mycobacteria (reviewed in (2)). Since these early studies, comparatively few biochemical studies have been performed on the electron transport components and the energetics of respiration in mycobacterial species. With the advent of microbial genome sequencing, sequence analyses have revealed that branched pathways exist in mycobacterial species for electron transfer from many low potential reductants via quinol, including H2to oxygen (Figure 1). Unlike other bacteria, there appears to be little redundancy in the transfer of electrons to oxygen during growth with only two terminal respiratory oxidases present in mycobacteria, an aa3-type cytochrome c oxidase (encoded by ctaBCDE) belonging to the heme-copper respiratory oxidase family and cytochrome bd-type menaquinol oxidase (cydABCD) (Figure 1). Mycobacteria coordinate the expression of these oxidases in response to oxygen supply to achieve maximal efficiency of oxygen utilization. The rationale for this coordinated expression is largely based on the assumption that the mycobacterial aa3-type cytochrome c oxidase and cytochrome bd-type have markedly different affinities for oxygen in mycobacteria, but this remains to be experimentally shown. Furthermore, the molecular mechanisms governing the regulation of terminal oxidase expression in mycobacterial species are unknown. In the absence of oxygen, mycobacterial growth is inhibited, even if alternative electron acceptors are present (e.g. nitrate, fumarate). Despite growth being inhibited, mycobacteria are able to metabolize exogenous and endogenous energy sources under low oxygen for maintenance functions. Whether mycobacteria utilize energy spilling or overflow metabolism (3) under these conditions to balance the rate of catabolic versus anabolic reactions remains to be determined. The electron acceptors and mechanisms to recycle reducing equivalents under these conditions are poorly understood. Irrespective of the oxygen concentration or the protonmotive force (PMF), ATP synthesis is obligatorily coupled to the electron transport chain and the F1Fo-ATP synthase, but the reasons for this remain unexplained. Research into mycobacterial respiration and oxidative phosphorylation has been energized by the discovery of a new drug (TMC207 – Sirturo - Bedaquiline) that targets the ATP synthase of mycobacteria, suggesting that inhibitors of respiration and ATP synthesis will provide the next generation of front line drugs to combat tuberculosis and nontuberculous mycobacterial disease. The aim of this chapter is to discuss recent advances in understanding the processes of respiration and oxidative phosphorylation in mycobacteria, with a particular focus on the obligate human pathogen M. tuberculosis.
Figure 1.
Organization and components of the electron transport chain in mycobacteria.
Energetics of mycobacteria during growth and non-growth conditions
Mycobacteria generally grow at neutral pH and under these conditions generate a PMF of approximately −180 mV (4). The PMF generated is used to drive proton-coupled energetic processes (e.g. ATP synthesis, solute transport, etc.) (4). The PMF-generating machinery in mycobacteria comprises three energy-conserving steps: complex I (4H+/2e−), complex III (menaquinol-cytochrome c oxidoreductase, 4H+/2e−) and complex IV (2H+/2e−) suggesting that the overall proton translocation stoichiometry for the transfer of 2e− from NADH to oxygen is 10H+/2e−. Based on a ratio of 3H+ utilized by the ATP synthase/ATP synthesized leads to a theoretical maximum P/O ratio (i.e. the number of moles of ADP phosphorylated to ATP per 2e− passing to oxygen) of approximately 3.3 (theoretical maximum). If complex I is bypassed by the non-proton translocating type II NADH:menaquinone oxidoreductase, the P/O ratio would be approximately 2. Electron flow from the menaquinone pool to the cytochrome bd oxidase branch (bypassing complex III and IV) would produce a P/O ratio of 0.67. Measured experimental values for mycobacteria oxidizing NADH or succinate yield P/O ratios of 0.52, and 0.36, respectively (5). Variations in theoretical P/O ratios versus those determined experimentally is well accepted and can be explained by pathways that involve proton leakage (i.e. bypass ATP synthase) or the PMF is used to drive reverse electron transport. When succinate oxidation is coupled to menaquinone reduction, the energetics suggest a reverse electron flow from succinate (lower midpoint redox potential Em = +30 mV) to menaquinone (Em = −74 mV) resulting in lower P/O ratios.
Growth of mycobacteria is sensitive to compounds that dissipate the membrane potential (e.g. protonophores and valinomycin) and these compounds are bactericidal towards growing and non-growing (aerobic or hypoxic) cells further highlighting the importance of the membrane potential in mycobacterial viability (4, 6). Growth is also sensitive to the ionophore monensin, however, the reasons for this are not clear. Interestingly, some solute transporters in mycobacteria are driven by a sodium-motive force (7), but the mechanism used for the generation of the primary sodium gradient in mycobacteria is not known. As the pH of the growth medium changes and becomes mildly acidic, mycobacteria are able to generate a considerable transmembrane pH gradient (ZΔpH) and maintain a constant PMF (4). While proton translocation via the respiratory chain generates the PMF during respiration with oxygen as the terminal electron acceptor, it is not clear how the PMF is established in the absence of oxygen under anaerobic growth conditions. Anaerobic bacteria are able to generate a significant PMF (−100 mV) using their membrane-bound F1FO -ATP synthase in the ATP hydrolysis direction (8). The ATPase activity (proton-pumping) of the enzyme is fuelled by ATP produced by substrate level phosphorylation. This mechanism does not appear to operate in mycobacterial cells where the F1FO-ATP synthase has been reported to have latent ATPase activity when measured in inverted membrane vesicles (9, 10). Whether the enzyme is also latent in actively growing cells is not known and therefore the potential exists for this enzyme to function as a primary proton pump in the absence of oxygen and a functional respiratory chain to generate the PMF. Rao et al. (6) have reported that hypoxic, non-replicating M. tuberculosis generate a total PMF of −113 mV, −73 mV of electrical potential (ΔΨ) and −41 mV of ZΔpH. The addition of thioridazine, a compound that targets NDH−2, results in dissipation of the ΔΨ and significant cell death suggesting that NADH is an important electron donor for the generation of the ΔΨ under hypoxic conditions. The addition of TMC207, a specific inhibitor of the F1FO-ATP synthase, was bactericidal against hypoxic, non-replicating M. tuberculosis, but had no effect on the ΔΨ (6).
Electron donors fueling respiration in mycobacteria
Mycobacterial species use a variety of primary dehydrogenases to deliver electrons from central metabolism into the respiratory chain to generate energy (Figure 1).
Proton-pumping type I NADH dehydrogenase and non-proton pumping type II NADH dehydrogenase
The major entry point is the transfer of electrons from NADH oxidation to quinone reduction (e.g. ubiquinone or menaquinone). In bacteria three different types of respiratory NADH dehydrogenases have been identified and characterized on the basis of reaction mechanism, subunit composition and protein architecture (11). These include the proton-pumping type I NADH dehydrogenase (NDH-1, complex I), non-proton pumping type II NADH dehydrogenase (NDH-2) and sodium-pumping NADH dehydrogenase (NQR). Weinstein et al. identified genes for two classes of NADH:menaquinone oxidoreductases in the genome of M. tuberculosis (12) (Table 1). NDH-1 is encoded by the nuoABCDEFGHIJKLMN operon and transfers electrons to menaquinone, conserving energy by translocating protons across the membrane to generate a PMF (Figure 2). The second class is NDH-2, a non-proton translocating type II NADH dehydrogenase that does not conserve energy and is present in two copies (Ndh and NdhA) in M. tuberculosis (12) (Table 1). NQR has not been reported in mycobacterial genomes.
Table 1.
Electron transport chain components and energy generating machinery of mycobacteria
| Operon/ Gene1 |
Subunits | Rv # | Enzyme Name | In vitro Essentiality | |
|---|---|---|---|---|---|
| Griffin et al2 | Zhang et al3 | ||||
| nuo | nuoABCDEFGHIJKLMN | Rv3145–Rv3158 | Type I NADH:Menaquinone oxidoreductase | nuoDFGHI = yes | nuoDFGL = yes |
| ndh | - | Rv1854c | Type II NADH:Menaquinone oxidoreductase | yes | no |
| ndhA | - | Rv0392c | Type II NADH:Menaquinone oxidoreductase | no | no |
| sdh1 |
sdh1CD sdh1A sdh1B |
Rv0249c Rv0248c Rv0247c |
Succinate:Menaquinone oxidoreductase I | yes | Only sdh1A |
| sdh2 |
sdh2C sdh2D sdh2A sdh2B |
Rv3316 Rv3317 Rv3318 Rv3319 |
Succinate:Menaquinone oxidoreductase II | no | no |
| mqo | - | Rv2852c | Malate:Menaquinone oxidoreductase | no | no |
| pru |
pruA pruB |
Rv1187 Rv1188 |
Proline dehydrogenase and pyrroline-5-carboxylate dehydrogenase | yes | yes |
| gpdA1 | - | Rv0564c | Glycerol-3-phosphase dehydrogenase A1 | no | no |
| gpdA2 | - | Rv2982c | Glycerol-3-phosphase dehydrogenase A2 | no | no |
| glpD1 | - - |
Rv2249c (Rv2250c) |
Glycerol-3-phosphase dehydrogenase D1 | no | yes |
| glpD2 |
glpD2 ldpA |
Rv3302c Rv3303c |
Glycerol-3-phosphase dehydrogenase D2 Dihydrolipoamide dehydrogenase |
yes | yes |
| cox | coxCMSLDEFG | Rv0376c–Rv0368c | Carbon monoxide dehydrogenase | coxLEF = yes | coxCL = yes |
| ald | - | Rv2780 | L-Alanine dehydrogenase | no | no |
| lldD1 |
? pqqE lldD1 |
Rv0692 Rv0693 Rv0694 |
L-Lactate dehydrogenase 1 | no | lldD1 = yes |
| lldD2 |
lldD2 - - |
Rv1872c Rv1871c Rv1870c |
L-Lactate dehydrogenase 2 | no | no |
| Rv0843 | - | (Rv0842) Rv0843 |
Pyruvate dehydrogenase E1 component alpha subunit | no | no |
| pdh |
pdhA pdhB pdhC |
Rv2497c Rv2496c Rv2495c |
Pyruvate dehydrogenase | pdhB = yes | pdhC =yes |
| ace | - | Rv2241 | Pyruvate dehydrogenase E1 component | yes | yes |
| lpd | - | Rv0462 | Dihydrolipoamide dehydrogenase | yes | yes |
| hyc | Rv0081 Rv0082 Rv0083 hycDPQE Rv0088 |
Rv0081-Rv0088 | Formate hydrogenlyase OR Energy-converting hydrogenase related complex (Ehr) (a.k.a HydTB) | hycE and Rv0088 = yes | hycQE = yes |
| qcr |
qcrC qcrA qcrB |
Rv2194 Rv2195 Rv2196 |
Cytochrome bc1 | yes | yes |
| ctaB | - | Rv1451 | Cytochrome c oxidase assembly factor | yes | yes |
| ctaC |
ctaC - |
Rv2200c (Rv2199c) |
Transmembrane Cytochrome C subunit II | yes | Rv2200c = yes |
| ctaD | - | Rv3043c | Cytochrome C oxidase polypeptide I | yes | yes |
| ctaE | - | Rv2193 | Cytochrome C oxidase subunit III | yes | yes |
| cyd |
cydA cydB cydD cydC |
Rv1623c Rv1622c Rv1621c Rv1620c |
Cytochrome bd oxidase | yes | cydBC = yes |
| nar |
narG narH narJ narI |
Rv1161 Rv1162 Rv1163 Rv1164 |
Menaquinone:Nitrate reductase | no | no |
| sirA |
sirA cysH che1 |
Rv2391 Rv2392 Rv2393 |
Sulfite reductase APS reductase ferrochelatase |
yes | Rv2391 and Rv2392 = yes |
| nirBD |
nirB nirD |
Rv0252 Rv0253 |
Nitrite reductase [NAD(P)H] | no | no |
| frd |
frdA frdB frdC frdD |
Rv1552 Rv1553 Rv1554 Rv1555 |
Menaquinone:Fumarate reductase | no | frdA = yes |
| atp | atpIBEFHAGDC and Rv1312 | Rv1303-Rv1312 | F1Fo ATP synthase operon | yes | yes except atpI and Rv1312 |
In the absence of operons confirmed by previous publications, the TB Database operon prediction algorithms (www.tbdb.org) were used to identify potential operons. Rv loci enclosed in parentheses signify weak operon predictions.
Griffin et al. (26)
Zhang et al. (129). N.B. Genes identified as containing both essential and non-essential regions (a “D call”) are referred to as essential here.
Figure 2.
