Abstract
Chronic morphine leads to compensatory up-regulation of cAMP signaling pathways in numerous brain regions. One potential consequence of up-regulated cAMP signaling is increased phosphorylation of cAMP response element binding protein (CREB), a transcription factor that may regulate neuroadaptations related to morphine dependence. Altered gene expression within the nucleus accumbens (NAc), a ventral component of the striatum that receives substantial dopaminergic input, may play a role in some of the motivational aspects of opiate withdrawal. To determine if morphine withdrawal leads to increased CREB phosphorylation in striatal tissues, we examined the effects of naloxone-precipitated morphine withdrawal on CREB phosphorylation in primary cultures of rat striatal neurons. Precipitated morphine withdrawal was associated with enhanced dopamine-, SKF 82958 (D1 receptor agonist)-, and forskolin-induced CREB phosphorylation. During precipitated withdrawal, D1 receptor-mediated CREB phosphorylation was dependent on cAMP-dependent protein kinase (PKA). Precipitated withdrawal also led to up-regulation of c-fos mRNA in response to SKF 82958. CREB protein levels were not altered by acute or chronic morphine. These results suggest that D1 receptor-mediated signal transduction is enhanced during morphine withdrawal. Furthermore, they are consistent with in vivo evidence suggesting that increased CREB activation in portions of the striatum (e.g. the NAc) is related to dysphoric states associated with drug withdrawal.
Keywords: c-fos, CREB, naloxone, nucleus accumbens, primary cell culture, SKF 82958
Opiates such as morphine are rewarding in animals including rodents, non-human primates, and humans. The mesolimbic dopamine system is important for the rewarding properties of morphine (for review, see Wise 1989). For example, rats will self-administer opiates directly into the ventral tegmental area (VTA) (Bozarth and Wise 1983), which contains dopaminergic cell bodies, and into the ventral striatum [nucleus accumbens (NAc)] (Olds 1982), which receives substantial dopaminergic input from the VTA. Repeated administration of morphine leads to behavioral and molecular adaptations that may contribute to drug dependence, which is characterized by a somatic abstinence syndrome and dysphoric or aversive states (for review, see Nestler 1992; Wise 1996). The persistence of these neuroadaptations may underlie the long-term behavioral abnormalities that accompany addiction (Nestler 2001). There is considerable evidence that the locus coeruleus and periaqueductal gray mediate many of the physical symptoms of opiate withdrawal (Rasmussen et al. 1990; Maldonado et al. 1992). The NAc may also mediate some of the physical symptoms of opiate withdrawal (Koob et al. 1992; Harris and Aston-Jones 1994). Importantly, however, the NAc appears to play a key role in mediating negative motivational aspects of withdrawal, including hypofunction of brain reward systems (Koob et al. 1989b). Presumably, these negative motivational aspects of drug withdrawal contribute to the maintenance of addictive behaviors (Koob et al. 1989a). Thus the NAc appears to be critically involved in multiple aspects of opiate dependence.
Morphine acts at μ opiate receptors (MOR) located in brain regions important for addiction, including the VTA and the NAc (Mansour et al. 1995). MORs are linked to inhibitory heterotrimeric guanosine triphosphate-binding (Gαi) proteins, and acute stimulation of MORs leads to a decrease in cAMP levels (Childers 1991). In the presence of chronic morphine, however, molecular adaptations occur such that adenylate cyclase activity increases and cAMP levels return to approximately normal; when morphine is discontinued or the opioid receptor antagonist naloxone is administered, cAMP levels dramatically increase (for review, see Nestler and Aghajanian 1997). This phenomenon of early inhibition and late positive regulation of adenylate cyclase by morphine has been described in many cell types and in many brain regions, including a neuroblastoma × glioma cell line (Sharma et al. 1975a), primary cultures of striatal neurons (Van Vliet et al. 1991), the locus coeruleus (Duman et al. 1988; Nestler and Tallman 1988), NAc (Terwilliger et al. 1991; Self et al. 1995; Shaw-Lutchman et al. 2002), and VTA (Bonci and Williams 1997). Up-regulation of the cAMP pathway observed during morphine withdrawal would be predicted to lead to a myriad of intracellular adaptations, including activation of cAMP-dependent protein kinase (PKA) and phosphorylation of cAMP response element binding protein (CREB) at Ser133. Indeed, increased CREB phosphorylation has been demonstrated in the locus coeruleus of rats after chronic morphine treatment (Guitart et al. 1992). Furthermore, precipitated morphine withdrawal has been shown to increase several indices of CREB function within the NAc of whole animals, including elevated c-fos expression in rats (Nye and Nestler 1996; Gracy et al. 2001) and CRE-mediated gene transcription in CRE-LacZ transgenic mice (Shaw-Lutchman et al. 2002). Correlations between the timing of up-regulated activity within cAMP pathways and signs of withdrawal raise the possibility that activation of cAMP-regulated molecules (such as CREB) may contribute to aversive or dysphoric states associated with drug withdrawal.
Increasing evidence suggests that CREB within the NAc regulates rewarding and aversive states. Stimulation of PKA and viral-mediated elevations of CREB within the NAc each decrease cocaine reward, whereas antagonism of PKA and viral-mediated overexpression of dominant-negative CREB within the NAc each increase cocaine reward (Carlezon et al. 1998; Self et al. 1998; Pliakas et al. 2001). Similarly, treatment regimens that result in increased CREB within the NAc are associated with aversive responses to cocaine (Andersen et al. 2002). Furthermore, mice with a targeted disruption of CREB α and Δ isoforms (CREBαΔ) show reductions in signs of precipitated withdrawal after chronic morphine (Maldonado et al. 1996; Walters and Blendy 2001). Finally, elevated expression of CREB in the NAc is associated with increased depressive-like behaviors that may be qualitatively similar to depressive-like behaviors and dysphoria observed during drug withdrawal (Pliakas et al. 2001). Considered together, these findings suggest that CREB activation within the NAc may oppose acute drug reward and lead to aversive motivational states that accompany drug withdrawal. There is also evidence that D1 receptors (which stimulate cAMP) play an important role in reward and reinforcement mechanisms (Shippenberg and Herz 1987; Self and Stein 1992; Gilliss et al. 2002), although the consequences of selective stimulation of D1 receptors in animals with opiate-induced up-regulation of cAMP systems within the NAc has never been addressed specifically.
Because whole animal studies demonstrate that precipitated morphine withdrawal increases expression of downstream targets of CREB in the striatum and NAc (Nye and Nestler 1996; Gracy et al. 2001) and triggers aversive abstinence symptoms, we designed the present studies to examine how precipitated opiate withdrawal affects the activation of CREB function in striatal-like tissue. To accomplish this, we used a simple cellular model: primary cultures of dissociated rat striata (which includes NAc tissue). Previous studies have shown that striatal cultures express dopamine receptors and MORs (Vaysse et al. 1990; Wong et al. 1999). Striatal neurons were exposed acutely or chronically to morphine, and withdrawal was precipitated with naloxone. Using this system, we were able to examine the effects of precipitated opiate withdrawal on both CREB activation [as measured by phospho-CREB (P-CREB)] and levels of CREB protein. We also determined the effects of stimulating cAMP systems with a D1 receptor agonist during morphine withdrawal on P-CREB and on a potential CREB-responsive target gene, c-fos. Finally, we examined potential intracellular mechanisms by which CREB could become activated during naloxone-precipitated withdrawal.
Materials and methods
Primary striatal cultures
Primary striatal cultures were prepared as previously described (Rajadhyaksha et al. 1999; Leveque et al. 2000). Briefly, striata were dissected from embryonic day 18 Sprague–Dawley rat fetuses in Hank’s balanced salt solution (HBSS) (Gibco, Gaithersburg, MD, USA) using a stereomicroscope. Striata were resuspended in 2 mL of defined medium [50% F12/DMEM and 50% DMEM (Cellgro, Herndon, VA, USA)] that contained the following supplements per liter of medium: 4 g dextrose, 1 × B27 (Invitrogen, Carlsbad, CA, USA), 10 mL of penicillin-streptomycin liquid (Sigma-Aldrich, St. Louis, MO, USA), and 7.5 mM HEPES (Sigma). Using a Pasteur pipette, the tissue was mechanically dissociated and the cells resuspended to 1 × 106 cells/mL. The cells were plated in 12-well plates (1 × 106 cells/well) for Western blot analysis or 6-well plates (2×106 cells/well) for Northern blot analysis. Plates (Becton Dickinson, Franklin Lakes, NJ, USA) were pretreated with 1 mL of sterile polyethylenimine (Sigma-Aldrich) 1 : 500 in H2O for 24 h, washed twice with sterile H2O, coated with 2.5% fetal bovine serum (Sigma-Aldrich) in phosphate-buffered saline (PBS) for 4 h, and aspirated just prior to plating. All experiments were performed with cells in culture for 7 days and were repeated at least once using cells obtained from an independent dissection.
