Ovarian cancers are the 5th leading cause of cancer-related deaths in women and the most lethal gynecologic malignancy. In 2013, it is estimated that 22,240 new cases of ovarian cancers will be diagnosed, with 14,030 associated deaths from this disease[1]. Ovarian epithelial cancers (OECs) account for 80~90% of all ovarian cancers. Among OECs, serous carcinomas are the most common, accounting for ~70% of all cases [2].
Serous ovarian cancers can be divided into two broad groups: type I or low-grade serous carcinomas (LG-SCs), and type II or high-grade serous carcinomas (HG-SCs). LG-SCs are generally present at early stages and clinically less aggressive; they rarely harbor TP53 mutations, instead, contain other specific mutations (e.g. KRAS and BRAF) and are genetically relatively stable. On the contrary, HG-SCs are clinically aggressive; they frequently display TP53 mutations and are genetically unstable [3]. HG-SCs account for the majority (~90%) of ovarian serous cancers while LG-SCs account for ~10%.
How do ovarian serous cancers originate and develop? Last decade has seen a paradigm shift in ovarian cancer carcinogenesis. Rather than starting from the ovarian surface, many ovarian HG-SCs have been surprisingly found to originate from the distal fallopian tube, possibly from expansion of secretory cells, as shown by a large body of recent clinical-pathological and molecular studies [4–9].
The cell of origin of LG-SCs is less clear compared with that of HG-SCs. LG-SCs are thought to evolve in a stepwise fashion, from ovarian epithelial inclusions (OEI) to benign cystadenomas and borderline tumors, and finally to LG-SCs [3, 10]. Li, et al. recently suggested that the majority of OEIs are derived from the fallopian tube rather than ovarian surface epithelium (OSE), and that the tubal secretory cells are likely the cell origin of LG-SCs [11]. This central role of the fallopian tube in LG-SC development has received further support [12, 13]. Here we summarize evidence for the fallopian tube as the site of origin for ovarian LG-SCs.
LG-SCs evolve from OEIs
The stepwise development of LG-SCs from OEIs is supported by several morphological and histological observations. First, the majority of benign cystadenomas seem to derive from OEIs, as cystadenomas display an epithelial lining similar to that of OEIs morphologically and immunophenotypically. Actually, the diagnostic criterion that separates cystadenomas from OEIs is merely an arbitrary threshold made at the 1 cm size [14]. Second, histological transitions from cystadenomas to borderline tumors are observed at high frequency in nearly 75% of cases [15]. Third, borderline tumors are found associated with the majority of LG-SCs [16]. It is seen that, foci of true early invasion in borderline tumors resemble LG-SCs [15, 17–19], and, invasive implants mostly associated with micropapillary serous borderline tumors, which has been recently defined as LS-SC [20–22], are histologically identical to LG-SCs [23, 24]. All these morphological and histological observations support a model wherein LG-SCs evolve from OEIs, via intermediate stages of serous cystadenomas and borderline tumors.
OEIs’ tubal origin
Since OEIs may represent the earliest putative precursor for LG-SCs, origination of OEIs may provide cues of the LG-SC origin. Recently, the morphologic and immunophenotypic features of OEIs, serous tumors (cystadenomas, borderline tumors, and LG-SCs), ovarian surface epithelium (OSE), and distal tubal epithelium were evaluated [11]. Two types of OSE were found: the vast majority of OSE displayed a mesothelial phenotype (calretinin+/PAX8−/tubulin−) and a low proliferative index (0.012), while about 4% of cases displayed foci with tubal phenotype (calretinin−/PAX8+/tubulin+). Although the OSE with tubal phenotype were found in only 4% of the cases, it did show that benign tubal epithelia can possibly implant on the ovarian surface and architecturally simulate ‘OSE’ microscopically. Meanwhile, there were also two types of OEIs: most (78%) of the OEIs displayed a tubal phenotype (vs. mesothelial phenotype) and had a significantly higher proliferative index than OSE’s, suggesting that OEIs and OSE are mostly of different cellular lineages. The fact that significantly more tubal-like epithelia were found in OEIs than in OSE argues that most OEIs are not derived from the OSE, rather, bear a tubal origin. One straightforward explanation is that the fallopian-derived OEIs represent intraovarian endosalpingiosis, which is well in line with the ideas expressed by Dubeau and Crum [25, 26]. Regarding the possibility that tubal-phenotype OEIs (78%) originate from mesothelium-derived OEIs through a müllerian metaplasia, if it were true, the metaplastic process must result in a hybrid type of OEIs in the ovary. The fact that the hybrid or intermediate type of OEIs with both mesothelial and tubal phenotypes were rarely found makes the müllerian metaplasia hypothesis unlikely. Furthermore, mesothelium-derived OEIs may not be able to grow into a tumor mass due to their extremely low cellular proliferative index (similar to OSE’s), while fallopian-derived OEIs showed proliferative activities and immuophenotypes similar to ovarian serous tumors. The above findings suggest that OEIs with tubal phenotype and increased proliferative index [11] are likely originated from tubal epithelia, and are likely the precursors of LG-SCs.