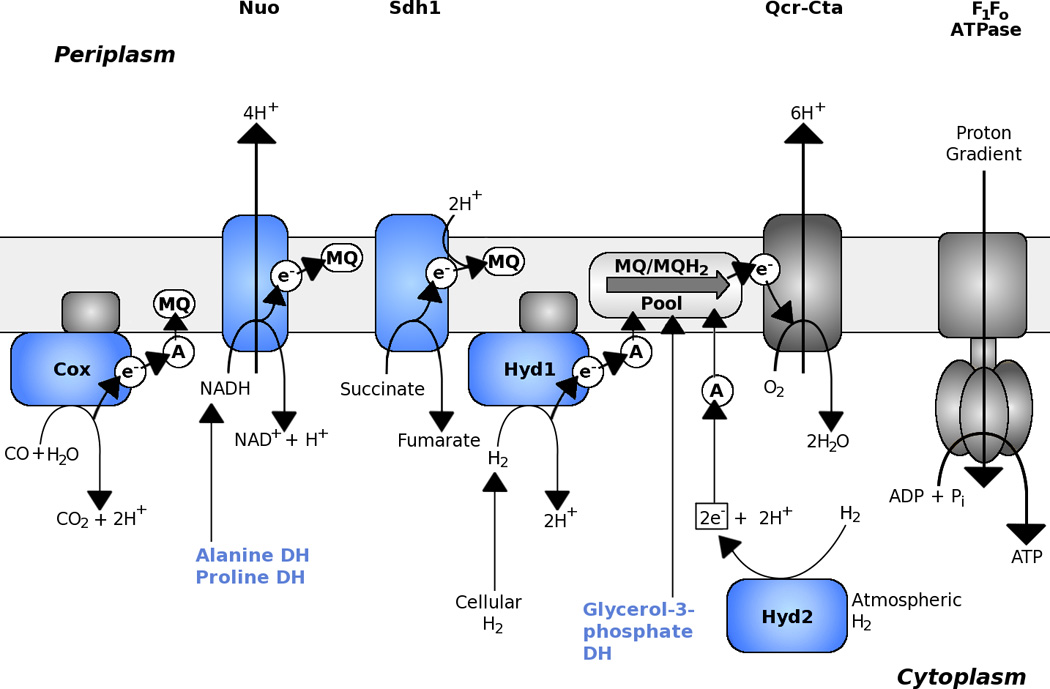
The core respiratory chain of Mycobacteria and components upregulated under energy-limiting conditions. During in vitro exponential growth, Mycobacteria use a classical respiratory chain composed of a Type I NADH:Menaquinone oxidoreductase (Nuo), Succinate:Menaquinone oxidoreductase 1 (SDH1), Cytochrome aa3-bc supercomplex (Qcr-Cta) and F1Fo ATPase. Menaquinone (MQ) is the only quinone present in mycobacterial membranes and reverse electron transport driven by the proton motive force is proposed to facilitate the function of SDH1 and similar enzymes (see text). Components coloured in light blue are upregulated in response to energy-limiting conditions (6). Catalysis and electron flow are indicated by arrows. Other acronyms: Cox – Carbon monoxide dehydrogenase; Hyd – Hydrogenase; DH – Dehydrogenase; A – Unidentified electron acceptor.
NDH-1 is composed of 14 subunits (nuoA-N), which represent a 15704 bp operon in M. tuberculosis (Rv3145 to Rv3158) (Table 1). NuoB, C, D, E, F, and G are peripheral membrane proteins located in the cytoplasmic side while NuoA, H, J, K, L, M, and N are in the membrane section of the complex with multiple predicted transmembrane domains (from 3 to 16). In contrast, the nuo operon has been lost from the genome of the intracellular parasite Mycobacterium leprae except for a single remaining nuoN pseudogene (13). NDH-1 uses flavin mononucleotide (FMN) and iron-sulfur clusters to transport electrons from NADH to the quinone pool (menaquinone). The release of the two electrons during the NADH oxidation produces enough energy to pump four protons across the membrane to generate a PMF (Figure 2). In M. tuberculosis, the nuo operon is neither essential for growth nor persistence in an in vitro Wayne model (6). Mycobacterium smegmatis also contains genes for a type I NADH:menaquinone oxidoreductase (nuoA-N). However, M. smegmatis NDH-I activity is very low, representing about 5% of the NDH-2 activity (14, 15). Rather, increased expression (15-fold) of the nuo operon in M. smegmatis was observed in a carbon-limited chemostat in response to a slow down in growth rate (16). In the slow-growing M. tuberculosis and M. bovis strains, NDH-1 activity is 28% to 50% lower than the NDH-2 activity when measured in membrane fractions of mycobacteria (Vilchèze and Jacobs, unpublished data.), suggesting that the major NADH oxidizing activity is mediated by NDH-2. This is supported by the observation that NADH oxidation by mycobacterial membranes is relatively insensitive to complex I inhibitors (12). Notwithstanding this, M. tuberculosis mutants lacking only one of the subunits of NDH-1, nuoG, have an in vivo phenotype, where this gene was critical for host macrophage apoptosis inhibition and mouse virulence (17). This implies that nuoG and potentially other subunits of NDH-1 are anti-apoptosis factors and are attractive candidates for vaccine development.
Several studies have reported that nuo is down-regulated in M. tuberculosis during mouse lung infection (18), survival in macrophages (19), in both non-replicating persistence (NRP)-1 (1% oxygen saturation) and NRP-2 (0.06% oxygen saturation) relative to aerated mid-log growth (18), and upon starvation in vitro (20). The transcription of NDH-2 (ndh) is also down-regulated in M. tuberculosis during mouse lung infection, but transcript levels for ndh peak during NRP-2 in vitro demonstrating that the pattern of ndh regulation is different between in vivo and in vitro conditions (19). These data are in contrast to Escherichia coli where NDH-1 is usually associated with anaerobic respiratory pathways (e.g. fumarate) and non-coupling dehydrogenases such as NDH-2 are synthesized aerobically (21).
The non-proton translocating NDH-2 is a small monotopic membrane protein (50–60 kDa) that catalyses electron transfer from NADH via FAD (non-covalently bound redox prosthetic group) to quinone. No tertiary structural information exists for either the bacterial, plant or protist NDH-2 enzymes, but the yeast NDH-2 structure was recently solved by two laboratories (22, 23). NDH-2 is widespread in bacteria, and the mitochondria of fungi, plants, and some protists. In some cases more than one copy is present (24). The role(s) of multiple type II NADH dehydrogenases in prokaryotes, plants and parasites is unclear. In some organisms with multiple type II NADH dehydrogenases, one copy appears more essential than the other pointing to non-redundant functional differences (25, 26). M. tuberculosis harbors two copies of NDH-2 (ndh Rv1854c and ndhA Rv0392c) (12) (Table 1), which are well conserved among slow-growing mycobacterial species. In M. tuberculosis, Ndh (1392 bp) and NdhA (1413 bp) share 65% identity, however the FAD and NADH binding motifs, G21SGFGG26 and G177AGPTG182 are highly conserved. Both proteins contain one transmembrane domain located at the amino acids 385 to 407 and 387 to 409, respectively. The Ndh and NdhA proteins of M. tuberculosis have been shown to be functional NADH dehydrogenases that transfer electrons to the quinone pool via a ping-pong reaction mechanism (27). NdhA is not present in M. smegmatis, yet the level of NADH oxidation by M. smegmatis NDH-2 is several fold higher than in M. tuberculosis or M. bovis and represents 95% of the total NADH oxidation measured (15). This might correlate with higher NAD+ concentrations in M. smegmatis compared to M. bovis (three times higher as shown in (15)). In addition, when M. smegmatis and M. bovis were transformed with a replicative plasmid containing ndh, the ratio NAD+/NADH doubled (15) further confirming the involvement of ndh in the oxidation of NADH into NAD+.
Several studies have suggested that ndh is essential for growth of M. tuberculosis (12, 26, 28, 29) (Table 1). Mutations in the ndh gene of M. smegmatis result in a pleitropic effect: temperature sensitivity, amino acid auxotrophy and resistance to the first-line anti-TB drug isoniazid (INH) and its analog and second-line anti-TB drug ethionamide (ETH) (14, 15). The ndh mutants had decreased NADH oxidase activity and increased NADH concentration (14, 15). Selection for ndh mutants grown on rich media (Mueller Hinton) led to the isolation of ndh mutants that were auxotrophic for serine and glycine; this auxotrophy was resolved with complementation by a wild-type copy of ndh. The correlation between ndh mutations and serine/glycine auxotrophy was attributed to the increase in NADH concentration, which might inhibit the first step in serine/glycine biosynthesis (14). The increase in NADH concentration was also responsible for the high resistance to INH and ETH by competitively inhibiting the binding of the INH-NAD or ETH-NAD adduct to the NADH-dependent enoyl-ACP reductase InhA (15). ndh mutants in both slow-growing (M. bovis BCG) and fast-growing (M. smegmatis) mycobacteria had no growth defect at permissive temperature although they had lost up to 90% of their ability to oxidize NADH. Interestingly, in M. smegmatis and M. bovis, the levels of NAD+ cofactor stayed relatively constant despite overexpression of ndh or mutations in ndh, which highly reduced their NADH oxidation capability (15), suggesting that the NAD+ pool is tightly regulated in mycobacteria to maintain essential biochemical functions.
NDH-2 has not been reported in mammalian mitochondria leading to the proposal that these enzymes may represent a potential drug target for the treatment of human pathogens (6, 12, 27, 30, 31) and intracellular parasites (32). Despite the potential of NDH-2 as drug target, no potent nanomolar inhibitors of NDH-2 are known. Several classes of compounds are proposed to target the enzyme at micromolar concentrations (e.g. phenothiazine analogues, platanetin, quinolinyl pyrimidines) (12, 27, 33), but the mechanism of inhibition remains unknown. Despite poor in vitro activity, drugs of the phenothiazine family (trifluoroperazine, chlorpromazine) have potent activity in vitro against drug-susceptible and drug-resistant M. tuberculosis strains (34, 35). A phenothiazine analog was also tested in a mouse model of acute M. tuberculosis infection and found to reduce by 90% the M. tuberculosis bacterial load in the lungs after 11 days of treatment compared to a 3 to 4-log reduction in colony-forming units (CFUs) with the INH or rifampicin control (12). From a library of microbial products, two new compounds, scopafungin and gramicidin S, were identified as inhibitors of M. smegmatis NDH-2 with IC50 values better than trifluoperazine (36). There is a crucial need for new drug targets to inhibit M. tuberculosis and the electron transport chain is a very attractive avenue. However, reduction in NDH-2 activity has been linked to INH and ETH resistance in both slow- and fast-growing mycobacteria (15) and phenothiazines have been shown to be antagonistic with INH (30). Therefore, the development of NDH-2 inhibitors will have to ascertain that interference with current drug therapy does not occur.
Some interesting questions arise from these observations. Why do mycobacteria use type II NADH dehydrogenases to recycle NADH, when they could continue to use the energy conserving and PMF generating NDH-I? One potential explanation is that because type II NADH dehydrogenases are non-proton translocating, they will not be impeded by a high PMF, which could ultimately slow down metabolic flux due to back-pressure on the system. This mechanism is akin to a “relief valve” that would allow for a higher metabolic flux and ultimately higher rates of ATP synthesis, at the expenses of low energetic efficiency of the respiratory chain. Secondly, why is ndh an essential gene when mycobacteria could also use nuo? The fact that ndh is essential implies that mycobacteria do not have another mechanism to recycle NADH during normal aerobic growth. Alternatively, this is the only NADH dehydrogenase that is operating under these growth conditions and the activity of this enzyme is essential for maintaining an energized membrane. Compounds that target NDH-2 are bactericidal towards hypoxic non-replicating M. tuberculosis suggesting that the respiratory chain is essential for the recycling of NADH under these conditions (6).
Multiple succinate dehydrogenases and fumarate reductase: an essential link between central metabolism and respiration in mycobacteria
Succinate dehydrogenase forms complex II of the respiratory chain and couples oxidative phosphorylation to central carbon metabolism by being an integral part of the TCA cycle (Figure 2). This enzyme catalyzes the oxidation of succinate to fumarate wherein two electrons are transferred to quinol. The reverse reaction can be catalyzed by fumarate reductase, which is involved in anaerobic respiration (Figure 3). Fumarate reductase and succinate dehydrogenase are closely related enzymes and the reaction catalyzed cannot be predicted based on the primary sequence alone. Most mycobacteria harbour two annotated succinate dehydrogenases, SDH1 and SDH2 (Table 1). SDH2 has high homology to SDH enzymes from other species and is encoded by four genes sdhC, sdhD, sdhA and sdhB. The genes sdhA and sdhB encode for the cytoplasmic part of the enzyme where the succinate to fumarate reaction takes place (SdhA) and electrons are shuttled via three iron-sulfur clusters (SdhB) to the membrane subunits SdhC and SdhD, which catalyze the electron transfer to menaquinone. SdhA (encoded by Rv0248c) and SdhB (Rv0247c) subunits of SDH1 are similar to the common SDH enzyme. However, the other two genes (Rv0250c and Rv0249c) in the operon show no homology to membrane-bound components of SDH and fumarate dehydrogenase FRD and are specific to the phylum Actinobacteria. Gene expression data show that all four genes of SDH1 in M. smegmatis are expressed in concert (16). As an exception among mycobacteria, M. tuberculosis encodes a third complex (frdABCD) that is annotated as a fumarate reductase (Table 1) and is absent in all other pathogenic strains like Mycobacterium avium paratuberculosis, Mycobacterium marinum, Mycobacterium ulcerans and M. leprae. Succinate dehydrogenase activity has been measured in M. tuberculosis as well as many other mycobacterial species (37, 38), but it is still unclear which of the three enzymes or if all are responsible for this activity.
