Abstract
Cytotoxic lymphocyte skill target cells by polarized release of the content of perforin-containing granules. In natural killer cells, the binding of β2 integrin to its ligand ICAM-1 is sufficient to promote not only adhesion but also lytic granule polarization. This provided a unique opportunity to study polarization in the absence of degranulation, and β2 integrin signaling independently of inside-out signals from other receptors. Using an unbiased proteomics approach we identified a signaling network centered on an integrin-linked kinase (ILK)–Pyk2–Paxillin core that was required for granule polarization. Downstream of ILK, the highly conserved Cdc42–Par6 signaling pathway that controls cell polarity was activated and required for granule polarization. These results delineate two connected signaling networks induced upon β2 integrin engagement alone, which are integrated to control polarization of the microtubule organizing center and associated lytic granules toward the site of contact with target cells during cellular cytotoxicity.
INTRODUCTION
Integrins bind to extracellular matrix and other proteins to regulate interactions among cells. They play an essential role in many processes, including cell adhesion, cell cycle, and cell migration. The high affinity conformation of β1 and β2 integrins is dependent on inside-out signals delivered by other receptors, which stimulate extension of the αβ heterodimer and exposure of the ligand binding site (1). In turn, integrin binding to ligand transduces outside-in signals, which contribute tolymphocyte motility, polarity, and adhesion. The β2 (CD18) family of integrins, present in leukocytes, includes four members associated with different α chains. CD18 deficiency results in Leukocyte Adhesion Deficiency syndrome type 1 (LAD-I), with severe abnormalities in adhesion-dependent functions of leukocytes (2). αLβ2 (CD11a/CD18, LFA-1), which binds to ICAM-1 and other ICAM molecules, is present in lymphocytes. Natural Killer (NK) cells contain also, at a lower abundance, αMβ2 (CD11b/CD18, Mac-1), which binds to ICAM-1 and other ligands.
In cytotoxic lymphocytes, including T cells and NK cells, LFA-1 is essential for firm adhesion to target cells and for effective cytotoxicity. Adhesion of T cells to endothelial cells, antigen presenting cells, and target cells relies on inside-out signals (1) from the T cell receptor (TCR) or chemokine receptors. This dependence on signals from other receptors has made it difficult to study outside-in signaling by integrins.
In contrast, LFA-1 on primary, resting NK cells binds to its ligands ICAM-1 and ICAM-2 in the absence of inside-out signals from other receptors (3). Binding of LFA-1 on NK cells to ICAM-1 provides not only adhesion but is also sufficient to promote the polarization of perforin-containing granules to the site of contact with cells that carry ICAM-1 (3, 4). Therefore, NK cells provide a unique opportunity to examine integrin signaling without interference from signaling by other receptors.
Signals that induce degranulation and granule polarization are uncoupled in NK cells: β2 integrin binding to ICAM-1 induces granule polarization, but not degranulation (4), whereas binding of FcγRIIIa (CD16) to IgG results in Ca2+ mobilization and degranulation without polarization (4). Therefore, NK cells provide also the opportunity to define signals that promote polarization of lytic granules, independently of degranulation.
We took advantage of these opportunities to study β2 integrin signaling in the absence of degranulation and of inside-out signals from other receptors, and used an unbiased mass spectrometry approach to identify signaling components in primary NK cells stimulated by purified ICAM-1. In a previous study, we had observed that stimulation of NK cells by ICAM-1 resulted in a pattern of protein tyrosine phosphorylation that was surprisingly similar to that obtained after stimulation by CD16, including phosphorylation of spleen tyrosine kinase (Syk) and Phospholipase C-γ (PLC-γ) (5). Therefore, we analyzed NK cells stimulated in parallel with ICAM-1 and human IgG1, and focused on signals that were induced selectively by stimulation with ICAM-1. Proteins identified by this approach were tested for their role in β2 integrin-dependent binding to ICAM-1 and in promoting granule polarization toward ICAM-1 positive cells.
Biochemical validation of the mass spectrometry results, imaging, and siRNA-mediated silencing of signaling components demonstrated that an ILK, Pyk2, Paxillin, and RhoGEF7 pathway was required for polarization of the microtubule organizing center (MTOC) and associated granules toward ICAM-1 positive cells. Distal signals, dependent on ILK, were transmitted by the GTPase Cdc42 and the microtubular network regulators Par6, APC, and CLIP-170. The results reveal how β2 integrin can signal on its own, using a highly conserved signaling pathway that establishes polarity during cell migration, to stimulate granule polarization in NK cells.
RESULTS
Components of β2 Integrin Outside-In Signaling Identified by Mass Spectrometry
To identify molecules involved in β2 integrin signaling in NK cells, tyrosine-phosphorylated proteins and associated proteins were isolated from lysates of primary NK cells that had been left unstimulated, or stimulated for 20 min through either ICAM-1, a ligand of β2 integrins LFA-1 and Mac-1, or human IgG1as a ligand for CD16. Immunoblots of proteins eluted with phenylphosphate from anti-phosphotyrosine (p-Tyr) mAb pull-downs revealed induction of tyrosine phosphorylation by both stimulations (Fig. 1A), as previously described (5). Pooled eluates from several experiments were separated by gel electrophoresis (fig. S1) and the entire protein content of each sample was analyzed by mass spectrometry. The complete data set, including spectral counts, and heat maps is available in Table S1.
Fig. 1. Components of β2 Integrin Outside-In Signaling Identified by Mass Spectrometry.
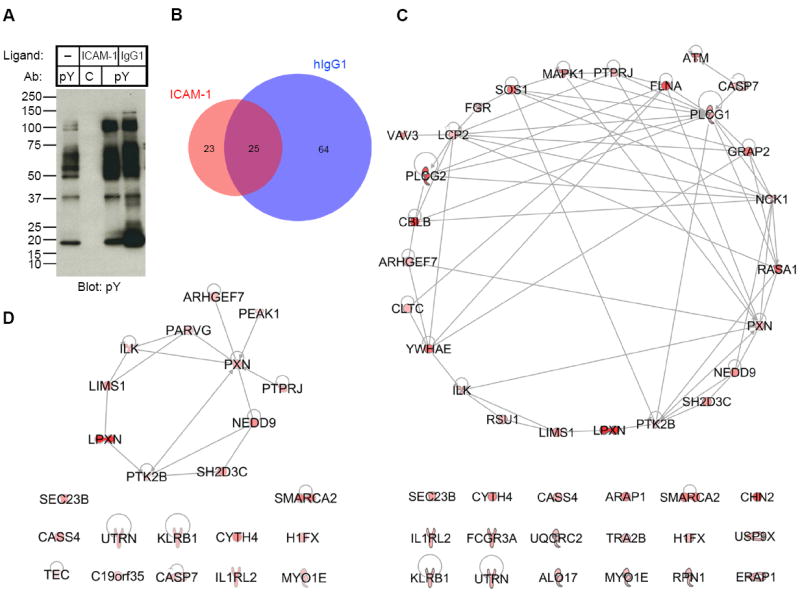
(A) Anti-phosphotyrosine (pY) immunoblot of samples used for mass spectrometry analysis. Tyrosine phosphorylated proteins and associated proteins were pulled-down with mAb 4G10 (pY) or isotype control IgG2b mAb MOPC141 (C) from lysates of primary NK cells stimulated on plates for 20 min with BSA only (–), ICAM-1, and human IgG1, as indicated. Samples were eluted with sodium phenyl phosphate, run on SDS-PAGE, and immunoblotted with mAb 4G10. (B) Venn diagram of the number of proteins identified by mass spectrometry in the samples stimulated with ICAM-1 or human IgG1, after subtraction of proteins in the control samples. Selection criteria are described in Table S4 to S6. (C) Ingenuity Pathway Analysis (IPA, http://www.ingenuity.com) of the proteins identified in the sample stimulated with ICAM-1 (Table S4). The intensity of the red color indicates the score from mass spectrometry analysis. (D) Network analysis of the 23 proteins in the sample stimulated with ICAM-1 that had higher scores than the sample stimulated with human IgG1 (Table S6).
Every one of the top 20 proteins, according to the number of spectral counts, in the isotype control pull-down of NK cell lysates was a component of the actin cytoskeleton (Table S2). Sequences in the 4G10 pull-down from lysates of IL-2 expanded, unstimulated NK cells included several molecules expected to be constitutively phosphorylated: Src-family kinases Lck, Lyn, and Fyn (6), c-Src tyrosine kinase Csk and its adapter Cbp (PAG1), and proteins in the IL-2 signaling pathway: Janus kinases JAK1 and JAK3, and their substrate Signal Transducer and Activator of Transcription (Stat5), and the p85 subunit of Phosphoinositide-3-Kinase (PI-3K) (Table S3).
The samples stimulated by ICAM-1 and human IgG1 were compared, after subtraction of proteins identified in the isotype control sample and in the 4G10 pull-down of NK cells incubated on control plates (Fig. 1B). Fourty four proteins were scored as positive in the ICAM-1 stimulated sample (Fig. 1C and Table S4), and 89 in the IgG1 stimulated sample (Table S5). Twenty five of those proteins were identified in both samples (Fig. 1B). This large overlap was consistent with the phosphotyrosine immunoblots (Fig. 1A). A direct comparison of samples stimulated with ICAM-1 and IgG1 identified another 4 proteins unique to ICAM-1 (Table S6), bringing the total number of proteins unique to the ICAM-1 stimulation to 23 (Fig. 1B). Phosphotyrosine protein complexes stimulated selectively through CD16 included a number of expected proteins, including TCR ζ chain, tyrosine kinase Zeta-chain Associated Protein Kinase of 70kDa (ZAP70), adapter Linker for Activation of T cells (LAT), guanine exchange factor Vav3 (7), and Munc13-4, a protein required for fusion of lytic granules with the plasma membrane, which is selectively recruited to perforin-containing granules in NK cells stimulated by CD16 but not LFA-1 (8). LAT is phosphorylated selectively after stimulation with CD16 and is not required for β2 integrin-dependent granule polarization (5). These results provided some confidence in our approach.
