Summary
The hippocampus is crucial for normal brain function, especially for the encoding and retrieval of multimodal sensory information. Neuropsychiatric disorders such as temporal lobe epilepsy, amnesia, and the dementias are associated with structural and functional abnormalities of specific hippocampal neurons. More recently we have also found evidence for a role of the hippocampus in the pathophysiology of schizophrenia. The most consistent finding is a subtle, yet significant volume difference in schizophrenia. Here we review the cellular and molecular basis of smaller hippocampal volume in schizophrenia. In contrast to neurodegenerative disorders, total hippocampal cell number is not markedly decreased in schizophrenia. However, the intriguing finding of a selective loss of hippocampal inter-neurons deserves further study. Two neurotransmitter receptors, the GABAA and AMPA/kainate glutamate receptors, appear to be abnormal, whereas changes of the NMDA glutamate receptor are less robust. The expression of several genes, including those related to the GABAergic system, neurodevelopment, and synaptic function, is decreased in schizophrenia. Taken together, recent studies of hippocampal cell number, protein expression, and gene regulation point towards an abnormality of hippocampal architecture in schizophrenia.
Keywords: Hippocampal volume, interneurons, GABA, glutamate
Introduction
Studies of the brain have contributed greatly to our current understanding of schizophrenia, but the details of the structural and functional basis of schizophrenia are not known yet. Among all the studies to date, abnormalities of hippocampal structure and function are now one of the most consistent findings (Arnold, 2000; Benes, 1999; Harrison, 1999; Harrison and Eastwood, 2001; Heckers, 2001; McCarley et al., 1999; Nelson et al., 1998a). The main finding is a subtle but significant decrease of hippocampal volume in schizophrenia. Post-mortem studies are needed to understand the cellular and molecular basis of smaller hippocampal volume in schizophrenia. Here we will review the volumetric studies and some aspects of recent neuropathological studies to develop a conceptual framework for further investigations.
Hippocampal volume in schizophrenia
The ventromedial area of the human temporal lobe contains the amygdala, the hippocampal region, and superficial cortical areas that cover the hippocampal region and form the parahippocampal gyrus (Van Hoesen, 1997). The hippocampal region can be subdivided into three subregions: the dentate gyrus, the Cornu Ammonis sectors, and the subiculum. Some authors have defined the hippocampal region and the closely connected entorhinal cortex, located on the anterior aspects of the parahippocampal gyrus, as hippocampal formation (Amaral and Insausti, 1990).
The first study of hippocampal structure in schizophrenia estimated volumes from area measurements of the hippocampus on serial sections taken from whole-hemisphere specimens of the Vogt brain collection (Bogerts et al., 1985). This study reported a 40% difference between a group of control subjects and patients with schizophrenia. Subsequently, several neuropathological and many neuroimaging studies have revisited the question: is hippocampal volume changed in schizophrenia? A recent meta-analysis (Nelson et al., 1998b) and several reviews (Dwork, 1997; Heckers, 2001; McCarley et al., 1999) have now concluded that hippocampal volume is about 5% smaller in schizophrenia, making it one of the most robust and replicated findings in all of the schizophrenia neuropathology and neuroimaging literature.
A considerable strength of structural neuroimaging studies is the ability to study hippocampal volume at the onset and throughout the course of the illness. Such studies have provided evidence that the hippocampus is smaller in schizophrenic patients early in their disease process (first-break psychosis) (Bogerts et al., 1990; Hirayasu et al., 1998; Velakoulis et al., 1999). This is consistent with the notion that schizophrenia is not a degenerative process, leading to marked cell loss and subsequent volume changes. However, the finding of a slow progression of hippocampal volume reduction throughout the disease process, found in some (Giedd et al., 1999; Velakoulis et al., 1999) but not all studies (Wood et al., 2000), can be interpreted as evidence for a degenerative process due to the disease (e.g., stress) or the treatment of schizophrenia.
Recent studies have reported that smaller hippocampal volume predicts whether at-risk children of schizophrenic parents will develop schizophrenia themselves (Lawrie et al., 1999; Pantelis et al., 2000). Furthermore, adult relatives of schizophrenic patients who do not develop the full-fledged picture of schizophrenia (but might show more subtle signs of psychopathology) still have smaller hippocampal volume (Seidman et al., 1999). These studies provide evidence for the hypothesis that a smaller hippocampus is an endophenotype (i.e., a brain abnormality linked to a genetic risk factor for schizophrenia) (Tsuang, 2000). However, this is in contrast to a study of monozygotic twins discordant for schizophrenia which showed that hippocampal volume was consistently smaller in the affected proband when compared with the unaffected co-twin (Suddath et al., 1990).
Despite these intriguing findings of hippocampal volume differences in schizophrenia, neuroimaging studies are limited by their spatial resolution. Anatomists can use staining techniques to delineate the hippocampus from surrounding areas (i.e., amygdala, parahippocampal gyrus, perirhinal cortex, fusiform/lingual gyrus). This allows for a reliable volume estimate of the human hippocampus, which is typically between 3–5cm3 (after correction for shrinkage of brain tissue post-mortem) (Heckers et al., 1991). Even when the best structural neuroimaging technology is combined with a keen understanding of the anatomy of the human hippocampus, these tools do not allow us to reliably delineate the hippocampus in the living human brain. Rather, we have to rely on arbitrary borders that are clearly visible to the human eye without the luxury of a microscope and a staining technique (Pruessner et al., 2000).