Development model from tubal epithelia to OEIs
Based on the connection between the tubal epithelium and LG-SCs, Li et al. proposed a two-step model of LG-SC development as following [11]: First, fallopian tubal epithelia, mostly from fimbriated end, may detach and implant on the ovarian surface. This step could occur via two possibilities: 1) the close spatial relationship between the tubal fimbriated end and the ovarian surface may allow detached tubal epithelium to implant in the ovarian stroma, especially when ovulation or non-ovulation induced disruption of the ovarian surface occurs [27]; and 2) adhesion of tubal epithelium on the ovarian surface (due to inflammation or other factors) and dynamic stromal growth around it may result in tubal derived OEIs formation. During the second step, the acquisition of mutations such as KRAS or BRAF in tubal derived OEIs results in their transformation to cystadenomas, borderline tumors and ultimately to LG-SCs [28–32]. Notably, the further acquisition of additional mutations such as TP53 in LG-SCs may contribute to the development of a small proportion of HG-SCs [27].
There are other recent studies linking LG-SC origin to the fallopian tube. Kurman et al identified a fallopian tube lesion, designated as papillary tubal hyperplasia (PTH) whose cytological appearance is essentially identical to that of atypical proliferating serous tumor (APST), non-invasive implants, and endosalpingiosis [13]. The authors hypothesized both a PTH-pathway and a non-PTH pathway model [9] for the origin and development of the pelvic low-grade serous proliferations. The PTH process begins with chronic inflammation, leading to tubal hyperplasia, which, if progresses to PTH, can shed and implant tubal epithelium on ovarian and peritoneal surfaces, resulting in a variety of low-grade serous proliferations including serous borderline tumors, noninvasive epithelial implants, and endosalpingiosis. In the non-PTH pathway, which is similar to the aforementioned model by Li et al., APST evolves from OEIs and normal tubal epithelium may form endosalpingiosis directly. APST and endosalpingiosis then can both form non-invasive implants. Another study by Laury et al showed that PAX2-null secretory cell outgrowths (SCOUTs) were more frequently found in the fallopian tubes of women with serous borderline tumors correlating with the loss of PAX2 expression found in most serous borderline tumors [12]. In particular, they identified two cases with discrete multifocal papillary SCOUTs that resemble PTH in the fallopian tubes, which were thought to associate with serous borderline tumors. These observations provided additional support for a tubal origin of LG-SCs.
Cell origin of LG-SCs
There are two types of epithelial cells within the fallopian tubal mucosa: ciliated and non-ciliated, the latter are also called secretory cells. Both types of the cells are also present in fallopian tube-derived OSE, fallopian tube-derived OEI, serous cystadenomas, and borderline tumors, with a significant increase in secretory-to-ciliated cell ratio (S/C ratio) observed during the apparent transition from the normal fallopian tube to fallopian tube-derived OEIs (P<0.001) [11]. Fallopian tube-derived OEIs and cystadenomas displayed very similar S/C ratios (consistent with their arbitrary pathologic difference, size threshold of 1 cm), while the S/C ratio of serous borderline tumors was slightly higher. In contrast, LG-SCs contained almost entirely secretory cells in their epithelial components and displayed a strikingly high S/C ratio [11]. These results suggested that LG-SCs are likely derived from the expansion [33] or outgrowth of tubal secretory cells, similar to HG-SCs [34, 35]. The significant increase of the S/C ratio between normal tubal epithelium to fallopian tube-derived OEIs suggests that during this transition step some molecular event(s) occurred, either facilitating secretory cell expansion or ciliated cell suppression. The reduction in cilia with advancing tumor development could indicate an impaired maturation program. Overall, the progressive increase of S/C ratio from fallopian tube-derived OEIs all the way to LG-SCs is consistent with the concept of a stepwise progression, and the secretory cells in fallopian tube appear to be the cell of origin of LG-SCs.