Bioinformatics analysis of the 44 proteins in the ICAM-1 stimulated sample resulted in a network of 26 proteins and left 18 orphan proteins (Fig. 1C). Analysis of the 23 proteins unique to ICAM-1 stimulation revealed a much simpler network of 11 proteins and 12 orphan proteins (Fig. 1D). Paxillin (PXN), connected to 7 other proteins, formed the main node in the network, followed by Pyk2 (PTK2B), with 4 connections. This result validated the approach, given that paxillin is required for β2 integrin-dependent granule polarization (5).
Signaling Components Required for Integrin-Dependent Granule Polarization
Proteins within the network of proteins that were unique to stimulation with ICAM-1 were validated by immunoblotting, and tested for their role in NK cellbinding to ICAM-1 and signaling for granule polarization aftergene silencing. ILKis a large scaffold protein and forms a stable complex with Pinch1 and one of the parvin molecules, which is called the IPP complex (9). Pinch1 (LIMS1) and γ-parvin (PARVG) molecules were indeed present in our analysis. γ-parvin, which is presentin leukocytes, controls spreading on fibronectin and cell polarity for migration (10). To validate the presence of ILK, NK cells were stimulated for 5 and 20 min on control plates and plates coated with ICAM-1 or human IgG1, and lysates were immunoprecipitated with 4G10. Samples eluted with phenyl-phosphate were immunoblotted for ILK. The ILK band intensity was stronger in the ICAM-1 stimulated sample than in the control stimulation and in the samples stimulated with human IgG1 (Fig. 2A). This experiment confirmed the mass spectrometry results and provided additional information. NK cells analyzed by mass spectsrometry had been stimulated for 20 min. Here, we compared the signal intensity after stimulation for 5 and 20 min. The signal for ILK after IgG1 stimulation was weaker than after ICAM-1 stimulation at both time points (Fig. 2A). This result indicated that the difference seen between ICAM-1 and IgG1 stimulation at 20 min, as in the mass spectrometry analysis, was not due to a faster response during stimulation of CD16, which could have decayed by the 20 min time point.
Fig. 2. ILK is Required for Granule Polarization Toward S2–ICAM-1 Cells.
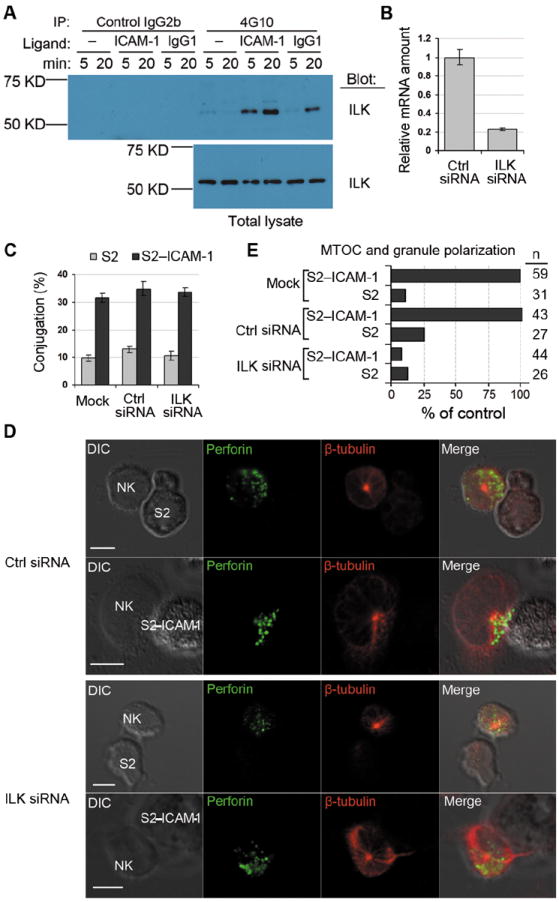
(A) Primary NK cells were stimulated with BSA only (–), ICAM-1, or human IgG1 for 5 and 20 min, as indicated. Cell lysates were immunoprecipitated with isotype control IgG2b agarose or with 4G10-agarose. Samples eluted with sodium phenyl phosphate (Top panel) and total cell lysates (Bottom panel) were immunoblotted with anti-ILK mAb. (B) ILK mRNA abundance 48 hours after silencing with siRNA, relative to ILK mRNA in cells treated with control (Ctrl) siRNA. Data shows 3 experiments with mean ± SD. (C) Conjugateformation of NK cells with S2 and S2–ICAM-1 cells after ILK silencing. Cells were either mock transfected or transfected with control (Ctrl) or ILK siRNA. Data shows 3 experiments with mean ± SD. (D) Representative images of MTOC and granule polarization toward S2 or S2–ICAM-1 cells. Cells were fixed, permeabilized and stained with mAb to perforin (IgG2b) and β-tubulin (IgG1) followed byisotype-specific Alexa Fluor 488-conjugated and Alexa Fluor 647-conjugatedsecondary antibodies. Scale bar = 5 μm. (E) Quantitative analysis of MTOC and granule polarization toward S2 and S2–ICAM-1 cells in NK cells treated as in (C). n represents individual NK–S2 cell contacts.
The role of ILK in the polarization of lytic granules induced by β2 integrin was tested by imaging, after silencing ILK with siRNA (Fig. 2B). As β2 integrin LFA-1 is essential for adhesion with target cells, we determined if ILK silencing had an effect on the ability of NK cells to bind to ICAM-1, before testing its effect on granule polarization. ILK silencing had no effect on the ability of NK cells to form conjugates with insect S2 cells transfected with ICAM-1 (S2–ICAM-1) (Fig. 2C). Polarization of the MTOC and of perforin-containing granules toward untransfected S2 cells or S2–ICAM-1 cells was imaged after staining fixed and permeabilized cells with Abs to perforin and β-tubulin (Fig. 2D). Polarization was scored as positive only when both the MTOC and the bulk of perforin-containing granules were in the first quarter of the NK cell diameter, closest to the target cell. Polarization was abolished after ILK silencing but not after mock transfection or transfection with control siRNA (Fig. 2E). We concluded that ILK is either directly tyrosine phosphorylated or recruited into phosphotyrosine protein complexes in NK cells stimulated through β2 integrin, more so than in NK cells stimulated by CD16, and that ILK is required in the signaling for granule polarization.
The same approach was used to test the role of γ-parvin, Pyk2, Leupaxin (LPXN), and RhoGEF7 (ARHGEF7) in β2 integrin-dependent functions. Biochemical validation of the mass spectrometry data was performed exactly as described for ILK in Fig. 2A. All four proteins were present in the 4G10 pull-downs after stimulation with ICAM-1 for 5 and 20 min (Fig. 3A). The band intensities were stronger than those in the CD16-stimulated cells at each time point. After silencing with siRNA (Fig. 3B), conjugation with S2–ICAM-1 cells and polarization of MTOC and granules toward synapses formed with S2–ICAM-1 cells were evaluated. Binding to S2–ICAM-1 cells was unaffected by the silencing of any of the proteins, except for Pyk2, which caused a small but significant reduction in conjugate formation (Fig. 3B). In contrast, silencing each of these four proteins significantly inhibited MTOC and granule polarization toward S2–ICAM-1 cells (Fig. 3B). We concluded that ILK, γ-parvin, Pyk2, Leupaxin, and RhoGEF7 all contribute to the polarization of lytic granules induced by β2 integrin upon binding to ICAM-1.
Fig. 3. Signaling Components Required for β2 Integrin-Dependent Granule Polarization.
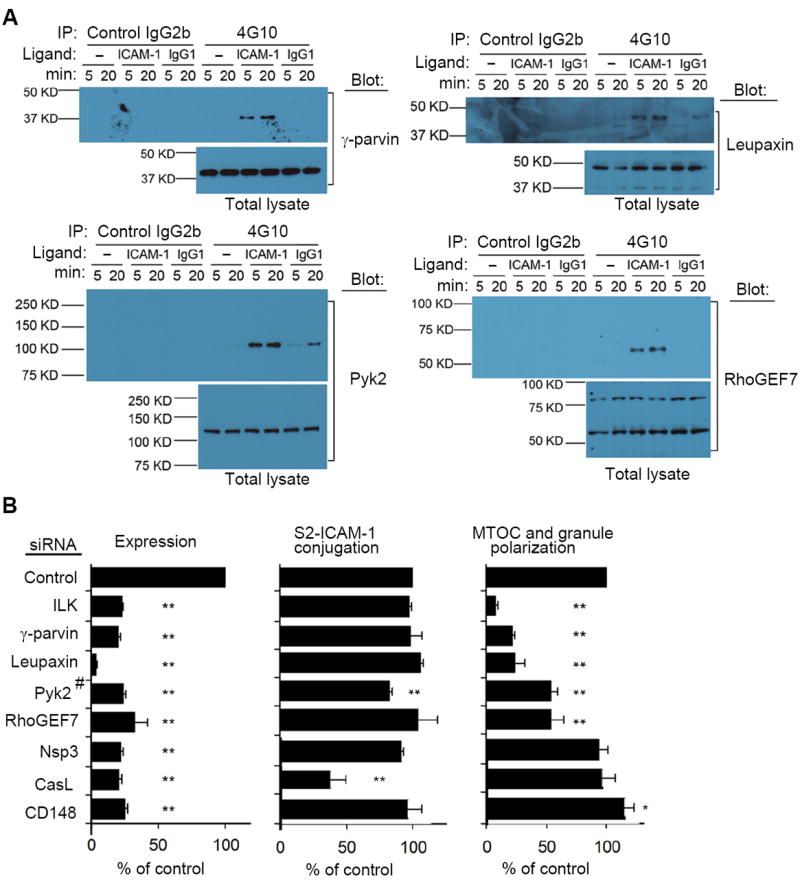
(A) NK cells treated as in Fig. 2A were tested for the presence of γ-parvin, Leupaxin, Pyk2 and RhoGEF7 in tyrosine-phosphorylated protein complexes. Representative immunoblots of 3 to 5 experiments are shown. (B) mRNA abundance, conjugate formation with S2–ICAM-1 cells, and MTOC and granule polarization, as indicated, of NK cells after silencing of the indicated genes by siRNA. (#Pyk2 protein was monitored by immunoblot.) Data are shown as percent of control siRNA. Conjugation with S2–ICAM-1 cells varied from 32% to 38%, and MTOC and granule polarization varied from 34% to 38% in the controls. Graphs show mean ± SEM from 3 experiments. *p<0.05, **p<0.01.