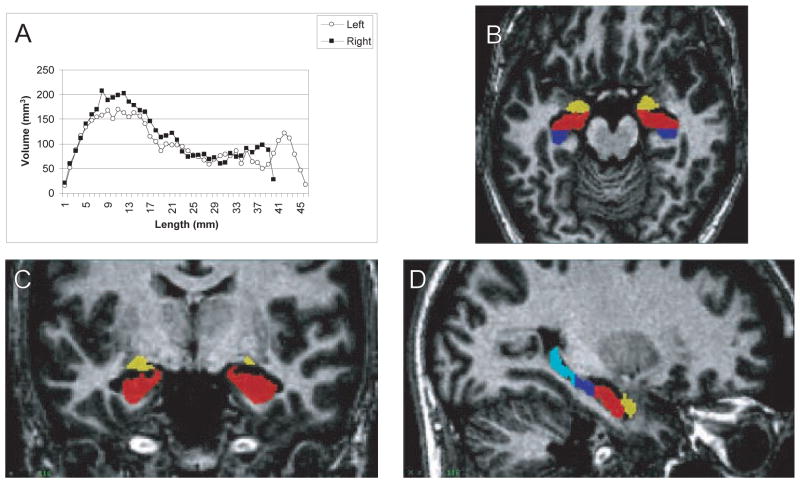
Two important questions can be addressed at the macroscopic level of hippocampal anatomy. First, are the anterior and posterior parts of the hippocampus differentially affected in schizophrenia? Some investigators have used structural MRI to study this question (Csernansky et al., 1998; Rossi et al., 1994; Suddath et al., 1990; Velakoulis et al., 2001). Considering the distinct afferent and efferent connections (Barbas and Blatt, 1995; Goldman-Rakic et al., 1984) and the recent evidence for a functional segregation of the anterior and posterior hippocampal formation (Lepage et al., 1998; Schacter and Wagner, 1999; Strange et al., 1999), this finding could indicate that some but not all hippocampal functions are impaired in schizophrenia. However, most previous studies of hippocampal volume have estimated hippocampal volume based on relatively thick slices (i.e., 3 mm or more). High-resolution structural MRI scans, providing 1 mm slices through the hippocampus, are necessary to reliably estimate regional volumes of the hippocampus. Figure 1 provides an example from our laboratory for such a volumetric analysis of amygdala and three hippocampal regions (uncus, body, tail) in the human brain.
Fig. 1.
High-resolution MRI of human hippocampus. A Histogram of hippocampal volume along the anterior-posterior axis. B–D Three orthogonal views of the human hippocampus in a normal subject. The amygdala (yellow) can be separated from the uncus (red), hippocampal body (dark blue) and hippocampal tail (light blue). The slices represent a horizontal (B), coronal (C), and sagittal (D) view
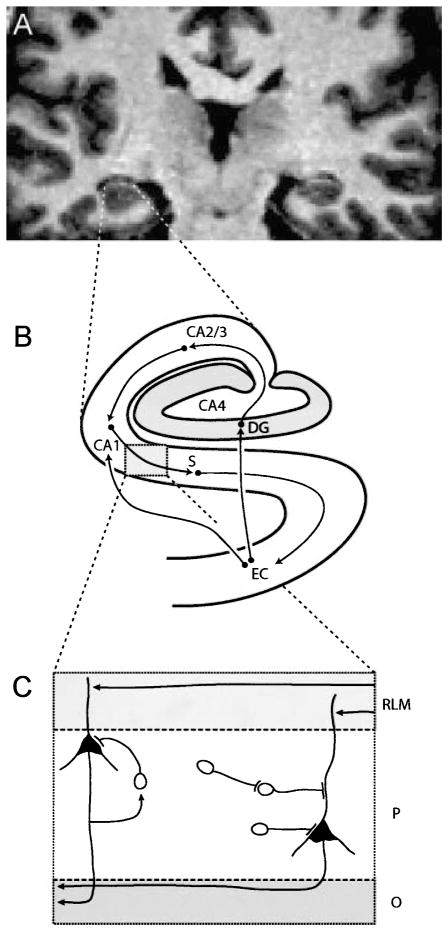
Second, is the hippocampal volume difference in schizophrenia selective for one hippocampal region (Dentate gyrus, Cornu ammonis sectors, Subiculum) (see Fig. 2)? The hippocampus processes multimodal sensory information via a circuit of glutamatergic neurons in the dentate gyrus, CA2/3, CA1, and subiculum. Deficits in each of these nodes of the hippocampal circuitry may produce quite different patterns of neural dysfunction. This requires the study of hippocampal anatomy in schizophrenia at the level of these regions. A recent study (Wang et al., 2001) reported a selective reduction of hippocampal volume in the subiculum, but this inference was not based on an identification of hippocampal subdivisions but rather on mapping the hippocampal volume maps on the expected map of the normal human hippocampus. With high-resolution structural MRI scans it might be possible to identify and quantify the regions of the human hippocampus (Zeineh et al., 2000). Such techniques would greatly advance our understanding of hippocampal volume differences in schizophrenia.
Fig. 2.
