Abstract
Background
Plasmodium falciparum is the parasite that causes the most severe form of malaria responsible for nearly a million deaths a year. Currently, science has been established about its cellular structures, its metabolic processes, and even the molecular structures of its intrinsic membrane proteins responsible for transporting water, nutrient, and waste molecules across the parasite plasma membrane (PPM).
Presentation of the hypothesis
I hypothesize that Plasmodium falciparum has an Achilles’ heel that can be attacked with erythritol, the well-known sweetener that is classified as generally safe. This hypothesis is based on the molecular structure of the parasite’s membrane and the quantitative mechanics of how erythritol interacts with the multi-functional channel protein expressed in the PPM. Most organisms have in their cell membrane two types of water-channel proteins: aquaporins to maintain hydro-homeostasis across the membrane and aquaglyceroporins to uptake glycerols etc. In contrast, P. falciparum has only one type of such proteins---the multi-functional aquaglyceroporin (PfAQP) expressed in the PPM---to do both jobs. Moreover, the parasite also uses PfAQP to excrete its metabolic wastes (ammonia included) produced at a very high rate in the blood stage. This extremely high efficiency of the bug using one protein for multiple essential tasks makes the parasite fatally vulnerable. Erythritol in the blood stream can kill the parasite by clogging up its PfAQP channel that needs to be open for maintaining hydro-homeostasis and for excreting toxic wastes across the bug’s PPM.
Testing the hypothesis
In vitro tests are to measure the growth/death rate of P. falciparum in blood with various erythritol concentrations. In vivo experiments are to administer groups of infected mice with various doses of erythritol and monitor the parasite growth levels from blood samples drawn from each group. Clinic trials can be performed to observe the added effects of administering to patients erythritol along with the known drugs because erythritol was classified as a safe food ingredient.
Implications of the hypothesis
If proven true, erythritol will cure the most severe form of malaria without significant side effects.
Keywords: Malaria, P. falciparum, Channel protein, Erythritol
Background
Does Plasmodium falciparum---the parasite that causes the most severe form of malaria leading to the death of a child every 60 second [1]---have an Achilles’ heel that can be exploited therapeutically? The answer seems to be yes even though the bug enjoys three layers of protection by the red cell membrane (RCM), the parasitophorous vacuole membrane (PVM), and the parasite plasma membrane (PPM). On the basis of the molecular structure of the bug’s PPM and the quantitative mechanics of its major intrinsic membrane protein interacting with erythritol, I hypothesize that erythritol [2]--- the generally considered safe sugar substitute [3]---could be the weapon needed to deliver a fatal attack on the heel of the parasite that is responsible for about a million deaths a year.
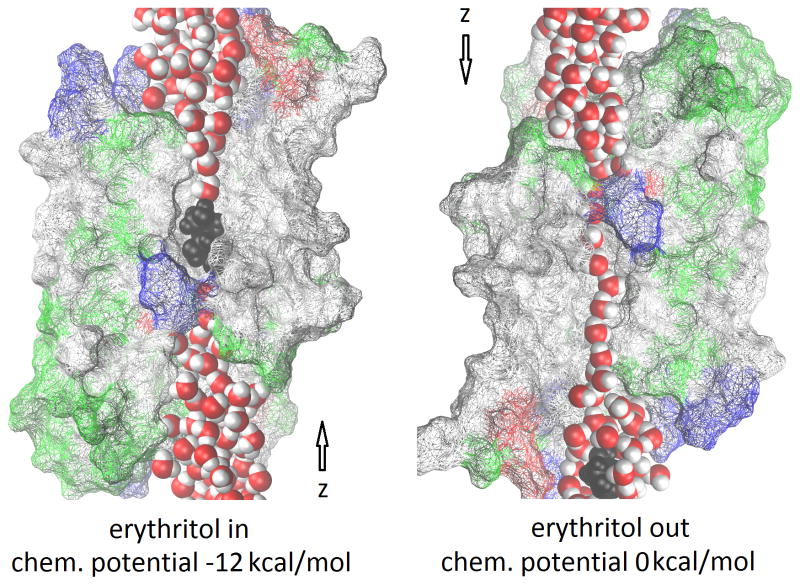
Currently, all malaria drugs used or researched for are intricate compounds that have strong side effects and induce drug-resistance in the parasites. [4–17] Researchers have not considered the possibility that the benign erythritol can kill or impede the growth of P. falciparum. In fact, erythritol can move across the cell membrane through the conducting pore of aquaglyceroporins just like glycerol which is a nutrient for the parasite’s growth [18] and up taken into the P. falciparum cell across, consecutively, the RCM, the PVM, and the PPM. [19, 20] The possibility of erythritol being an inhibitor of P. falciparum is visible only in the light of the parasite’s molecular structure available recently in the literature [20–22] and the quantitative determination [23–25] of how erythritol interacts with the channel protein in the PPM illustrated in Figure 1.