Silencing of Nsp3 (SH2D3C) had no effect on either binding to or granule polarization toward S2–ICAM-1 cells, and silencing of CasL (NEDD9) significantly reduced conjugation with, but had no effect on granule polarization towardS2-ICAM-1 cells, among those NK cells that had formed conjugates despite CasL silencing (Fig. 3B). These results do not exclude a role of these proteins in β2 integrin signaling. The importance of paxillin in ICAM-1 stimulated granule polarization in NK cells has been demonstrated previously (5). Silencing of CD148 (PTPRJ) had no effect on conjugate formation, and caused a small but significant increase in granule polarization toward S2-ICAM-1 cells. As CD148 is a receptor-type protein tyrosine phosphatase, which induces dephosphorylation of paxillin (11), it may dampen paxillin-dependent signals for polarization. The remaining 2 proteins in the network, Pinch1 and Sgk269 (PEAK1) could not be evaluated due to inefficient silencing with siRNA. Pinch1 may be required for granule polarization, as it is an obligate member of the IPP complex, and provides IPP protein stability (9). Sgk269 is a pseudo-kinase involved in the actin cytoskeleton and focal adhesion in migrating cells, via regulation of paxillin signaling (12, 13). Our analysis has identified a signaling network, centered around paxillin and ILK (Fig. 4A), which is required for granule polarization induced by β2 integrin.
Fig. 4. A Conserved Signaling Pathway for Cell Polarity is Used by β2 Integrin to Induce Granule Polarization.
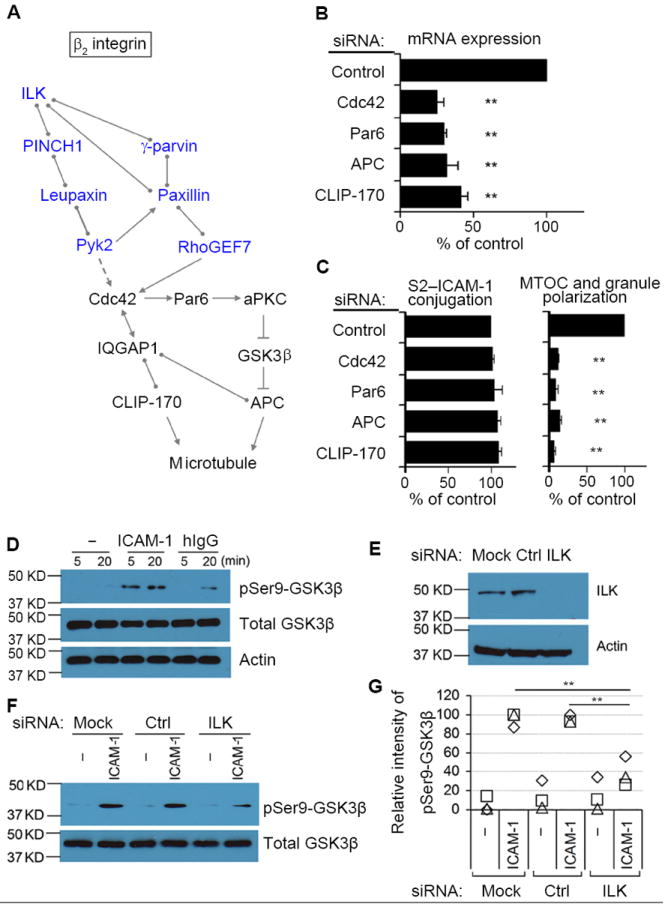
(A) Potential pathway for β2 integrin-dependent granule polarization in NK cells. Molecules in blue have been identified by mass spectrometry. Cdc42, known as a master regulator of microtubule-dependent cell polarity, can be activated by RhoGEF7 and Pyk2. Cdc42 controls cell polarity through both CLIP-170 and APC. Interactions in the diagram indicate direct binding (circles), activation (arrows), indirect activation (dashed arrow), or inhibition (cross bar), according to Ingenuity Pathway Analysis. (B) mRNA abundance after silencing of the indicated proteins. (C) Conjugation with S2–ICAM-1 cells and MTOC and granule polarization after silencing of the indicated proteins. Conjugation varied from 30% to 36%, and MTOC and granule polarization varied from 33% to 37% in the controls. Graphs show mean ± SEM from 3 experiments. **p<0.01. (D) Primary NK cells stimulated with BSA only (–), ICAM-1, or human IgG1 for the indicated times. Cell lysates were immunoblotted with antibodies to Ser9 phosphorylated GSK3β, total GSK3β, and actin. (E) ILK abundance monitored by immunoblot after silencing with siRNA. (F) After stimulation with BSA only (–) or ICAM-1 for 5 min, lysates were immunoblotted as in (D). (G) Intensity of GSK3β Ser9 phosphorylation, relative to phosphorylation in siRNA controls. Squares, triangles, and diamonds represent data from, from 3 independent experiments. **p<0.01.
A Conserved Signaling Pathway for Cell Polarity is Used by β2 Integrin to Induce Granule Polarization
A candidate effector downstream of the paxillin–ILK network is the small GTPase Cdc42, known as a master regulator of cell polarity (14). Potential connections with Cdc42 include RhoGEF7, which is a direct Cdc42 activator, and Pyk2, which contributes indirectly to Cdc42 activation by inhibition of a RhoGAP protein (15). Cdc42 activity at NK immunological synapses oscillates, and this oscillation requires RhoGEF6 and RhoGEF7 (16). The Cdc42 pathway, which controls cell polarity during cell migration and cell division, is conserved from C. elegans to man. It involves activation of CLIP-170, which mediates attachment to microtubules (17, 18), and Par6-dependent activation of APC, which promotes microtubule stability (19). Cdc42 is connected to CLIP-170 through IQGAP1 (Fig. 4A), a large scaffold protein, which contributes to granule polarization in NK cells, but not in T cells (20). To test the involvement of this pathway (Fig. 4A) in controlling granule polarization induced by β2 integrin, we reduced the abundance of mRNA for Cdc42, Par6, APC, and CLIP-170 by siRNA (Fig. 4B). Silencing of any one of the four genes had no effect on conjugate formation with S2–ICAM-1 cells but strongly inhibited MTOC and granule polarization (Fig. 4C). These results demonstrated that the Cdc42 pathway, known to control polarity mostly during cell migration and cell division (14, 19), is triggered by β2 integrin signaling to control polarization of NK cell granules toward target cells.
We tested whether the ILK and Cdc42 pathways, required for granule polarization induced by β2 integrin alone, could be bypassed in NK cells that are bound to sensitive human target cells, which present a multitude of ligands for NK cell receptors. Polarization of MTOC and granules toward the MHC-I deficient cell line 721.221 was imaged after silencing ILK, RhoGEF7, Cdc42, APC, and CLIP-170. In all cases, MTOC and granule polarization toward 721. 221 cells was greatly reduced (fig. S2). Therefore, components of the pathway identified here that were required for polarization toward S2–ICAM-1 cells were also required in the context of NK cell conjugates with NK-sensitive human cells.
The Cdc42 pathway relies on phosphorylation of kinase GSK3β on Ser9 by an a typical protein kinase C (aPKC), which inhibits GSK3β and reverses its inhibitory effect on APC (14). Therefore, we tested whether binding of NK cells to ICAM-1 alone was sufficient to induce GSK3β phosphorylation. Ser9 phosphorylation of GSK3β was detected in NK cells that had been stimulated for 5 or 20 min with ICAM-1 on plates (Fig. 4D). pSer9-GSK3β was not detected in control cells and was weaker in NK cells stimulated with human IgG1. ILK silencing (Fig. 4E) resulted in diminished GSK3β phosphorylation on Ser9 in primary NK cells stimulated with ICAM-1 (Fig. 4, F and G). These data showed that the ILK pathway controls GSK3βphosphorylation on Ser9.