Three levels of hippocampal anatomy. A High resolution coronal MRI of the human hippocampus at the level of the hippocampal body. B Schematic diagram of the regions of the human hippocampal formation and their major connections. The entorhinal cortex (EC) sends direct projections and indirect projections via the dentate gyrus (DG) and cornu Ammonis sector CA2/3 to CA1, from which output is relayed via the subiculum (S) back to EC. C The laminar organization of the human hippocampus shown for cornu Ammonis sector CA2/3. Input arrives at dendrites of pyramidal cells in the stratum radiatum/lacunosum/moleculare (RLM), output leaves via fibers in the stratum oriens (O). The two neuronal types in the pyramidal cell layer are the pyramidal shaped principal cells and the nonpyramidal shaped interneuron. At least three types of inhibitory projections can arise from hippocampal interneurons: recurrent inhibition (driven by a collateral of a glutamatergic axon), direct inhibition of a pyramidal cell, and disinhibition of a pyramidal cell (via interneuron-interneuron projection)
In summary, reduced hippocampal volume is now established as one of the most robust brain abnormalities in schizophrenia (McCarley et al., 1999; Nelson et al., 1998a). It is therefore of great importance to understand the cellular basis of hippocampal pathology in schizophrenia. Is schizophrenia associated with a selective loss of cells, an abnormal expression of proteins, or an alteration at the level of gene expression (i.e., abnormal levels of mRNA) in the hippocampus? These questions have been studied in post-mortem preparations of the hippocampus.
Hippocampal neurons in schizophrenia
There are two types of neurons in the human hippocampus: large, pyramidal-shaped neurons (principal cells) and smaller, non-pyramidal neurons (nonprincipal cells). These two cell types have a different neurochemical signature: the principal cells are glutamatergic and the vast majority of nonprincipal cells are GABAergic. The class of GABAergic nonprincipal cells is also referred to as hippocampal interneuron (Freund and Buzsaki, 1996). The hippocampal interneurons can be subdivided further based on their colocalization with proteins such as the calcium-binding proteins parvalbumin, calbindin, and calretinin. These subclasses of GABAergic neurons in the human hippocampus are distributed differentially in the hippocampal subsectors and in their respective three layers (stratum oriens, stratum pyramidale, stratum radiatum/lacunosum/moleculare) (Freund and Buzsaki, 1996).
Decreased hippocampal volume could be due to a loss of neurons, which prompted several investigators to estimate the distribution, density, and number of hippocampal neurons in schizophrenia. Most studies of hippocampal neurons have relied on single or selected sections through the hippocampus. Such estimates might not reflect the true density or number of hippocampal cells. Furthermore, studies of cell density are vulnerable to the “reference trap”, i.e., it is impossible to know the true number of cells in a brain region for which there is no reliable estimate of the total volume (Braendgaard and Gundersen, 1986). For example, a true loss of hippocampal neurons could be associated with a finding of decreased neuronal density (in the setting of no significant change of hippocampal volume) or a finding of normal density (in the setting of decreased hippocampal volume). This makes it difficult to interpret cell density studies, since a negative finding (normal density) could lead to the incorrect conclusion that total cell number is unchanged.
Bearing this in mind, a review of the literature shows that most studies of hippocampal cells in schizophrenia have reported no significant change in cell density (Arnold, 2000; Dwork, 1997; Harrison, 1999). The only study that provided estimates of total cell number found a subtle reduction of volume (5% on the left, 2% on the right) and no change in neuronal density, resulting in estimates of total cell number that were not different between schizophrenia and control subjects (Heckers et al., 1991). This scenario, i.e., decreased volume in the context of overall normal cell number, raises two important questions.
First, is the decrease of hippocampal volume due to changes in the grey matter (pyramidal cell layer) or the white matter (stratum oriens and stratum radiatum/lacunosum/moleculare) compartments of the hippocampus (see Fig. 2)? Heckers et al. found that the subtle volume reduction of the hippocampus in their cohort was entirely due to volume reduction of the hippocampal white matter compartments, possibly indicating the loss of intrinsic or extrinsic hippocampal connections (Heckers et al., 1991). This is similar to the finding by Colter et al. (1987) who reported decreased white matter in the parahippocampal gyrus in schizophrenia.
Second, are subsets of hippocampal neurons selectively affected in schizophrenia? Such a deficit might not be detectable in studies of total cell number. Benes et al. distinguished hippocampal neurons based on their morphology into pyramidal and nonpyramidal cells and reported a selective decrease of the density of nonpyramidal cells, but no change in the density of pyramidal cells (Benes et al., 1998). Since the vast majority of neurons in the human hippocampus are pyramidal cells, a selective loss of nonpyramidal cells might go unnoticed in studies of total neuron number. Studies of protein and gene expression, which allow us to label subsets of hippocampal neurons, provide the opportunity to test the hypothesis of a selective loss of hippocampal neurons in schizophrenia. These studies will be reviewed in the next section.
Hippocampal proteins and genes in schizophrenia
The metabolic and signaling functions of neurons rely on a vast number of proteins. Some proteins are expressed constitutively whereas others undergo modulation, typically in response to environmental stimuli. A small number of proteins have been visualized in sections of post-mortem human brain tissue to characterize neurons using the techniques of immunohistochemistry and high-resolution receptor binding.
Each hippocampal cell is also endowed with the full set of genetic information in the form of DNA. However, only a fraction of the DNA is transcribed into mRNA and an even smaller fraction is expressed constitutively. The expression profile of genes is regulated by several factors, including the pattern of connectivity and the amount and type of sensory information arriving from the cortex. Recent studies have used in-situ hybridization to characterize neurons based on their gene expression profile.