Figure 1.
PfAQP channel occluded (left) and open (right) for water/solute permeation. The luminal residues of the protein are shown in wireframes colored by residue types, the waters in red (oxygen) and white (hydrogen) balls, and the erythritol in black balls (hydrogen, oxygen, and carbon). Not all luminal residues of PfAQP are shown for the purpose of fully exposing the waters and erythritol that line up in single file inside the conducting pore. The z-axis points from the extracellular to the cytoplasmic bulk. Graphics rendered with Virtual Molecular Dynamics. [30]
Presentation of the hypothesis
The hypothesis is that erythritol in the blood stream will kill P. falciparum or, at the least, impede the parasite’s growth in the human blood stage. The rationale is the following:
Recent in vitro studies concluded that P. falciparum has only one type of water-channel proteins, aquaporins (AQPs), expressed in its plasma membrane---Plasmodium Aquaporin (PfAQP). [21, 22] This is in contrast with other organisms that all have two types of water-channel proteins in their cell membranes---aquaporins dedicated for water transport and aquaglycero-porins that conduct water, glycerol, ammonia and other small solutes. [26] P. falciparum relies on PfAQP for glycerol uptake, water conduction, and excretion of its metabolic wastes produced at high speed in the blood-stage. [27] The waste molecules, if not excreted, would intoxicate the bug itself within minutes. [20]
Needless to say, a living cell needs its waste-excretion channel unclogged in order to not be intoxicated by its own metabolism. Less obvious but equally true, a living cell needs its water channel unclogged as well to maintain hydro-homeostasis across its cell membrane. Otherwise, it will not be able to survive the severe osmotic stress during renal circulation.
The conducting channel of PfAQP, like all other aquaporins, is single file in nature. [22] As shown in Fig. 1, water and solute molecules line up in single file throughout the conducting pore. When an erythritol molecule dwells inside the pore instead of passing through the channel instantly, it clogs it up, inhibiting the transport of water, glycerol, and waste molecules across the cell membrane.
The chemical-potential profile of erythritol in PfAQP tells us that there will always be a erythritol dwelling inside the channel when erythritol is administered to a patient at a rather low dosage. The binding affinity of erythritol to PfAQP corresponds to a half-maximal inhibitory concentration of IC50=786 nM [25]. Therefore, when the erythritol concentration in the blood is in the high μM range, the binding site inside PfAQP will be saturated with erythritol. The PfAQP channel will be clogged up nearly all the time for transporting water, glycerol, and waste molecules. Consequently, P. falciparum will lose its ability to excrete wastes and to survive osmotic stress.
Now, what the advantages does a permeant of the channel protein have over a total channel blocker which were suggested to be ineffective [28]? First, a total blocker of the PfAQP channel probably inhibits other members of the aquaglyceroporin subfamily including AQP3 and AQP9 that are expressed in the red cell and other human cells. Indiscriminate inhibition of aquaglyceroporins in all red cells alone (infested with P. falciparum or not) will certainly cause undesirable side effects, not counting other cells. In contrast, erythritol permeates aquaglyceroporins in the RCM, PVM, and PPM. Consequently, equilibrium of erythritol concentration is expected between the parasite’s cytosol and the serum outside the red cells, assuming that the parasite does not consume erythritol. If the parasite does metabolize erythritol, depending on the rate of consumption, a concentration gradient would exist in the cytoplasmic direction. Even in such a case, the PfAQP pore will still be occupied and thus occluded by an erythritol with a probability of nearly 100% if the extracellular concentration of erythritol is far above the IC50 of 786 nM. Moreover, erythritol taken orally enters quickly into the blood stream and has a long biological half-life [29]. Therefore, any amount of erythritol would practically inhibit the highly efficient functions of PfAQP in facilitating transport of water, ammonia, urea, and glycerol. Finally, erythritol is known to be safe, not causing side effects.