Distinct Requirements for β2 Integrin-Dependent MTOC Polarization and Granule Convergence to the MTOC
Polarization of lytic granules to cytotoxic immunological synapses requires two distinct steps: MTOC polarization toward the synapse, and convergence of lytic granules to the MTOC (21, 22). To use a stringent definition of granule polarization, we have so far defined it as the combined polarization of MTOC and of granules toward ICAM-1 positive cells. To delineate the requirements for granule convergence to the MTOC, in the absence of MTOC polarization, we used the NK cell line KHYG-1, in which granules are constitutively retained near the MTOC (23). It was therefore possible to examine granule convergence to the MTOC in KHYG-1 cells in the absence of conjugate formation with target cells. Furthermore, the large size of KHYG-1 cells facilitated the quantitative measurements of granule convergence to the MTOC (Fig. 5). Silencing of leupaxin, but not ILK, resulted in diminished granule retention at the MTOC (Fig. 5, A and B). This result showed that granule retention near the MTOC is an active process, which was disrupted in the absence of leupaxin. The proteins required for MTOC and granule polarization in primary NK cells were silenced in KHYG-1 cells to examine their role in granule convergence to the MTOC. Leupaxin and Pyk2 silencing reduced the amount of perforin located near the MTOC, and the fraction of KHYG-1 cells in which granules were retained near the MTOC (Fig. 5, B and C). CLIP-170 silencing resulted in a significant reduction of perforin density near the MTOC (Fig. 5, D and E). There was no change in granule retention near the MTOC after Par6 and APC silencing, and a small but significant reduction after Cdc42 silencing (Fig. 5F). These results showed that Pyk2, leupaxin, Cdc42 and CLIP-170 are required for granule retention near the MTOC in KHYG-1 cells.
Fig. 5. Convergence and Retention of Granules at the MTOC Require Leupaxin, Pyk2, and CLIP-170.
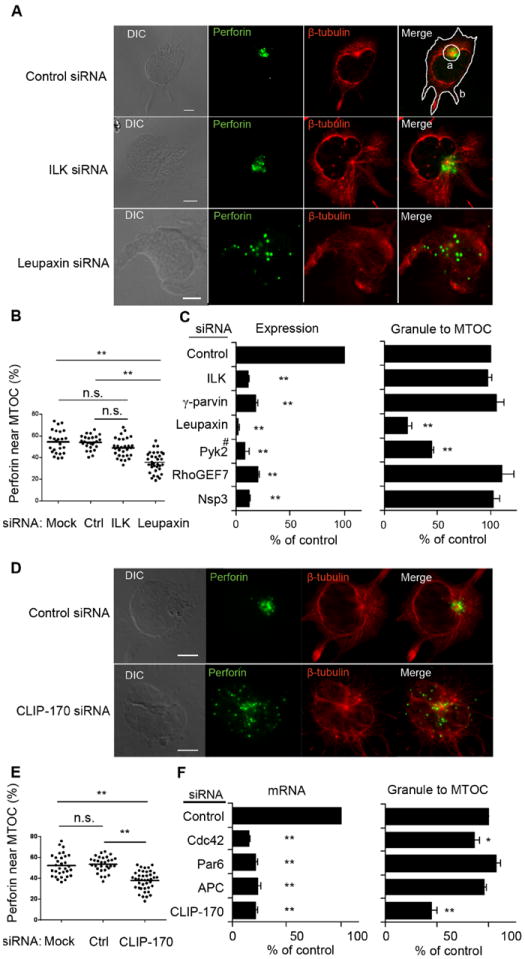
(A) Staining of perforin and β-tubulin in KHYG-1 cells after ILK or Leupaxin silencing. The contours in the merged panel of the control indicate a 7.5 μm circle centered over the MTOC (a), and the whole cell (b). (B) Fraction of perforin located near the MTOC relative to perforin in the whole cell after ILK or Leupaxin silencing. (C) mRNA abundance and fraction of cells with perforin near the MTOC after silencing of the indicated genes. (#Pyk2 abundance was monitored by immunoblot.) The fraction of NK cells in which perforin was near the MTOC in the controls (control siRNA) varied from 71% to 78%, and is set to 100% for each experiment. Graphs show mean ± SEM from 3 experiments. **p<0.01. (D-F) KHYG-1 cells analyzed after silencing of the indicated genes, exactly as described for A-C. The fraction of NK cells in which perforin was near the MTOC in the controls (control siRNA) varied from 71% to 78%, and is set to 100% for each experiment. Graphs show mean ± SEM from 3 experiments. *p<0.05, **p<0.01.
This information, obtained with KHYG-1 cells, suggested that the established role of Pyk2, leupaxin, Cdc42, and CLIP-170 in granule polarization in primary NK cells bound to ICAM-1 positive cells could be due mainly to their role in granule convergence to the MTOC, rather than in MTOC polarization. Therefore, we tested whether the inhibition of granule polarization toward S2–ICAM-1 cells observed after siRNA silencing of these 4 proteins in primary NK cells involved a direct inhibition of MTOC polarization. Pyk2, leupaxin, Cdc42, and CLIP-170 silencing in primary NK cells resulted in strong inhibition of MTOC polarization (Fig. 6A). We concluded that these four proteins contribute to both granule convergence to the MTOC and MTOC polarization.
Fig. 6. β2 Integrin Signaling Controls MTOC Polarization and Granule Convergence to the MTOC.
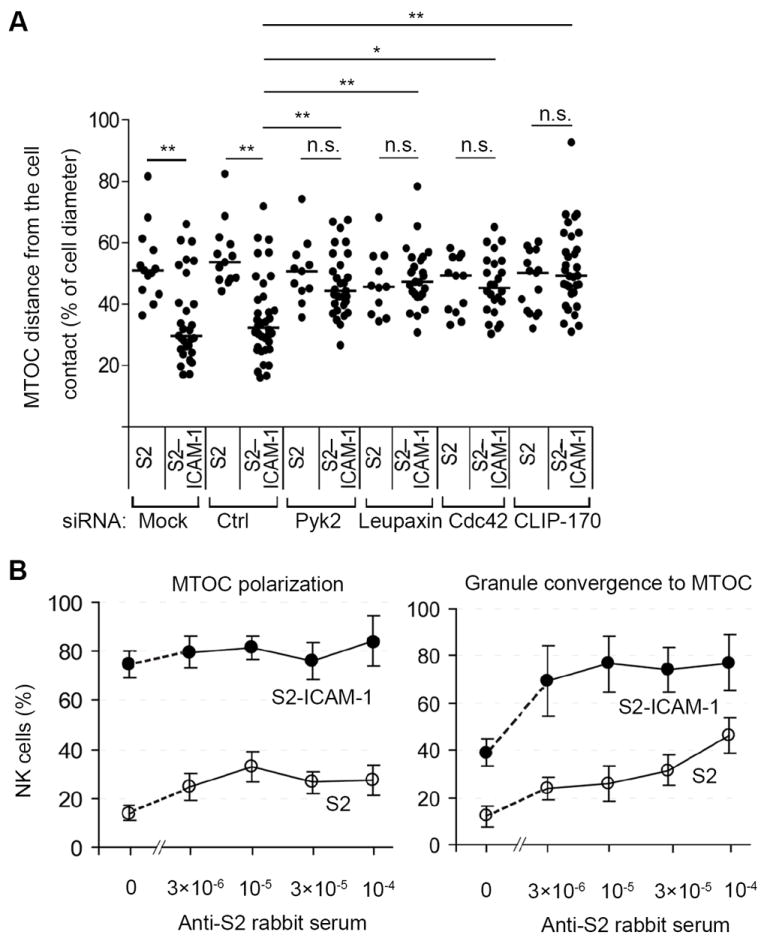
(A) Distance of the MTOC in NK cells from the site of contact with S2 or S2–ICAM-1 cells, relative to the cell diameter, after silencing of the indicated genes. Each dot represents a measurement from a single cell. The graph shows the median from measurements collected in 3 experiments. N. s., not significant; *p<0.05; **p<0.01. (B) Polarization of the MTOC toward S2 and S2–ICAM-1 cells and granule convergence to the MTOC, as indicated, were scored in the absence or presence of an anti-S2 rabbit serum. A minimum of 34 conjugates were analyzed.
The fraction of NK cells conjugated with S2–ICAM-1 cells in which the MTOC was polarized (75% ± 6%) was greater than that of NK cells in which granules had converged to the MTOC (39% ± 6%). To test whether this was due top referential signaling by β2 integrin for MTOC polarization, or to an intrinsic property of granule polarization, we examined the contribution of CD16 to these two processes by mixing NK cells with S2 cells coated with anti-S2 rabbit serum. Stimulation of CD16 with rabbit IgG on S2 cells induced MTOC polarization in a smaller fraction of NK cells (33% ± 7%) than stimulation by S2–ICAM-1 cells (75 % ± 6), and rabbit IgG did not enhance MTOC polarization toward S2–ICAM-1 cells (Fig. 6B). Furthermore, CD16 stimulation with rabbit IgG induced granule convergence to the MTOC in 46% ± 8% of NK cells, and enhanced granule convergence to S2–ICAM-1 cells from 39% ± 6% to 76% ± 12% of NK cells (Fig. 6B). Therefore, signaling by β2 integrin is sufficient for strong MTOC polarization, and for partial granule convergence to the MTOC. We conclude that granule convergence to the MTOC and MTOC polarization have overlapping but distinct signaling requirements, that β2 integrin contributes to both processes, and that β2 integrin signaling is sufficient for strong MTOC polarization.
β2 Integrin–ILK Complexes at the Synapse with ICAM-1 Positive Cells
ILK has been linked to β1 and β3, but not β2 integrin (9). Recruitment of ILK to synapses formed by NK cells bound to S2–ICAM-1 cells was tested by immunofluorescence with fixed and permeabilized cell conjugates. Accumulation of ILK at the synapse, where LFA-1 was also accumulated was evident in most of the synapses (fig. S3). To test whether ILK was recruited to close proximity with β2 integrin, proximity ligation assays (PLA) were performed, using a rabbit polyclonal serum to the cytoplasmic tail of CD18 in combination with mAbs to other molecules. PLA give positive signals if two DNA strands, each one attached to a different secondary Ab, are within 30 nm of each other (24). Two proteins within this range are either confined in the same tight space or associated to the same molecular complex. As positive control for the PLA, association of the β2 integrin tail with talin (25) was examined. PLA signals were evident in NK cells mixed with untransfected S2 cells, and were not polarized toward S2 cells (Fig. 7, A and B). A greater number of PLA signals were detected in NK cells mixed with S2–ICAM-1 cells, and 64% of them were polarized toward the synapse (Fig. 7, A and B). Therefore, this assay detected the constitutive association of talin with the β2 cytoplasmic tail, and showed an enhanced association when β2 integrin bound to ICAM-1. PLA with a mAb to ILK showed a few signals in NK cells mixed with S2 cells, and a greater number of signals in NK cells mixed with S2–ICAM-1 cells, 76% of which were polarized to the synapse (Fig. 7, A and B). These results suggested that ILK was actively recruited to close proximity of β2 integrin when NK cells bound to ICAM-1.
