Significance
PDGF-CC plays critical roles in many biological processes, such as development, tumor growth, and angiogenesis. However, its role in blood vessel survival/regression and the underlying mechanisms remain unknown. Here, using different loss- and gain-of-function assays and multiple model systems, we show that PDGF-CC is a critical vascular protective factor required to maintain blood vessel survival. Mechanistically, we found that heme oxygenase-1 (HMOX1) activity is crucial for the vascular protective/survival effect of PDGF-CC. Given the general involvement of vascular degeneration in most degenerative diseases, PDGF-CC may be of therapeutic use in treating different types of degenerative disorders. Our findings point out that the PDGF-CC level should be monitored closely in various pathological conditions to ensure normal blood vessel survival.
Keywords: PDGF-CC, vasoprotective effect, blood vessel regression, HMOX1, retinal degeneration
Abstract
Blood vessel degeneration is critically involved in nearly all types of degenerative diseases. Therefore strategies to enhance blood vessel protection and survival are highly needed. In this study, using different animal models and cultured cells, we show that PDGF-CC is a potent vascular protective and survival factor. PDGF-CC deficiency by genetic deletion exacerbated blood vessel regression/degeneration in various animal models. Importantly, treatment with PDGF-CC protein not only increased the survival of retinal blood vessels in a model of oxygen-induced blood vessel regression but also markedly rescued retinal and blood vessel degeneration in a disease model of retinitis pigmentosa. Mechanistically, we revealed that heme oxygenase-1 (HMOX1) activity is critically required for the vascular protective/survival effect of PDGF-CC, because blockade of HMOX1 completely abolished the protective effect of PDGF-CC in vitro and in vivo. We further found that both PDGF receptors, PDGFR-β and PDGFR-α, are required for the vasoprotective effect of PDGF-CC. Thus our data show that PDGF-CC plays a pivotal role in maintaining blood vessel survival and may be of therapeutic value in treating various types of degenerative diseases.
Blood vessel degeneration and regression are vital pathologies in numerous human diseases and are associated with nearly all types of degenerative diseases, such as retinitis pigmentosa (RP), diabetic retinopathy, age-related macular degeneration, Alzheimer’s disease, Parkinson's disease, and amyotrophic lateral sclerosis (1–3). RP is a retinal degenerative disorder in which blood vessel degeneration contributes significantly to retinal atrophy, ultimately leading to loss of vision. In addition, recent studies have shown that prolonged treatment with antiangiogenic drugs may cause tissue degeneration (4–6). Given the increasing incidence of many degenerative diseases in an aging population and the rapidly growing clinical use of antiangiogenic drugs, there is an urgent need for strategies promoting blood vessel survival and protection. Because the pathological process of vascular degeneration involves complex mechanisms (7), treating such diseases remains challenging. Identifying effective vascular protective factors and the underlying mechanisms therefore is highly warranted.
The PDGF family plays important roles in the vascular system (8–11). PDGF-CC was the third of the four PDGF family members discovered (12, 13), long after the finding of PDGF-AA and PDGF-BB. PDGF-CC is highly expressed in the vascular system (8, 14) and is produced as a secreted homodimer that binds to and activates the PDGF receptors PDGFR-α and PDGFR-β (12, 15). PDGF-CC is a critical survival/protective factor for neuronal cells (8, 9, 16) and macrophages (17) and has been shown to be a potent angiogenic factor (10, 15, 18–20). However, whether PDGF-CC plays a role in the survival/regression of blood vessels remains unknown thus far.
In this study we used different animal models and cultured cells and investigated the potential effect of PDGF-CC on blood vessel survival. We found that PDGF-CC is a potent vasoprotective factor that rescues blood vessels from degeneration/regression under developmental and pathological conditions. Mechanistically, we show that heme oxygenase-1 (HMOX1), a potent antioxidative and anti-inflammatory factor, is required for the vasoprotective effect of PDGF-CC. Our data indicate that PDGF-CC may be of therapeutic use in treating different types of degenerative diseases in which blood vessel survival is impaired.