Testing the hypothesis
In vitro experiments
Adding erythritol to non-immune human whole blood, prepare 10 culture media with erythritol concentrations ranging from 0·0 mM to 0·9 mM by increment of 0·1 mM, nine media from 1·0 mM to 9·0 mM by increment of 1·0 mM, and 10 media from 10 mM to 100 mM by increment of 10 mM. Measure the growth and death rates of P. falciparum in these 29 media. Repeat these experiments multiple times to minimize the statistical uncertainties.
In vivo experiments
Divide mice infected with P. falciparum into 20 groups. Give each group a different daily dose of erythritol. Group One, 0 mg/kg, Group Two, 10 mg/kg, Group Three, 20 mg/kg, ……, Group 10, 90 mg/kg, Group 11, 100 mg/kg, Group 12, 200 mg/kg, ……, and Group 20, 1000 mg/kg. Observe the survival rate and time of each group. During the course of time, take blood samples and measure the parasite growth in each group. Repeat the experiments to minimize the statistical uncertainties. Note that even the highest dosage suggested here does not have any adverse effects to the physiology of humans.
Implications of the hypothesis
Finally, a physician can safely administer a range of doses of erythritol along with other anti-malaria drugs to controlled patient groups and observe the added effectiveness due to erythritol at various dosages. If the hypothesis is proven true, we will soon see the eradication of the most severe form of malaria.
Acknowledgments
The author acknowledges support from the NIH (Grant #GM084834) and the Texas Advanced Computing Center.
List of abbreviations
- AQP
Aquaporin/Aquaglyceroporin
- PfAQP
Plasmodium falciparum aquaglyceroporin
- PPM
Parasite plasma membrane
- PVM
Parasitophorous vacuole membrane
- RCM
Red cell membrane
Footnotes
Competing interests
The author declares that no competing interests of any type exist.
References
- 1.WHO. World Malaria Report 2013. Available from: http://www.who.int/malaria/publications/world_malaria_report_2013/en/
- 2.Stenhouse J. Examination of the Proximate Principles of Some of the Lichens. Philosophical Transactions of the Royal Society of London. 1848;138:63–89. [Google Scholar]
- 3.Munro IC, et al. Erythritol: an interpretive summary of biochemical, metabolic, toxicological and clinical data. Food and Chemical Toxicology. 1998;36(12):1139–1174. doi: 10.1016/s0278-6915(98)00091-x. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, et al. Malaria in South Asia: Prevalence and control. Acta Tropica. 2012;121(3):246–255. doi: 10.1016/j.actatropica.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pongtavornpinyo W, et al. ORIGINAL ARTICLE: Probability of emergence of antimalarial resistance in different stages of the parasite life cycle. Evolutionary Applications. 2009;2(1):52–61. doi: 10.1111/j.1752-4571.2008.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerb R, et al. Pharmacogenetics of antimalarial drugs: effect on metabolism and transport. The Lancet Infectious Diseases. 2009;9(12):760–774. doi: 10.1016/S1473-3099(09)70320-2. [DOI] [PubMed] [Google Scholar]
- 7.Fairhurst RM, et al. Artemisinin-resistant malaria: research challenges, opportunities, and public health implications. Am J Trop Med Hyg. 2012;87(2):231–41. doi: 10.4269/ajtmh.2012.12-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guiguemde WA, et al. Global phenotypic screening for antimalarials. Chem Biol. 2012;19(1):116–29. doi: 10.1016/j.chembiol.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilgeroth A, Hemmer M, Coburger C. The impact of the induction of multidrug resistance transporters in therapies by used drugs: recent studies. Mini Rev Med Chem. 2012;12(11):1127–34. doi: 10.2174/138955712802762130. [DOI] [PubMed] [Google Scholar]
- 10.Miller LH, et al. Malaria biology and disease pathogenesis: insights for new treatments. Nat Med. 2013;19(2):156–67. doi: 10.1038/nm.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mita T, Tanabe K. Evolution of Plasmodium falciparum drug resistance: implications for the development and containment of artemisinin resistance. Jpn J Infect Dis. 2012;65(6):465–75. doi: 10.7883/yoken.65.465. [DOI] [PubMed] [Google Scholar]