Fig. 7. Proximity of β2 Integrin with ILK at the Site of Contact with S2–ICAM-1 Cells.
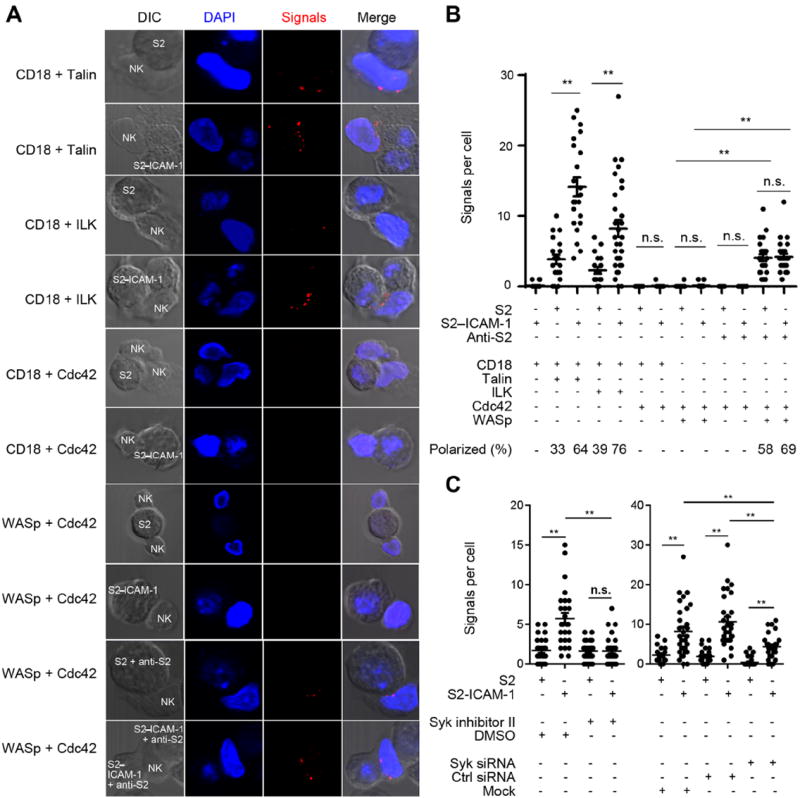
(A) PLAs were performed with primary NK cells mixed with S2 or S2–ICAM-1 cells for 20 min, fixed, permeabilized, and incubated with rabbit polyclonal Abs to the CD18 cytoplasmic tail or to WASp, together with mouse mAbs to talin, ILK, or Cdc42, as indicated on the left. In the last two panels, S2 and S2–ICAM-1 cells were pre-loaded with rabbit anti-S2 serum. (B) Quantitative analysis of PLAs. Each dot represents an NK cell. A minimum of 19 contacts with S2 cells and 22 contacts with S2–ICAM-1 cells were analyzed. The combination of S2 cells and Abs used are indicated below the graph. The fraction of PLA signals that were polarized toward S2 cells is indicated at the bottom of each column. (C) PLAs for CD18 with ILK in NK cells treated with 1 μM Syk inhibitor II, or with Syk siRNA, as indicated. A minimum of 19 contacts with S2 cells and 26contacts with S2–ICAM-1 cells were analyzed. **p<0.01.
To validate the specificity of the method, we examined association of the β2 cytoplasmic tail with other molecules. PLA with a mAb to Cdc42 gave almost no signal in NK cells mixed with S2 or S2–ICAM-1 cells, indicating that the rabbit polyclonal serum to the cytoplasmic tail of CD18 did not give spurious PLA signals with mouse IgG (Fig. 7, A and B). As a positive control for the mAb to Cdc42, PLA was performed with a rabbit polyclonal serum to the Wiscott-Aldrich Syndrome protein (WASp). Cdc42-GTP binds to WASp after activation of T cells through the TCR (26). NK cells were also stimulated through CD16 by rabbit anti-S2 cell serum. First, we showed that rabbit IgG at the surface of S2 cells did not result in PLA signals when combined with a mAb to intracellular Cdc42 (Fig. 7B and fig. S4). Interestingly, PLA signals for Cdc42–WASp interaction were detected after stimulation of NK cells through CD16 but not β2 integrin alone (Fig. 7, A and B). Furthermore, the presence of ICAM-1 did not enhance the Cdc42–WASp association induced by CD16 (Fig. 7, A and B). This result provided the positive control we were seeking for the mAb to Cdc42, and suggested that the role of Cdc42 in the granule polarization induced in NK cells bound to ICAM-1 did not require Cdc42–WASp association. It also confirmed that signals through β2 integrin (leading to granule polarization) and through CD16 (resulting in degranulation) are clearly distinct in NK cells (4). These results showed that ILK is actively recruited to immunological synapses formed by NK cells bound to ICAM-1, as part of a β2 integrin outside-in signaling complex.
We showed previously that granule polarization and paxillin phosphorylation induced by β2 integrin binding to ICAM-1 were blocked by an inhibitor of tyrosine kinase Syk (5). We tested whether ILK recruitment to β2 integrin was proximal or distal to Syk activity. The proximity of ILK and β2 integrin was tested after Syk inhibition and Syk silencing using siRNA. PLA signals for β2 integrin and ILK in NK cells bound to S2–ICAM-1 cells were completely blocked by the Syk inhibitor, and partially but significantly blocked by siRNA-mediated Syk silencing (Fig. 7C). These results showed that the β2 integrin–ILK pathway is dependent on tyrosine kinase Syk.
DISCUSSION
To study ligand-induced signaling by an integrin, without the complication of inside-out signals from other receptors, we took advantage of the ability of human NK cells to bind ICAM-1 directly via β2 integrin. As this interaction controls polarization of lytic granules, we had an opportunity to determine signals required for this polarization. We performed an unbiased mass spectrometry analysis of proteins that were tyrosine phosphorylated or associated with tyrosine phosphorylated proteins in primary NK cells bound to ICAM-1. The impetus behind this approach was that β2 integrin binding to ICAM-1 induces strong overall protein tyrosine phosphorylation in primary NK cells, similar to that induced by binding to human IgG1, a ligand for CD16 (5). This similarity is surprising, given that binding to IgG1 leads to degranulation but not granule polarization (4). To narrow the focus on proteins that are unique to β2 integrin signaling, proteins identified in unstimulated NK cells, as well as in NK cells stimulated through CD16, were subtracted from the proteins identified in the ICAM-1 stimulated sample. A signaling network emerged from a bioinformatics analysis.
Six proteins (ILK, Paxillin, Pyk2, γ-parvin, Leupaxin, and RhoGEF7) in the network clearly contributed to polarization, while silencing of others (e. g. CasL, Nsp3, and CD148) did not impair polarization. Except for CasL, silencing each one of these proteins had no effect on ICAM-1 dependent adhesion, ruling out defective adhesion as a cause of impaired polarization. The presence of Pyk2 and RhoGEF7 pointed to the conserved Cdc42–Par6 pathway for cell polarity as a potential downstream effector (27, 28). All four of the tested components of this pathway (Cdc42, Par6, APC, and CLIP-170) were required for polarization. In addition, GSK3β phosphorylation on Ser9, which results in APC activation in the Cdc42–Par6 pathway, occurred in NK cells bound to ICAM-1, and was dependent on ILK. Thus, we have identified and connected two pathways required for granule polarization induced by β2 integrin. The strengths of this study are the specific focus on independent signaling by an integrin, the reliance on an unbiased proteomics approach, and the integration of several protein networks into two connected pathways. Several studies have implicated a number of individual proteins in granule polarization in cytotoxic lymphocytes, but not in the specific context of integrin-dependent signals (20, 29). It is remarkable that engagement of an integrin by its ligand is sufficient to trigger an entire program for granule convergence to the MTOC, followed by MTOC relocation to the site of integrin engagement.
Despite its name, ILK was not an obvious candidate for signaling by β2 integrin. It has been linked to β1 and β3 integrin, and to the associated kindlin-2, but not to β2 integrin or to the associated kindlin-3 (9, 25). ILK is a pseudokinase and a large scaffold protein that regulates several integrin-dependent processes, from cell attachment in C. elegans to polarity of mammary epithelial cells (9, 30). Here, we provide evidence for Syk-dependent ILK recruitment to β2 integrin upon binding to ICAM-1 and for a functional role of ILK in β2 integrin-dependent granule polarization in human NK cells.
MTOC polarization in T cells requires Cdc42 activity and it is well established that activated Cdc42 binds WASp to regulate actin remodeling in T cells (31). However, in NK cells, the contribution of Cdc42 to β2 integrin signaling for polarization may not occur through WASp, as a Cdc42–WASp association was detected only after stimulation with CD16, and not with β2 integrin. Paxillin is a cytoskeletal scaffold protein with multiple interaction domains, which contributes to β2 integrin-dependent granule polarization in NK cells (5). In T cells, paxillin localizes to the peripheral region of the immunological synapse, binds to microtubules, and contributes to MTOC reorientation (32). Therefore, paxillin may also provide a connection with the microtubular network in NK cells stimulated by β2 integrin.