Drug treatments
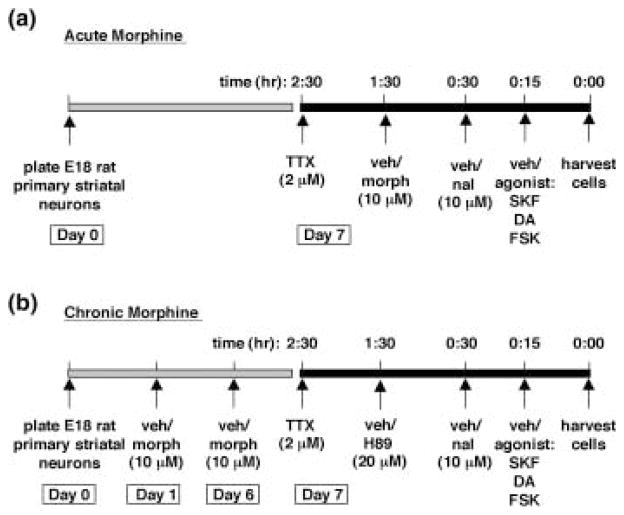
Morphine sulfate, naloxone hydrochloride dihydrate, tetrodotoxin (TTX), forskolin, dopamine hydrochloride, chloro-APB hydrobromide (SKF 82958), and H89 were purchased from Sigma-Aldrich. Morphine, naloxone, and dopamine were dissolved in PBS; TTX was dissolved in citrate buffer, pH 4.8; SKF 82958 was dissolved in H2O; and forskolin and H89 were dissolved in dimethyl sulfoxide (DMSO). All drugs were made at 100 × final concentrations, with 0.01 mL of drug (100) added per 1 mL of media. For experiments in which primary striatal cultures were treated acutely with morphine (10 μM), TTX (2 μM) was added 2.5 h prior to harvesting the cells. TTX is a sodium-channel blocker that prevents the generation of action potentials that could lead to release of endogenous neuro-transmitters into the culture medium. Either vehicle or morphine (10 μM) was added to the media for 1.5 h; naloxone (10 μM) was added for 30 min; and the agonists DA (50 μM), forskolin (2 μM), and SKF 82958 (50 μM) were added 15 min prior to harvesting the cells (Fig. 1a). For experiments in which primary striatal cultures were treated chronically with morphine, morphine (10 μM) was added to the media on day 1 and on day 6 of culture (media changed on day 6). On day 7, TTX (2 μM) was added 2.5 h prior to harvesting the cells; H89 (20 μM) was added 1.5 h; naloxone (10 μM) was added 30 min; and the agonists DA (50 μM), forskolin (2 μM), and SKF 82958 (50 μM) were added 15 min prior to harvesting the cells (unless otherwise indicated) (Fig. 1b).
Fig. 1.
Timeline for drug treatments. (a) For experiments involving acute morphine treatment, primary cultures of rat striatal neurons were harvested and plated on day 0. On day 7, TTX (2 μM) was added to the cultures 2.5 h prior to harvesting. Vehicle or morphine (10 μM) was added 1.5 h prior to harvesting, vehicle or naloxone (10 μM) was added 30 min prior to harvesting, and vehicle or the agonists SKF, dopamine (DA), or forskolin (FSK) was added 15 min prior to harvesting. (b) For experiments involving chronic morphine treatment, primary cultures of rat striatal neurons were harvested and plated on day 0. On days 1 and 6, cells were treated with vehicle or morphine (10 μM). On day 7, TTX (2 μM) was added to the cultures 2.5 h prior to harvesting. Vehicle or H89 (20 μM) was added 1.5 h prior to harvesting, vehicle or naloxone (10 μM) was added 30 min prior to harvesting, and vehicle or the agonists SKF, dopamine (DA), or forskolin (FSK) was added 15 min prior to harvesting. TTX, tetrodotoxin; morph, morphine; veh, vehicle; SKF, SKF 82958; DA, dopamine; FSK, forskolin.
Western blot analyses
Primary rat striatal cultures were harvested in NuPAGE LDS (lithium dodecyl sulfate) sample buffer (Invitrogen) and 50 mM dithiothreitol. Cell lysates were sonicated at 4°C, and then heated to 70°C for 10 min prior to gel electrophoresis. Equal volumes of each sample were loaded onto NuPAGE Novex 4–12% Bis-Tris gels (Invitrogen) for separation by gel electrophoresis. Proteins were subsequently transferred to polyvinylidene fluoride (PVDF) membrane (Perkin-Elmer Life Sciences, Boston, MA, USA). Nonspecific binding sites on the membranes were blocked for 2 h at room temperature in blocking buffer [5% non-fat dry milk in PBS and 0.1% Tween 20 (PBS-T)]. Blots were then incubated in primary antibody [1 : 4000 monoclonal anti-Ser133-phospho-CREB (P-CREB) or 1 : 4000 anti-CREB (Cell Signaling Technology, Beverly, MA, USA)] in PBS-T for 2 h at room temperature. Blots were washed 4 × 15 min in PBS-T and then incubated in secondary antibody [1 : 5000 goat anti-rabbit (or anti-mouse) horseradish peroxidase-linked IgG (Vector Laboratories, Burlingame, CA, USA)] for 2 h at room temperature. Blots were washed 4 × 15 min in PBS-T, followed by immunological detection using Chemiluminescence Reagent Plus (Perkin-Elmer Life Sciences). P-CREB or CREB antibodies were stripped from the blots by incubation with stripping buffer (62.5 mM Tris, 2% sodium dodecyl sulfate [SDS], 100 mM β-mercaptoethanol, pH 6.8) for 15 min at 50°C. Blots were subsequently re-blocked and probed with 1 : 20 000 anti-actin (Sigma). SeeBlue Plus 2 (Invitrogen) pre-stained standards were run for molecular weight estimation. P-CREB and CREB bands were detected at 43 kDa, and actin was detected at 42 kDa.
Northern blot analyses
Total RNA was prepared from primary rat striatal cultures using RNAgents Total RNA Isolation System (Promega, Madison, WI, USA). Briefly, medium was aspirated and cells were lysed in 0.5 mL denaturing solution for 10 min on ice. Sodium acetate (2 M) was added followed by a phenol/chloroform extraction. After centrifugation, total RNA was collected, precipitated with isopropanol, and resuspended in RNA loading buffer [25% formamide, 3-(N-morpholino) propanesulfonic acid (MOPS) buffer (20 mM MOPS, pH 7.0, 5 mM sodium acetate, and 1 mM EDTA), 1 M formaldehyde, 5% glycerol, 0.4 mg/mL bromophenol blue]. RNA was size-separated on a 1.2% denaturing agarose gel (1 M paraformaldehyde) in MOPS buffer and electroblotted onto a nylon membrane (GeneScreen, DuPont, Billerica, MA, USA). Blots were hybridized with a 32P-labeled c-fos RNA probe using Riboprobe in vitro Transcription Systems (Promega) and with a 32P-labeled cyclophilin DNA probe using Prime-It II (Stratagene, La Jolla, CA, USA). Cyclophilin mRNA was used as an unregulated internal reference probe to control for loading differences. Membranes were exposed to Kodak X-Omat Blue XB-1 film (Kodak, Rochester, NY, USA) at −80°C for varying times before developing.
Statistical analyses
For all experiments involving protein analysis, striatal cultures were treated in triplicate for each drug condition. For mRNA analysis by Northern blot, striatal cultures were treated at least in pairs for each drug condition. Protein immunoblots and Northern blots were analyzed using Kodak 1D Image Analysis software (Kodak). Relative optical densities were determined for each band of interest. To control for loading differences of protein or RNA, the optical density of each band was normalized with the corresponding optical density of actin or cyclophilin, respectively. To allow for comparisons of blots from independent dissections, data were normalized to the vehicle-treated controls in each experiment. One-way ANOVAs were used to analyze densitometry data. Significant effects were further analyzed using Newman-Keuls post hoc tests.