Emerging concept of mesothelial-müllerian junction as a potential source of LG-SC origination
Another model for the origination of ovarian serous carcinomas has been gradually emerging [36–39] in light of the recent discovery of a cancer-prone stem cell niche at the junction area of OSE, mesothelium and tubal epithelium [40], as well as the knowledge advancement in cervical cancer origin [41–44], which suggests that Krt7+ embryonic progenitors give rise to immuno-phenotypically distinct progeny under stromal influences via a ‘top-down’ differentiation. The ovarian surface and müllerian cortical inclusion cysts have been implicated to give rise to borderline tumors, LG- and HG-SCs [27]. Since the residual multipotential embryonic epithelial cells in cervix squamo-columnar junction can be transformed and differentiate into either squamous or columnar neoplasms, the same scenario might happen as well in the remainder of the female genital tract (Krt7+ cells extends from the fallopian tube to the extra tubal mesothelium and ovarian surface epithelium/mesothelium), thus potentially allowing LG-SC to arise from the OSE, in particular within the potentially vulnerable mesothelial-müllerian junction under neoplastic stimuli.
Conclusion
Our understanding of the origin of LG-SCs has important clinical significance. A clear role of the fallopian tube in ovarian cancer origin has been suggested by a rapidly increasing body of studies, as summarized here in this review for LG-SCs and in several recent reviews for HG-SCs [45–47] (Figure 1). This paradigm shift in the understanding of ovarian cancer origin provides exciting opportunities for identifying precursor lesions and early-stage malignancies, as well as for developing screening methods and early-detection biomarkers. Fallopian tube and tubal epithelia cells are becoming the direction for future ovarian cancer early detection and prevention.
Figure 1. LG-SC development with a tubal origin.
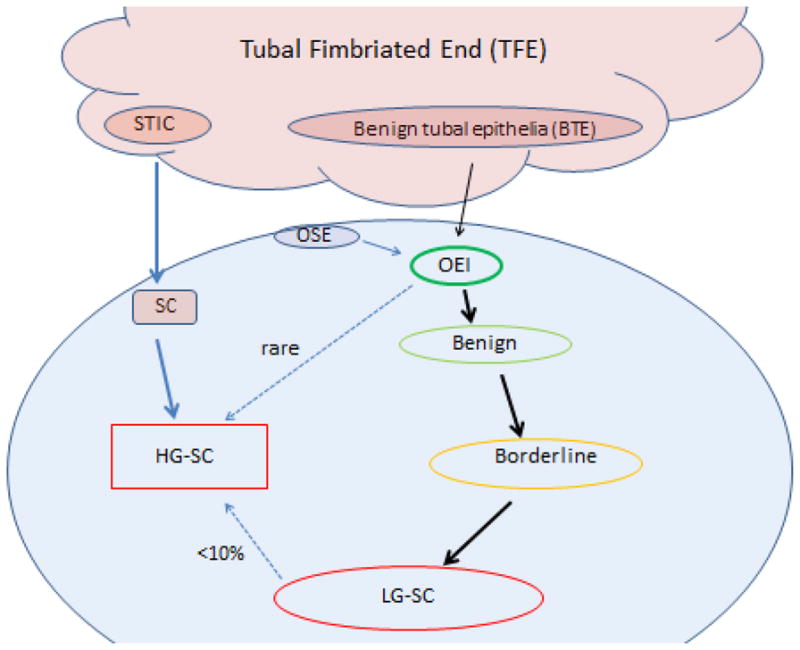
Serous cancers, including HG-SCs and LG-SCs, originate mostly from the tubal fimbriated end (TFE). For LG-SCs, benign tubal epithelia (BTE) are able to form OEIs through an unclear pathway, then evolve into benign cystadenomas, borderline tumors and eventually LG-SCs. Some OEIs may derive from OSE. However, those OSE-derived OEIs are thought to have limited proliferative index and rarely develop into LG-SCs. Less than 10% of LG-SCs might develop into HG-SCs upon the acquisition of p53 mutation. HG-SCs also mainly originate from the TFE, starting from serous tubal intraepithelial carcinoma (STIC). This figure is adapted from REF [48].