Pyk2 phosphorylation in NK cellsis induced by engagement of β2 integrin, but not CD16 (5, 33), and Pyk2 contributes to MTOC polarization in NK cells stimulated by sensitive target cells (33, 34). We show here that Pyk2 is also required for granule polarization induced by β2 integrin on its own. Little is known about leupaxin function, besides suppression of paxillin phosphorylation in fibronectin-induced focal adhesions (35) and negative regulation of BCR signaling and adhesion in B cells (36). These negative regulatory roles of leupaxin are in contrast to the combined requirement of paxillin and leupaxin for β2 integrin dependent granule polarization in NK cells.
Our study has also contributed to the understanding of granule convergence to the MTOC, the requirements for which differ in NK cells and T cells. Stronger TCR signals are required for granule convergence than for MTOC relocation to the immunological synapse (37, 38). In contrast, in NK cells, convergence occurs rapidly and precedes MTOC polarization (39). This convergence is dependent on Src-family kinase activity and LFA-1 (39). Here we show that Pyk2, leupaxin, and CLIP-170 are also required for integrin-dependent granule convergence. While convergence is induced by signals from either CD16 or β2 integrin, it is clearly enhanced when both receptors are engaged. In contrast, β2 integrin signaling is sufficient to promote maximal MTOC polarization, independently of CD16.
TCR signaling induces granule polarization through the classical Lck, ZAP70, LAT and SLP-76 pathway (40). TCR signaling provides also strong inside-out signals to LFA-1, and co-engagement of LFA-1 with TCR enhances granule polarization (41, 42). However, it is not known whether LFA-1 receives TCR inside-out signals merely to promote adhesion, or provides essential signals for polarization, as it is difficult to disentangle inside-out signals to LFA-1 from outside-in signals for polarization. The unique ability of LFA-1 to stimulate granule polarization in NK cells but not in T cells may be due simply to its independence from inside-out signals. Alternatively, LFA-1 may be connected to different signaling complexes in NK cells and in T cells. The data presented here provide a framework to begin to address this question. Towards this end, NK cells have proven useful in the analysis of outside-in signaling by LFA-1 independently of other signals. In conclusion, this work has revealed a greater signaling capacity of β2 integrin than previously appreciated, and identified two connected signaling networks that control integrin-dependent granule polarization in NK cells.
MATERIALS AND METHODS
Cells
Primary human NK cells were isolated and expanded as described (5) with minor modification. Briefly, NK cells isolated from peripheral blood using negative NK selection kit (Stemcell Technologies Inc., Vancouver BC, Canada), were cultured with irradiated autologous peripheral blood leukocytes at 37°C and 5% CO2 for 1 week in serum-free OpTmizer T cell expansion medium (GIBCO) supplemented with 10% purified human IL-2 (Hemagen Diagnostics), 100 U/ml recombinant human IL-2 (National Cancer Institute-FCRDCM, Frederick MD, USA) and 5 μg/ml PHA (Sigma-Aldrich). NK cells were then expanded in the same medium without PHA and feeders, and used after 4 to 5 weeks. KHYG-1 cells were cultured in RPMI1640 supplemented with 10% FBS (Atlanta biologicals, Lawrenceville GA, USA), 100 U/ml recombinant human IL-2 and 50 μM 2-Mercaptoethanol (Sigma) at 37°C and 5% CO2. Culture and transfection of Drosophila Schneider line 2 (S2) cells were performed as described (43, 44).
Reagents
PKH67 green was from Sigma. Colloidal blue was from Invitrogen. Syk inhibitor II (2-(2-Aminoethylamino)-4-(3-trifluoromethylanilino)-pyrimidine-5-carboxamide) was from EMD Biosciences. 4G10-conjugated agarose was from Millipore. His-tagged mouse ICAM-1 was prepared as described (5). Primer sequences for Real-time RT-PCR (Table S7) were from PrimerBank (http://pga.mgh.harvard.edu/primerbank) and were synthesized by IDT. All antibodies used are listed in Table S8.
NK Cell Stimulation and Isolation of Phosphotyrosine Protein Complexes
Primary NK cell stimulation using purified ICAM-1 or human IgG1, were performed as described (5). Briefly, 10 μg/ml purified ICAM-1 or purified human IgG1 were coated on 10 cm diameter Petri dishes overnight at 37°C in 5 ml of 50 mM sodium carbonate solution, or same Petri dishes treated with 5 ml of 50 mM sodium carbonate solution without protein overnight at 37°C as control, washed twice with PBS, blocked with 5% BSA in PBS at 4°C for 30 min, and washed twice with PBS. NK cells were collected, washed with PBS, and suspended in cold serum-free IMDM. 5 ml of twenty million NK cells were added per dish. The dishes were placed at 4°C for 15 min, and incubated at 37°C for 5 or 20 min. Cells were collected and lysed in 1 ml lysis buffer for each dish (50 mM Tris, 150 mM NaCl, 10 mM Sodium Pyrophosphate, 1 mM Phenylmethylsulfonyl Fluoride, 1 mM Sodium Metavanadate, 2 mM Sodium Fluoride, 0. 5% Triton X-100 and protease inhibitor cocktail (Roche)). Dishes were rocking at 4°C for 10 min. Lysates were collected and nuclei were removed by a 10 min centrifugation at 16,100× g. Cell lysates were incubated with agarose-conjugated 4G10 or isotype control IgG2b mAb for 1 hour at 4°C, washed three times with lysis buffer, and bound proteins were eluted with 100 mM sodiumphenyl phosphate in PBS, via rotating for 1 hour at 4°C and centrifuging 16,100× g for 1 min at 4°C. The eluted samples were resolved by gel electrophoresis and transferred to PVDF membrane and immunoblotted, as described (5). Samples for mass spectrometry analysis were scaled up to 280 million NK cells for each stimulation condition, and eluates from 5 independent experiments with NK cells from different individuals were pooled and concentrated with Amicon Ultra Centrifugal Filters (3K) (Millipore) prior to gel electrophoresis. The gel was stained with colloidal blue (fig. S1), and destained with water. Each lane was cut into 7 slices and sent for mass spectrometry analysis.
Mass Spectrometry Analysis
Peptide sequence analysis was performed by microcapillary reverse-phase HPLC nano-electrospray tandem mass spectrometry (μLC/MS/MS) on a Thermo LTQ-Orbitrap mass spectrometer. The instruments are capable of acquiring individual sequence (MS/MS) spectra online at high mass accuracy (<2 ppm) and sensitivity (<1 femtomole) for multiple peptides in the chromatographic run. These MS/MS spectra are then correlated with known sequences using the algorithm Sequest developed programs (45-47). MS/MS peptide sequences are then reviewed for consensus with known proteins and the results confirmed for fidelity.
Silencing by siRNA
siRNA oligonucleotides (Table S9) were synthesized by Dharmacon. Five million primary NK cells or 2 million KHYG-1 cells were transfected by electroporation with 300 pmol siRNA duplexes in 0. 4 ml of complete culture medium in 0.4 cm gap cuvettes (Bio-Rad, Hercules, CA), and pulsed once for 15 msat260 V with a BTX electroporator (EMC830, Harvard Aparatus). Electroporated cells were transferred into pre-warmed complete culture media, and incubated at 37°C and 5% CO2. Forty-eight hours later, electroporation of siRNA was repeated. Cells were used 48 hours later. Real-time PCR or scans of immunoblots were used to quantitate silencing. Immunoblots were developed with SuperSignal West Substrate (Thermo Scientific). Images were acquired and analyzed with FluorChem-Q imager (Protein Simple) and Alpha View software (version 3. 3).
S2–ICAM-1 Conjugation Assay
Conjugation of NK cells with S2 or S2–ICAM-1 cells was performed as described with minor modification (3, 48). Briefly, S2 cells were suspended in HBSS with 5% FBS, and mixed with PKH67 Green stained NK cells in a ratio of 4×105 to 1×105. Cells were centrifuged at 20 g for 3 min, incubated at 37°C for 20 min, fixed with 4% paraformaldehyde, and analyzed by flow cytometry according to PKH67 Green and side scatter scale.
Confocal Microscopy Analysis
Image analysis of NK–S2 cell contacts was as described (49) with minor modification. Briefly, S2 or S2–ICAM-1 cells were washed with PBS and incubated on poly-L-lysine-coated slides. After washing, NK cells were added, centrifuged at 20 g for 3 min, incubated at 37°C for 20 min, and fixed with IC Fixation Buffer (eBioscience) and permeabilized with Permeabilization Buffer (eBioscience). For polarization assays, cells were stained with anti-perforin mouse IgG2b mAb and anti-β-tubulin mouse IgG1 mAb, and revealed with isotype-specific Alexa Fluor 488- and Alexa Fluor 647-conjugated secondary antibodies (Invitrogen). For staining of ILK and CD11a, fixed slides were stained with a mouse IgG2b mAb to ILK and a rabbit IgG mAb to CD11a, and revealed with Alexa Fluor 488- and Alexa Fluor 647-conjugated secondary antibodies. KHYG-1 cells were incubated on poly-L-lysine-coated slides for 30 min, fixed, permeabilized, stained with anti-perforin and anti-β-tubulin Abs, and revealed with Alexa Fluor-conjugated secondary antibodies. Images were acquired on a Zeiss LSM780 Meta Confocal microscope and analyzed using Zeiss 2010 software. Differential interference contrast (DIC) images were collected simultaneously with the fluorescence. Multi-track acquisition was used to avoid crosstalk between different fluorophores.