Figure 3.
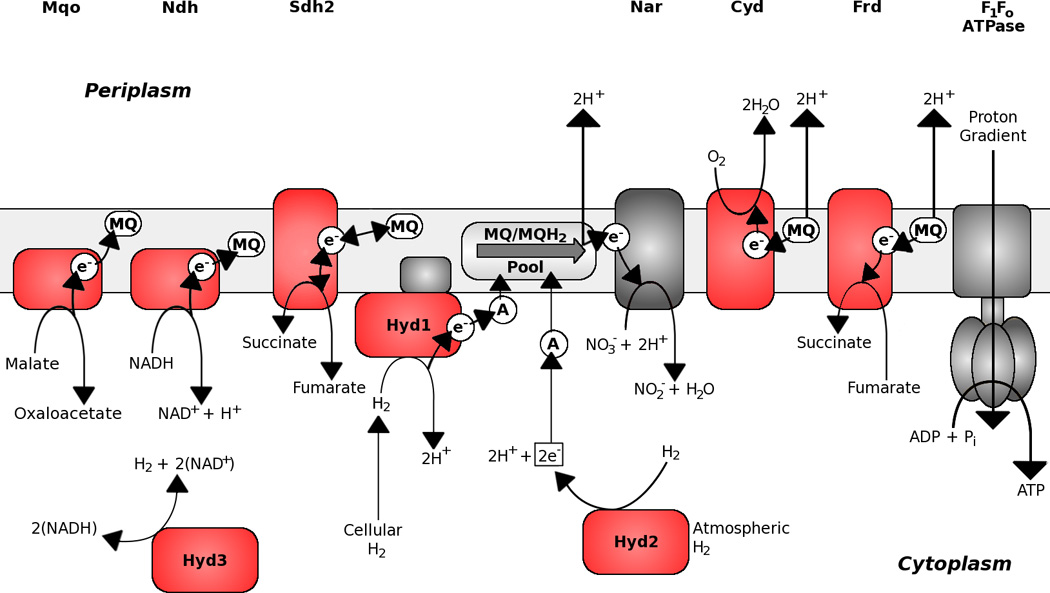
The preferential respiratory chain of an oxygen-limited mycobacterial cell. Under low oxygen-conditions a diverse response utilizing alternate electron donors and acceptors, energy-conserving enzymes and a high affinity terminal oxidase permit survival under hypoxic conditions. Components coloured in red are upregulated under microaerobic conditions (6). Catalysis and electron flow are indicated by arrows. The possible PMF-driven reverse electron flow of Sdh2 is not shown for clarity. Acronyms: Mqo – Malate:Menaquinone oxidoreductase; Ndh – Type II NADH:Menaquinone oxidoreductase; Sdh2 – Succinate:Menaquinone oxidoreductase 2; Nar – Nitrate reductase; Cyd – Cytochrome bd oxidase; Frd – Fumarate reductase; Hyd – Hydrogenase; MQ – Menaquinone; A – Unidentified electron acceptor.
Mycobacteria utilize menaquinone/menaquinol (MQ/MQH2) as a conduit between electron-donating and -accepting reactions (Figure 1). Menaquinone has a lower midpoint redox potential (Em = −74 mV) compared to ubiquinone (Em = +113 mV) and is ideally poised to donate electrons to fumarate during anaerobic conditions (39). This means that the SDH reaction in mycobacteria (succinate oxidation to fumarate) should have an unfavourable free energy due to reverse electron flow and proton uptake to drive this reaction. It is tempting to propose that the unusual subunits of SDH1 could be the result of structural specializations in the transmembrane region to facilitate reverse electron flow from succinate to MQ. In fact, SDH1 and SDH2 have been shown to be differentially expressed under energy- or oxygen-limiting conditions in M. smegmatis (16). Under energy-limiting conditions SDH1 was upregulated 4-fold while SDH2 was downregulated 3-fold. Under oxygen-limiting conditions, SDH1 was downregulated 30-fold while SDH2 was upregulated 2-fold. This indicates that SDH1 could be the dedicated succinate dehydrogenase and SDH2 catalyses fumarate reductase activity, which is important for survival under hypoxia. This hypothesis is supported by several reports in the recent literature. A recent transposon-site hybridization (TraSH) screen in M. tuberculosis suggested that SDH1 but not SDH2 was essential for growth under aerobic conditions on standard medium (26). On the contrary, in a TraSH screen selecting for mutants that continue to replicate under hypoxic conditions Baek and coworkers (40) found SDH2 mutants overrepresented. These results suggest that SDH2 has a pivotal role in the transition of M. tuberculosis from aerobic to hypoxic conditions and supports the hypothesis of it being a fumarate reductase. In fact, it has been proposed that fumarate may be an important endogenous electron acceptor for energy production and maintenance of redox balance (oxidation of NADH to NAD+) in hypoxic non-replicating mycobacteria (6). Interestingly, the use of fumarate as an electron acceptor in E. coli requires complex I, and expression of the nuo operon is stimulated by the presence of fumarate (41). This stands in direct contrast to M. tuberculosis, where the nuo operon seems to be silent under anaerobic conditions (18). In a recent study it was shown by 13C flux analysis that M. tuberculosis grown under hypoxia metabolizes glucose through a reverse TCA cycle to generate succinate as an excreted fermentation end product (42). However the metabolic flux from fumarate to succinate was unchanged in an M. tuberculosis FRD deletion mutant, suggesting that one of the remaining putative succinate dehydrogenases (most likely SDH2) could catalyze this reaction. A more recent study suggests that the glyoxylate shunt, and not the reverse TCA, is used to metabolize both glycolytic and fatty acid carbon sources in response to oxygen limitation and this route also produces succinate as its metabolic end product (43). The authors propose that during oxygen limitation large amounts of succinate are produced by this pathway that are used to sustain the membrane potential, ATP synthesis and TCA cycle precursors akin to a “metabolic battery” (43). Moreover, because of the near neutral mid-point potential of succinate/fumarate redox couple (+30 mV), succinate is able to bridge both oxidative and fermentative metabolic schemes depending on electron acceptor availability (43).
The role of the frdABCD operon in the M. tuberculosis complex is not clear. Increased expression of this operon has been shown during carbon starvation (20), oxygen depletion (44) and in macrophages (19) suggesting a role for this enzyme in persistence.
Alternative electron donor utilization during starvation and hypoxia
During carbon starvation and slow growth, mycobacteria switch to alternative electron donors (16, 20, 45) (Figure 2). Proline dehydrogenase is upregulated under both energy-limiting conditions and hypoxia (7, 16) and is increasingly being recognized as a critical amino acid in cellular bioenergetics and redox control (46). Proline can be utilized as an electron donor as well as a carbon and nitrogen source (Figure 2). The degradation of proline occurs by means of two enzymes: proline dehydrogenase (PRODH) and pyrroline-5-carboxylate dehydrogenase (P5CDH). These two enzymes catalyze the oxidation of proline to glutamate with four electrons transferred to the respiratory chain (46). In the first step FAD is reduced to FADH2 while in the second step NAD+ is reduced to NADH. In some bacteria, PRODH and P5CDH are monofunctional enzymes but in the majority of bacterial species they are fused into one protein called proline utilization A flavoenzyme PutA (47).
In mycobacteria, PRODH and P5CDH are predicted to be monofunctional enzymes (46). The genes encoding PRODH (putB, Rv1188) and P5CDH (putA, rocA, Rv1187) are expressed as part of an operon (7). It has been shown that M. smegmatis can grow on proline as the sole carbon and energy source and also that PRODH is an important electron donor, under both energy-limiting conditions and hypoxia (7, 16). The same authors show that proline metabolism in mycobacteria is regulated by a unique membrane-associated transcriptional regulator called PruC (Rv1186c), encoded upstream of pruA. The pruAB operon with its upstream regulator pruC is highly conserved in mycobacteria with the exception of M. leprae. Recent proteomics data on M. avium paratuberculosis (MAP) show that protein levels of RocA (PutA) are elevated in the intestinal tissues of cows (48). The authors propose that MAP has adapted to utilize proline as a carbon and nitrogen source due to the high abundance of this amino acid in the plant material that is eaten by such ruminants. Mycobacteria also encode pyrroline-5-carboxylate reductase (encoded by proC) that catalyses the reverse reaction of putA converting pyrroline-5-carboxylate to proline. Interestingly, a proC mutant of M. tuberculosis was avirulent in immunocompetent mice, however this was not due to its inability to proliferate intracellulary because bacterial loads increased in the mouse lungs after 20 days post infection (49).
Hydrogenases catalyze the reversible oxidation of molecular hydrogen: 2H+ + 2e−→ H2 and play a central role in energy metabolism of bacteria, archaea and eukarya (50). Under physiological conditions, hydrogenases couple H2 oxidation to respiration (Knallgas reaction) or reduce protons as a way to dispose of surplus reducing equivalents. Four different types of hydrogenases are found in mycobacteria and are annotated to be of the NiFe type. M. smegmatis harbours three (designated Hyd1, 2 and 3) of the four hydrogenase complexes (16), Hyd1 aligns closely with Group 2a uptake hydrogenases of the cyanobacteria such as Nostoc, indicating it oxidises H2 (51). In contrast, Hyd3 is closely related to the Group 3 cytoplasmic bidirectional hydrogenases. Hyd2 appears to be a founding member of the Group 5 high-affinity hydrogenases (52, 53). Hyd1, Hyd2 and Hyd3 are all soluble hydrogenases and found in mycobacteria of the slow-growing and fast-growing type, as well as pathogenic and non-pathogenic mycobacteria (16). However, the fourth putative hydrogenase is only found in pathogenic mycobacteria (including M. tuberculosis complex) and seems to be restricted to slow-growers (Table 1). It shows homology to Group 4 membrane-bound H2 evolving hydrogenases. It has been shown that M. smegmatis, among other mycobacterial species, can oxidize molecular hydrogen in the presence of carbon monoxide (CO), implying that M. smegmatis expresses a functional hydrogenase (54). To date no studies have reported on the ability of mycobacteria to produce hydrogen. Gene expression studies suggest that Hyd1 and Hyd2 are used during nutrient starvation as an alternative electron source (Figure 2) while Hyd3 and the membrane-bound hydrogenase are more likely to have a function in disposing electrons under anaerobic conditions (16) (Figure 3). A knockout mutant of Hyd2 in M. smegmatis showed reduced biomass production when grown on complex medium under atmospheric conditions (16) and its homolog in Streptomyces sp. was shown to facilitate hydrogen oxidation (53). These data suggest that Hyd2 oxidizes hydrogen at very low concentrations, which fits with its purported role as a high-affinity hydrogenase (52).
Carbon monoxide dehydrogenase (CO-DH) is responsible for the oxidation of CO to carbon dioxide (CO2) in carboxydobacteria, which grow on CO as a sole source of carbon and energy (55). Carboxydobacteria catalyze the oxidation of CO to CO2 by the following reaction: CO + H2O → CO2 + 2H+ + 2e−. Several pathogenic and nonpathogenic mycobacteria including M. tuberculosis are known to possess CO-DH genes. It has been shown that M. tuberculosis H37Ra, which possesses CO-DH activity, can grow on CO as a sole source of carbon and fuel for energy generation (56) (Figure 2).
Glycerol-3-phosphate dehydrogenase catalyzes the oxidation of glycerol-3-phosphate to dihydroxy-acetone phosphate and reduces either quinone or NADP (57). In E. coli, glycerol 3-phosphate is either used as precursor in the biosynthesis of phospholipids or as a carbon source for energy supply (58). M. tuberculosis possesses genes for four predicted glycerol-3-phosphate dehydrogenases, but their role and function in mycobacterial respiration remain unknown (Table 1).
The membrane-associated malate quinone oxidoreductase (MQO) oxidizes malate to oxaloacetate and transfers the reducing equivalents to menaquinone (MK) (59, 60) (Figure 3). M. tuberculosis harbors a copy of MQO and a cytoplasmic NAD+-dependent malate dehydrogenase (MDH) (61, 62). The function and role of MQO in mycobacterial respiration is unknown. M. smegmatis lacks an MDH homologue and it was shown that MQO responded to low oxygen concentration with 4-fold increase in gene expression (16). Mutants of Corynebacterium glutamicum defective in NDH-2 activity are able to grow despite the loss of all membrane-bound NADH dehydrogenase activity (59). The authors propose a reaction scheme whereby electron transfer from NADH to MK is catalyzed by the sequential action of malate dehydrogenase (MDH) and malate:quinone oxidoreductase (MQO) (membrane-bound). MDH can reduce oxaloacetate with NADH to malate, and then malate is reoxidized to oxaloacetate by MQO using MK as an electron acceptor (59, 60). Support for this model comes from the observation that a Δmqo/ndh double mutant failed to grow under conditions where the Δndh mutant grew. Furthermore, M. smegmatis ndh mutants could be complemented by M. bovis BCG mdh suggesting that such a reaction scheme might operate in mycobacteria (64).