Here we will briefly review the evidence for an abnormality of the GABAergic neuron, the glutamatergic neuron, and of the synaptic organization of hippocampal neurons [for more detailed reviews see: (Arnold, 2000; Benes, 1999; Benes and Berretta, 2001; Harrison and Eastwood, 2001)]. When reviewing this literature we have to bear in mind that postmortem studies cannot avoid the confounding effects of pharmacological treatment. Some investigators have studied the effect of long-term treatment with anti-psychotic drugs on protein and gene expression in the schizophrenic brain using statistical methods [e.g., see Kornhuber et al. (1989b), Benes and Todtenkopf (1999), Benes et al. (2000)]. The study of antipsychotic drugs in animals complements these studies and helps to elucidate the mechanism of drug effects [e.g., Harrison and Roberts (2000)]. For this review we will focus only on the studies of the human hippocampus.
GABAergic neurons
Initial studies of the hippocampal GABA system in schizophrenia focused on the targets of GABAergic neurons, i.e., GABAergic receptors. These studies revealed a regionally-specific upregulation of GABA(A) receptor binding in sectors CA2-4, but not CA1 (Benes et al., 1996, 1997). This could indicate a compensatory upregulation of GABA(A) receptors in neurons in response to decreased GABAergic input. Most of the GABAergic receptors in the human hippocampus are expressed on glutamatergic principal cells, but a substantial proportion is also expressed on interneurons. It is of interest that a marked increase of the GABA(A) receptor in CA2/3 was found on interneurons, which indicates a decreased GABAergic regulation of other interneurons (Benes, 1999). In contrast to the upregulation of GABA(A) receptors, a study of the benzodiazepine receptor (located on the same GABA(A)/BZD receptor complex) revealed minimal changes in the hippocampus in schizophrenia (Benes et al., 1997). This is consistent with a differential regulation of the two subunits of the receptor complex responsible for GABA and BZD binding respectively.
The signature enzymes of GABAergic neurons are the two isoforms of glutamic acid decarboxylase, GAD65 and GAD67, i.e., the enzymes that synthesize GABA. Studies have assessed the level of protein and gene expression of GAD65 and GAD67 in the hippocampus in schizophrenia. One study of GAD65 immunoreactive terminals in the hippocampus revealed no altered regulation in schizophrenia, but a significant positive correlation of GAD65 immunoreactivity with the dose of antipsychotic medication (Todtenkopf and Benes, 1998). This provides evidence that GAD65 protein expression is regulated by antipsychotic drugs. Heckers et al. recently completed a study of glutamic acid decarboxylase (GAD) mRNA expression in the hippocampus in normal controls, patients with schizophrenia, and patients with bipolar disorder (Heckers et al., 2002). This study revealed significant decreases of GAD65 (and to a lesser degree GAD67) mRNA expression in bipolar disorder and less significant changes in schizophrenia. The changes were most pronounced in sectors CA2/3 and CA4. This is in contrast to studies of cortical GAD mRNA expression in schizophrenia, which have shown changes primarily of GAD67 mRNA expression (Akbarian et al., 1995; Guidotti et al., 2000; Volk et al., 2000). It is not clear yet whether these changes of GAD mRNA expression translate into changes of GAD protein expression and whether the decreased levels of mRNA are related to the previously described loss of nonpyramidal cells in schizophrenia.
GABAergic neurons can also be studied via the expression of the GABA-reuptake transporter protein. One study found the GABAergic uptake sites to be decreased in the hippocampus in schizophrenia (Reynolds et al., 1990). This could be due to an overall decrease in the number of GABAergic neurons or due to a decreased level of expression of the GABA transporter.
The calcium binding proteins parvalbumin, calbindin, and calretinin are expressed in essentially non-overlapping subpopulations of interneurons in the human hippocampus (Seress et al., 1993). Therefore, the study of these proteins permits inferences about selective alterations of GABAergic neurotransmission in schizophrenia. Previous studies of parvalbumin-positive neurons in the cerebral cortex in schizophrenia have found them to be decreased (Reynolds et al., 2000; Reynolds and Beasley, 2001) or unchanged (Woo et al., 1997), whereas calretinin-positive cells (Reynolds et al., 2000; Daviss and Lewis, 1995) were not changed. A recent study of the hippocampus found a significantly decreased density of parvalbumin-positive neurons in all hippocampal regions, while the density of calretinin-positive cells was normal (Zhang and Reynolds, 2000). This provides the first evidence for a specific deficit of a subset of hippocampal interneurons in schizophrenia.
Taken together, there is now good evidence for an abnormality of hippocampal interneurons in schizophrenia. Future studies need to address the details of the anatomical organization of these neurons in schizophrenia.
Glutamatergic neurons
The vast majority of hippocampal neurons are glutamatergic. Glutamate controls the excitation of neurons and glia through the activation of various glutamate receptors. Most studies of glutamatergic neurons in schizophrenia have focused on the expression of these receptor complexes. Glutamate receptors include ion channels (ionotropic glutamate receptors) and G-protein coupled receptors (metabotropic glutamate receptors) (Ozawa et al., 1998). Ionotropic glutamate receptors are named after their distinguishing ligands and classified into NMDA receptors (N-methyl-D-aspartate), AMPA receptors (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid), and kainate receptors.
The expression of various glutamate receptor subtypes is altered in the hippocampus in schizophrenia (Meador-Woodruff and Healy, 2000). In a number of studies, the AMPA subunits GluR1 and GluR2 were decreased in the hippocampus and the parahippocampal gyrus (Eastwod et al., 1995, 1997; Harrison et al., 1991). In concordance, ligand binding to AMPA receptors was decreased (Kerwin et al., 1990). The kainate receptor subtypes GluR6 and KA2 were also significantly reduced in the schizophrenic hippocampus (Porter et al., 1997). Studies on kainate receptor density, conducted with radiolabeled kainate, demonstrated a decrease in the hippocampus (Deakin et al., 1989; Kerwin et al., 1990).