Results
Effects of morphine and precipitated morphine withdrawal on CREB
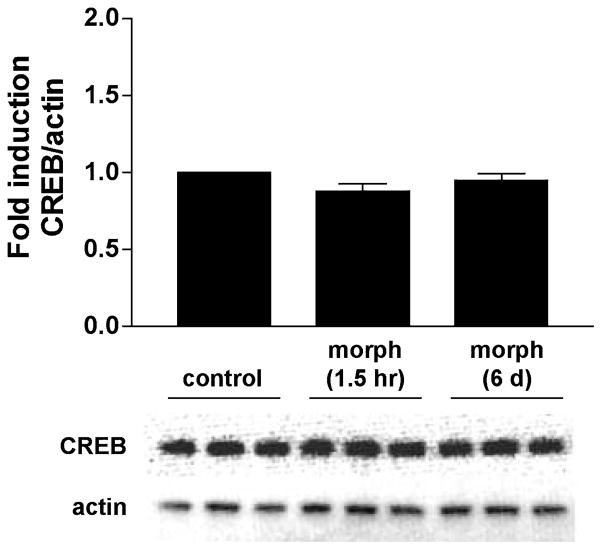
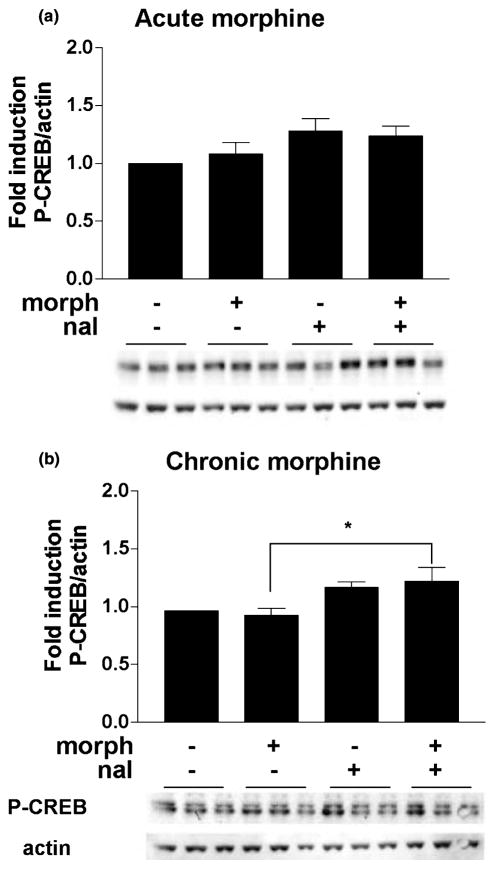
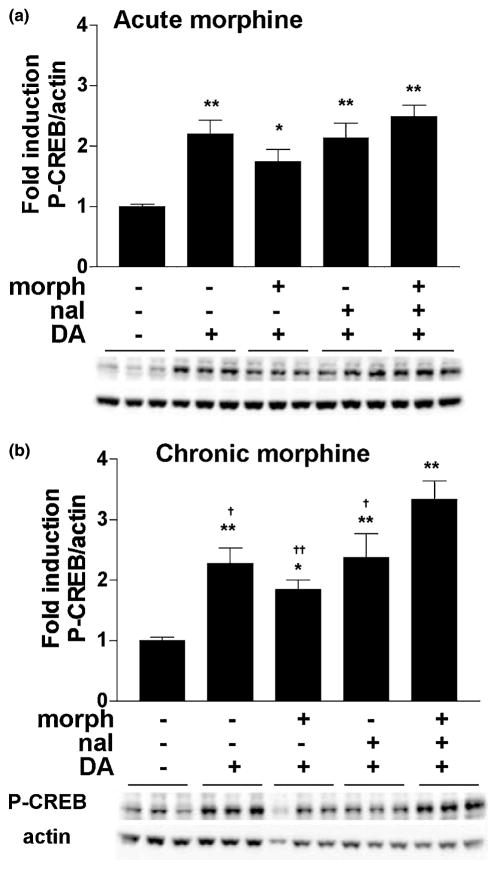
To study the effects of acute and chronic morphine on the induction of CREB phosphorylation, we first determined how morphine affected basal CREB and P-CREB protein levels. Morphine (10 μM) was added to the medium of the rat striatal cultures for either 1.5 h (acute treatment) or 6 days (chronic treatment), after which the cells were harvested and proteins size-separated and immunoblotted (see Fig. 1 for drug treatment timeline). No changes in CREB levels were observed after either acute or chronic morphine (F2,30 = 2.44, n.s.) (Fig. 2). For assessment of CREB phosphorylation, which can occur within minutes of a stimulus (Lonze and Ginty 2002), striatal cultures were treated with morphine for either 1 h or 6 days and with naloxone (10 μM) for 30 min. Using an antibody specific for CREB phosphorylated at Ser133, we found no significant effect of treatment on P-CREB levels with acute morphine (F3,41 = 2.19, n.s.) (Fig. 3a). There was a significant effect of treatment on P-CREB levels after chronic morphine (F3,55 = 3.65, p < 0.02) (Fig. 3b), but post hoc analyses revealed that this was due to a difference between cultures treated with chronic morphine and those treated with chronic morphine and naloxone, rather than differences compared with control treated cells.
Fig. 2.
CREB protein levels remain unchanged after acute or chronic morphine. Primary striatal cultures were treated with morphine (morph, 10 μM) for 1.5 h (acute) or 6 days (chronic) prior to harvesting the cells for analysis by western blot. The ratio of CREB/actin was determined for each sample and normalized to the control group ratio to yield a fold induction. Data are plotted as the mean fold induction ± SEM. N = 3 experiments with treatments in triplicate. Representative blots are shown.
Fig. 3.
CREB phosphorylation is slightly increased during naloxone-precipitated withdrawal from chronic morphine. Primary striatal cultures were treated with either vehicle or morphine (morph, 10 μM) for (a) 1.5 h (acute) or (b) 6 days (chronic) followed by 30-min incubation with vehicle or naloxone (nal, 10 μM). The ratio of P-CREB/actin was determined for each sample and normalized to the control group ratio to yield a fold induction. Data are plotted as the mean fold induction ± SEM. *p < 0.05 compared with vehicle plus chronic morphine. N = 4 experiments with treatments in triplicate (a) and five experiments with treatments in triplicate (b). Representative blots are shown.
Effects of SKF 82958, dopamine, and forskolin on CREB during precipitated withdrawal
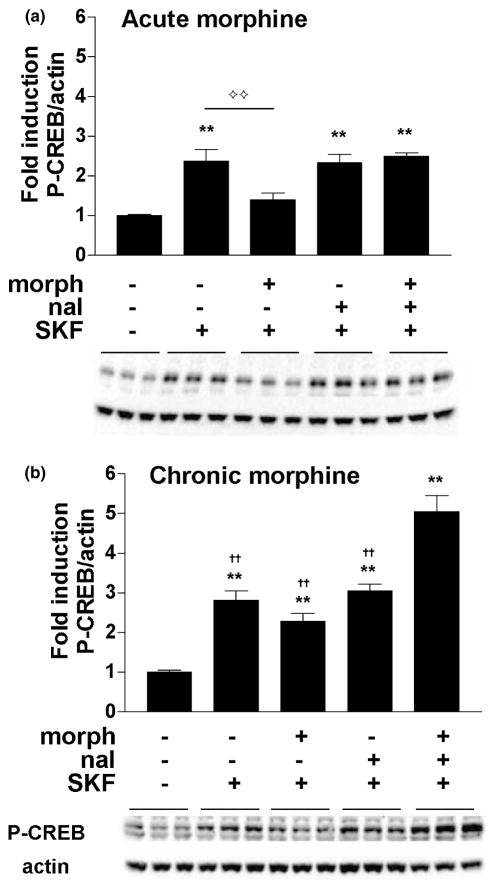
To determine if stimulation of cAMP pathways leads to abnormal CREB phosphorylation during withdrawal from chronic morphine, we treated primary striatal cultures with 10 μM morphine for 1.5 h or 6 days, precipitated withdrawal with the addition of 10 μM naloxone, and administered a D1 receptor agonist, SKF 82958 (50 μM), 15 min prior to harvesting the cells for western blot analyses (see Fig. 1 for drug treatment timeline). There was a significant effect of treatment after both acute morphine (F4,52 = 13.18, p < 0.01) (Fig. 4a) and chronic morphine (F4,55 = 35.90, p < 0.01) (Fig. 4b). In the acute studies (Fig. 4a) SKF 82958 alone increased CREB phosphorylation approximately 2.5-fold compared with vehicle-treated cells (p < 0.01, Newman–Keuls). Acute morphine blocked SKF 82958-mediated CREB phosphorylation (p < 0.01), and this inhibitory effect of morphine was reversed by naloxone. In contrast, the induction of P-CREB by SKF 82958 was not significantly reduced in the presence of chronic morphine treatment (Fig. 4b). Furthermore, naloxone-precipitated withdrawal from chronic morphine resulted in a fivefold increase in CREB phosphorylation by SKF 82958 (p < 0.01). This degree of CREB activation represents a ‘super-induction’, because the amount of CREB phosphorylation in response to SKF 82958 in the presence of naloxone and chronic morphine is significantly greater than that seen in the presence of chronic vehicle, naloxone and SKF 82958 (Fig. 4b).