Terminal Electron Acceptor Utilization
During aerobic respiration, energy is conserved by the generation of a PMF across a proton-impermeable membrane. The electron transport chain components are membrane-bound and asymmetrically arranged across the membrane to achieve net consumption of protons from the cytoplasm and net release of protons on the outside of the cell. An important part of all electron transport chains are the terminal respiratory oxidases. In order for mycobacteria to utilize oxygen efficiently and obtain the maximum growth yield on a particular carbon and energy source, there must be co-ordinate regulation of terminal respiratory oxidase expression/activity. For example, in E. coli, cytochrome bo (Km for oxygen in the micromolar range) and cytochrome bd (Km for oxygen in the nanomolar range) (63, 64) are coordinately regulated by the ArcBA system and transcriptional regulator FNR (65). Cytochrome bo is synthesized at high oxygen tension (optimal between 15 to 100% air saturation) and repressed as the oxygen concentration decreases (66). This coincides with the induction of cytochrome bd at 7% air saturation, which is turned off (FNR-mediated repression) once the cells enter anaerobiosis (65, 66). Mycobacteria adopt regulation of oxidase expression to match oxygen supply. Under conditions of low oxygen tension (ca. 1 % air saturation), cytochrome bd is induced in M. smegmatis as the transition to anaerobiosis is approached (67), a value that is 10-fold lower than that observed in E. coli (ca. 10% air saturation). In M. tuberculosis, cytochrome bd is upregulated in the early stages of NRP-1 (i.e. decreasing oxygen) (68), in NRP-2 (18) and in response to nitric oxide (NO) (18). Intriguingly mRNA levels of cydA increased transiently about 7-fold after 30 days in mouse lungs (18). Mycobacteria appear to adopt a strategy whereby a down-regulation or a slowing of metabolism occurs as cells enter NRP-1 and NRP-2. Transcriptional analysis of M. tuberculosis in the macrophage (phagosomal environment) has revealed that NDH-1, menaquinol-cytochrome c oxidoreductase and the ATP synthase are all down-regulated when compared to cells growing exponentially, suggesting that there is a reduced need for energy generation during bacteriostasis i.e., the growth state of intraphagosomal M. tuberculosis (19). Consistent with these observations is the repression of these operons during starvation. In contrast, fumarate reductase, nitrate reductase and NDH-2 are all upregulated under these conditions (19). Whilst these proteins do not appear to contribute to increased energy conservation, it has been suggested that they may play a pivotal role in the recycling of NAD+ as a result of β-oxidation of fatty acids (19).
Complex III and IV of the M. tuberculosis electron transport chain
M. tuberculosis harbours genes for a cytochrome c pathway consisting of a menaquinol-cytochrome c oxidoreductase termed the bc1 complex (encoded by the qcrCAB operon, complex III) and an aa3-type cytochrome c oxidase (encoded by the genes ctaBCDE, complex IV) belonging to the heme-copper respiratory oxidase family (69–71) (Table 1). The bc1 complex (menaquinol-cytochrome c oxidoreductase) consists of redox groups comprising a 2Fe/2S centre, located on a Rieske protein (QcrA); two b-type haems (low and high potential) located on a single polypeptide (QcrB); and the haem of cytochrome c1 (QcrC). For every two electrons passing from quinol to cytochrome c, complex III releases four protons into the periplasmic side of the membrane (4H+/2e−) (Figure 2). The iron sulfur subunit QcrA has three transmembrane helices and sequence motifs characteristic of 2Fe-2S Rieske iron-sulfur proteins (viz. CSHLGC and CPCH). In contrast to bovine heart cytochrome b, QcrB from M. tuberculosis has a 120 amino acid extension at the C-terminus as noted for C. glutamicum and Streptomyces species (72). The QcrB subunit has been recently shown to be the target of novel imidazo[1,2-a]pyridine inhibitors that are active against M. tuberculosis at low micromolar concentrations (73). As first reported by Niebisch and Bott (72) the QcrC subunit (cytochrome c1) harbours two haem-binding signatures (CXXCH motifs) for c-type cytochromes (viz. CVSCH and CASCH) suggesting a covalent di-haem (bcc) configuration. Mycobacterial genomes do not appear to harbor genes for either a soluble or membrane-bound cytochrome c suggesting the bcc complex interacts directly with the aa3-type cytochrome c oxidase to achieve electron transfer and function as a supercomplex. Niebisch and Bott (72, 74) suggest a supercomplex mechanism for C. glutamicum in which the haem group of QcrC takes over the function of a separate cytochrome c in electron transfer to cytochrome aa3 oxidase forging a close contact relationship between cytochrome c1 and the CuA site in the subunit II of cytochrome aa3 oxidase. Supercomplexes between bc1 and aa3 have been reported for C. glutamicum (72), Paracoccus denitrificans (75) and thermophilic strain PS3 (76). A functional association between bcc and aa3 has been shown for the complex in M. smegmatis family (71).
The aa3-type cytochrome c oxidase in M. tuberculosis appears to be encoded by four genes ctaBCDE (Table 1). The ctaE gene is located immediately upstream of the qcrCAB operon, but the other three genes are located in three different locations on the chromosome and in some cases appear to be in operons with other genes (Table 1). The four subunits of the M. tuberculosis aa3-type cytochrome c oxidase include: CtaB (a cytochrome c oxidase assembly factor); the catalytic subunits CtaD (cytochrome c oxidase subunit I containing haem a, a3, and CuB) and CtaC (cytochrome c oxidase, subunit II location of copper A (CuA), and CtaE (subunit III). For every two electrons passing through complex IV, four protons are taken up from the cytoplasm and two are released into the periplasm. The oxidase acts as a proton pump with a stoichiometry of 2H+/2e− (Figure 2).
The bc1-aa3 pathway appears to be the major respiratory route in mycobacteria under standard aerobic culturing conditions (70). Matsoso et al. (70) have demonstrated that disruption of this pathway in M. smegmatis is accompanied by a constitutive upregulation of the cytochrome bd-type menaquinol oxidase. In M. tuberculosis, the bc1-aa3 pathway is essential for growth suggesting an inability of this bacterium to adapt in a manner analogous to M. smegmatis (70). The aa3 branch is proposed to contain three ctaD alleles in M. smegmatis versus the one in M. tuberculosis, suggesting the existence of alternate isoforms of cytochrome c oxidase in M. smegmatis (67).
Cytochrome bd-type oxidase
M. tuberculosis and other mycobacterial species harbour genes for the cytochrome bd-type menaquinol oxidase (cydAB) (67) (Table 1). The cytochrome bd branch is bioenergetically less efficient (non-proton translocating) and is synthesized at low oxygen tensions in mycobacteria (67). In addition to cytochrome bd, the M. smegmatis respiratory chain has been proposed to contain a third possible respiratory branch terminating in the YthAB (bd-type) menaquinol oxidase (67). The existence of two cytochrome bd-type oxidases (I and II) is not unprecedented in bacteria (77), and recent work had reported that cytochrome bd-II in E. coli is able to generate a PMF with a H+/e− ratio of 1.0 (78). Cytochrome bd mutants of E. coli have been shown to have a pleiotropic phenotype: they are sensitive to H2O2nitric oxide, temperature, and iron (III) chelators; and are unable to exit from stationary phase and resume aerobic growth at 37°C (77). A cydA mutant of M. smegmatis has been generated, but the phenotypes are very subtle compared to E. coli. The M. smegmatis cydA mutant showed a reduced growth rate at 0.5–1 % air saturation and also a 10-fold difference in CFU after 140 h of growth in the presence of cyanide at 21% air saturation when co-cultured with wild-type M. smegmatis (67).
The cydAB genes appear to be in an operon with cydDC in mycobacterial species (Table 1), an arrangement similar to that found in Bacillus. In E. coli, cydAB and cydDC form two discrete operons and cydDC mutants are defective in cytochrome bd assembly and the periplasmic space is more oxidized in the mutant versus wild-type (79). In E. coli, the CydDC protein (ABC transporter) has been reported to pump glutathione and cysteine into the periplasm to maintain redox homeostasis (80). The role of the cydDC genes in mycobacteria is unknown, however, evidence exists that CydDC plays a role during mycobacterial persistance in vivo. A cydC mutant of M. tuberculosis showed reduced ability to survive the transition from acute to chronic infection in mice (18) and Dhar and McKinney have reported that CydC plays a role in mycobacterial persistence in isoniazid-treated mice (81).
Electron acceptor utilization under hypoxia
In the absence of oxygen, alternative electron acceptors (e.g. nitrate and fumarate) are available for mycobacterial metabolism, but none of these electron acceptors have been shown to support growth of mycobacterial species. These potential electron acceptors may play an important role in the disposal of reducing equivalents in the absence of oxygen.
NO is generated in large amounts within the macrophages and restricts the growth of M. tuberculosis. Nitrate can be produced by oxidation of NO and is an alternative source of nitrogen for bacteria within the human host. Early work in E. coli had suggested that narK was involved only in nitrite export (82), and so the homologous narK2 in M. tuberculosis was annotated as a “nitrite extrusion protein”. More recent work with an E. coli narK narU double mutant indicated that the two proteins could transport nitrate into and nitrite out of the cell (83, 84). In M. tuberculosis, four genes, narK1 through narK3 and narU are homologous to narK and narU (Table 1). Since M. tuberculosis is unable to reduce nitrite, which could accumulate to toxic levels, it must be exported out of the cell. The M. tuberculosis narK2 was shown to complement this E. coli double mutant, supporting a role for narK2 in nitrate reduction by coding for a transporter of nitrate into and nitrite out of the cell (85). Nitrate reduction by M. tuberculosis is regulated by control of nitrate transport into the cell by NarK2. It is proposed that NarK2 senses the redox state of the cell, possibly by monitoring the flow of electrons to cytochrome oxidase, and adjusts its activity so that nitrate is transported under reducing, but not under oxidizing conditions (86). Inhibition of nitrate transport by oxygen has been documented in other bacteria (87). It is intriguing that M. tuberculosis, classified as an obligate aerobe, should have such intricate control of an anaerobic enzyme system. Transcription of narK2 is controlled by DosR/DevR, which responds to O2NO, and CO (88–90). Both the transcription of the narK2 gene and the activity of NarK2 are controlled by similar signals (86).
M. tuberculosis contains genes (narGHJI, Rv1161–1164) that code for a putative membrane-bound molybdenum-containing nitrate reductase complex similar to the corresponding narGHJI operon of E. coli (Table 1). Moreover, the narGHJI operon of M. tuberculosis is able to functionally complement a nar mutant of E. coli to grow on glycerol and reduce nitrate anaerobically (85). Importantly however, the expression of the narGHJI operon in M. tuberculosis is not upregulated in response to either hypoxia or stationary phase (85). Sohaskey and Wayne demonstrate that overexpression of recombinant M. tuberculosis nitrate reductase in either M. tuberculosis or M. smegmatis (low nitrate reductase activity) does not confer the ability of these cells to grow anaerobically i.e. no growth of either species is observed with nitrate anaerobically even though the nitrate reductase activity of whole cells increases (85). The genome of M. tuberculosis also lacks orthologs of transcription regulator FNR which, in combination with NarL, are responsible for the transcriptional activation of the narGHJI operon by anaerobic conditions in E. coli (21). A putative NarL (Rv0884c) has been indentifed in M. tuberculosis but the promoter of the narGHJI lacks consensus-like binding sites for this regulatory protein. Based on these observations, it is apparent that this enzyme does not support anaerobic growth of mycobacteria and therefore the role of this enzyme in the physiology of mycobacteria is unclear. Given the proposed membrane-bound location of the enzyme and the proton-translocating activity via a redox loop of the E. coli enzyme, perhaps the primary role of the mycobacterial enzyme is to generate a PMF when the concentration of oxygen is low, and hence its activity increases but not its expression.
An alternative role for nitrate reductase may be maintaining the redox balance of the cell during conditions of hypoxia. Sohaskey (91) has reported that exogenously supplied nitrate has no effect on long-term persistence during gradual oxygen depletion, but played an important role during rapid adaptation to hypoxia (< 18 h). This effect required a functional nitrate reductase, suggesting that nitrate reduction may play a role in protecting cells during sudden changes in oxygen concentration leading to disruption of aerobic respiration. The same author (86) has proposed a role for NarK2 in sensing the redox state of the cell such that nitrate is transported into the cell under reducing, but not oxidizing conditions. Lastly, Tan et al. (92) propose a role for nitrate reductase in protecting M. tuberculosis from mild acid challenge under hypoxic conditions.