Initial studies of the NMDA receptor, which focused on the PCP binding site located inside the ion channel, found no marked changes in the hippocampus in schizophrenia (Kornhuber et al., 1989a; Meador-Woodruff and Healy, 2000). A recent study of the NMDA receptor subunits NR1, NR2A, and NR2B found an increase of NR2B mRNA and a decrease of NR1 mRNA in the hippocampus in schizophrenia (Gao et al., 2000).
Taken together, these studies provide good evidence for selective changes of glutamatergic receptors in the hippocampus in schizophrenia. In the context of the morphometric studies estimating cell number in schizophrenia, these changes do not appear to indicate a loss of glutamatergic neurons, but rather a functional adaptation.
Synaptic organization
The most important function of neurons is the transmission of signals via the release of neurotransmitters. The anatomical organization of the synapse is crucial for an optimal signal transfer from the presynaptic to the postsynaptic neuron. Several studies have investigated the expression of proteins and genes involved in the construction of functional synapses in schizophrenia [for review see: Harrison and Eastwood (2001); Honer et al. (2000)]. The most frequently studied proteins are synapsin and synaptophysin, which are involved in the formation of synaptic vesicles. The majority of studies have reported a decreased gene and protein expression of these two synaptic proteins in schizophrenia (Harrison and Eastwood, 2001).
One intriguing finding stems from studies of two synaptic proteins, complexin I and complexin II. In the hippocampus, complexin I mRNA is expressed primarily in interneurons, whereas complexin II mRNA is expressed primarily in pyramidal neurons (Eastwood et al., 2000; Harrison and Eastwood, 1998). In schizophrenia, both complexin mRNAs were found to be reduced, but more so for complexin II. Furthermore, at the level of protein expression, only complexin II was decreased. This has been interpreted as evidence for a preferential involvement of excitatory neurons in the medial temporal lobe in schizophrenia (Harrison and Eastwood, 1998).
The interpretation of the studies investigating the expression of genes and proteins involved in synaptic organization has to take into consideration several scenarios that could explain the changes seen in post-mortem human brain tissue. For example, synaptic organization could have been abnormal early during brain development, could have occurred after onset of the illness, or only during the final stages of life. The preferred inference would be that such changes can tell us something about the pathophysiology and ultimately the psychopathology of schizophrenia [e.g., schizophrenia is a disorder of connectivity (Feinberg, 1982; Friston, 1998; McGlashan and Hoffman, 2000)], but there are several less far reaching alternatives (Harrison and Eastwood, 2001). The combined approach of quantitative neuroanatomy, proteinomics, and gene expression profiling could provide some compelling new data to better understand these changes of synaptic organization in the hippocampus.
The role of the hippocampus in schizophrenia
Here we have reviewed three lines of evidence of hippocampal pathology in schizophrenia: decreased hippocampal volume, selective decrease of hippocampal neurons, and decreased expression of proteins and genes associated with GABAergic neurons, glutamatergic neurons, and synaptic organization. Although it is premature to draw any conclusions about the cellular pathology of the hippocampus in schizophrenia, it seems safe to make the following statements:
The hippocampus is smaller in schizophrenia.
The total number of hippocampal neurons is not reduced to the degree seen in Alzheimer’s disease or temporal lobe epilepsy.
The number of interneurons appears to be more reduced than the number of principal cells.
Changes of GABAergic and glutamatergic neurotransmission indicate decreased activity of GABAergic neurons and decreased function of the AMPA glutamate receptor.
Abnormalities of synaptic proteins indicate abnormal synaptic function in the hippocampus.
What does this evidence tell us about the role of the hippocampus in the pathophysiology of schizophrenia?
The human hippocampus receives highly-processed, multimodal sensory information via two inputs from the entorhinal cortex (one direct pathway to CA1, another via DG → CA2/3 → CA1), compares the two inputs, and sends information back to the cortex via the entorhinal cortex and to limbic structures via direct projections. This unique pattern of connections appears crucial for at least two brain functions, i.e., memory (in concert with association cortices) and affect regulation (in concert with the hypothalamus, thalamus, amygdala, and anterior cingulate cortex), both of which are abnormal in the majority of patients with schizophrenia. It is less clear, however, which role the hippocampus plays in the pathogenesis of the cognitive and affective changes in schizophrenia.
It is unlikely that the hippocampus is the sole or even primary locus of pathology in schizophrenia. There is compelling evidence, that all three regions closely associated with the hippocampus (i.e., entorhinal cortex, multimodal association cortex, and limbic system) are abnormal in schizophrenia. For example, a disturbed architecture of the entorhinal cortex neurons could lead to abnormal hippocampal projection patterns (Jakob and Beckmann, 1986). Furthermore, projections to the medial temporal lobe could be affected by a primary cortical abnormality (Lewis and Lieberman, 2000). Lastly, hippocampal dysfunction could be due to primary changes in the amygdala, cingulate cortex, or thalamus (Benes, 1998; Benes and Berretta, 2000). Only the integrated approach of studying more than one brain region (in both neuroimaging and postmortem studies) will be able to shed light on the role of each of these nodes of a widely distributed circuit.
Abnormalities of hippocampal structure in schizophrenia could be primary for the features of schizophrenia, or they could be secondary due to the disease process, the stress associated with a life-long disabling mental illness, or the various treatments of schizophrenia. Postmortem studies will not be in a strong position to address these concerns. Neuroimaging studies, however, can follow patients over time and can map out the time course of the hippocampal changes in schizophrenia.