Results
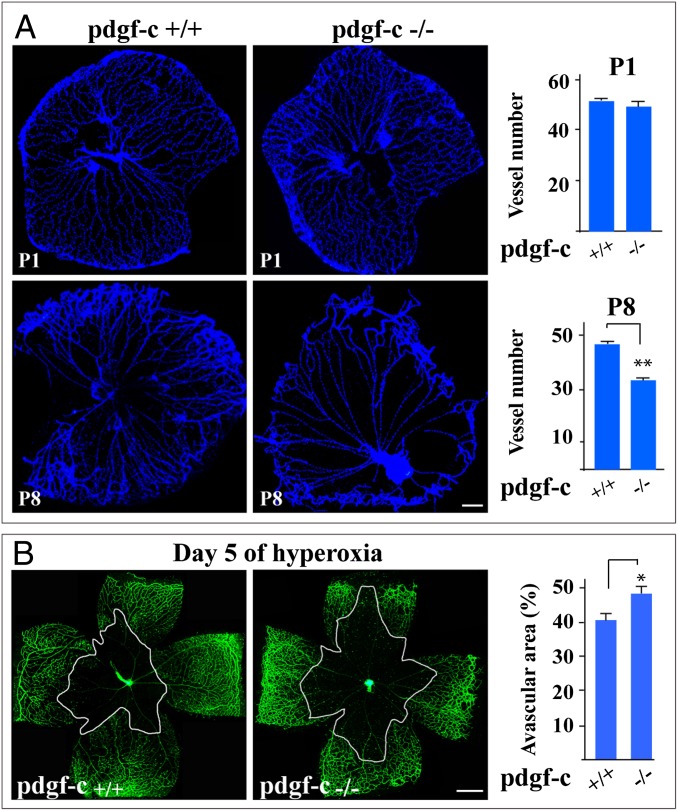
pdgf-c Deficiency Increases Blood Vessel Regression.
Although PDGF-CC is highly expressed in the vascular system (8, 19), its role in blood vessel survival is unclear. We therefore used pdgf-c–deficient mice (Fig. S1A) and various model systems to investigate this role. First, a model of hyaloid blood vessel regression showed lower hyaloid vessel densities at postnatal day 3 (P3) and P8 in pdgf-c–deficient mice (n = 8, P < 0.01, Fig. 1A; n = 8, P < 0.05, Fig. S1B), demonstrating that pdgf-c deficiency increases hyaloid vessel regression. It is noteworthy that no difference in hyaloid vessel density was observed at P1 (n = 8, P > 0.05) (Fig. 1A), suggesting the unlikelihood of a developmental defect. Secondly, a model of oxygen-induced retinal blood vessel regression (OIR) showed that pdgf-c deficiency led to larger avascular areas 5 d after hyperoxia (n = 8, P < 0.05) (Fig. 1B), demonstrating that pdgf-c deficiency accelerates oxygen-induced retinal blood vessel regression. Again, no difference in retinal blood vessel density was observed in neonatal pdgf-c–deficient mice (D n = 8, P > 0.05) (Fig. S1C), suggesting the unlikelihood of a developmental defect.
Fig. 1.
Increased blood vessel regression in pdgf-c–deficient mice. (A, Upper) At P1, no difference in hyaloid vessel density was observed between wild-type and pdgf-c–deficient mice. (Lower) At P8, the density of hyaloid vessels in the pdgf-c−/− mice was lower, demonstrating poorer hyaloid blood vessel survival. (B) pdgf-c deficiency led to larger avascular areas, demonstrating more retinal blood vessel regression 5 d after hyperoxia in an OIR model measured by Alexa 488-IB4 staining. Each image of a whole-mount retina shown in B represents a mosaic of several individual images. (Scale bars: 200 µm in A; 300 µm in B.) *P < 0.05, **P < 0.01.
PDGF-CC Protects Retinal Blood Vessels from Oxygen-Induced Regression.