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.McCluggage WG. Morphological subtypes of ovarian carcinoma: a review with emphasis on new developments and pathogenesis. Pathology. 2011;43:420–432. doi: 10.1097/PAT.0b013e328348a6e7. [DOI] [PubMed] [Google Scholar]
- 3.Kurman RJ, Shih Ie M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol. 2011;42:918–931. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medeiros F, Muto MG, Lee Y, Elvin JA, Callahan MJ, Feltmate C, Garber JE, Cramer DW, Crum CP. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30:230–236. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 5.Crum CP, Drapkin R, Kindelberger D, Medeiros F, Miron A, Lee Y. Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res. 2007;5:35–44. doi: 10.3121/cmr.2007.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levanon K, Crum C, Drapkin R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol. 2008;26:5284–5293. doi: 10.1200/JCO.2008.18.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, Callahan MJ, Garner EO, Gordon RW, Birch C, Berkowitz RS, Muto MG, Crum CP. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 8.Carlson JW, Miron A, Jarboe EA, Parast MM, Hirsch MS, Lee Y, Muto MG, Kindelberger D, Crum CP. Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J Clin Oncol. 2008;26:4160–4165. doi: 10.1200/JCO.2008.16.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vang R, Shih Ie M, Kurman RJ. Fallopian tube precursors of ovarian low- and high-grade serous neoplasms. Histopathology. 2013;62:44–58. doi: 10.1111/his.12046. [DOI] [PubMed] [Google Scholar]
- 10.Shih Ie M, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–1518. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Abushahin N, Pang S, Xiang L, Chambers SK, Fadare O, Kong B, Zheng W. Tubal origin of ‘ovarian’ low-grade serous carcinoma. Mod Pathol. 2011;24:1488–1499. doi: 10.1038/modpathol.2011.106. [DOI] [PubMed] [Google Scholar]
- 12.Laury AR, Ning G, Quick CM, Bijron J, Parast MM, Betensky RA, Vargas SO, McKeon FD, Xian W, Nucci MR, Crum CP. Fallopian tube correlates of ovarian serous borderline tumors. Am J Surg Pathol. 2011;35:1759–1765. doi: 10.1097/PAS.0b013e318233b0f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurman RJ, Vang R, Junge J, Hannibal CG, Kjaer SK, Shih Ie M. Papillary tubal hyperplasia: the putative precursor of ovarian atypical proliferative (borderline) serous tumors, noninvasive implants, and endosalpingiosis. Am J Surg Pathol. 2011;35:1605–1614. doi: 10.1097/PAS.0b013e318229449f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scully RE. Pathology of ovarian cancer precursors. J Cell Biochem Suppl. 1995;23:208–218. doi: 10.1002/jcb.240590928. [DOI] [PubMed] [Google Scholar]
- 15.Smith Sehdev AE, Sehdev PS, Kurman RJ. Noninvasive and invasive micropapillary (low-grade) serous carcinoma of the ovary: a clinicopathologic analysis of 135 cases. Am J Surg Pathol. 2003;27:725–736. doi: 10.1097/00000478-200306000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Malpica A, Deavers MT, Lu K, Bodurka DC, Atkinson EN, Gershenson DM, Silva EG. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28:496–504. doi: 10.1097/00000478-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 17.McKenney JK, Balzer BL, Longacre TA. Patterns of stromal invasion in ovarian serous tumors of low malignant potential (borderline tumors): a reevaluation of the concept of stromal microinvasion. Am J Surg Pathol. 2006;30:1209–1221. doi: 10.1097/01.pas.0000213299.11649.fa. [DOI] [PubMed] [Google Scholar]