Fig. 4.
CREB phosphorylation is super-induced by the D1 agonist SKF 82958 during naloxone-precipitated withdrawal from chronic morphine. Primary striatal cultures were treated with either vehicle or morphine (morph, 10 μM) for (a) 1.5 h (acute) or (b) 6 days (chronic) followed by 30 min incubation with vehicle or naloxone (nal, 10 μM) and 15 min incubation with vehicle or SKF 82958 (SKF, 50 μM). The ratio of P-CREB/actin was determined for each sample and normalized to the control group ratio to yield a fold induction. Data are plotted as the mean fold induction ± SEM. **p < 0.01 compared with control. ††p < 0.01 compared with the chronic morphine plus naloxone plus SKF 82958 treatment group. ⟡⟡p < 0.05 between acute vehicle plus SKF 82958 and acute morphine plus SKF 82958. N = 4 experiments with treatments in triplicate (a,b). Representative blots are shown.
To explore whether the super-induction of P-CREB observed with D1 receptor activation would also occur in response to an endogenous transmitter (dopamine), we treated striatal neurons with dopamine (50 μM) after either acute or chronic morphine administration. The results were qualitatively similar to those seen with SKF 82958: there was a significant effect of treatment on P-CREB induction after both acute morphine (F4,52 = 9.11, p < 0.01) (Fig. 5a) and chronic morphine (F4,53 = 9.76, p < 0.01) (Fig. 5b). In cells treated acutely (Fig. 5a), dopamine caused increases in CREB phosphorylation that were approximately 2.0-fold greater than those seen in vehicle-treated cells (p < 0.01). Acute morphine did not significantly attenuate dopamine-induced P-CREB. In cells treated chronically with morphine (Fig. 5b), dopamine caused a super-induction of P-CREB during naloxone-precipitated withdrawal (p < 0.05), similar to the effect seen with SKF 82958.
Fig. 5.
CREB phosphorylation is super-induced by dopamine during naloxone-precipitated withdrawal from chronic morphine. Primary striatal cultures were treated with either vehicle or morphine (morph, 10 μM) for (a) 1.5 h (acute) or (b) 6 days (chronic) followed by 30 min incubation with vehicle or naloxone (nal, 10 μM) and 15 min incubation with vehicle or dopamine (DA, 50 μM). The ratio of P-CREB/actin was determined for each sample and normalized to the control group ratio to yield a fold induction. Data are plotted as the mean fold induction ± SEM. * = p < 0.05; **p < 0.01 compared with control. †p < 0.05; ††p < 0.01 compared with the chronic morphine plus naloxone plus dopamine treatment group. N = 4 experiments with treatments in triplicate (a,b). Representative blots are shown.
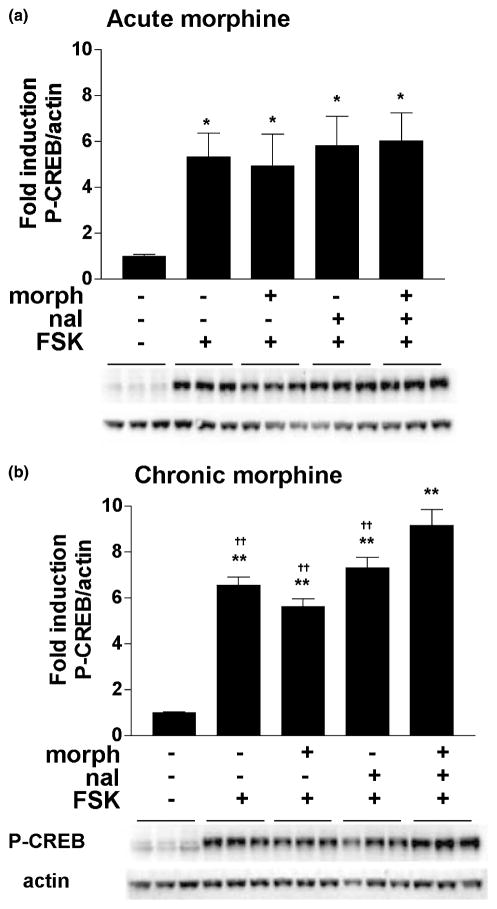
To determine if the mechanisms underlying D1 receptor-mediated super-induction of P-CREB during morphine withdrawal require activation of D1 receptors specifically or activation of adenylate cyclase in general, the direct cyclase activator forskolin (2 μM) was administered to striatal cultures after either acute or chronic morphine. There was a significant effect of treatment on P-CREB induction after both acute morphine (F4,25 = 3.45, p < 0.05) (Fig. 6a) and chronic morphine (F4,55 = 49.17, p < 0.01) (Fig. 6b). In cells treated either acutely or chronically, forskolin dramatically increased the level of CREB phosphorylation (4.5–6.5-fold compared with control) (p < 0.05), and this was not reduced by either acute or chronic morphine. During naloxone-precipitated withdrawal from chronic morphine, forskolin also led to a super-induction of CREB phosphorylation (p < 0.01) (Fig. 6b).
Fig. 6.
CREB phosphorylation is super-induced by forskolin during naloxone-precipitated withdrawal from chronic morphine. Primary striatal cultures were treated with either vehicle or morphine (morph, 10 μM) for (a) 1.5 h (acute) or (b) 6 days (chronic) followed by 30 min incubation with vehicle or naloxone (nal, 10 μM) and 15 min incubation with vehicle or forskolin (FSK, 2 μM). The ratio of P-CREB/actin was determined for each sample and normalized to the control group ratio to yield a fold induction. Data are plotted as the mean fold induction ± SEM. * = p < 0.05; **p < 0.01 compared with control. ††p < 0.01 compared with the chronic morphine plus naloxone plus forskolin treatment group. N = 2 experiments with treatments in triplicate (a) and N = 4 experiments with treatments in triplicate (b). Representative blots are shown.
Effect of PKA blockade on SKF 82958-mediated CREB phosphorylation during naloxone-precipitated withdrawal
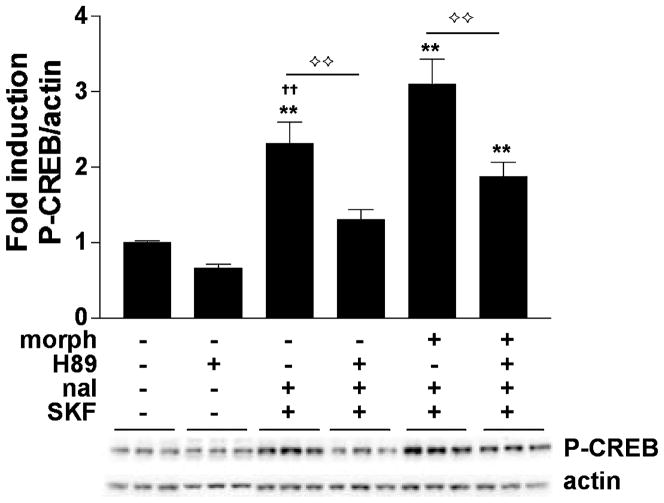
Phosphorylation of CREB at Ser133 can occur through several different signaling pathways, including via Ca2+- and cAMP-dependent kinases (e.g. PKA). To determine if PKA mediates the super-induction of P-CREB by SKF 82958 during naloxone-precipitated morphine withdrawal, the PKA inhibitor H89 was applied to the striatal cultures. Cells were treated chronically (6 days) with vehicle or morphine (10 μM) and then treated with vehicle (DMSO) or H89 (20 μM) for 1.5 h prior to harvesting the cells, vehicle or naloxone (10 μM) 30 min, and finally vehicle or SKF 82958 (50 μM) 15 min before harvesting (See Fig. 1 for drug treatment timeline). Under these conditions, there was a significant effect of treatment on P-CREB induction (F5,57 = 25.16, p < 0.01) (Fig. 7). The addition of DMSO (the vehicle for H89) did not affect the ability of SKF 82958 to super-induce P-CREB during naloxone-precipitated withdrawal from chronic morphine compared with cultures treated with chronic vehicle, naloxone, and SKF 82958 (p < 0.01). In vehicle-treated control cultures, H89 tended to cause small decreases in P-CREB that did not reach statistical significance. H89 fully blocked SKF 82958-induced P-CREB in chronic vehicle plus naloxone-treated cells (p < 0.01). Furthermore, it attenuated SKF 82958-induced P-CREB during naloxone-precipitated morphine withdrawal (p < 0.01), although P-CREB levels in these cells remained significantly higher than those seen in control cells (p < 0.01).