We subsequently investigated whether PDGF-CC could protect blood vessels from regression using an OIR model (21, 22), which includes two stages to assess the degree of vessel regression. In the first stage (75% O2), hyperoxia induced retinal blood vessel regression from P7 to P12 (Fig. 2A, Fig. S2A, BSA-treated, and Fig. S2B, Upper Left). In the second stage (room air), severe retinal hypoxia caused by the preceding retinal vessel regression led to retinal neovascularization at P17 (Fig. 2B, Upper Left and Fig. S2A, BSA-treated). Therefore, if a potent vasoprotective factor was supplied in the first stage to prevent retinal blood vessels from regression in the first place, retinal neovascularization would not occur in the second stage, as indeed was the case for PDGF-CC. In the first stage of this model, under this specific experimental condition, intravitreal injection of PDGF-CC (500 ng per eye) decreased oxygen-induced retinal blood vessel regression by more than 50% at various time points (n = 8, P < 0.001) (Fig. 2A and Fig. S2B), whereas PDGF-AA, another ligand of PDGFR-α, had little effect, and PDGF-BB and PDGF-DD had weaker effects (n = 8, P < 0.001 and P < 0.01, respectively) (Fig. 2A and Fig. S2B). In the second stage of this model (after 5 d of room air), PDGF-CC–treated retinas displayed little hypoxia-induced retinal neovascularization and smaller avascular areas than the BSA-treated control (n = 8, P < 0.001 and P < 0.01, respectively) (Fig. 2B), but in retinae treated with PDGF-BB and PDGF-DD, these two parameters were more similar to those in the BSA control. Immunoprecipitation assays and immunofluorescent staining showed that the PDGF proteins activated PDGFR-α and PDGFR-β in cultured vascular smooth muscle cells (SMCs) and endothelial cells (ECs), respectively (Fig. S3). In addition, PDGF-CC treatment led to more CD31+, neural/glial antigen 2 (NG2)+, and smooth muscle actin (SMA)+ vessels at both P8 and P12 (Fig. 2C and Fig. S1E) but did not result in more blood vessels than what can be seen in normal retinae (n = 8, P < 0.001) (Fig. S2C). In addition, the ratios of the staining intensities of CD31/NG2 and CD31/SMA show no difference between BSA- and PDGF-CC–treated samples (Fig. 2C), indicating that PDGF-CC seems to affect all the vascular cells.
Fig. 2.
PDGF-CC protects retinal blood vessels from oxygen-induced regression. (A) Intravitreal injection of PDGF-CC protein (500 ng per eye) inhibited blood vessel regression, leading to a smaller avascular area as measured by IB4 staining after 5 d of hyperoxia at P12. PDGF-BB and PDGF-DD had some effect, but PDGF-AA had no effect. (B) After 5 d of room air at P17, retinae treated with PDGF-CC displayed better retinal blood vessel regrowth with fewer avascular areas and less hypoxia-induced neovascularization than in the BSA control. Retinae treated with PDGF-BB and PDGF-DD had somewhat reduced avascular areas and hypoxia-induced neovascularization. Each image of a whole-mount retina shown in A and B represents a mosaic of several individual images. (C) Immunofluorescent staining using markers for ECs, pericytes, and SMCs showed that PDGF-CC treatment decreased vessel regression, leading to more CD31+, NG2+, and SMA+ blood vessels at both P8 and P12. The ratios of the staining intensities of CD31/NG2 and CD31/SMA did not differ in the BSA- and PDGF-CC–treated samples. (Scale bars: 300 µm in A and B; 50 µm in C; 10 µm in C, Insets.) **P < 0.01, ***P < 0.001.
PDGF-CC Up-Regulates HMOX1 Expression in Vitro and in Vivo.