Proximity Ligation Assay
Proximity ligation assay kits were from Olink Bioscience. NK cells and S2 or S2–ICAM-1 cells were co-incubated, fixed, permeabilized, and stained with two primary Abs (one rabbit IgG and amouse IgG, each one specific for a different protein), as described above. The slides were washed and incubated with PLA probes (secondary Abs coupled to DNA), and DNA was ligated and amplified following the supplier’s instructions. Slides were analyzed under confocal microscope and scored for the number of discrete PLA signals. PLA give positive fluorescent signals if the two DNA strands, each one attached to a different secondary Ab, are within 30 nm of each other (24). Two proteins within this range are either confined in the same tight space or associated to the same molecular complex.
Statistical Analysis
Each graph was generated from at least three independent experiments. For normally distributed data, mean±SEM was shown unless otherwise stated. Individual data between two groups were analyzed by two-tailed Student’s t test, and multiple groups were statistically analyzed by one-way analysis of variance (ANOVA).
Supplementary Material
Fig. S1. Preparative Gel for Phosphotyrosine Protein Complexes Analyzed by Mass Spectrometry
Fig. S2. Granule Polarization toward 721.221 Cells
Fig. S3. ILK Recruitment to the Site of Contact with S2–ICAM-1 Cells
Fig. S4. Rabbit Anti-S2 Cell Antibodies Do not Generate Proximity Ligation Signals with Mouse mAb to Cdc42
Table S1. Spectral Count HeatMap of Mass Spectrometry Data. (See the supplemental excel file)
Table S2. Top Spectral Counts in Isotype Control Pull-down.
Table S3. Constitutive Phosphotyrosine Protein Complexes in NK Cells.
Table S4. Top Spectral Counts in 4G10 Pull-down from ICAM-1 Stimulated NK Cells.
Table S5. Top Spectral Counts in 4G10 Pull-down from Human IgG1 Stimulated NK Cells.
Table S6. Phosphotyrosine Protein Complexes Selectively Enriched in ICAM-1 Stimulated over Human IgG1 Stimulated NK Cells.
Table S7. Primers Used in Experiments.
Table S8. Antibodies Used in Experiments.
Table S9. siRNA Used in Experiments.
Acknowledgments
We thank S. Rajagopalan and M. Peterson for advice, comments, and help, and J. Brzostowski for help in imaging analysis. Funding: This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Footnotes
Author contributions: MZ, MEM and EOL designed the experiments. MZ performed the experiments. MZ and EOL analyzed the data. WSL performed mass spectrometry and data analysis. MZ and EOL wrote the paper.
Competing interests: The authors have declared that no competing interests exist.
REFERENCES AND NOTES
- 1.Springer TA, Dustin ML. Integrin inside-out signaling and the immunological synapse. Curr Opin Cell Biol. 2012;24:107. doi: 10.1016/j.ceb.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson DC, Springer TA. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- 3.Barber DF, Faure M, Long EO. LFA-1 contributes an early signal for NK cell cytotoxicity. J Immunol. 2004;173:3653. doi: 10.4049/jimmunol.173.6.3653. [DOI] [PubMed] [Google Scholar]
- 4.Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202:1001. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.March ME, Long EO. beta2 integrin induces TCRzeta-Syk-phospholipase C-gamma phosphorylation and paxillin-dependent granule polarization in human NK cells. J Immunol. 2011;186:2998. doi: 10.4049/jimmunol.1002438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nika K, Soldani C, Salek M, Paster W, Gray A, Etzensperger R, Fugger L, Polzella P, Cerundolo V, Dushek O, Hofer T, Viola A, Acuto O. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity. 2010;32:766. doi: 10.1016/j.immuni.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cella M, Fujikawa K, Tassi I, Kim S, Latinis K, Nishi S, Yokoyama W, Colonna M, Swat W. Differential requirements for Vav proteins in DAP10- and ITAM-mediated NK cell cytotoxicity. J Exp Med. 2004;200:817. doi: 10.1084/jem.20031847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood SM, Meeths M, Chiang SC, Bechensteen AG, Boelens JJ, Heilmann C, Horiuchi H, Rosthoj S, Rutynowska O, Winiarski J, Stow JL, Nordenskjold M, Henter JI, Ljunggren HG, Bryceson YT. Different NK cell-activating receptors preferentially recruit Rab27a or Munc13-4 to perforin-containing granules for cytotoxicity. Blood. 2009;114:4117. doi: 10.1182/blood-2009-06-225359. [DOI] [PubMed] [Google Scholar]
- 9.Widmaier M, Rognoni E, Radovanac K, Azimifar SB, Fassler R. Integrin-linked kinase at a glance. J Cell Sci. 2012;125:1839. doi: 10.1242/jcs.093864. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimi R, Yamaji S, Suzuki A, Mishima W, Okamura M, Obana T, Matsuda C, Miwa Y, Ohno S, Ishigatsubo Y. The gamma-parvin-integrin-linked kinase complex is critically involved in leukocyte-substrate interaction. J Immunol. 2006;176:3611. doi: 10.4049/jimmunol.176.6.3611. [DOI] [PubMed] [Google Scholar]
- 11.Barr AJ, Ugochukwu E, Lee WH, King ON, Filippakopoulos P, Alfano I, Savitsky P, Burgess-Brown NA, Muller S, Knapp S. Large-scale structural analysis of the classical human protein tyrosine phosphatome. Cell. 2009;136:352. doi: 10.1016/j.cell.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bristow JM, Reno TA, Jo M, Gonias SL, Klemke RL. Dynamic phosphorylation of tyrosine 665 in pseudopodium-enriched atypical kinase 1 (PEAK1) is essential for the regulation of cell migration and focal adhesion turnover. J Biol Chem. 2013;288:123. doi: 10.1074/jbc.M112.410910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Kelber JA, Tran Cao HS, Cantin GT, Lin R, Wang W, Kaushal S, Bristow JM, Edgington TS, Hoffman RM, Bouvet M, Yates JR, 3rd, Klemke RL. Pseudopodium-enriched atypical kinase 1 regulates the cytoskeleton and cancer progression. Proc Natl Acad Sci U S A. 2010;107:10920. doi: 10.1073/pnas.0914776107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etienne-Manneville S. Cdc42--the centre of polarity. J Cell Sci. 2004;117:1291. doi: 10.1242/jcs.01115. [DOI] [PubMed] [Google Scholar]
- 15.Ren XR, Du QS, Huang YZ, Ao SZ, Mei L, Xiong WC. Regulation of CDC42 GTPase by proline-rich tyrosine kinase 2 interacting with PSGAP, a novel pleckstrin homology and Src homology 3 domain containing rhoGAP protein. J Cell Biol. 2001;152:971. doi: 10.1083/jcb.152.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlin LM, Evans R, Milewicz H, Fernandes L, Matthews DR, Perani M, Levitt J, Keppler MD, Monypenny J, Coolen T, Barber PR, Vojnovic B, Suhling K, Fraternali F, Ameer-Beg S, Parker PJ, Thomas NS, Ng T. A targeted siRNA screen identifies regulators of Cdc42 activity at the natural killer cell immunological synapse. Sci Signal. 2011;4:ra81. doi: 10.1126/scisignal.2001729. [DOI] [PubMed] [Google Scholar]
- 17.Fukata M, Watanabe T, Noritake J, Nakagawa M, Yamaga M, Kuroda S, Matsuura Y, Iwamatsu A, Perez F, Kaibuchi K. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell. 2002;109:873. doi: 10.1016/s0092-8674(02)00800-0. [DOI] [PubMed] [Google Scholar]
- 18.Lansbergen G, Komarova Y, Modesti M, Wyman C, Hoogenraad CC, Goodson HV, Lemaitre RP, Drechsel DN, Munster van E, Gadella TW, Grosveld F, Jr, Galjart N, Borisy GG, Akhmanova A. Conformational changes in CLIP-170 regulate its binding to microtubules and dynactin localization. J Cell Biol. 2004;166:1003. doi: 10.1083/jcb.200402082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Etienne-Manneville S, Manneville JB, Nicholls S, Ferenczi MA, Hall A. Cdc42 and Par6-PKCzeta regulate the spatially localized association of Dlg1 and APC to control cell polarization. J Cell Biol. 2005;170:895. doi: 10.1083/jcb.200412172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ham H, Billadeau DD. Human Immunodeficiency Syndromes Affecting Human Natural Killer Cell Cytolytic Activity. Front Immunol. 2014;5:2. doi: 10.3389/fimmu.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- 22.Mentlik AN, Sanborn KB, Holzbaur EL, Orange JS. Rapid lytic granule convergence to the MTOC in natural killer cells is dependent on dynein but not cytolytic commitment. Mol Biol Cell. 2010;21:2241. doi: 10.1091/mbc.E09-11-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suck G, Branch DR, Aravena P, Mathieson M, Helke S, Keating A. Constitutively polarized granules prime KHYG-1 NK cells. Int Immunol. 2006;18:1347. doi: 10.1093/intimm/dxl071. [DOI] [PubMed] [Google Scholar]
- 24.Soderberg O, Gullberg M, Jarvius M, Ridderstrale K, Leuchowius KJ, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson LG, Landegren U. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006;3:995. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 25.Lefort CT, Rossaint J, Moser M, Petrich BG, Zarbock A, Monkley SJ, Critchley DR, Ginsberg MH, Fassler R, Ley K. Distinct roles for talin-1 and kindlin-3 in LFA-1 extension and affinity regulation. Blood. 2012;119:4275. doi: 10.1182/blood-2011-08-373118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng R, Cannon L, Abraham RT, Way M, Billadeau DD, Bubeck-Wardenberg J, Burkhardt JK. SLP-76 coordinates Nck-dependent Wiskott-Aldrich syndrome protein recruitment with Vav-1/Cdc42-dependent Wiskott-Aldrich syndrome protein activation at the T cell-APC contact site. J Immunol. 2003;171:1360. doi: 10.4049/jimmunol.171.3.1360. [DOI] [PubMed] [Google Scholar]