Fig. 7.
PKA is required for super-induction of CREB phosphorylation during naloxone-precipitated morphine withdrawal. Primary striatal cultures were treated chronically with either vehicle or morphine (morph, 10 μM) for 6 days, followed by a 1.5-h treatment with vehicle (DMSO) or H89 (20 μM), followed by a 30-min incubation with vehicle or naloxone (nal, 10 μM), and SKF 82958 (SKF, 50 μM) for 15 min. The ratio of P-CREB/actin was determined for each sample and normalized to the control group ratio to yield a fold induction. Data are plotted as the mean fold induction ± SEM. **p < 0.01 compared with control. ††p < 0.01 compared with the chronic morphine plus naloxone plus SKF 82958 treatment group. ⟡⟡p < 0.01 comparing groups designated by solid lines. N = 3 experiments with treatments in triplicate. Representative blots are shown.
Time course of SKF 82958-mediated CREB phosphorylation during naloxone-precipitated withdrawal
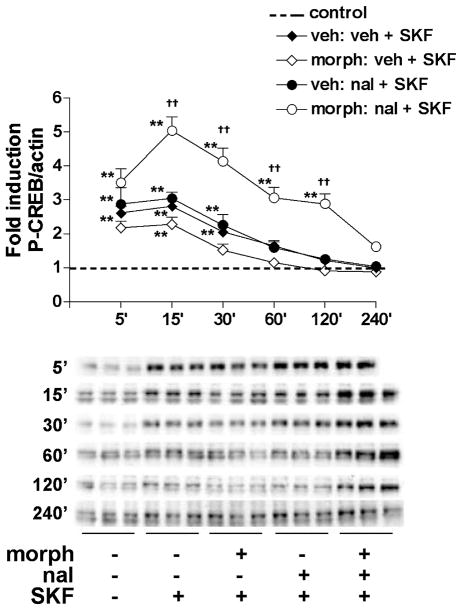
To further characterize D1 receptor-mediated P-CREB induction during withdrawal from chronic morphine, we examined the time course of CREB activation. Striatal cultures were treated chronically (6 days) with vehicle or morphine (10 μM) and then treated with naloxone (10 μM) for 30 min, followed by SKF 82958 (50 μM) for different lengths of time (5–240 min). Figure 8 shows that the effects of SKF 82958 on P-CREB were treatment- and time-dependent (treatment time interaction: F4,350 = 9.81, p < 0.01). In cultures never treated with morphine, P-CREB levels remained significantly greater than control from 5 to 30 min (p < 0.01) after SKF 82958 administration. In cells treated chronically with morphine, SKF 82958-induced P-CREB remained significantly greater than control for less time (5–15 min) (p < 0.01). In contrast, during naloxone-precipitated withdrawal from chronic morphine, SKF 82958-induced CREB phosphorylation was significantly greater than control from 5 to 120 min (p < 0.01). Interestingly, super-induction of CREB phosphorylation by SKF 82958 during naloxone-precipitated withdrawal from chronic morphine (compared with during naloxone treatment of vehicle-treated cells) was not observed until 15 min but was sustained until at least 120 min after SKF 82958 (p < 0.01). Using an antibody specific for CREB, we found that CREB levels did not change in striatal cultures treated with SKF 82958 for 5–240 min, either in the presence or absence of morphine (data not shown), suggesting that the sustained elevations in SKF 82958-induced CREB phosphorylation during naloxone-precipitated withdrawal were not due to increases in levels of CREB protein.
Fig. 8.
Time course of SKF 82958-mediated CREB phosphorylation in the presence of chronic morphine. Primary striatal cultures were treated chronically with either vehicle or morphine (morph, 10 μM) for 6 days, followed by 30-min incubation with vehicle or naloxone (nal, 10 μM), and followed by SKF 82958 (SKF, 50 μM) for different lengths of time (5–240 min). The ratio of P-CREB/actin was determined for each sample and normalized to the control group ratio to yield a fold induction. Data are plotted as the mean fold induction ± SEM. The dashed line represents the average fold induction for all vehicle-treated cultures. **p < 0.01 compared with control for each time point. ††p < 0.01 compared with the chronic vehicle plus naloxone plus SKF 82958 treatment group. N = 3–4 experiments with treatments in triplicate per time point. Representative P-CREB blots are shown.
Effect of naloxone-precipitated withdrawal on induction of c-fos by SKF 82958
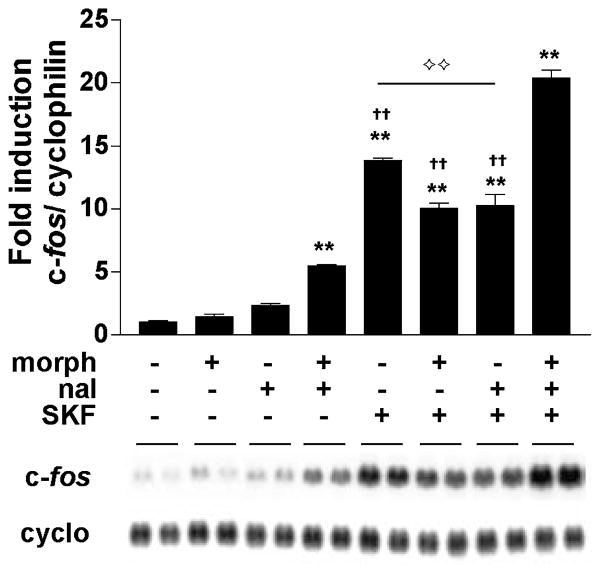
CREB phosphorylated at Ser133 can activate the transcription of genes such as c-fos. Using northern blot analysis, we measured the induction of c-fos mRNA in striatal cultures treated chronically (6 days) with vehicle or morphine (10 μM) and found a significant effect of treatment on c-fos induction (F7,10 = 203.41, p < 0.01) (Fig. 9). c-fos expression was significantly elevated after naloxone-precipitated withdrawal from chronic morphine, even in the absence of D1 receptor stimulation (p < 0.01). SKF 82958 (50 μM) strongly induced c-fos (p < 0.01), and this induction was attenuated in the presence of chronic morphine (p < 0.01). During naloxone-precipitated withdrawal, SKF 82958-mediated c-fos expression was super-induced relative to all other treatment groups administered SKF 82958 (p < 0.01).
Fig. 9.
C-fos mRNA is super-induced by the D1 agonist SKF 82958 during naloxone-precipitated withdrawal from chronic morphine. Primary striatal cultures were treated chronically (6 days) with either vehicle or morphine (morph, 10 μM) followed by 30 min incubation with vehicle or naloxone (nal, 10 μM) and 30 min incubation with vehicle or SKF 82958 (SKF, 50 μM). The ratio of c-fos/cyclophilin was determined for each sample and normalized to the control group ratio to yield a fold induction. Data are plotted as the mean fold induction ± SEM. **p < 0.01 compared with control. ††p < 0.01 compared with the chronic morphine plus naloxone plus SKF 82958 treatment group. ⟡⟡p < 0.05 comparing chronic vehicle plus SKF82958 to groups under solid line. N = 2 experiments with treatments in duplicate. Representative blots are shown.
Discussion
Precipitated withdrawal from chronic morphine affects CREB activation in cultures of dissociated rat striatal cells. We found that P-CREB activation was slightly enhanced during naloxone-precipitated withdrawal from chronic morphine. However, P-CREB activation was profoundly increased by stimulation of cAMP pathways during morphine withdrawal. Dopamine, the D1 receptor agonist SKF 82958, and the adenylate cyclase activator forskolin each increased P-CREB relative to vehicle-treated control cells. In cells undergoing naloxone-precipitated morphine withdrawal, induction of P-CREB by these dopamine-related agonists was further increased, or ‘super-induced’, and this was dependent upon activation of PKA. Furthermore, D1 receptor-mediated c-fos mRNA expression was also super-induced during morphine withdrawal, demonstrating that the enhanced activation of CREB had functional consequences on a known CREB-regulated target gene (Lonze and Ginty 2002).