M. tuberculosis is unable to reduce nitrite and the genome was proposed to harbor genes for a putative ferredoxin-dependent nitrite reductase (NirA) and a NAD(P)H nitrite reductase (NirBD). However, a subsequent study has reported that the nirA gene is misannotated and is in fact sirA, a sulfite reductase shown to be essential for growth on sulfate or sulfite as the sole sulfur source (93) (Table 1). The nirBD genes are operonic and non-essential for growth, and unlike the narGHJI genes, no role in resistance to acid stress under hypoxia was noted for NirBD (92). These data support the proposal that nitrite produced by nitrate reductase is not converted to ammonium, but instead is exported from the cell by NarK2.
The evolution of H2 is a mechanism commonly employed by anaerobic bacteria to recycle reducing equivalents obtained from anaerobic degradation of organic substrates. However, even strictly aerobic bacteria have been shown to produce H2 under anaerobic conditions (94). In the family of Mycobacteriaceae, two potential hydrogen-evolving hydrogenases are present. One belongs to Group 2 cytoplasmic bidirectional hydrogenases and can be found in some slow-growing (e.g. M. marinum and M. kansasii) and fast-growing (e.g. M. smegmatis) mycobacteria. In M. smegmatis, this enzyme is termed Hyd3 and has been shown to respond strictly to oxygen-limiting conditions with up to 50-fold increase in gene expression (16). The second type is a yet uncharacterized enzyme that shows homology to membrane-bound Group 4 hydrogenases. This putative formate-hydrogen lyase is enocoded in M. tuberculosis (viz. Rv0082 to Rv0087) and is upregulated during infection of human macrophage-like THP-1 cells (95). Moreover, the transcription of the early genes of the hycP/hycQ containing operon was shown to be upregulated during anaerobic adaptation in several studies (44, 68, 90, 96, 97). This gene cluster shows homology to components of hydrogenase 4 and 3 complexes of E. coli, the latter of which has been shown to catalyze hydrogen evolution at acidic pH (98). Upon closer inspection, the predicted [NiFe]-hydrogenase of M. tuberculosis appears to form part of an Ehr (Ech hydrogenase-related) complex (99, 100). Ehr enzymes lack the cysteine residues needed to ligate a [NiFe] center, which is the catalytic center of hydrogenases. Additionally, no maturation factors to synthesize these complex centers are present in M. tuberculosis. Recently, it was shown that the operon Rv0081-Rv0087 is positively regulated by the two two-component signal transduction systems DosRS-DosT and MprAB and negatively regulated by Rv0081 a member of the ArsR/SmtB family of metal-dependent transcriptional regulators (101). DosRS-DosT is a redox sensing regulatory system that is important during the adaptation to hypoxic conditions. No function has yet been proposed for Ehr enzymes, but its homology to hydrogenases and to proton-pumping type I NADH dehydrogenases points to a role in energy metabolism.
ATP Synthesis by the F1Fo ATP Synthase
In M. tuberculosis and other mycobacterial species, ATP is synthesized via substrate level phosphorylation and oxidative phosphorylation using the membrane-bound F1Fo-ATP synthases (encoded by the atpBEFHAGDC operon, Rv1304–1311) (Table 1). In M. tuberculosis, the atp operon is downregulated during growth in macrophages (19), the mouse lung and in cells exposed to NO or hypoxia (18). The atp operon of M. bovis BCG and M. smegmatis is downregulated in response to slow growth rate (16, 45). When slow growing cells of M. smegmatis (70 h doubling time), with low levels of atp operon expression, are exposed to hypoxia (0.6% oxygen saturation), the atp operon is upregulated 3-fold suggesting an important role for this enzyme during adaptation to hypoxia (16).
The F1Fo-ATP synthase catalyzes ATP synthesis by utilizing the electrochemical gradient of protons to generate ATP from ADP and inorganic phosphate (Pi) and operates under conditions of a high PMF and low intracellular ATP. The enzyme is also capable of working as an ATPase under conditions of high intracellular ATP and an overall low PMF (102). As an ATPase, the enzyme hydrolyzes ATP, while pumping protons from the cytoplasm to the outside of the cell. The ATP synthase of mycobacteria has been studied in detail at a biochemical level in M. phlei and shown to exhibit latent ATPase activity (10). ATPase activity could be activated by trypsin treatment and magnesium ions, but the mechanism of activation was not elucidated. Recent experiments with inverted membrane vesicles of M. bovis BCG and M. smegmatis demonstrate latent ATPase activity that could be activated by methanol and the PMF, suggesting regulation by the epsilon subunit and ADP inhibition (9). The reason for the extreme latency in ATP hydrolysis of the mycobacterial ATP synthase is unknown, but may represent an adaptation to function at low PMF and under hypoxia. Hypoxic non-replicating cells of M. tuberculosis generate a PMF in the order of −100 mV and the ATP synthase inhibitor TMC207 is bactericidal towards these cells demonstrating that the ATP synthase still continues to function at relatively low PMF (6).
The F1Fo-ATP synthase in M. tuberculosis and M. smegmatis has been shown to be essential for optimal growth (29, 103). In other bacteria, the F1Fo-ATP synthase is dispensable for growth on fermentable carbon sources (104, 105), where increased glycolytic flux can compensate for the loss of oxidative phosphorylation. This strategy does not appear to be exploited by M. smegmatis: the F1Fo-ATP synthase is essential for growth even on fermentable substrates, suggesting that ATP production from substrate level phosphorylation alone, despite increased glycolytic flux, may be insufficient to sustain growth of these bacteria (103). This may be due to an extraordinarily high value for the amount of ATP required to synthesize a mycobacterial cell, a possibility that requires further investigation (106). Alternatively, in conjunction with a high ATP demand for growth, the ATP synthase may be an obligatory requirement for the oxidation of NADH by providing a sink for translocated protons during NADH oxidation coupled to oxygen reduction (103). Such strict coupling would imply that mycobacteria do not support uncoupled respiration; either they lack a conduit for proton re-entry in the absence of the F1Fo-ATP synthase or they are unable to adjust the proton permeability of the cytoplasmic membrane to allow a futile cycle of protons to operate. In this context, the cytoplasmic membrane of M. smegmatis has been shown to be extremely impermeable to protons (107). The ATP synthase of the close mycobacterial phylogenetic relative C. glutamicum is non-essential for growth on fermentable carbon sources and Δatp mutants of this bacterium show enhanced rates of glucose uptake, oxygen consumption and excretion of pyruvate into the growth medium, suggesting that substrate level phosphorylation alone can sustain growth of this bacterium (108).
Several new anti-tubercular compounds have been reported that target oxidative phosphorylation in mycobacteria (12, 109, 110). The most promising compounds clinically, the diarylquinolines, have been shown to target the F1Fo-ATP synthase and inhibit ATP synthesis (109, 111, 112). Genome sequencing of both M. tuberculosis and M. smegmatis mutants that are resistant to diarylquinolines (i.e. TMC207) revealed that the target of these compounds is the oligomeric c ring (encoded by atpE) of the enzyme (109, 113, 114). The purified c ring from M. smegmatis binds TMC207 with a KD of 500 nm, and modelling/docking and kinetic studies suggest that TMC207 blocks rotary movement of the c-ring during catalysis by mimicking key residues in the proton transfer chain (115, 116). Further investigations with inverted membrane vesicles of M. smegmatis and TMC207 have revealed that TMC207 acts independently of the PMF, and that electrostatic forces play an important role in the interaction of the drug with the ATP synthase (116).
When mycobacterial cells (growing or non-growing) are treated with TMC207, time-dependent (not dose-dependent) killing is observed (109). The mechanism of killing is not clear, but does not involve the dissipation of the membrane potential, which is lethal to all living cells. A dose-dependent decrease in intracellular ATP has been observed when M. tuberculosis cells are treated with TMC207 (111, 112), but these data do not explain cell death because mycobacterial cells can be depleted of ATP and yet remain viable (117). TMC207 is bactericidal towards most species of mycobacteria (109), but is only bacteriostatic against M. avium (118) and M. smegmatis (Berney and Cook, unpublished data). Even when M. smegmatis is grown at a doubling time of 70 h in glycerol-limited continuous culture, TMC207 was bacteriostatic (Berney and Cook, unpublished data). The identification of the mechanisms underlying this sensitivity will be important in understanding how TMC207 exerts its inhibitory effects on mycobacterial cells.
Control of electron transport chain expression and oxidative phosphorylation
Mycobacteria are obligate aerobes and as such have to possess mechanisms to detect the ambient oxygen tension to enable them to adapt to changes in oxygen availability by adjusting their metabolism accordingly. Furthermore, gradual depletion of oxygen has been implicated in entry of M. tuberculosis into non-replicating persistence and latency (119). The mechanisms by which mycobacteria sense oxygen have been extensively studied, and the two major sensory systems known to date are the DosT/DosS/DosR system and WhiB3.
The dormancy survival, or Dos, system consists of the three proteins DosR (or DevR), DosS (or DevS) and DosT. DosR has been shown to act as a transcriptional regulator, which is responsible for the induction of nearly all hypoxia-induced genes of M. tuberculosis (96). In addition to hypoxia, the Dos-regulon has also be shown to be activated by exposure of M. tuberculosis to NO or CO (88, 90, 120).
WhiB3 has been implicated in sensing of oxygen and redox state by mycobacteria. Singh and colleagues report that WhiB3 contains a 4Fe-4S cluster, which binds NO (121). Furthermore, in the presence of oxygen, the WhiB3 [4Fe-4S]2+ cluster is degraded first to a [3Fe-4S]+ cluster, then a [2Fe-2S]2+ and subsequently lost altogether, in a mechanism reminiscent of the one found in the E. coli oxygen sensor FNR (121, 122). Apo-WhiB3 was shown to have protein disulfide reductase activity, and it has been proposed that loss of the Fe-S cluster is required to gain this activity (123). WhiB3-mediated response to the presence of oxygen therefore may occur through direct control of the activity of metabolic proteins or through modification of transcriptional regulators (123). A role of WhiB3 in regulation of the transcriptional machinery in mycobacteria may be supported by the finding that WhiB3 interacts with the major sigma factor, SigA (RpoV) (124), but the effect of this interaction on SigA activity is not known, and further study is required to understand the precise role of WhiB3 in mediating any adaptation of mycobacteria to changes in oxygen tension.
A striking observation is that neither Dos nor WhiB3 controls any component of the electron transport machinery in M. tuberculosis. While the Dos system and WhiB3 are the most studied regarding the perception of oxygen tension by mycobacteria, they are likely not the only systems used by these bacteria. For example, DosR of M. tuberculosis H37Rv was shown not to be strictly required for survival of hypoxia in vitro (125). The authors further found that expression of Dos regulon genes in response to hypoxia was transient, with expression of about half of the 50 DosR-dependent genes returning to baseline levels within 24 h of hypoxia. In contrast, a set of 230 genes was significantly up-regulated at four and seven days of hypoxia, but not initially, and were termed the enduring hypoxic response (EHR) (125). Induction of EHR was independent of DosR, suggesting that other sensory and regulatory mechanisms must exist to signal prolonged exposure to hypoxia. Strikingly, the EHR genes contained an unusually high number of regulatory genes (FurA, FurB, PhoP, three WhiB family members and two ECF sigma factors, SigH and SigE), but it is not yet known which of these, if any, are involved in entry into EHR (125). A study into the effect of addition of cAMP to growing cultures of M. bovis BCG or M. tuberculosis H37Rv found that the number of genes affected by cAMP was larger under low oxygen, CO2-enriched conditions than under ambient air (126). The authors proposed that cAMP may be used by mycobacteria as a signaling molecule in response to hypoxic conditions. However, the signal leading to cAMP synthesis or which adenylyl cyclase might catalyse this reaction under hypoxia remains unknown. The regulator of cydABDC expression in mycobacteria has not been identified. Several regulators have been implicated in controlling the expression of cyd expression i.e. SenX3-RegX3 (127) and cAMP receptor protein (CRP) (128), but the signal transduction pathway or signals sensed remain to be elucidated.
Acknowledgments
Research in the authors’ laboratory is funded by Health Research Council, Lottery Health, Marsden Fund, Royal Society New Zealand, the Maurice Wilkins Centre and National Institute of Health grant AI26170.
Contributor Information
Gregory M. Cook, Email: gregory.cook@otago.ac.nz, University of Otago, Department of Microbiology and Immunology, Dunedin, New Zealand.
Kiel Hards, University of Otago, Department of Microbiology and Immunology, Dunedin, New Zealand.
Catherine Vilchèze, Department of Microbiology and Immunology, Albert Einstein College of Medicine, Bronx, New York, USA.
Travis Hartman, Department of Microbiology and Immunology, Albert Einstein College of Medicine, Bronx, New York, USA.