- 27.Jones NP, Katan M. Role of phospholipase Cgamma1 in cell spreading requires association with a beta-Pix/GIT1-containing complex, leading to activation of Cdc42 and Rac1. Mol Cell Biol. 2007;27:5790. doi: 10.1128/MCB.00778-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filipenko NR, Attwell S, Roskelley C, Dedhar S. Integrin-linked kinase activity regulates Rac- and Cdc42-mediated actin cytoskeleton reorganization via alpha-PIX. Oncogene. 2005;24:5837. doi: 10.1038/sj.onc.1208737. [DOI] [PubMed] [Google Scholar]
- 29.Mace EM, Dongre P, Hsu HT, Sinha P, James AM, Mann SS, Forbes LR, Watkin LB, Orange JS. Cell biological steps and checkpoints in accessing NK cell cytotoxicity. Immunol Cell Biol. 2014;92:245. doi: 10.1038/icb.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akhtar N, Streuli CH. An integrin-ILK-microtubule network orients cell polarity and lumen formation in glandular epithelium. Nat Cell Biol. 2013;15:17. doi: 10.1038/ncb2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cannon JL, Burkhardt JK. The regulation of actin remodeling during T-cell-APC conjugate formation. Immunol Rev. 2002;186:90. doi: 10.1034/j.1600-065x.2002.18609.x. [DOI] [PubMed] [Google Scholar]
- 32.Robertson LK, Ostergaard HL. Paxillin associates with the microtubule cytoskeleton and the immunological synapse of CTL through its leucine-aspartic acid domains and contributes to microtubule organizing center reorientation. J Immunol. 2011;187:5824. doi: 10.4049/jimmunol.1003690. [DOI] [PubMed] [Google Scholar]
- 33.Gismondi A, Jacobelli J, Mainiero F, Paolini R, Piccoli M, Frati L, Santoni A. Cutting edge: functional role for proline-rich tyrosine kinase 2 in NK cell-mediated natural cytotoxicity. J Immunol. 2000;164:2272. doi: 10.4049/jimmunol.164.5.2272. [DOI] [PubMed] [Google Scholar]
- 34.Sancho D, Nieto M, Llano M, Rodriguez-Fernandez JL, Tejedor R, Avraham S, Cabanas C, Lopez-Botet M, Sanchez-Madrid F. The tyrosine kinase PYK-2/RAFTK regulates natural killer (NK) cell cytotoxic response, and is translocated and activated upon specific target cell recognition and killing. J Cell Biol. 2000;149:1249. doi: 10.1083/jcb.149.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka T, Moriwaki K, Murata S, Miyasaka M. LIM domain-containing adaptor, leupaxin, localizes in focal adhesion and suppresses the integrin-induced tyrosine phosphorylation of paxillin. Cancer Sci. 2010;101:363. doi: 10.1111/j.1349-7006.2009.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chew V, Lam KP. Leupaxin negatively regulates B cell receptor signaling. J Biol Chem. 2007;282:27181. doi: 10.1074/jbc.M704625200. [DOI] [PubMed] [Google Scholar]
- 37.Beal AM, Anikeeva N, Varma R, Cameron TO, Vasiliver-Shamis G, Norris PJ, Dustin ML, Sykulev Y. Kinetics of early T cell receptor signaling regulate the pathway of lytic granule delivery to the secretory domain. Immunity. 2009;31:632. doi: 10.1016/j.immuni.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenkins MR, Tsun A, Stinchcombe JC, Griffiths GM. The strength of T cell receptor signal controls the polarization of cytotoxic machinery to the immunological synapse. Immunity. 2009;31:621. doi: 10.1016/j.immuni.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.James AM, Hsu HT, Dongre P, Uzel G, Mace EM, Banerjee PP, Orange JS. Rapid activation receptor- or IL-2-induced lytic granule convergence in human natural killer cells requires Src, but not downstream signaling. Blood. 2013;121:2627. doi: 10.1182/blood-2012-06-437012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuhne MR, Lin J, Yablonski D, Mollenauer MN, Ehrlich LI, Huppa J, Davis MM, Weiss A. Linker for activation of T cells, zeta-associated protein-70, and Src homology 2 domain-containing leukocyte protein-76 are required for TCR-induced microtubule-organizing center polarization. J Immunol. 2003;171:860. doi: 10.4049/jimmunol.171.2.860. [DOI] [PubMed] [Google Scholar]
- 41.Anikeeva N, Somersalo K, Sims TN, Thomas VK, Dustin ML, Sykulev Y. Distinct role of lymphocyte function-associated antigen-1 in mediating effective cytolytic activity by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 2005;102:6437. doi: 10.1073/pnas.0502467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yi J, Wu X, Chung AH, Chen JK, Kapoor TM, Hammer JA. Centrosome repositioning in T cells is biphasic and driven by microtubule end-on capture-shrinkage. J Cell Biol. 2013;202:779. doi: 10.1083/jcb.201301004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barber DF, Long EO. Coexpression of CD58 or CD48 with intercellular adhesion molecule 1 on target cells enhances adhesion of resting NK cells. J Immunol. 2003;170:294. doi: 10.4049/jimmunol.170.1.294. [DOI] [PubMed] [Google Scholar]
- 44.March ME, Gross CC, Long EO. Use of transfected Drosophila S2 cells to study NK cell activation. Methods Mol Biol. 2010;612:67. doi: 10.1007/978-1-60761-362-6_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taniguchi T, Garcia-Higuera I, Xu B, Andreassen PR, Gregory RC, Kim ST, Lane WS, Kastan MB, D’Andrea AD. Convergence of the fanconi anemia and ataxia telangiectasia signaling pathways. Cell. 2002;109:459. doi: 10.1016/s0092-8674(02)00747-x. [DOI] [PubMed] [Google Scholar]
- 46.Chittum HS, Lane WS, Carlson BA, Roller PP, Lung FD, Lee BJ, Hatfield DL. Rabbit beta-globin is extended beyond its UGA stop codon by multiple suppressions and translational reading gaps. Biochemistry. 1998;37:10866. doi: 10.1021/bi981042r. [DOI] [PubMed] [Google Scholar]
- 47.Eng JK, McCormack AL, Yates JR. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:976. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 48.Burshtyn DN, Shin J, Stebbins C, Long EO. Adhesion to target cells is disrupted by the killer cell inhibitory receptor. Curr Biol. 2000;10:777. doi: 10.1016/s0960-9822(00)00568-6. [DOI] [PubMed] [Google Scholar]
- 49.Liu D, Bryceson YT, Meckel T, Vasiliver-Shamis G, Dustin ML, Long EO. Integrin-dependent organization and bidirectional vesicular traffic at cytotoxic immune synapses. Immunity. 2009;31:99. doi: 10.1016/j.immuni.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Durbin AD, Somers GR, Forrester M, Pienkowska M, Hannigan GE, Malkin D. JNK1 determines the oncogenic or tumor-suppressive activity of the integrin-linked kinase in human rhabdomyosarcoma. J Clin Invest. 2009;119:1558. doi: 10.1172/JCI37958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Natarajan M, Stewart JE, Golemis EA, Pugacheva EN, Alexandropoulos K, Cox BD, Wang W, Grammer JR, Gladson CL. HEF1 is a necessary and specific downstream effector of FAK that promotes the migration of glioblastoma cells. Oncogene. 2006;25:1721. doi: 10.1038/sj.onc.1209199. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi T, Takahashi K, Mernaugh RL, Tsuboi N, Liu H, Daniel TO. A monoclonal antibody against CD148, a receptor-like tyrosine phosphatase, inhibits endothelial-cell growth and angiogenesis. Blood. 2006;108:1234. doi: 10.1182/blood-2005-10-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hadjihannas MV, Bruckner M, Jerchow B, Birchmeier W, Dietmaier W, Behrens J. Aberrant Wnt/beta-catenin signaling can induce chromosomal instability in colon cancer. Proc Natl Acad Sci U S A. 2006;103:10747. doi: 10.1073/pnas.0604206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watson P, Stephens DJ. Microtubule plus-end loading of p150(Glued) is mediated by EB1 and CLIP-170 but is not required for intracellular membrane traffic in mammalian cells. J Cell Sci. 2006;119:2758. doi: 10.1242/jcs.02999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng S, Coffey G, Zhang XH, Shaknovich R, Song Z, Lu P, Pandey A, Melnick AM, Sinha U, Wang YL. SYK inhibition and response prediction in diffuse large B-cell lymphoma. Blood. 2011;118:6342. doi: 10.1182/blood-2011-02-333773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Preparative Gel for Phosphotyrosine Protein Complexes Analyzed by Mass Spectrometry
Fig. S2. Granule Polarization toward 721.221 Cells
Fig. S3. ILK Recruitment to the Site of Contact with S2–ICAM-1 Cells
Fig. S4. Rabbit Anti-S2 Cell Antibodies Do not Generate Proximity Ligation Signals with Mouse mAb to Cdc42
Table S1. Spectral Count HeatMap of Mass Spectrometry Data. (See the supplemental excel file)
Table S2. Top Spectral Counts in Isotype Control Pull-down.
Table S3. Constitutive Phosphotyrosine Protein Complexes in NK Cells.
Table S4. Top Spectral Counts in 4G10 Pull-down from ICAM-1 Stimulated NK Cells.
Table S5. Top Spectral Counts in 4G10 Pull-down from Human IgG1 Stimulated NK Cells.
Table S6. Phosphotyrosine Protein Complexes Selectively Enriched in ICAM-1 Stimulated over Human IgG1 Stimulated NK Cells.
Table S7. Primers Used in Experiments.
Table S8. Antibodies Used in Experiments.
Table S9. siRNA Used in Experiments.