If we can establish that the hippocampus is a crucial locus of pathology that gives rise to, rather then results from, schizophrenia, we still have to link the structural and functional abnormalities to the clinical features of schizophrenia. We also have to investigate whether hippocampal abnormalities are found in all patients with schizophrenia or only in a subgroup. Furthermore, if hippocampal abnormalities can be linked to psychotic symptoms, we have to study other psychotic disorders (e.g., bipolar disorder, depression with psychotic features) for abnormalities of the hippocampus. Neuroimaging and electrophysiological studies of hippocampal function are necessary for this last piece of the puzzle. Such studies have demonstrated a role of the hippocampus for disorders such as epilepsy and dementia. We now have the tools for such studies in schizophrenia (Heckers, 2001).
Acknowledgments
This work was supported by the National Alliance for Research on Schizophrenia and Depression (CK, SH), a Stanley Foundation Research Award (SH), a National Institute of Mental Health grant MH01763 (SH), and a National Institute of Drug Abuse grant DA07134 (CK).
References
- Akbarian S, Kim JJ, Potkin SG, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–66. doi: 10.1001/archpsyc.1995.03950160008002. discussion 267–278. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Insausti R. Hippocampal formation. In: Paxinos G, editor. The human nervous system. Academic Press; San Diego: 1990. [Google Scholar]
- Arnold SE. Hippocampal pathology. In: Harrison PJ, Roberts GW, editors. The neuropathology of schizophrenia. Progress and interpretation. Oxford University Press; Oxford: 2000. pp. 57–80. [Google Scholar]
- Barbas H, Blatt GJ. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus. 1995;5:511–533. doi: 10.1002/hipo.450050604. [DOI] [PubMed] [Google Scholar]
- Benes FM. Model generation and testing to probe neural circuitry in the cingulate cortex of postmortem schizophrenic brain. Schizophr Bull. 1998;24:219–230. doi: 10.1093/oxfordjournals.schbul.a033322. [DOI] [PubMed] [Google Scholar]
- Benes FM. Evidence for altered trisynaptic circuitry in schizophrenic hippocampus. Biol Psychiatry. 1999;46:589–599. doi: 10.1016/s0006-3223(99)00136-5. [DOI] [PubMed] [Google Scholar]
- Benes FM, Todtenkopf MS. Effect of age and neuroleptics on tyrosine hydroxylase-IR in sector CA2 of schizophrenics. Neuroreport. 1999;10:3527–3530. doi: 10.1097/00001756-199911260-00012. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. Amygdalo-entorhinal inputs to the hippocampal formation in relation to schizophrenia. Ann NY Acad Sci. 2000;911:293–304. doi: 10.1111/j.1749-6632.2000.tb06733.x. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. Gabaergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Benes FM, Khan Y, Vincent SL, Wickramasinghe R. Differences in the subregional and cellular distribution of GABAA receptor binding in the hippocampal formation of schizophrenic brain. Synapse. 1996;22:338–349. doi: 10.1002/(SICI)1098-2396(199604)22:4<338::AID-SYN5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Benes FM, Wickramasinghe R, Vincent SL, Khan Y, Todtenkopf M. Uncoupling of GABA(A) and benzodiazepine receptor binding activity in the hippocampal formation of schizophrenic brain. Brain Res. 1997;755:121–129. doi: 10.1016/s0006-8993(97)00113-3. [DOI] [PubMed] [Google Scholar]
- Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives [see comments] Biol Psychiatry. 1998;44:88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- Benes FM, Todtenkopf MS, Logiotatos P, Williams M. Glutamate decarboxylase(65)-immunoreactive terminals in cingulate and prefrontal cortices of schizophrenic and bipolar brain. J Chem Neuroanat. 2000;20:259–269. doi: 10.1016/s0891-0618(00)00105-8. [DOI] [PubMed] [Google Scholar]
- Bogerts B, Meertz E, Schonfeldt-Bausch R. Basal ganglia and limbic system pathology in schizophrenia. A morphometric study of brain volume and shrinkage. Arch Gen Psychiatry. 1985;42:784–791. doi: 10.1001/archpsyc.1985.01790310046006. [DOI] [PubMed] [Google Scholar]
- Bogerts B, Ashtari M, Degreef G, Alvir JM, Bilder RM, Lieberman JA. Reduced temporal limbic structure volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res. 1990;35:1–13. doi: 10.1016/0925-4927(90)90004-p. [DOI] [PubMed] [Google Scholar]
- Braendgaard H, Gundersen HJG. The impact of recent stereological advances on quantitative studies of the nervous system. J Neurosci Methods. 1986;18:39–78. doi: 10.1016/0165-0270(86)90112-3. [DOI] [PubMed] [Google Scholar]
- Colter N, Battal S, Crow TJ, Johnstone EC, Brown R, Bruton C. White matter reduction in the parahippocampal gyrus of patients with schizophrenia. Arch Gen Psychiatry. 1987;44:1023. doi: 10.1001/archpsyc.1987.01800230103016. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Joshi S, Wang L, et al. Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proc Natl Acad Sci USA. 1998;95:11406–11411. doi: 10.1073/pnas.95.19.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviss SR, Lewis DA. Local circuit neurons of the prefrontal cortex in schizophrenia: selective increase in the density of calbindin-immunoreactive neurons. Psychiatry Res. 1995;59:81–96. doi: 10.1016/0165-1781(95)02720-3. [DOI] [PubMed] [Google Scholar]
- Deakin JFW, Slater P, Simpson MDC, et al. Frontal cortical and left temporal glutamatergic dysfunction in schizophrenia. J Neurochem. 1989;52:1781–1786. doi: 10.1111/j.1471-4159.1989.tb07257.x. [DOI] [PubMed] [Google Scholar]