To explore the mechanism underlying the vasoprotective/survival effect of PDGF-CC, we investigated the genes regulated by PDGF-CC. Real-time PCR showed that PDGF-CC treatment up-regulated the expression of HMOX1, a potent anti-oxidative gene (23), and other prosurvival genes in the retinae 24 h after hyperoxia (Fig. 3A and Fig. S4A). Indeed, PDGF-CC inhibition or blocking PDGFR-α, the receptor for PDGF-CC, using neutralizing antibodies markedly decreased HMOX1 expression (Fig. 3 B and C) and inhibited PDGF-CC–induced PDGFR-α activation in the retinae (Fig. S4F). At a protein level, Western blot showed higher HMOX1 levels after PDGF-CC treatment in different vascular cells, whereas pdgf-c deficiency decreased HMOX1 expression in the retinae (Fig. 3D). PDGF-AA, another ligand of PDGFR-α, did not up-regulate HMOX1 expression (Fig. S4E), and PDGF-BB and PDGF-DD had a weaker effect than PDGF-CC (Fig. S4 B and C). Thus, PDGF-CC is a potent inducer of HMOX1.
Fig. 3.
PDGF-CC up-regulates HMOX1 expression and promotes survival of vascular ECs and SMCs. (A) PDGF-CC treatment up-regulated the expression of HMOX1 in the retinae 24 h after hyperoxia, as measured by real-time PCR. (B) PDGF-CC inhibition by neutralizing antibody in the retinae down-regulated HMOX1 expression, as shown by real-time PCR. (C) PDGFR-α inhibition by a neutralizing antibody down-regulated HMOX1expression in the retinae, as shown by real-time PCR. (D) Western blot showed up-regulated expression of HMOX1 by PDGF-CC in human dermal microvascular endothelial cells (HMEC-1s) and human brain vascular smooth muscle cells (HBVSMCs). pdgf-c deficiency decreased HMOX1 expression in the retinae of pdgf-c−/− mice. (E–H) PDGF-CC treatment increased survival of vascular ECs [human retinal endothelial cells (HRECs) and HMEC-1s] and SMCs [human aortic smooth muscle cells (HASMCs) and HBVSMCs] cultured in serum-free medium. The survival effect of PDGF-CC was abolished by ZnPP IX, a HMOX1 inhibitor. (I and J) HMOX1 siRNA1 and siRNA2 transfection decreased HMOX1 expression and abolished the survival effect of PDGF-CC in vascular ECs and SMCs. *P < 0.05, **P < 0.01, ***P < 0.001.
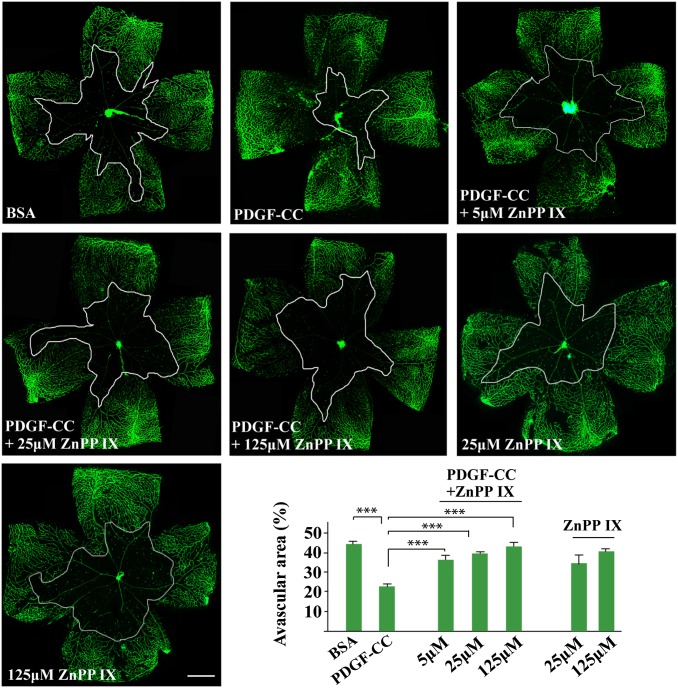
Vasoprotection by PDGF-CC via HMOX1 in Vitro and in Vivo.