- 18.Seidman JD, Soslow RA, Vang R, Berman JJ, Stoler MH, Sherman ME, Oliva E, Kajdacsy-Balla A, Berman DM, Copeland LJ. Borderline ovarian tumors: diverse contemporary viewpoints on terminology and diagnostic criteria with illustrative images. Hum Pathol. 2004;35:918–933. doi: 10.1016/j.humpath.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Burks RT, Sherman ME, Kurman RJ. Micropapillary serous carcinoma of the ovary. A distinctive low-grade carcinoma related to serous borderline tumors. Am J Surg Pathol. 1996;20:1319–1330. doi: 10.1097/00000478-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Seidman JD, Kurman RJ. Subclassification of serous borderline tumors of the ovary into benign and malignant types. A clinicopathologic study of 65 advanced stage cases. Am J Surg Pathol. 1996;20:1331–1345. doi: 10.1097/00000478-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 21.May T, Virtanen C, Sharma M, Milea A, Begley H, Rosen B, Murphy KJ, Brown TJ, Shaw PA. Low malignant potential tumors with micropapillary features are molecularly similar to low-grade serous carcinoma of the ovary. Gynecol Oncol. 2010;117:9–17. doi: 10.1016/j.ygyno.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Curry EW, Stronach EA, Rama NR, Wang YY, Gabra H, El-Bahrawy MA. Molecular subtypes of serous borderline ovarian tumor show distinct expression patterns of benign tumor and malignant tumor-associated signatures. Mod Pathol. 2013 doi: 10.1038/modpathol.2013.130. [DOI] [PubMed] [Google Scholar]
- 23.Longacre TA, McKenney JK, Tazelaar HD, Kempson RL, Hendrickson MR. Ovarian serous tumors of low malignant potential (borderline tumors): outcome-based study of 276 patients with long-term (> or =5-year) follow-up. Am J Surg Pathol. 2005;29:707–723. doi: 10.1097/01.pas.0000164030.82810.db. [DOI] [PubMed] [Google Scholar]
- 24.Bell DA, Longacre TA, Prat J, Kohn EC, Soslow RA, Ellenson LH, Malpica A, Stoler MH, Kurman RJ. Serous borderline (low malignant potential, atypical proliferative) ovarian tumors: workshop perspectives. Hum Pathol. 2004;35:934–948. doi: 10.1016/j.humpath.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Crum CP. Intercepting pelvic cancer in the distal fallopian tube: theories and realities. Mol Oncol. 2009;3:165–170. doi: 10.1016/j.molonc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubeau L. The cell of origin of ovarian epithelial tumours. Lancet Oncol. 2008;9:1191–1197. doi: 10.1016/S1470-2045(08)70308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singer G, Oldt R, 3rd, Cohen Y, Wang BG, Sidransky D, Kurman RJ, Shih Ie M. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95:484–486. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez AA, Moore WF, Robboy SJ, Bentley RC, Gumbs C, Futreal PA, Berchuck A. K-ras mutations in Mullerian inclusion cysts associated with serous borderline tumors of the ovary. Gynecol Oncol. 2001;80:201–206. doi: 10.1006/gyno.2000.6066. [DOI] [PubMed] [Google Scholar]
- 30.Haas CJ, Diebold J, Hirschmann A, Rohrbach H, Lohrs U. In serous ovarian neoplasms the frequency of Ki-ras mutations correlates with their malignant potential. Virchows Arch. 1999;434:117–120. doi: 10.1007/s004280050314. [DOI] [PubMed] [Google Scholar]
- 31.Mayr D, Hirschmann A, Lohrs U, Diebold J. KRAS and BRAF mutations in ovarian tumors: a comprehensive study of invasive carcinomas, borderline tumors and extraovarian implants. Gynecol Oncol. 2006;103:883–887. doi: 10.1016/j.ygyno.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 32.Sieben NL, Macropoulos P, Roemen GM, Kolkman-Uljee SM, Jan Fleuren G, Houmadi R, Diss T, Warren B, Al Adnani M, De Goeij AP, Krausz T, Flanagan AM. In ovarian neoplasms, BRAF, but not KRAS, mutations are restricted to low-grade serous tumours. J Pathol. 2004;202:336–340. doi: 10.1002/path.1521. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Ning Y, Abushahin N, Yuan Z, Wang Y, Yuan B, Cragun JM, Chambers SK, Hatch K, Kong B, Zheng W. Secretory cell expansion with aging: Risk for pelvic serous carcinogenesis. Gynecol Oncol. 