- Dwork AJ. Postmortem studies of the hippocampal formation in schizophrenia. Schizophr Bull. 1997;23:403–421. doi: 10.1093/schbul/23.3.385. [DOI] [PubMed] [Google Scholar]
- Eastwod SL, McDonald B, Burnet PWJ, Beckwith JP, Kerwin RW, Harrison PJ. Decreased expression of mRNAs encoding non-NMDA glutamate receptors GluR1 and GluR2 in medial temporal lobe neurons in schizophrenia. Mol Brain Res. 1995;29:211–223. doi: 10.1016/0169-328x(94)00247-c. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Burnet PW, Harrison PJ. GluR2 glutamate receptor subunit flip and flop isoforms are decreased in the hippocampal formation in schizophrenia: a reverse transcriptase-polymerase chain reaction (RT-PCR) study. Brain Res Mol Brain Res. 1997;44:92–98. doi: 10.1016/s0169-328x(96)00195-7. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Burnet PW, Harrison PJ. Expression of complexin I and II mRNAs and their regulation by antipsychotic drugs in the rat forebrain. Synapse. 2000;36:167–177. doi: 10.1002/(SICI)1098-2396(20000601)36:3<167::AID-SYN2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res [Suppl] 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Friston KJ. The disconnection hypothesis. Schizophr Res. 1998;30:115–125. doi: 10.1016/s0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- Gao X-M, Sakai K, Roberts RC, Conley RR, Dean B, Tamminga CA. Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am J Psychiatry. 2000;157:1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Jeffries NO, Blumenthal J, et al. Childhood-onset schizophrenia: progressive brain changes during adolescence. Biol Psychiatry. 1999;46:892–898. doi: 10.1016/s0006-3223(99)00072-4. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus cortex. Neuroscience. 1984;12:719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Eastwood SL. Preferential involvement of excitatory neurons in medial temporal lobe in schizophrenia. Lancet. 1998;352:1669–1673. doi: 10.1016/S0140-6736(98)03341-8. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Roberts GW. The neuropathology of schizophrenia. 1. Oxford University Press; Oxford: 2000. p. 374. [Google Scholar]
- Harrison PJ, Eastwood SL. Neuropathological studies of synaptic connectivity in the hippocampal formation in schizophrenia. Hippocampus. 2001;11:508–519. doi: 10.1002/hipo.1067. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, McLaughlin D, Kerwin RW. Decreased hippocampal expression of a glutamate receptor gene in schizophrenia. Lancet. 1991;337:450–452. doi: 10.1016/0140-6736(91)93392-m. [DOI] [PubMed] [Google Scholar]
- Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11:520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- Heckers S, Heinsen H, Geiger B, Beckmann H. Hippocampal neuron number in schizophrenia. A stereological study. Arch Gen Psychiatry. 1991;48:1002–1008. doi: 10.1001/archpsyc.1991.01810350042006. [DOI] [PubMed] [Google Scholar]
- Heckers S, Stone D, Walsh J, Shick J, Koul P, Benes FM. Differential hippocampal expression of glutamic acid decarboxylase 65 and 67 messenger RNA in bipolar disorder and schizophrenia. Arch Gen Psychiatry. 2002 doi: 10.1001/archpsyc.59.6.521. in press. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, Shenton ME, Salisbury DF, et al. Lower left temporal lobe MRI volumes in patients with first-episode schizophrenia compared with psychotic patients with first-episode affective disorder and normal subjects. Am J Psychiatry. 1998;155:1384–1391. doi: 10.1176/ajp.155.10.1384. [DOI] [PubMed] [Google Scholar]
- Honer WG, Young C, Falkai P. Synaptic pathology. In: Harrison PJ, Roberts GW, editors. The neuropathology of schizophrenia. Progress and interpretation. Vol. 1. Oxford University Press; Oxford: 2000. pp. 105–136. [Google Scholar]
- Jakob H, Beckmann H. Prenatal developmental disturbances in the limbic allocortex in schizophrenics. J Neural Transm. 1986;65:303–326. doi: 10.1007/BF01249090. [DOI] [PubMed] [Google Scholar]
- Kerwin R, Patel S, Meldrum B. Quantitative autoradiographic analysis of glutamate binding sites in the hippocampal formation in normal and schizophrenic brain post mortem. Neuroscience. 1990;39:25–32. doi: 10.1016/0306-4522(90)90219-t. [DOI] [PubMed] [Google Scholar]
- Kornhuber J, Mack-Burkhardt F, Riederer P, et al. [3H]MK-801 binding sites in postmortem brain regions of schizophrenic patients. J Neural Transm [Gen Sect] 1989a;77:231–236. doi: 10.1007/BF01248936. [DOI] [PubMed] [Google Scholar]
- Kornhuber J, Riederer P, Reynolds GP, Beckmann H, Jellinger K, Gabriel E. 3H-spiperone binding sites in post-mortem brains from schizophrenic patients: relationship to neuroleptic drug treatment, abnormal movements, and positive symptoms. J Neural Transm [Gen Sect] 1989b;75:1–10. doi: 10.1007/BF01250639. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Whalley H, Kestelman JN, et al. Magnetic resonance imaging of brain in people at high risk of developing schizophrenia. Lancet. 1999;353:30–33. doi: 10.1016/S0140-6736(98)06244-8. [DOI] [PubMed] [Google Scholar]