We next investigated the role of HMOX1 in PDGF-CC-mediated survival of vascular cells. Different vascular cells were cultured in serum-free medium so that their survival (rather than proliferation) could be investigated. We found that PDGF-CC increased the survival of both vascular ECs and SMCs (n = 6, P < 0.05, P < 0.01, P < 0.001) (Fig. 3 E–H). Importantly, cotreatment with an HMOX1 inhibitor, zinc protoporphyrin (ZnPP IX) (n = 6, P < 0.05, P < 0.01, P < 0.001) (Fig. 3 E–H), or HMOX1 siRNA (n = 6, P < 0.05) (Fig. 3 I and J and Fig. S5A) abolished the survival effect of PDGF-CC. In an OIR model in vivo, intravitreal injection of PDGF-CC protected retinal vessels from regression and markedly reduced avascular areas (n = 6, P < 0.001) (Fig. 4). Importantly, coadministration of the HMOX1 inhibitor ZnPP IX completely abolished the vasoprotective effect of PDGF-CC in vivo in a dose-dependent manner (n = 6, P < 0.001) (Fig. 4), demonstrating that the vascular protective/survival effect of PDGF-CC requires HMOX1.
Fig. 4.
HMOX1 blockade diminished the vasoprotective effect of PDGF-CC in the retinae in vivo. In an OIR model, intravitreal injection of PDGF-CC protein protected retinal blood vessels from regression, leading to smaller avascular areas. Coadministration of the HMOX1 inhibitor ZnPP IX abolished the vasoprotective effect of PDGF-CC. The avascular areas in the ZnPP IX-treated retinae were similar to those in BSA-treated retinae, suggesting that HMOX1 mediates the effect of PDGF-CC. Each image of a whole-mount retina represents a mosaic of several individual images. (Scale bar: 300 µm.) ***P < 0.001.
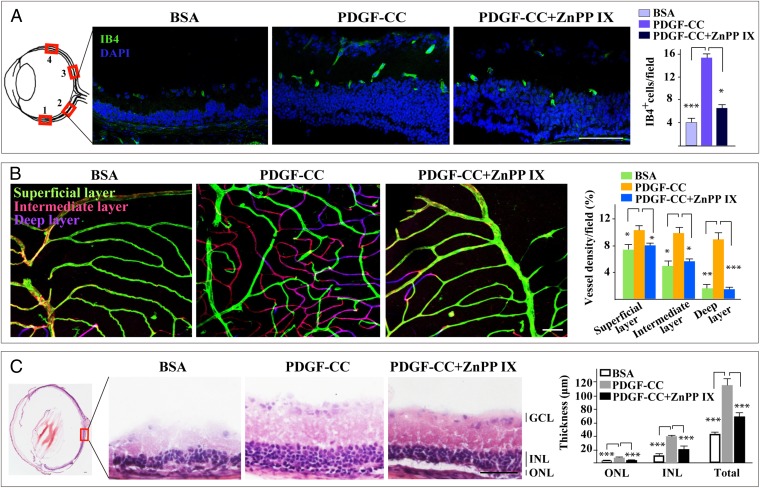
PDGF-CC Rescues Blood Vessel and Retinal Degeneration via HMOX1 in an RP Model.