- 12.Muregi FW, Wamakima HN, Kimani FT. Novel drug targets in malaria parasite with potential to yield antimalarial drugs with long useful therapeutic lives. Curr Pharm Des. 2012;18(24):3505–21. [PubMed] [Google Scholar]
- 13.Nevin RL. Mefloquine gap junction blockade and risk of pregnancy loss. Biol Reprod. 2012;87(3):65. doi: 10.1095/biolreprod.112.099614. [DOI] [PubMed] [Google Scholar]
- 14.Price RN, et al. Phenotypic and genotypic characterisation of drug-resistant Plasmodium vivax. Trends Parasitol. 2012;28(11):522–9. doi: 10.1016/j.pt.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qidwai T, Khan F. Antimalarial drugs and drug targets specific to fatty acid metabolic pathway of Plasmodium falciparum. Chem Biol Drug Des. 2012;80(2):155–72. doi: 10.1111/j.1747-0285.2012.01389.x. [DOI] [PubMed] [Google Scholar]
- 16.The mal, E.R.A.C.G.o.D. A Research Agenda for Malaria Eradication: Drugs. PLoS Med. 2011;8(1):e1000402. doi: 10.1371/journal.pmed.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tschan S, Kremsner PG, Mordmuller B. Emerging drugs for malaria. Expert Opin Emerg Drugs. 2012;17(3):319–33. doi: 10.1517/14728214.2012.702754. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, et al. Aquaporin 9 is the major pathway for glycerol uptake by mouse erythrocytes, with implications for malarial virulence. Proceedings of the National Academy of Sciences. 2007;104(30):12560–12564. doi: 10.1073/pnas.0705313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Promeneur D, et al. Aquaglyceroporin PbAQP during intraerythrocytic development of the malaria parasite Plasmodium berghei. Proceedings of the National Academy of Sciences. 2007;104(7):2211–2216. doi: 10.1073/pnas.0610843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beitz E. Jammed traffic impedes parasite growth. Proceedings of the National Academy of Sciences. 2007;104(35):13855–13856. doi: 10.1073/pnas.0706632104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen M, et al. A Single, Bi-functional Aquaglyceroporin in Blood-stage Plasmodium falciparum Malaria Parasites. Journal of Biological Chemistry. 2002;277(7):4874–4882. doi: 10.1074/jbc.M110683200. [DOI] [PubMed] [Google Scholar]
- 22.Newby ZER, et al. Crystal structure of the aquaglyceroporin PfAQP from the malarial parasite Plasmodium falciparum. Nat Struct Mol Biol. 2008;15(6):619–625. doi: 10.1038/nsmb.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen LY. Glycerol inhibits water permeation through Plasmodium Falciparum aquaglyceroporin. Journal of Structural Biology. 2013;181(1):71–76. doi: 10.1016/j.jsb.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen LY. Glycerol modulates water permeation through Escherichia coli aquaglyceroporin GlpF. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2013;1828(8):1786–1793. doi: 10.1016/j.bbamem.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen LY. Healthy sweet inhibitor of Plasmodium falciparum aquaglyceroporin. 2013 eprint arXiv:1305.1267. [Google Scholar]
- 26.Zeuthen T, et al. Ammonia permeability of the aquaglyceroporins from Plasmodium falciparum, Toxoplasma gondii and Trypansoma brucei. Molecular Microbiology. 2006;61(6):1598–1608. doi: 10.1111/j.1365-2958.2006.05325.x. [DOI] [PubMed] [Google Scholar]
- 27.King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2004;5(9):687–698. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- 28.Kun JF, de Carvalho EG. Novel therapeutic targets in Plasmodium falciparum: aquaglyceroporins. Expert Opin Ther Targets. 2009;13(4):385–94. doi: 10.1517/14728220902817839. [DOI] [PubMed] [Google Scholar]
- 29.Ishikawa M, et al. Effects of Oral Administration of Erythritol on Patients with Diabetes. Regulatory Toxicology and Pharmacology. 1996;24(2):S303–S308. doi: 10.1006/rtph.1996.0112. [DOI] [PubMed] [Google Scholar]
- 30.Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. Journal of Molecular Graphics. 1996;14(1):33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]