- Lepage M, Habib R, Tulving E. Hippocampal PET activations of memory encoding and retrieval: the HIPER model. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Lewis D, Lieberman JA. Catching up on schizophrenia: natural history and neurobiology. Neuron. 2000;28:325–334. doi: 10.1016/s0896-6273(00)00111-2. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Wible CG, Frumin M, et al. MRI anatomy of schizophrenia. Biol Psychiatry. 1999;45:1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Healy DJ. Glutamate receptor expression in schizophrenic brain. Brain Res Brain Res Rev. 2000;31:288–294. doi: 10.1016/s0165-0173(99)00044-2. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry. 1998a;55:433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study [see comments] Arch Gen Psychiatry. 1998b;55:433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog Neurobiol. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, Suckling J, et al. Left medial temporal volume reduction occurs during the transition from high-risk to first-episode psychosis. Schizophr Res. 2000;41(1):35. [Google Scholar]
- Porter RH, Eastwood SL, Harrison PJ. Distribution of kainate receptor subunit mRNAs in human hippocampus, neocortex and cerebellum, and bilateral reduction of hippocampal GluR6 and KA2 transcripts in schizophrenia. Brain Res. 1997;751:217–231. doi: 10.1016/s0006-8993(96)01404-7. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, et al. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cerebral Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Beasley CL. GABAergic neuronal subtypes in the human frontal cortex – development and deficits in schizophrenia. J Chem Neuroanat. 2001;22:95–100. doi: 10.1016/s0891-0618(01)00113-2. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Czudek C, Andrews HB. Deficit and hemispheric asymmetry of GABA uptake sites in the hippocampus in schizophrenia. Biol Psychiatry. 1990;27:1038–1044. doi: 10.1016/0006-3223(90)90039-5. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Zhang ZJ, Patten I, Beasley CL. Selective deficits of frontal cortical GABAergic neuronal subtypes defined by calcium binding proteins in psychotic illness. Schizophr Res. 2000;41:255. (Abs) [Google Scholar]
- Rossi A, Stratta P, Mancini F, et al. Magnetic resonance imaging findings of amygdala-anterior hippocampus shrinkage in male patients with schizophrenia. Psychiatry Res. 1994;52:43–53. doi: 10.1016/0165-1781(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Faraone SV, Goldstein JM, et al. Thalamic and amygdala-hippocampal volume reductions in first-degree relatives of patients with schizophrenia: an MRI-based morphometric analysis. Biol Psychiatry. 1999;46:941–954. doi: 10.1016/s0006-3223(99)00075-x. [DOI] [PubMed] [Google Scholar]
- Seress L, Gulyas AI, Ferrer I, Tunon T, Soriano E, Freund TF. Distribution, morphological features, and synaptic connections of parvalbumin- and calbindin D28k-immunoreactive neurons in the human hippocampal formation. J Comp Neurol. 1993;337:208–230. doi: 10.1002/cne.903370204. [DOI] [PubMed] [Google Scholar]
- Strange BA, Fletcher PC, Henson RN, Friston KJ, Dolan RJ. Segregating the functions of human hippocampus. Proc Natl Acad Sci USA. 1999;96:4034–4039. doi: 10.1073/pnas.96.7.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR. Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med. 1990;322:789–794. doi: 10.1056/NEJM199003223221201. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Benes FM. Distribution of glutamate decarboxylase65 immunoreactive puncta on pyramidal and nonpyramidal neurons in hippocampus of schizophrenic brain. Synapse. 1998;29:323–332. doi: 10.1002/(SICI)1098-2396(199808)29:4<323::AID-SYN4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Tsuang M. Schizophrenia: genes and environment. Biol Psychiatry. 2000;47:210–220. doi: 10.1016/s0006-3223(99)00289-9. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW. Ventromedial temporal lobe anatomy, with comments in Alzheimer’s disease and temporal injury. J Neuropsychiatr Clin Neurosci. 1997;9:331–341. doi: 10.1176/jnp.9.3.331. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Pantelis C, McGorry PD, et al. Hippocampal volume in first-episode psychoses and chronic schizophrenia: a high-resolution magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56:133–141. doi: 10.1001/archpsyc.56.2.133. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Stuart GW, Wood SJ, et al. Selective bilateral hippocampal volume loss in chronic schizophrenia. Biol Psychiatry. 2001;50:531–539. doi: 10.1016/s0006-3223(01)01121-0. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase 67 messenger RNA expression in a subset of prefrontal cortical γ-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Wang L, Joshi SC, Miller MI, Csernansky JG. Statistical analysis of hippocampal asymmetry in schizophrenia. Neuroimage. 2001;14:531–545. doi: 10.1006/nimg.2001.0830. [DOI] [PubMed] [Google Scholar]
- Woo TU, Miller JL, Lewis DA. Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. Am J Psychiatry. 1997;154:1013–1015. doi: 10.1176/ajp.154.7.1013. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Velakoulis D, Smith DJ, et al. A longitudinal study of hippocampal volume in first episode psychosis and chronic schizophrenia. Schizophr Res. 2000;52:37–46. doi: 10.1016/s0920-9964(01)00175-x. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Bookheimer SY. Application of cortical unfolding techniques to functional MRI of the human hippocampal region. NeuroImage. 2000;11:668–683. doi: 10.1006/nimg.2000.0561. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Reynolds GP. A selective deficit in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res. 2000;49:65. doi: 10.1016/s0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]