Michael Berney, Department of Microbiology and Immunology, Albert Einstein College of Medicine, Bronx, New York, USA.
References
- 1.Segal W, Bloch H. Biochemical differentiation of Mycobacterium tuberculosis grown in vivo and in vitro. J Bacteriol. 1956;72:132–141. doi: 10.1128/jb.72.2.132-141.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brodie AF, Gutnik DL. Electron transport and oxidative phosphorylation in microbial systems. In: King TE, Klingenberg M, editors. Electron and coupled energy transfer systems. 1B. New York: Marcel Dekker Inc: 1972. pp. 599–681. [Google Scholar]
- 3.Russell JB, Cook GM. Energetics of bacterial growth: balance of anabolic and catabolic reactions. Microbiol Rev. 1995;59:48–62. doi: 10.1128/mr.59.1.48-62.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao M, Streur TL, Aldwell FE, Cook GM. Intracellular pH regulation by Mycobacterium smegmatis and Mycobacterium bovis BCG. Microbiology. 2001;147:1017–1024. doi: 10.1099/00221287-147-4-1017. [DOI] [PubMed] [Google Scholar]
- 5.Ishaque M. Energy generation mechanisms in the in vitro-grown Mycobacterium lepraemurium. Int J Lepr Other Mycobact Dis. 1992;60:61–70. [PubMed] [Google Scholar]
- 6.Rao SP, Alonso S, Rand L, Dick T, Pethe K. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2008;105:11945–11950. doi: 10.1073/pnas.0711697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berney M, Weimar MR, Heikal A, Cook GM. Regulation of proline metabolism in mycobacteria and its role in carbon metabolism under hypoxia. Mol Microbiol. 2012;84:664–681. doi: 10.1111/j.1365-2958.2012.08053.x. [DOI] [PubMed] [Google Scholar]
- 8.Dimroth P, Cook GM. Bacterial Na+ - or H+ -coupled ATP synthases operating at low electrochemical potential. Adv Microb Physiol. 2004;49:175–218. doi: 10.1016/S0065-2911(04)49004-3. [DOI] [PubMed] [Google Scholar]
- 9.Haagsma AC, Driessen NN, Hahn MM, Lill H, Bald D. ATP synthase in slowand fast-growing mycobacteria is active in ATP synthesis and blocked in ATP hydrolysis direction. FEMS Microbiol Lett. 2010;313:68–74. doi: 10.1111/j.1574-6968.2010.02123.x. [DOI] [PubMed] [Google Scholar]
- 10.Higashi T, Kalra VK, Lee SH, Bogin E, Brodie AF. Energy-transducing membrane-bound coupling factor-ATPase from Mycobacterium phlei. I. Purification, homogeneity, and properties. J Biol Chem. 1975;250:6541–6548. [PubMed] [Google Scholar]
- 11.Kerscher S, Drose S, Zickermann V, Brandt U. The three families of respiratory NADH dehydrogenases. Results Probl Cell Differ. 2008;45:185–222. doi: 10.1007/400_2007_028. [DOI] [PubMed] [Google Scholar]
- 12.Weinstein EA, Yano T, Li LS, Avarbock D, Avarbock A, Helm D, McColm AA, Duncan K, Lonsdale JT, Rubin H. Inhibitors of type II NADH:menaquinone oxidoreductase represent a class of antitubercular drugs. Proc Natl Acad Sci U S A. 2005;102:4548–4553. doi: 10.1073/pnas.0500469102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole ST, Eiglmeier K, Parkhill J, James KD, Thomson NR, Wheeler PR, Honore N, Garnier T, Churcher C, Harris D, Mungall K, Basham D, Brown D, Chillingworth T, Connor R, Davies RM, Devlin K, Duthoy S, Feltwell T, Fraser A, Hamlin N, Holroyd S, Hornsby T, Jagels K, Lacroix C, Maclean J, Moule S, Murphy L, Oliver K, Quail MA, Rajandream MA, Rutherford KM, Rutter S, Seeger K, Simon S, Simmonds M, Skelton J, Squares R, Squares S, Stevens K, Taylor K, Whitehead S, Woodward JR, Barrell BG. Massive gene decay in the leprosy bacillus. Nature. 2001;409:1007–1011. doi: 10.1038/35059006. [DOI] [PubMed] [Google Scholar]
- 14.Miesel L, Weisbrod TR, Marcinkeviciene JA, Bittman R, Jacobs WR., Jr NADH dehydrogenase defects confer isoniazid resistance and conditional lethality in Mycobacterium smegmatis. J Bacteriol. 1998;180:2459–2467. doi: 10.1128/jb.180.9.2459-2467.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vilcheze C, Weisbrod TR, Chen B, Kremer L, Hazbon MH, Wang F, Alland D, Sacchettini JC, Jacobs WR., Jr Altered NADH/NAD+ ratio mediates coresistance to isoniazid and ethionamide in mycobacteria. Antimicrob Agents Chemother. 2005;49:708–720. doi: 10.1128/AAC.49.2.708-720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berney M, Cook GM. Unique flexibility in energy metabolism allows mycobacteria to combat starvation and hypoxia. PLoS ONE. 2010;5:e8614. doi: 10.1371/journal.pone.0008614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velmurugan K, Chen B, Miller JL, Azogue S, Gurses S, Hsu T, Glickman M, Jacobs WR, Jr, Porcelli SA, Briken V. Mycobacterium tuberculosis nuoG is a virulence gene that inhibits apoptosis of infected host cells. PLoS Pathog. 2007;3:e110. doi: 10.1371/journal.ppat.0030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi L, Sohaskey CD, Kana BD, Dawes S, North RJ, Mizrahi V, Gennaro ML. Changes in energy metabolism of Mycobacterium tuberculosis in mouse lung and under in vitro conditions affecting aerobic respiration. Proc Natl Acad Sci U S A. 2005;102:15629–15634. doi: 10.1073/pnas.0507850102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnappinger D, Ehrt S, Voskuil MI, Liu Y, Mangan JA, Monahan IM, Dolganov G, Efron B, Butcher PD, Nathan C, Schoolnik GK. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J Exp Med. 2003;198:693–704. doi: 10.1084/jem.20030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol. 2002;43:717–731. doi: 10.1046/j.1365-2958.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- 21.Unden G, Bongaerts J. Alternative respiratory pathways of Escherichia coli : energetics and transcriptional regulation in response to electron acceptors. Biochim Biophys Acta. 1997;1320:217–234. doi: 10.1016/s0005-2728(97)00034-0. [DOI] [PubMed] [Google Scholar]
- 22.Iwata M, Lee Y, Yamashita T, Yagi T, Iwata S, Cameron AD, Maher MJ. The structure of the yeast NADH dehydrogenase (Ndi1) reveals overlapping binding sites for water- and lipid-soluble substrates. Proc Natl Acad Sci USA. 2012;109:15247–15252. doi: 10.1073/pnas.1210059109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng Y, Li WF, Li J, Wang JW, Ge JP, Xu D, Liu YJ, Wu KQ, Zeng QY, Wu JW, Tian CL, Zhou B, Yang MJ. Structural insight into the type-II mitochondrial NADH dehydrogenases. Nature. 2012;491:478–482. doi: 10.1038/nature11541. [DOI] [PubMed] [Google Scholar]
- 24.Melo AMP, Bandeiras TM, Teixeira M. New insights into Type II NAD(P)H : quinone oxidoreductases. Microbiol Mol Biol Rev. 2004;68:603–616. doi: 10.1128/MMBR.68.4.603-616.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin SS, Gross U, Bohne W. Two internal type II NADH dehydrogenases of Toxoplasma gondii are both required for optimal tachyzoite growth. Mol Microbiol. 2011;82:209–221. doi: 10.1111/j.1365-2958.2011.07807.x. [DOI] [PubMed] [Google Scholar]
- 26.Griffin JE, Gawronski JD, Dejesus MA, Ioerger TR, Akerley BJ, Sassetti CM. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog. 2011;7:e1002251. doi: 10.1371/journal.ppat.1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yano T, Li LS, Weinstein E, Teh JS, Rubin H. Steady-state kinetics and inhibitory action of antitubercular phenothiazines on Mycobacterium tuberculosis type-II NADH-menaquinone oxidoreductase (NDH-2) J Biol Chem. 2006;281:11456–11463. doi: 10.1074/jbc.M508844200. [DOI] [PubMed] [Google Scholar]
- 28.McAdam RA, Quan S, Smith DA, Bardarov S, Betts JC, Cook FC, Hooker EU, Lewis AP, Woollard P, Everett MJ, Lukey PT, Bancroft GJ, Jacobs WR, Jr, Duncan K., Jr Characterization of a Mycobacterium tuberculosis H37Rv transposon library reveals insertions in 351 ORFs and mutants with altered virulence. Microbiology. 2002;148:2975–2986. doi: 10.1099/00221287-148-10-2975. [DOI] [PubMed] [Google Scholar]
- 29.Sassetti CM, Boyd DH, Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol. 2003;48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 30.Warman AJ, Rito TS, Fisher NE, Moss DM, Berry NG, O'Neill PM, Ward SA, Biagini GA. Antitubercular pharmacodynamics of phenothiazines. J Antimicrob Chemother. 2013;68:869–880. doi: 10.1093/jac/dks483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teh JS, Yano T, Rubin H. Type II NADH: menaquinone oxidoreductase of Mycobacterium tuberculosis. Infectious Disorders Drug Targets. 2007;7:169–181. doi: 10.2174/187152607781001781. [DOI] [PubMed] [Google Scholar]
- 32.Biagini GA, Viriyavejakul P, O'Neill PM, Bray PG, Ward SA. Functional characterization and target validation of alternative complex I of Plasmodium falciparum mitochondria. Antimicrob Agents Chemother. 2006;50:1841–1851. doi: 10.1128/AAC.50.5.1841-1851.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shirude PS, Paul P, Choudhury NR, Kedari C, Bandodkar B, Ugarkar BG. Quinolinyl pyrimidines: potent inhibitors of NDH-2 as a novel class of anti-TB agents. ACS Medicinal Chemistry Letters. 2012;3:736–740. doi: 10.1021/ml300134b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ordway D, Viveiros M, Leandro C, Bettencourt R, Almeida J, Martins M, Kristiansen JE, Molnar J, Amaral L. Clinical concentrations of thioridazine kill intracellular multidrug-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2003;47:917–922. doi: 10.1128/AAC.47.3.917-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amaral L, Kristiansen JE, Abebe LS, Millett W. Inhibition of the respiration of multi-drug resistant clinical isolates of Mycobacterium tuberculosis by thioridazine: potential use for initial therapy of freshly diagnosed tuberculosis. J Antimicrob Chemother. 1996;38:1049–1053. doi: 10.1093/jac/38.6.1049. [DOI] [PubMed] [Google Scholar]
- 36.Mogi T, Matsushita K, Murase Y, Kawahara K, Miyoshi H, Ui H, Shiomi K, Omura S, Kita K. Identification of new inhibitors for alternative NADH dehydrogenase (NDH-II) FEMS Microbiol Lett. 2009;291:157–161. doi: 10.1111/j.1574-6968.2008.01451.x. [DOI] [PubMed] [Google Scholar]
- 37.Tian J, Bryk R, Itoh M, Suematsu M, Nathan C. Variant tricarboxylic acid cycle in Mycobacterium tuberculosis : identification of alpha-ketoglutarate decarboxylase. Proc Natl Acad Sci USA. 2005;102:10670–10675. doi: 10.1073/pnas.0501605102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Youmans AS, Millman I, Youmans GP. The oxidation of compounds related to the tricarboxylic acid cycle by whole cells and enzyme preparations of Mycobacterium tuberculosis var. hominis. J Bacteriol. 1956;71:565–570. doi: 10.1128/jb.71.5.565-570.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cecchini G, Schroder I, Gunsalus RP, Maklashina E. Succinate dehydrogenase and fumarate reductase from Escherichia coli. Biochimica et Biophysica Acta. 2002;1553:140–157. doi: 10.1016/s0005-2728(01)00238-9. [DOI] [PubMed] [Google Scholar]