We next tested whether PDGF-CC could ameliorate blood vessel degeneration under pathological conditions using rd1 mice, a commonly accepted model for retinitis pigmentosa, in which retinal and blood vessel degeneration represents a serious pathology by displaying few blood vessels and thin retinae (Fig. 5 A and C, BSA-treated). In this model, intravitreal injection of PDGF-CC protein rescued retinal blood vessels from degeneration and resulted in higher blood vessel densities throughout the retinae (n = 4, P < 0.001 or P <0.05) (Fig. 5A and Fig. S5B). The effect of PDGF-CC was particularly striking in the deep retinal layer where blood vessel density increased by about eight-fold (n = 4, P < 0.01) (Fig. 5B). Importantly, apart from the increased survival of retinal blood vessels, PDGF-CC treatment also rescued retinal degeneration, as shown by the increased thickness of different retinal layers (n = 4, P < 0.05, P < 0.01, P < 0.001) (Fig. 5C and Fig. S5C). It is noteworthy that coadministration of the HMOX1 inhibitor ZnPP IX abolished the effect of PDGF-CC nearly completely (n = 4, P < 0.05, P <0.01, P <0.001) (Fig. 5 A–C and Figs. S5 B and C and S6 A and B). GFAP staining showed no significant difference in overall astrocyte activation/density between PDGF-CC– and BSA-treated retinae even though there seemed to be a tendency of increased GFAP staining in the nerve fiber layer (Fig. S4G). Thus, PDGF-CC rescued both retinal and blood vessel degeneration via HMOX1 under pathological conditions.
Fig. 5.
PDGF-CC treatment rescues blood vessel and retinal degeneration via HMOX1 in an RP model. (A) In an RP model (rd1 mice) with serious retinal and blood vessel degeneration, IB4 staining showed few retinal blood vessels in the BSA-treated retinae. Intravitreal injection of PDGF-CC protein rescued retinal blood vessels from degeneration, leading to more IB4+ vessels throughout the retinae. The effect of PDGF-CC was abolished nearly completely by a HMOX1, inhibitor ZnPP IX. (B) IB4 staining of whole-mount retinae showed few blood vessels in the intermediate (pink) and deep (purple) layers of the BSA-treated retinae. PDGF-CC rescued retinal blood vessels from degeneration, leading to more vessels in the intermediate (pink) and deep (purple) retinal layers. The effect of PDGF-CC was abolished by ZnPP IX. (C) H&E staining showed severe retinal degeneration in the BSA-treated retinae. PDGF-CC treatment rescued retinal degeneration and markedly increased the thickness of different retinal layers. The effect of PDGF-CC was abolished nearly completely by ZnPP IX. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. (Scale bars: 50 μm in A and C; 100 μm in B.) *P < 0.05, **P < 0.01, ***P < 0.001.
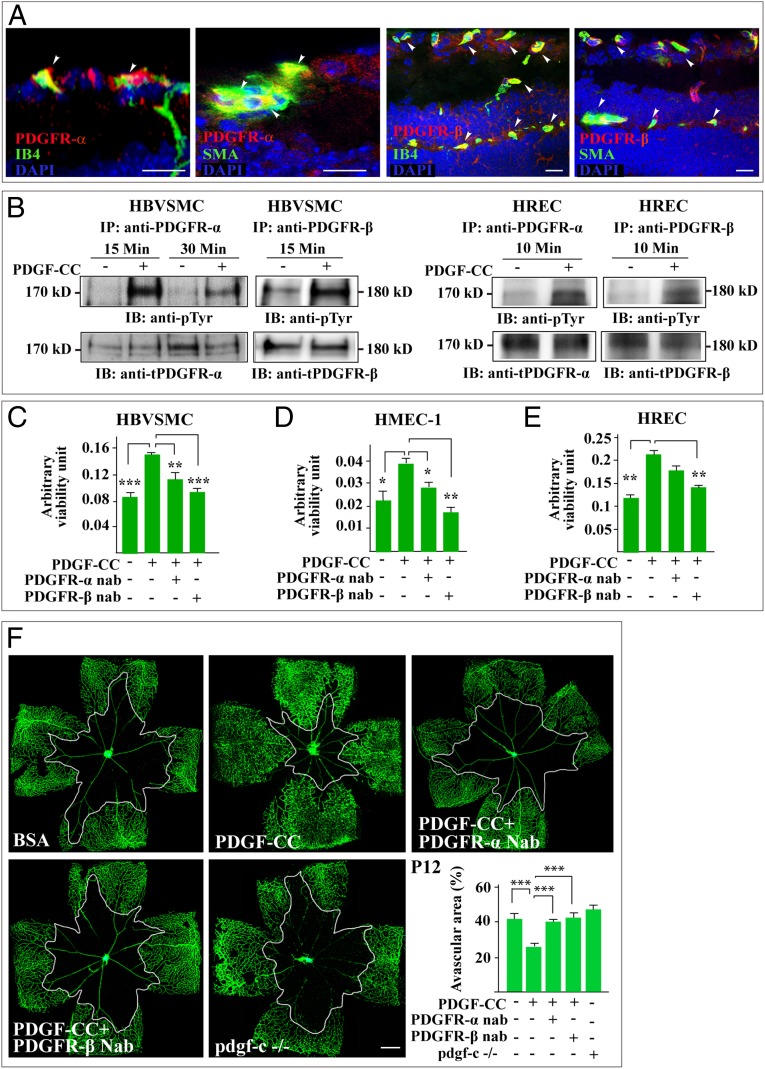
PDGFR-α and PDGFR-β Are Required for the Vasoprotective Effect of PDGF-CC.