This is the first demonstration of up-regulated CREB phosphorylation in striatal cells in response to morphine withdrawal. Our findings are consistent with earlier in vitro and in vivo studies indicating that cAMP systems are increased in striatum during opiate withdrawal. For example, profound increases in D1 receptor-stimulated cAMP production were observed in dissociated rat striatal cells undergoing morphine withdrawal (Van Vliet et al. 1991). Also, increases in forskolin-stimulated adenylate cyclase and PKA activity were reported in the NAc after chronic morphine (Terwilliger et al. 1991). Moreover, increases in cAMP response element (CRE)-dependent reporter gene expression were observed in numerous regions of mouse brain during naltrexone-precipitated morphine withdrawal (Shaw-Lutchman et al. 2002). Finally, c-fos expression is enhanced in striatal regions of whole animals during precipitated opiate withdrawal (Nye and Nestler 1996; Gracy et al. 2001). Our data show that, as might be expected, morphine withdrawal-induced up-regulation of cAMP systems leads to enhanced activation of CREB, as indicated by elevated P-CREB.
The use of dissociated striatal cultures as a model system
Morphine exerts a variety of effects on mesolimbic neuro-transmission. For example, acute morphine administration leads to increased dopamine release in the NAc via activation of MORs located on inhibitory GABAergic interneurons within the VTA (Johnson and North 1992). During morphine withdrawal, dopamine release is decreased due to increased inhibition of dopaminergic neurons in the VTA (Rossetti et al. 1992; Diana et al. 1995; Bonci and Williams 1997). Concurrently, glutamate and acetylcholine release in the NAc is increased (Tjon Tien Ril et al. 1993; Rada et al. 1996). These changes in neurotransmitter function — due to actions of morphine on independent populations of MORs located within the midbrain and within the NAc — presumably affect intracellular signaling in the NAc. The use of dissociated primary striatal cultures allowed us to distinguish morphine-mediated molecular adaptations at the level of striatal circuits from adaptations in intracellular signaling specifically at the level of individual striatal neurons. Additionally, tetrodotoxin was added to the cultures hours prior to harvesting the cells in order to block endogenously generated action potentials that might lead to the release of neurotransmitters into the culture medium. Together, these conditions were designed to minimize endogenous activation of G protein-coupled receptors, thereby maximizing experimental control over the factors that contribute to CREB activation.
Regulation of CREB levels by morphine
CREB proteins are constitutively expressed, and can interact with CRE elements on the promoter regions of select genes. CREB becomes transcriptionally active when the Ser133 residue in the kinase inducible domain (KID) is phosphorylated in response to various stimuli, including adenylate cyclase-mediated increases in cAMP (Lonze and Ginty 2002). CREB function can also be increased if the levels of CREB protein are up-regulated, thereby providing more substrate for phosphorylation and increasing the likelihood of interaction at CRE sites. It has been shown previously that chronic treatment with morphine increases CREB immunoreactivity in the locus coeruleus (Widnell et al. 1994), decreases CREB immunoreactivity in the NAc, and has no effect on CREB immunoreactivity in the striatum (Widnell et al. 1996). In our studies, neither acute (1.5 h) nor chronic (6 days) morphine administration had any effect on CREB protein levels assessed by western blot in dissociated striatal cultures. Furthermore, CREB levels were also not changed by acute (30 min) naloxone treatment, in the presence or absence of morphine (data not shown). Finally, CREB levels were unaltered in the cultures even after incubation with naloxone and SKF 82958 for 4 h (data not shown). The lack of an effect of chronic morphine on CREB protein could be a result of the fact that the striatal cultures contain neurons from both the NAc and the striatum proper. Any effect specific to the NAc may have been diluted out by the presence of these different cell types. Another potentially important difference is that the rats in the previous studies were treated with sustained-release subcutaneous morphine pellets, and thus were not in morphine withdrawal at the time when the these brain regions were obtained.
Regulation of P-CREB by morphine
P-CREB activation was unaltered in the striatal cultures after acute (1.5 h) morphine. There was a slight trend for naloxone itself to increase basal P-CREB levels. One possible explanation for these results is that they may indicate blockade of low-level endogenous opioid activity within the primary cultures. There was a significant increase in P-CREB levels in cells treated with naloxone in the presence of chronic morphine compared with cultures treated with vehicle in the presence of chronic morphine. This is consistent with the slight increase in cAMP levels observed in neuroblastoma × glioma hybrid cells grown in the presence of morphine and treated acutely with naloxone (Sharma et al. 1975a). The inability to detect a super-induction (e.g. an increase relative to vehicle plus naloxone) of P-CREB after naloxone-precipitated withdrawal was most likely due to a lack of endogenous excitatory G protein-coupled receptor inputs within our cultures of dissociated striatal cells. In order to ascertain whether acute or chronic morphine could modulate receptor-induced CREB activation, we briefly applied selective activators of various components of cAMP pathways to the dissociated striatal cultures. Non-selective activation of dopamine receptors with dopamine robustly activated P-CREB. This effect was mimicked by SKF 82958, suggesting a specific role of D1 receptors in the activation of P-CREB. Acute administration of morphine inhibited SKF 82958-mediated P-CREB induction, and had a qualitatively similar effect on dopamine-mediated P-CREB induction. These inhibitory effects of morphine were reversed by naloxone. These findings are consistent with the notion that acute morphine inhibits cAMP pathways, whereas chronic morphine causes a late positive regulation of cAMP systems (Sharma et al. 1975a). Because dopamine is a general agonist at D1 and D2 dopamine receptors, it is possible that it has stimulatory actions on different cohorts of cells than SKF 82958. The colocalization of MORs and dopamine receptors may be such that morphine is more efficacious at inhibiting SKF 82958-induced P-CREB than dopamine-induced P-CREB. Indeed, MORs are colocalized with D1 receptors on dynorphin-containing striatonigral neurons (Georges et al. 1999). Interestingly, acute morphine failed to inhibit forskolin-induced P-CREB. One explanation for this finding could be that the induction of P-CREB by forskolin was so robust (five- to sevenfold) that the concentration of morphine (10 μM) was insufficient to cause a detectable reduction. This concentration of morphine has been shown to maximally inhibit basal adenylate cyclase activity and cAMP formation in a neuroblastoma × glioma cell line (Sharma et al. 1975b). Another possibility is that, as a direct and potent activator of the catalytic subunit of adenylate cyclase, forskolin stimulates cAMP formation in all cells, independent of their Gαi/Gαs-coupled receptor profiles. As such, any effect of acute MOR activation on forskolin-stimulated P-CREB may have been masked by generalized adenylate cyclase activation.
The induction of P-CREB by SKF 82958 and dopamine was less sensitive to attenuation after chronic administration of morphine than after acute morphine treatment. Although not addressed explicitly in the present study, the underlying assumption is that components of the cAMP signaling pathway were up-regulated to compensate for the persistent MOR inhibition of adenylate cyclase in the presence of chronic morphine, as has been demonstrated previously (Nestler and Aghajanian 1997; Watts 2002). P-CREB levels were super-induced when SKF 82958 and dopamine were added to cultures treated chronically with morphine and then administered naloxone to precipitate withdrawal. These data suggest that, at minimum, D1 receptor-mediated pathways are augmented with chronic morphine. Although not tested in the present studies, it is possible that activation of P-CREB by all Gαs-coupled receptors in striatal neurons would be enhanced. This is supported by previous research showing that both D1 receptor- and β-adrenoceptor-stimulated cAMP production were increased with chronic morphine treatment of dissociated striatal cells (Van Vliet et al. 1991). Furthermore, we show that P-CREB levels were super-induced when forskolin was added to cultures undergoing naloxone-precipitated morphine withdrawal. This suggests that at least part of the mechanism underlying CREB super-activation occurs at the level of, or downstream from, adenylate cyclase.