- 40.Baek SH, Li AH, Sassetti CM. Metabolic regulation of mycobacterial growth and antibiotic sensitivity. PLoS Biol. 2011;9:e1001065. doi: 10.1371/journal.pbio.1001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Unden G, Schirawski J. The oxygen-responsive transcriptional regulator FNR of Escherichia coli: the search for signals and reactions. Mol Microbiol. 1997;25:205–210. doi: 10.1046/j.1365-2958.1997.4731841.x. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe S, Zimmermann M, Goodwin MB, Sauer U, Barry CE, 3rd, Boshoff HI. Fumarate reductase activity maintains an energized membrane in anaerobic Mycobacterium tuberculosis. PLoS Pathog. 2011;7:e1002287. doi: 10.1371/journal.ppat.1002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eoh H, Rhee KY. Multifunctional essentiality of succinate metabolism in adaptation to hypoxia in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2013;110:6554–6559. doi: 10.1073/pnas.1219375110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bacon J, James BW, Wernisch L, Williams A, Morley KA, Hatch GJ, Mangan JA, Hinds J, Stoker NG, Butcher PD, Marsh PD. The influence of reduced oxygen availability on pathogenicity and gene expression in Mycobacterium tuberculosis. Tuberculosis (Edinb) 2004;84:205–217. doi: 10.1016/j.tube.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 45.Beste DJ, Laing E, Bonde B, Avignone-Rossa C, Bushell ME, McFadden JJ. Transcriptomic analysis identifies growth rate modulation as a component of the adaptation of mycobacteria to survival inside the macrophage. J Bacteriol. 2007;189:3969–3976. doi: 10.1128/JB.01787-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanner JJ. Structural biology of proline catabolism. Amino Acids. 2008;35:719–730. doi: 10.1007/s00726-008-0062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menzel R, Roth J. Purification of the putA gene product. A bifunctional membrane-bound protein from Salmonella typhimurium responsible for the two-step oxidation of proline to glutamate. J Biol Chem. 1981;256:9755–9761. [PubMed] [Google Scholar]
- 48.Weigoldt M, Meens J, Bange FC, Pich A, Gerlach GF, Goethe R. Metabolic adaptation of Mycobacterium avium subsp. paratuberculosis to the gut environment. Microbiology. 2013;159:380–391. doi: 10.1099/mic.0.062737-0. [DOI] [PubMed] [Google Scholar]
- 49.Smith DA, Parish T, Stoker NG, Bancroft GJ. Characterization of auxotrophic mutants of Mycobacterium tuberculosis and their potential as vaccine candidates. Infect Immun. 2001;69:1142–1150. doi: 10.1128/IAI.69.2.1142-1150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vignais PM, Colbeau A. Molecular biology of microbial hydrogenases. Curr Issues Mol Biol. 2004;6:159–188. [PubMed] [Google Scholar]
- 51.Tamagnini P, Leitao E, Oliveira P, Ferreira D, Pinto F, Harris DJ, Heidorn T, Lindblad P. Cyanobacterial hydrogenases: diversity, regulation and applications. FEMS Microbiol Rev. 2007;31:692–720. doi: 10.1111/j.1574-6976.2007.00085.x. [DOI] [PubMed] [Google Scholar]
- 52.Constant P, Chowdhury SP, Hesse L, Pratscher J, Conrad R. Genome data mining and soil survey for the novel group 5 [NiFe]-hydrogenase to explore the diversity and ecological importance of presumptive high-affinity H(2)-oxidizing bacteria. Appl Environ Microbiol. 2011;77:6027–6035. doi: 10.1128/AEM.00673-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Constant P, Chowdhury SP, Pratscher J, Conrad R. Streptomycetes contributing to atmospheric molecular hydrogen soil uptake are widespread and encode a putative high-affinity [NiFe]-hydrogenase. Environ Microbiol. 2010;12:821–829. doi: 10.1111/j.1462-2920.2009.02130.x. [DOI] [PubMed] [Google Scholar]
- 54.King GM. Uptake of carbon monoxide and hydrogen at environmentally relevant concentrations by mycobacteria. Appl Environ Microbiol. 2003;69:7266–7272. doi: 10.1128/AEM.69.12.7266-7272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim YM, Hegeman GD. Oxidation of carbon monoxide by bacteria. Int Rev Cytol. 1983;81:1–32. doi: 10.1016/s0074-7696(08)62333-5. [DOI] [PubMed] [Google Scholar]
- 56.Park SW, Hwang EH, Park H, Kim JA, Heo J, Lee KH, Song T, Kim E, Ro YT, Kim SW, Kim YM. Growth of mycobacteria on carbon monoxide and methanol. J Bacteriol. 2003;185:142–147. doi: 10.1128/JB.185.1.142-147.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schryvers A, Lohmeier E, Weiner JH. Chemical and functional properties of the native and reconstituted forms of the membrane-bound, aerobic glycerol-3-phosphate dehydrogenase of Escherichia coli. J Biol Chem. 1978;253:783–788. [PubMed] [Google Scholar]
- 58.Boos W. Binding protein-dependent ABC transport system for glycerol 3- phosphate of Escherichia coli. Methods Enzymol. 1998;292:40–51. doi: 10.1016/s0076-6879(98)92006-7. [DOI] [PubMed] [Google Scholar]
- 59.Molenaar D, van der Rest ME, Drysch A, Yucel R. Functions of the membrane-associated and cytoplasmic malate dehydrogenases in the citric acid cycle of Corynebacterium glutamicum. J Bacteriol. 2000;182:6884–6891. doi: 10.1128/jb.182.24.6884-6891.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Molenaar D, van der Rest ME, Petrovic S. Biochemical and genetic characterization of the membrane-associated malate dehydrogenase (acceptor) from Corynebacterium glutamicum. Eur J Biochem. 1998;254:395–403. doi: 10.1046/j.1432-1327.1998.2540395.x. [DOI] [PubMed] [Google Scholar]
- 61.Prasada Reddy TL, Suryanarayana Murthy P, Venkitasubramanian TA. Respiratory chains of Mycobacterium smegmatis Indian. J Biochem Biophys. 1975;12:255–259. [PubMed] [Google Scholar]
- 62.Prasada Reddy TL, Suryanarayana Murthy P, Venkitasubramanian TA. Variations in the pathways of malate oxidation and phosphorylation in different species of Mycobacteria. Biochim Biophys Acta. 1975;376:210–218. doi: 10.1016/0005-2728(75)90012-2. [DOI] [PubMed] [Google Scholar]
- 63.D'Mello R, Hill S, Poole RK. The oxygen affinity of cytochrome bo' in Escherichia coli determined by the deoxygenation of oxyleghemoglobin and oxymyoglobin: Km values for oxygen are in the submicromolar range. J Bacteriol. 1995;177:867–870. doi: 10.1128/jb.177.3.867-870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.D'Mello R, Hill S, Poole RK. The cytochrome bd quinol oxidase in Escherichia coli has an extremely high oxygen affinity and two oxygen-binding haems: implications for regulation of activity in vivo by oxygen inhibition. Microbiology. 1996;142:755–763. doi: 10.1099/00221287-142-4-755. [DOI] [PubMed] [Google Scholar]
- 65.Cotter PA, Melville SB, Albrecht JA, Gunsalus RP. Aerobic regulation of cytochrome d oxidase (cydAB ) operon expression in Escherichia coli : roles of Fnr and ArcA in repression and activation. Mol Microbiol. 1997;25:605–615. doi: 10.1046/j.1365-2958.1997.5031860.x. [DOI] [PubMed] [Google Scholar]
- 66.Tseng CP, Hansen AK, Cotter P, Gunsalus RP. Effect of cell growth rate on expression of the anaerobic respiratory pathway operons frdABCD dmsABC and narGHJI of Escherichia coli. J Bacteriol. 1994;176:6599–6605. doi: 10.1128/jb.176.21.6599-6605.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kana BD, Weinstein EA, Avarbock D, Dawes SS, Rubin H, Mizrahi V. Characterization of the cydAB -encoded cytochrome bd oxidase from Mycobacterium smegmatis. J Bacteriol. 2001;183:7076–7086. doi: 10.1128/JB.183.24.7076-7086.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Voskuil MI, Visconti KC, Schoolnik GK. Mycobacterium tuberculosis gene expression during adaptation to stationary phase and low-oxygen dormancy. Tuberculosis (Edinb) 2004;84:218–227. doi: 10.1016/j.tube.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 69.Boshoff HI, Barry CE., 3rd Tuberculosis - metabolism and respiration in the absence of growth. Nat Rev Microbiol. 2005;3:70–80. doi: 10.1038/nrmicro1065. [DOI] [PubMed] [Google Scholar]
- 70.Matsoso LG, Kana BD, Crellin PK, Lea-Smith DJ, Pelosi A, Powell D, Dawes SS, Rubin H, Coppel RL, Mizrahi V. Function of the cytochrome bc1-aa3 branch of the respiratory network in mycobacteria and network adaptation occurring in response to its disruption. J Bacteriol. 2005;187:6300–6308. doi: 10.1128/JB.187.18.6300-6308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Megehee JA, Hosler JP, Lundrigan MD. Evidence for a cytochrome bcc-aa3 interaction in the respiratory chain of Mycobacterium smegmatis. Microbiology. 2006;152:823–829. doi: 10.1099/mic.0.28723-0. [DOI] [PubMed] [Google Scholar]
- 72.Niebisch A, Bott M. Molecular analysis of the cytochrome bc1-aa3 branch of the Corynebacterium glutamicum respiratory chain containing an unusual diheme cytochrome c1. Arch Microbiol. 2001;175:282–294. doi: 10.1007/s002030100262. [DOI] [PubMed] [Google Scholar]
- 73.Abrahams KA, Cox JA, Spivey VL, Loman NJ, Pallen MJ, Constantinidou C, Fernandez R, Alemparte C, Remuinan MJ, Barros D, Ballell L, Besra GS. Identification of novel imidazo[1,2-a]pyridine inhibitors targeting M. tuberculosis QcrB. PLoS ONE. 2012;7:e52951. doi: 10.1371/journal.pone.0052951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Niebisch A, Bott M. Purification of a cytochrome bc-aa3 supercomplex with quinol oxidase activity from Corynebacterium glutamicum Identification of a fourth subunity of cytochrome aa3 oxidase and mutational analysis of diheme cytochrome c1. J Biol Chem. 2003;278:4339–4346. doi: 10.1074/jbc.M210499200. [DOI] [PubMed] [Google Scholar]
- 75.Berry EA, Trumpower BL. Isolation of ubiquinol oxidase from Paracoccus denitrificans and resolution into cytochrome bc1 and cytochrome c-aa3 complexes. J Biol Chem. 1985;260:2458–2467. [PubMed] [Google Scholar]
- 76.Sone N, Sekimachi M, Kutoh E. Identification and properties of a quinol oxidase super-complex composed of a bc1 complex and cytochrome oxidase in the thermophilic bacterium PS3. J Biol Chem. 1987;262:15386–15391. [PubMed] [Google Scholar]
- 77.Poole RK, Cook GM. Redundancy of aerobic respiratory chains in bacteria? Routes, reasons and regulation. Adv Microb Physiol. 2000;43:165–224. doi: 10.1016/s0065-2911(00)43005-5. [DOI] [PubMed] [Google Scholar]
- 78.Borisov VB, Murali R, Verkhovskaya ML, Bloch DA, Han H, Gennis RB, Verkhovsky MI. Aerobic respiratory chain of Escherichia coli is not allowed to work in fully uncoupled mode. Proc Natl Acad Sci USA. 2011;108:17320–17324. doi: 10.1073/pnas.1108217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goldman BS, Gabbert KK, Kranz RG. The temperature-sensitive growth and survival phenotypes of Escherichia coli cydDC and cydAB strains are due to deficiencies in cytochrome bd and are corrected by exogenous catalase and reducing agents. J Bacteriol. 1996;178:6348–6351. doi: 10.1128/jb.178.21.6348-6351.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pittman MS, Robinson HC, Poole RK. A bacterial glutathione transporter (Escherichia coli CydDC) exports reductant to the periplasm. J Biol Chem. 2005;280:32254–32261. doi: 10.1074/jbc.M503075200. [DOI] [PubMed] [Google Scholar]
- 81.Dhar N, McKinney JD. Mycobacterium tuberculosis persistence mutants identified by screening in isoniazid-treated mice. Proc Natl Acad Sci U S A. 2010;107:12275–12280. doi: 10.1073/pnas.1003219107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rowe JJ, Ubbink-Kok T, Molenaar D, Konings WN, Driessen AJ. NarK is a nitrite-extrusion system involved in anaerobic nitrate respiration by Escherichia coli. Mol Microbiol. 1994;12:579–586. doi: 10.1111/j.1365-2958.1994.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 83.Clegg S, Yu F, Griffiths L, Cole JA. The roles of the polytopic membrane proteins NarK, NarU and NirC in Escherichia coli K-12: two nitrate and three nitrite transporters. Mol Microbiol. 2002;44:143–155. doi: 10.1046/j.1365-2958.2002.02858.x. [DOI] [PubMed] [Google Scholar]
- 84.Jia W, Cole JA. Nitrate and nitrite transport in Escherichia coli. Biochem Soc Trans. 2005;33:159–161. doi: 10.1042/BST0330159. [DOI] [PubMed] [Google Scholar]
- 85.Sohaskey CD, Wayne LG. Role of narK2X and narGHJI in hypoxic upregulation of nitrate reduction by Mycobacterium tuberculosis. J Bacteriol. 2003;185:7247–7256. doi: 10.1128/JB.185.24.7247-7256.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sohaskey CD. Regulation of nitrate reductase activity in Mycobacterium tuberculosis by oxygen and nitric oxide. Microbiology. 2005;151:3803–3810. doi: 10.1099/mic.0.28263-0. [DOI] [PubMed] [Google Scholar]