PDGF-CC binds to and activates PDGFR-α and PDGFR-β (12, 15). We therefore investigated their potential role in the vasoprotective effect of PDGF-CC. Immunofluorescent staining detected PDGFR-α and PDGFR-β expression on retinal blood vessels (Fig. 6A and Fig. S7). Immunoprecipitation and immunoblotting showed activation of PDGFR-α and PDGFR-β by PDGF-CC in vascular SMCs and ECs (Fig. 6B). PDGFR-α and PDGFR-β activation in the PDGF-CC–treated retinae also was detected by fluorescent staining in both the OIR and RP models in vivo (Fig. S8 A and B). Moreover, the survival/protective effect of PDGF-CC was abolished by PDGFR-β and PDGFR-α neutralizing antibodies in cultured vascular SMCs and ECs (n = 5, P < 0.05, P < 0.01, P < 0.001) (Fig. 6 C–E). Furthermore, in the OIR model, the vasoprotective effect of PDGF-CC was largely abolished by neutralizing antibodies against PDGFR-α and PDGFR-β (n = 6, P < 0.001). The effect of blocking the PDGFR pathways was similar to pdgf-c deletion in pdgf-c knockout mice (n = 6, P < 0.001) (Fig. 6F), demonstrating the requirement of PDGFR-β and PDGFR-α for the survival/protective effect of PDGF-CC on blood vessels.
Fig. 6.
PDGFR-α and PDGFR-β mediate the vasoprotective effect of PDGF-CC. (A) Immunofluorescent staining detected PDGFR-α and PDGFR-β expression on retinal blood vessels using IB4 and SMA as markers for vascular ECs and SMCs, respectively. (B) Immunoprecipitation (IP) followed by immunoblotting (IB) showed PDGFR-α and PDGFR-β activation by PDGF-CC in HBVSMCs, HRECs, and HMEC-1s. (C–E) A 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide] (MTT) survival assay showed increased survival of HBVSMC, HMEC-1, and HREC treated with PDGF-CC. Inhibition of PDGFR-α and PDGFR-β using neutralizing antibodies abolished the survival effect of PDGF-CC. nab, neutralizing antibody. (F) An OIR model showed that PDGF-CC protected retinal blood vessels from regression leading to smaller avascular areas. Blockade of PDGFR-α and PDGFR-β using neutralizing antibodies abolished the vasoprotective effect of PDGF-CC, leading to larger avascular areas similar to those seen in pdgf-c–deficient mice. Each image of a whole-mount retina shown in F represents a mosaic of several individual images. (Scale bars: 20 μm in A; 300 µm in F.) *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Blood vessel degeneration and regression have become an important focus in biomedical research because of their critical involvement in nearly all types of degenerative diseases. Therefore, there is an urgent need to identify effective molecules for vascular protection and survival. In this study we show that PDGF-CC is a potent vascular protective/survival factor that rescues retinal and blood vascular degeneration, whereas its deficiency exacerbates blood vessel regression in various animal models. Importantly, we revealed a previously unidentified mechanism underlying the function of PDGF-CC by showing that HMOX1 is critically required for the vascular survival effect of PDGF-CC.