Potential mechanisms for super-induction of P-CREB during withdrawal
A straightforward explanation for enhanced CREB activation during morphine withdrawal is that D1 receptor activation stimulates PKA, which then phosphorylates CREB. Indeed, inhibition of PKA activity with H89 attenuates the induction of P-CREB by SKF 82958 during morphine withdrawal. However, it is not known which components of signal transduction pathways downstream of PKA activation are involved in — or are even necessary for — CREB super-induction. Ca2+ influx via ionotropic glutamate receptors and voltage sensitive calcium channels is a major factor in CREB activation in vivo (Kornhauser et al. 2002). Future studies in whole animals will address whether Ca2+ and other intracellular factors (e.g. PKA) regulate P-CREB during morphine withdrawal. Interestingly, we found that P-CREB levels were not super-induced during naloxone-precipitated morphine withdrawal after 5 min of SKF 82958 treatment, but were by 15 min. However, SKF 82958-induced P-CREB was significantly greater than vehicle-treated cultures at both 5 and 15 min. This time lag to P-CREB super-induction suggests that PKA may activate CREB while concurrently inducing a distinct parallel pathway that further activates CREB or prevents de-phosphorylation of CREB. As one example, it has been shown that dopamine-dependent activation of CREB requires NMDA receptors and Ca2+ (Konradi et al. 1996). PKA can phosphorylate glutamate-gated ionotropic receptors, which has been reported to potentiate their responses (Greengard et al. 1991). This cascade of intracellular events may facilitate the influx of Ca2+ and activation of calcium/calmodulin-dependent protein kinases, which can phosphorylate CREB at Ser133 (Lonze and Ginty 2002). Another possibility is that PKA can activate DARPP-32, functionally reducing the de-phosphorylation of CREB by protein phosphatases (Greengard et al. 1999).
Another consequence of the prolonged, D1 receptor-mediated P-CREB expression during morphine withdrawal may be activation of CREB-regulated target genes. Along similar lines, chronic cocaine treatment has been shown to lead to a long-lasting increase in Fos-mediated transcriptional activity in the NAc (Hope et al. 1994). When phosphorylated at Ser133, CREB regulates the transcription of many genes (for review, see Lonze and Ginty 2002). The immediate early gene c-fos contains several CREs in its promoter (Sassone-Corsi et al. 1988) and can be induced by dopamine in a CREB-dependent manner (Konradi et al. 1994). In dissociated cultures of striatal cells undergoing naloxone-precipitated morphine withdrawal, we observed a super-induction of SKF 82958-mediated c-fos expression. In the presence of chronic morphine, c-fos was also significantly induced by naloxone itself. It is possible that the slight elevation in CREB phosphorylation during naloxone-precipitated morphine withdrawal in the absence of D1 receptor activation was sufficient to induce c-fos expression. These data are consistent with the notion that late positive regulation of cAMP-mediated pathways by chronic morphine may have functional consequences in the NAc, including altered gene expression.
Conclusions
We have demonstrated in dissociated cultures of rat striatal cells that D1 receptor-stimulation during morphine withdrawal leads not only to heightened activation of the transcription factor CREB, but also to activation of a CREB-responsive gene, c-fos. This raises the possibility that CREB and c-fos-regulated target genes, such as dynorphin (Cole et al. 1995), may be up-regulated in the striatum and NAc during morphine withdrawal. These downstream effects may be critical factors in mediating withdrawal-associated aversive states. The molecular adaptations described in this study occurred by direct actions of morphine on striatal neurons, without the influence of afferent input, lending support to the notion that morphine has functional impact in the NAc as well as on dopaminergic neurons of the VTA (Koob et al. 1992; De Vries and Shippenberg 2002). Future studies are underway to evaluate whether D1 receptor-mediated signaling is also enhanced during morphine withdrawal in vivo and to determine what effect D1 receptor activation has on morphine withdrawal-associated behaviors. Regardless, these data add further support to the hypothesized connections between CREB function within the NAc-related tissues and aversive or dysphoric states (Carlezon et al. 1998; Pliakas et al. 2001; Walters and Blendy 2001; Barrot et al. 2002).
Acknowledgments
Funded by grants DA12736 (to WC) and DA07134 (to CK) from the National Institute on Drug Abuse, and an unrestricted grant from Johnson & Johnson (to WC). We thank Brian Gilliss for superb technical assistance.
Abbreviations used
- CREB
cAMP response element binding protein
- DMSO
dimethyl sulfoxide
- HBSS
Hank’s balanced salt solution
- LDS
lithium dodecyl sulfate
- MOR
μ opiate receptor
- NAc
nucleus accumbens
- PBS
phosphate-buffered saline
- P-CREB
phosphorylated CREB
- PKA
cAMP dependent protein kinase
- PVDF
polyvinylidene fluoride
- SDS
sodium dodecyl sulfate
- TTX
tetrodotoxin
- VTA
ventral tegmental area
References
- Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA., Jr Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci. 2002;5:13–14. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, Zachariou V, Nestler EJ. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci USA. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A, Williams JT. Increased probability of GABA release during withdrawal from morphine. J Neurosci. 1997;17:796–803. doi: 10.1523/JNEUROSCI.17-02-00796.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Neural substrates of opiate reinforcement. Prog Neuro-Psychopharmacol Biol Psychiatry. 1983;7:569–575. doi: 10.1016/0278-5846(83)90027-1. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Childers SR. Opioid receptor-coupled second messenger systems. Life Sci. 1991;48:1991–2003. doi: 10.1016/0024-3205(91)90154-4. [DOI] [PubMed] [Google Scholar]
- Cole RL, Konradi C, Douglass J, Hyman SE. Neuronal adaptation to amphetamine and dopamine: molecular mechanisms of prodynorphin gene regulation in rat striatum. Neuron. 1995;14:813–823. doi: 10.1016/0896-6273(95)90225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, Shippenberg TS. Neural systems underlying opiate addiction. J Neurosci. 2002;22:3321–3325. doi: 10.1523/JNEUROSCI.22-09-03321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Pistis M, Muntoni A, Gessa G. Profound decrease of mesolimbic dopaminergic neuronal activity in morphine withdrawn rats. J Pharmacol Exp Ther. 1995;272:781–785. [PubMed] [Google Scholar]
- Duman RS, Tallman JF, Nestler EJ. Acute and chronic opiate-regulation of adenylate cyclase in brain: specific effects in locus coeruleus. J Pharmacol Exp Ther. 1988;246:1033–1039. [PubMed] [Google Scholar]
- Georges F, Stinus L, Bloch B, Le Moine C. Chronic morphine exposure and spontaneous withdrawal are associated with modifications of dopamine receptor and neuropeptide gene expression in the rat striatum. Eur J Neurosci. 1999;11:481–490. doi: 10.1046/j.1460-9568.1999.00462.x. [DOI] [PubMed] [Google Scholar]
- Gilliss B, Malanga CJ, Pieper JO, Carlezon WA., Jr Cocaine and SKF-82958 potentiate brain stimulation reward in Swiss-Webster mice. Psychopharmacology (Berl) 2002;163:238–248. doi: 10.1007/s00213-002-1153-8. [DOI] [PubMed] [Google Scholar]
- Gracy KN, Dankiewicz LA, Koob GF. Opiate withdrawal-induced fos immunoreactivity in the rat extended amygdala parallels the development of conditioned place aversion. Neuro-psychopharmacology. 2001;24:152–160. doi: 10.1016/S0893-133X(00)00186-X. [DOI] [PubMed] [Google Scholar]
- Greengard P, Jen J, Nairn AC, Stevens CF. Enhancement of the glutamate response by cAMP-dependent protein kinase in hippocampal neurons. Science. 1991;253:1135–1138. doi: 10.1126/science.1716001. [DOI] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Guitart X, Thompson MA, Mirante CK, Greenberg ME, Nestler EJ. Regulation of cyclic AMP response element-binding protein (CREB) phosphorylation by acute and chronic morphine in the rat locus coeruleus. J Neurochem. 1992;58:1168–1171. doi: 10.1111/j.1471-4159.1992.tb09377.x. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Involvement of D2 dopamine receptors in the nucleus accumbens in the opiate withdrawal syndrome. Nature. 1994;371:155–157. doi: 10.1038/371155a0. [DOI] [PubMed] [Google Scholar]
- Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Leveque JC, Hyman SE. Amphetamine and dopamine-induced immediate early gene expression in striatal neurons depends on postsynaptic NMDA receptors and calcium. J Neurosci. 1996;16:4231–4239. doi: 10.1523/JNEUROSCI.16-13-04231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Cole RL, Heckers S, Hyman SE. Amphetamine regulates gene expression in rat striatum via transcription factor CREB. J Neurosci. 1994;14:5623–5634. doi: 10.1523/JNEUROSCI.14-09-05623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Stinus L, Le Moal M, Bloom FE. Opponent process theory of motivation: neurobiological evidence from studies of opiate dependence. Neurosci Biobehav Rev. 1989a;13:135–140. doi: 10.1016/s0149-7634(89)80022-3. [DOI] [PubMed] [Google Scholar]
- Koob GF, Wall TL, Bloom FE. Nucleus accumbens as a substrate for the aversive stimulus effects of opiate withdrawal. Psychopharmacologia. 1989b;98:530–534. doi: 10.1007/BF00441954. [DOI] [PubMed] [Google Scholar]
- Koob GF, Maldonado R, Stinus L. Neural substrates of opiate withdrawal. Trends Neurosci. 1992;15:186–191. doi: 10.1016/0166-2236(92)90171-4. [DOI] [PubMed] [Google Scholar]
- Kornhauser JM, Cowan CW, Shaywitz AJ, Dolmetsch RE, Griffith EC, Hu LS, Haddad C, Xia Z, Greenberg ME. CREB transcriptional activity in neurons is regulated by multiple, calcium-specific phosphorylation events. Neuron. 2002;34:221–233. doi: 10.1016/s0896-6273(02)00655-4. [DOI] [PubMed] [Google Scholar]
- Leveque JC, Macias W, Rajadhyaksha A, Carlson RR, Barczak A, Kang S, Li XM, Coyle JT, Huganir RL, Heckers S, Konradi C. Intracellular modulation of NMDA receptor function by antipsychotic drugs. J Neurosci. 2000;20:4011–4020. doi: 10.1523/JNEUROSCI.20-11-04011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Stinus L, Gold LH, Koob GF. Role of different brain structures in the expression of the physical morphine withdrawal syndrome. J Pharmacol Exp Ther. 1992;261:669–677. [PubMed] [Google Scholar]
- Maldonado R, Blendy JA, Tzavara E, Gass P, Roques BP, Hanoune J, Schutz G. Reduction of morphine abstinence in mice with a mutation in the gene encoding CREB. Science. 1996;273:657–659. doi: 10.1126/science.273.5275.657. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Akil H, Watson SJ. Immunohistochemical localization of the cloned mu opioid receptor in the rat CNS. J Chem Neuroanat. 1995;8:283–305. doi: 10.1016/0891-0618(95)00055-c. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. J Neurosci. 1992;12:2439–2450. doi: 10.1523/JNEUROSCI.12-07-02439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Tallman JF. Chronic morphine treatment increases cyclic AMP-dependent protein kinase activity in the rat locus coeruleus. Mol Pharmacol. 1988;33:127–132. [PubMed] [Google Scholar]
- Nye HE, Nestler EJ. Induction of chronic Fos-related antigens in rat brain by chronic morphine administration. Mol Pharmacol. 1996;49:636–645. [PubMed] [Google Scholar]
- Olds ME. Reinforcing effects of morphine in the nucleus accumbens. Brain Res. 1982;237:429–440. doi: 10.1016/0006-8993(82)90454-1. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada PV, Mark GP, Taylor KM, Hoebel BG. Morphine and naloxone, i. p or locally, affect extracellular acetylcholine in the accumbens and prefrontal cortex. Pharmacol Biochem Behav. 1996;53:809–816. doi: 10.1016/0091-3057(95)02078-0. [DOI] [PubMed] [Google Scholar]
- Rajadhyaksha A, Barczak A, Macias W, Leveque JC, Lewis SE, Konradi C. L-Type Ca(2+) channels are essential for glutamate-mediated CREB phosphorylation and c-fos gene expression in striatal neurons. J Neurosci. 1999;19:6348–6359. doi: 10.1523/JNEUROSCI.19-15-06348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen K, Beitner-Johnson DB, Krystal JH, Aghajanian GK, Nestler EJ. Opiate withdrawal and the rat locus coeruleus: behavioral, electrophysiological, and biochemical correlates. J Neurosci. 1990;10:2308–2317. doi: 10.1523/JNEUROSCI.10-07-02308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol. 1992;221:227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P, Visvader J, Ferland L, Mellon PL, Verma IM. Induction of proto-oncogene fos transcription through the adenylate cyclase pathway: characterization of a cAMP-responsive element. Genes Dev. 1988;2:1529–1538. doi: 10.1101/gad.2.12a.1529. [DOI] [PubMed] [Google Scholar]
- Self DW, Stein L. The D1 agonists SKF 82958 and SKF 77434 are self-administered by rats. Brain Res. 1992;582:349–352. doi: 10.1016/0006-8993(92)90155-3. [DOI] [PubMed] [Google Scholar]
- Self DW, McClenahan AW, Beitner-Johnson D, Terwilliger RZ, Nestler EJ. Biochemical adaptations in the mesolimbic dopamine system in response to heroin self-administration. Synapse. 1995;21:312–318. doi: 10.1002/syn.890210405. [DOI] [PubMed] [Google Scholar]
- Self DW, Genova LM, Hope BT, Barnhart WJ, Spencer JJ, Nestler EJ. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. J Neurosci. 1998;18:1848–1859. doi: 10.1523/JNEUROSCI.18-05-01848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Klee WA, Nirenberg M. Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. Proc Natl Acad Sci USA. 1975a;72:3092–3096. doi: 10.1073/pnas.72.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Nirenberg M, Klee WA. Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci USA. 1975b;72:590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw-Lutchman TZ, Barrot M, Wallace T, Gilden L, Zachariou V, Impey S, Duman RS, Storm D, Nestler EJ. Regional and cellular mapping of cAMP response element-mediated transcription during naltrexone-precipitated morphine withdrawal. J Neurosci. 2002;22:3663–3672. doi: 10.1523/JNEUROSCI.22-09-03663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A. Place preference conditioning reveals the involvement of D1-dopamine receptors in the motivational properties of mu- and kappa-opioid agonists. Brain Res. 1987;436:169–172. doi: 10.1016/0006-8993(87)91571-x. [DOI] [PubMed] [Google Scholar]
- Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 1991;548:100–110. doi: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- Tjon Tien Ril HK, De Vries TJ, Wardeh G, Hogenboom F, Mulder AH, Schoffelmeer AN. Long-lasting reciprocal changes in striatal dopamine and acetylcholine release upon morphine withdrawal. Eur J Pharmacol. 1993;235:321–322. doi: 10.1016/0014-2999(93)90155-b. [DOI] [PubMed] [Google Scholar]
- Van Vliet BJ, De Vries TJ, Wardeh G, Mulder AH, Schoffelmeer AN. mu-Opioid receptor-regulated adenylate cyclase activity in primary cultures of rat striatal neurons upon chronic morphine exposure. Eur J Pharmacol. 1991;208:105–111. doi: 10.1016/0922-4106(91)90060-u. [DOI] [PubMed] [Google Scholar]
- Vaysse PJ, Zukin RS, Fields KL, Kessler JA. Characterization of opioid receptors in cultured neurons. J Neurochem. 1990;55:624–631. doi: 10.1111/j.1471-4159.1990.tb04179.x. [DOI] [PubMed] [Google Scholar]
- Walters CL, Blendy JA. Different requirements for cAMP response element binding protein in positive and negative reinforcing properties of drugs of abuse. J Neurosci. 2001;21:9438–9444. doi: 10.1523/JNEUROSCI.21-23-09438.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts VJ. Molecular mechanisms for heterologous sensitization of adenylate cyclase. J Pharmacol Exp Ther. 2002;302:1–7. doi: 10.1124/jpet.302.1.1. [DOI] [PubMed] [Google Scholar]
- Widnell KL, Russell DS, Nestler EJ. Regulation of expression of cAMP response element-binding protein in the locus coeruleus in vivo and in a locus coeruleus-like cell line in vitro. Proc Natl Acad Sci USA. 1994;91:10947–10951. doi: 10.1073/pnas.91.23.10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widnell KL, Self DW, Lane SB, Russell DS, Vaidya VA, Miserendino MJ, Rubin CS, Duman RS, Nestler EJ. Regulation of CREB expression: in vivo evidence for a functional role in morphine action in the nucleus accumbens. J Pharmacol Exp Ther. 1996;276:306–315. [PubMed] [Google Scholar]
- Wise RA. Opiate reward: sites and substrates. Neurosci Bio-behav Rev. 1989;13:129–133. doi: 10.1016/s0149-7634(89)80021-1. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Wong AC, Shetreat ME, Clarke JO, Rayport S. D1- and D2-like dopamine receptors are co-localized on the presynaptic varicosities of striatal and nucleus accumbens neurons in vitro. Neuroscience. 1999;89:221–233. doi: 10.1016/s0306-4522(98)00284-x. [DOI] [PubMed] [Google Scholar]