2013 doi: 10.1016/j.ygyno.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Chen EY, Mehra K, Mehrad M, Ning G, Miron A, Mutter GL, Monte N, Quade BJ, McKeon FD, Yassin Y, Xian W, Crum CP. Secretory cell outgrowth, PAX2 and serous carcinogenesis in the Fallopian tube. J Pathol. 2010;222:110–116. doi: 10.1002/path.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jarboe E, Folkins A, Nucci MR, Kindelberger D, Drapkin R, Miron A, Lee Y, Crum CP. Serous carcinogenesis in the fallopian tube: a descriptive classification. Int J Gynecol Pathol. 2008;27:1–9. doi: 10.1097/pgp.0b013e31814b191f. [DOI] [PubMed] [Google Scholar]
- 36.Crum CP, Herfs M, Ning G, Bijron JG, Howitt BE, Jimenez CA, Hanamornroongruang S, McKeon FD, Xian W. Through the glass darkly: intraepithelial neoplasia, top-down differentiation and the road to ovarian cancer. J Pathol. 2013 doi: 10.1002/path.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Auersperg N. The origin of ovarian carcinomas: a unifying hypothesis. Int J Gynecol Pathol. 2011;30:12–21. doi: 10.1097/PGP.0b013e3181f45f3e. [DOI] [PubMed] [Google Scholar]
- 38.Auersperg N, Woo MM, Gilks CB. The origin of ovarian carcinomas: a developmental view. Gynecol Oncol. 2008;110:452–454. doi: 10.1016/j.ygyno.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 39.Seidman JD, Yemelyanova A, Zaino RJ, Kurman RJ. The fallopian tube-peritoneal junction: a potential site of carcinogenesis. Int J Gynecol Pathol. 2011;30:4–11. doi: 10.1097/PGP.0b013e3181f29d2a. [DOI] [PubMed] [Google Scholar]
- 40.Flesken-Nikitin A, Hwang CI, Cheng CY, Michurina TV, Enikolopov G, Nikitin AY. Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature. 2013;495:241–245. doi: 10.1038/nature11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Ouyang H, Yamamoto Y, Kumar PA, Wei TS, Dagher R, Vincent M, Lu X, Bellizzi AM, Ho KY, Crum CP, Xian W, McKeon F. Residual embryonic cells as precursors of a Barrett’s-like metaplasia. Cell. 2011;145:1023–1035. doi: 10.1016/j.cell.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xian W, Ho KY, Crum CP, McKeon F. Cellular origin of Barrett’s esophagus: controversy and therapeutic implications. Gastroenterology. 2012;142:1424–1430. doi: 10.1053/j.gastro.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herfs M, Yamamoto Y, Laury A, Wang X, Nucci MR, McLaughlin-Drubin ME, Munger K, Feldman S, McKeon FD, Xian W, Crum CP. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc Natl Acad Sci U S A. 2012;109:10516–10521. doi: 10.1073/pnas.1202684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herfs M, Vargas SO, Yamamoto Y, Howitt BE, Nucci MR, Hornick JL, McKeon FD, Xian W, Crum CP. A novel blueprint for ‘top down’ differentiation defines the cervical squamocolumnar junction during development, reproductive life, and neoplasia. J Pathol. 2013;229:460–468. doi: 10.1002/path.4110. [DOI] [PubMed] [Google Scholar]
- 45.Nik NN, Vang R, Shih IM, Kurman RJ. Origin and Pathogenesis of Pelvic (Ovarian, Tubal, and Primary Peritoneal) Serous Carcinoma. Annu Rev Pathol. 2013 doi: 10.1146/annurev-pathol-020712-163949. [DOI] [PubMed] [Google Scholar]
- 46.Erickson BK, Conner MG, Landen CN., Jr The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013 doi: 10.1016/j.ajog.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehra K, Mehrad M, Ning G, Drapkin R, McKeon FD, Xian W, Crum CP. STICS, SCOUTs and p53 signatures; a new language for pelvic serous carcinogenesis. Front Biosci (Elite Ed) 2011;3:625–634. doi: 10.2741/e275. [DOI] [PubMed] [Google Scholar]
- 48.Li J, Fadare O, Xiang L, Kong B, Zheng W. Ovarian serous carcinoma: recent concepts on its origin and carcinogenesis. J Hematol Oncol. 2012;5:8. doi: 10.1186/1756-8722-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