- 87.Moir JW, Wood NJ. Nitrate and nitrite transport in bacteria. Cell Mol Life Sci. 2001;58:215–224. doi: 10.1007/PL00000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kumar A, Deshane JS, Crossman DK, Bolisetty S, Yan BS, Kramnik I, Agarwal A, Steyn AJ. Heme oxygenase-1-derived carbon monoxide induces the Mycobacterium tuberculosis dormancy regulon. J Biol Chem. 2008;283:18032–18039. doi: 10.1074/jbc.M802274200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ohno H, Zhu G, Mohan VP, Chu D, Kohno S, Jacobs WR, Jr, Chan J. The effects of reactive nitrogen intermediates on gene expression in Mycobacterium tuberculosis. Cell Microbiol. 2003;5:637–648. doi: 10.1046/j.1462-5822.2003.00307.x. [DOI] [PubMed] [Google Scholar]
- 90.Voskuil MI, Schnappinger D, Visconti KC, Harrell MI, Dolganov GM, Sherman DR, Schoolnik GK. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J Exp Med. 2003;198:705–713. doi: 10.1084/jem.20030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sohaskey CD. Nitrate enhances the survival of Mycobacterium tuberculosis during inhibition of respiration. J Bacteriol. 2008;190:2981–2986. doi: 10.1128/JB.01857-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tan MP, Sequeira P, Lin WW, Phong WY, Cliff P, Ng SH, Lee BH, Camacho L, Schnappinger D, Ehrt S, Dick T, Pethe K, Alonso S. Nitrate respiration protects hypoxic Mycobacterium tuberculosis against acid- and reactive nitrogen species stresses. PLoS ONE. 2010;5:e13356. doi: 10.1371/journal.pone.0013356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pinto R, Harrison JS, Hsu T, Jacobs WR, Jr, Leyh TS. Sulfite reduction in mycobacteria. J Bacteriol. 2007;189:6714–6722. doi: 10.1128/JB.00487-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kuhn M, Steinbuchel A, Schlegel HG. Hydrogen evolution by strictly aerobic hydrogen bacteria under anaerobic conditions. J Bacteriol. 1984;159:633–639. doi: 10.1128/jb.159.2.633-639.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fontan P, Aris V, Ghanny S, Soteropoulos P, Smith I. Global transcriptional profile of Mycobacterium tuberculosis during THP-1 human macrophage infection. Infect Immun. 2008;76:717–725. doi: 10.1128/IAI.00974-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park HD, Guinn KM, Harrell MI, Liao R, Voskuil MI, Tompa M, Schoolnik GK, Sherman DR. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol Microbiol. 2003;48:833–843. doi: 10.1046/j.1365-2958.2003.03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sherman DR, Voskuil M, Schnappinger D, Liao R, Harrell MI, Schoolnik GK. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha -crystallin. Proc Natl Acad Sci U S A. 2001;98:7534–7539. doi: 10.1073/pnas.121172498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mnatsakanyan N, Bagramyan K, Trchounian A. Hydrogenase 3 but not hydrogenase 4 is major in hydrogen gas production by Escherichia coli formate hydrogenlyase at acidic pH and in the presence of external formate. Cell Biochem Biophys. 2004;41:357–366. doi: 10.1385/CBB:41:3:357. [DOI] [PubMed] [Google Scholar]
- 99.Coppi MV. The hydrogenases of Geobacter sulfurreducens : a comparative genomic perspective. Microbiology. 2005;151:1239–1254. doi: 10.1099/mic.0.27535-0. [DOI] [PubMed] [Google Scholar]
- 100.Marreiros BC, Batista AP, Duarte AM, Pereira MM. A missing link between complex I and group 4 membrane-bound [NiFe] hydrogenases. Biochimica et Biophysica Acta. 2013;1827:198–209. doi: 10.1016/j.bbabio.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 101.He H, Bretl DJ, Penoske RM, Anderson DM, Zahrt TC. Components of the Rv0081-Rv0088 locus, which encodes a predicted formate hydrogenlyase complex, are coregulated by Rv0081, MprA, and DosR in Mycobacterium tuberculosis. J Bacteriol. 2011;193:5105–5118. doi: 10.1128/JB.05562-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.von Ballmoos C, Cook GM, Dimroth P. Unique rotary ATP synthase and its biological diversity. Annu Rev Biophys. 2008;37:43–64. doi: 10.1146/annurev.biophys.37.032807.130018. [DOI] [PubMed] [Google Scholar]
- 103.Tran SL, Cook GM. The F1Fo-ATP synthase of Mycobacterium smegmatis is essential for growth. J Bacteriol. 2005;187:5023–5028. doi: 10.1128/JB.187.14.5023-5028.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Friedl P, Hoppe J, Gunsalus RP, Michelsen O, von Meyenburg K, Schairer HU. Membrane integration and function of the three F0 subunits of the ATP synthase of Escherichia coli K12. EMBO J. 1983;2:99–103. doi: 10.1002/j.1460-2075.1983.tb01388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Santana M, Ionescu MS, Vertes A, Longin R, Kunst F, Danchin A, Glaser P. Bacillus subtilis F0F1 ATPase: DNA sequence of the atp operon and characterization of atp mutants. J Bacteriol. 1994;176:6802–6811. doi: 10.1128/jb.176.22.6802-6811.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cox RA, Cook GM. Growth regulation in the mycobacterial cell. Curr Mol Med. 2007;7:231–245. doi: 10.2174/156652407780598584. [DOI] [PubMed] [Google Scholar]
- 107.Tran SL, Rao M, Simmers C, Gebhard S, Olsson K, Cook GM. Mutants of Mycobacterium smegmatis unable to grow at acidic pH in the presence of the protonophore carbonyl cyanide m-chlorophenylhydrazone. Microbiology. 2005;151:665–672. doi: 10.1099/mic.0.27624-0. [DOI] [PubMed] [Google Scholar]
- 108.Koch-Koerfges A, Kabus A, Ochrombel I, Marin K, Bott M. Physiology and global gene expression of a Corynebacterium glutamicum DeltaF1Fo-ATP synthase mutant devoid of oxidative phosphorylation. Biochimica et Biophysica Acta. 2012;1817:370–380. doi: 10.1016/j.bbabio.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 109.Andries K, Verhasselt P, Guillemont J, Gohlmann HW, Neefs JM, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, Williams P, de Chaffoy D, Huitric E, Hoffner S, Cambau E, Truffot-Pernot C, Lounis N, Jarlier V. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science. 2005;307:223–227. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 110.Dhiman RK, Mahapatra S, Slayden RA, Boyne ME, Lenaerts A, Hinshaw JC, Angala SK, Chatterjee D, Biswas K, Narayanasamy P, Kurosu M, Crick DC. Menaquinone synthesis is critical for maintaining mycobacterial viability during exponential growth and recovery from non-replicating persistence. Mol Microbiol. 2009;72:85–97. doi: 10.1111/j.1365-2958.2009.06625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koul A, Dendouga N, Vergauwen K, Molenberghs B, Vranckx L, Willebrords R, Ristic Z, Lill H, Dorange I, Guillemont J, Bald D, Andries K. Diarylquinolines target subunit c of mycobacterial ATP synthase. Nat Chem Biol. 2007;3:323–324. doi: 10.1038/nchembio884. [DOI] [PubMed] [Google Scholar]
- 112.Koul A, Vranckx L, Dendouga N, Balemans W, Van den Wyngaert I, Vergauwen K, Gohlmann HW, Willebrords R, Poncelet A, Guillemont J, Bald D, Andries K. Diarylquinolines are bactericidal for dormant mycobacteria as a result of disturbed ATP homeostasis. J Biol Chem. 2008;283:25273–25280. doi: 10.1074/jbc.M803899200. [DOI] [PubMed] [Google Scholar]
- 113.Huitric E, Verhasselt P, Andries K, Hoffner SE. In vitro antimycobacterial spectrum of a diarylquinoline ATP synthase inhibitor. Antimicrob Agents Chemother. 2007;51:4202–4204. doi: 10.1128/AAC.00181-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huitric E, Verhasselt P, Koul A, Andries K, Hoffner S, Andersson DI. Rates and mechanisms of resistance development in Mycobacterium tuberculosis to a novel diarylquinoline ATP synthase inhibitor. Antimicrob Agents Chemother. 2010;54:1022–1028. doi: 10.1128/AAC.01611-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.de Jonge MR, Koymans LH, Guillemont JE, Koul A, Andries K. A computational model of the inhibition of Mycobacterium tuberculosis ATPase by a new drug candidate R207910. Proteins. 2007;67:971–980. doi: 10.1002/prot.21376. [DOI] [PubMed] [Google Scholar]
- 116.Haagsma AC, Podasca I, Koul A, Andries K, Guillemont J, Lill H, Bald D. Probing the interaction of the diarylquinoline TMC207 with its target mycobacterial ATP synthase. PLoS ONE. 2011;6:e23575. doi: 10.1371/journal.pone.0023575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Frampton R, Aggio RB, Villas-Boas SG, Arcus VL, Cook GM. Toxin-antitoxin systems of Mycobacterium smegmatis are essential for cell survival. J Biol Chem. 2012;287:5340–5356. doi: 10.1074/jbc.M111.286856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lounis N, Gevers T, Van den Berg J, Vranckx L, Andries K. ATP synthase inhibition of Mycobacterium avium is not bactericidal. Antimicrob Agents Chemother. 2009;53:4927–4929. doi: 10.1128/AAC.00689-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wayne LG, Sohaskey CD. Nonreplicating persistence of mycobacterium tuberculosis. Annu Rev Microbiol. 2001;55:139–163. doi: 10.1146/annurev.micro.55.1.139. [DOI] [PubMed] [Google Scholar]
- 120.Shiloh MU, Manzanillo P, Cox JS. Mycobacterium tuberculosis senses hostderived carbon monoxide during macrophage infection. Cell Host Microbe. 2008;3:323–330. doi: 10.1016/j.chom.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Singh A, Guidry L, Narasimhulu KV, Mai D, Trombley J, Redding KE, Giles GI, Lancaster JR, Jr, Steyn AJ. Mycobacterium tuberculosis WhiB3 responds to O2 and nitric oxide via its [4Fe-4S] cluster and is essential for nutrient starvation survival. Proc Natl Acad Sci USA. 2007;104:11562–11567. doi: 10.1073/pnas.0700490104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Crack J, Green J, Thomson AJ. Mechanism of oxygen sensing by the bacterial transcription factor fumarate-nitrate reduction (FNR) J Biol Chem. 2004;279:9278–9286. doi: 10.1074/jbc.M309878200. [DOI] [PubMed] [Google Scholar]
- 123.Suhail Alam M, Agrawal P. Matrix-assisted refolding and redox properties of WhiB3/Rv3416 of Mycobacterium tuberculosis H37Rv. Protein Expr Purif. 2008;61:83–91. doi: 10.1016/j.pep.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 124.Steyn AJ, Collins DM, Hondalus MK, Jacobs WR, Jr, Kawakami RP, Bloom BR. Mycobacterium tuberculosis WhiB3 interacts with RpoV to affect host survival but is dispensable for in vivo growth. Proc Natl Acad Sci U S A. 2002;99:3147–3152. doi: 10.1073/pnas.052705399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rustad TR, Harrell MI, Liao R, Sherman DR. The enduring hypoxic response of Mycobacterium tuberculosis. PLoS ONE. 2008;3:e1502. doi: 10.1371/journal.pone.0001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gazdik MA, McDonough KA. Identification of cyclic AMP-regulated genes in Mycobacterium tuberculosis complex bacteria under low-oxygen conditions. J Bacteriol. 2005;187:2681–2692. doi: 10.1128/JB.187.8.2681-2692.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Roberts G, Vadrevu IS, Madiraju MV, Parish T. Control of CydB and GltA1 expression by the SenX3 RegX3 two component regulatory system of Mycobacterium tuberculosis. PLoS ONE. 2011;6:e21090. doi: 10.1371/journal.pone.0021090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rickman L, Scott C, Hunt DM, Hutchinson T, Menendez MC, Whalan R, Hinds J, Colston MJ, Green J, Buxton RS. A member of the cAMP receptor protein family of transcription regulators in Mycobacterium tuberculosis is required for virulence in mice and controls transcription of the rpfA gene coding for a resuscitation promoting factor. Mol Microbiol. 2005;56:1274–1286. doi: 10.1111/j.1365-2958.2005.04609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang YJ, Ioerger TR, Huttenhower C, Long JE, Sassetti CM, Sacchettini JC, Rubin EJ. Global assessment of genomic regions required for growth in Mycobacterium tuberculosis. PLoS Pathog. 2012;8:e1002946. doi: 10.1371/journal.ppat.1002946. [DOI] [PMC free article] [PubMed] [Google Scholar]