Even though PDGF-CC has been shown to be a potent angiogenic factor (10, 11, 15, 18–20), its role in blood vessel survival and protection remained unclear. The receptors for PDGF-CC, PDGFR-α, and PDGFR-β, are thought to be expressed mainly by vascular SMCs, pericytes, and other mesenchymal cells and have been shown to be important for vascular survival because their inhibition led to vascular degeneration (24). However, it remained unknown whether PDGF-CC plays a role in this process and whether it has a direct effect on vascular ECs. In this study, we found expression and activation of PDGFR-β and PDGFR-α by PDGF-CC not only in vascular SMCs but also in ECs. Indeed, neutralizing antibodies against PDGFR-β and PDGFR-α abolished the survival effects of PDGF-CC nearly completely, demonstrating that a direct survival effect of PDGF-CC on vascular SMCs and ECs is a major factor in PDGFR-β– and PDGFR-α–mediated blood vessel survival and protection.
The genes downstream of the PDGF-CC pathway are not well studied thus far. In this study, we found that PDGF-CC markedly up-regulated the expression of HMOX1 in different vascular cells, whereas inhibition of PDGF-CC or its receptors suppressed its expression in vitro and in vivo. Importantly, treatment with an HMOX1 inhibitor completely abolished the PDGF-CC–mediated vasoprotective effect on vascular SMCs and ECs in vitro and in various animal models in vivo. HMOX1 is a potent vasoprotective and survival factor because its robust antioxidative, antiapoptotic, and anti-inflammatory effects protect cells and tissues from injury (23). Given that oxidative stress, apoptosis, and inflammation are critically involved in most degenerative diseases, induction of HMOX1 expression has been considered a promising strategy for the treatment of degenerative diseases. As a potent HMOX1 inducer, PDGF-CC achieves its vasoprotective effect, at least partially, through HMOX1.
It is noteworthy that PDGF-CC protein treatment in the RP model of rd1 mice rescued not only blood vessel but also retinal degeneration, as shown by the improved retinal vessel density and retinal thickness. RP is a retinal degenerative disease characterized by the death of retinal photoreceptor cells and blood vessel degeneration (3). To mitigate retinal degeneration in RP effectively, both retinal blood vessels and photoreceptors must be protected. In this study, the rescue effect of PDGF-CC may have several aspects. First, PDGF-CC has a direct vasoprotective effect on retinal blood vessels, which subsequently provide better blood perfusion and thus better photoreceptor survival. Second, PDGF-CC also may have a direct neuroprotective effect on retinal photoreceptor cells, which express the PDGFRs (8, 9). Indeed, we have shown previously that PDGF-CC is a potent neuronal survival factor (9). One potential issue that remains to be defined better in future studies is to investigate astrocyte activation in detail after PDGF-CC treatment, because there seems to be a tendency for increased GFAP staining in the nerve fiber layer in the PDGF-CC–treated retinae. Thus, PDGF-CC appears to be a potent survival factor that protects the retina from degeneration via multiple mechanisms.
In summary, using different loss- and gain-of-function assays and multiple model systems, we demonstrate here that PDGF-CC functions as a critical vascular protective factor that is required to maintain blood vessel survival. PDGFR-β and PDGFR-α mediate the vascular survival effect of PDGF-CC on multiple vascular cells by up-regulating HMOX1 expression. Given the general involvement of blood vessel defects in most degenerative disorders, PDGF-CC may be of therapeutic use in treating such diseases.
Materials and Methods
Models of Blood Vessel Regression/Degeneration.
All animal experiments were approved by the Animal Use and Care Committee of Zhongshan Ophthalmic Center at the Sun Yat-Sen University, Guangzhou, People’s Republic of China. pdgf-c–deficient mice, the OIR model, the hyaloid blood vessel regression model, and the RP model of rd1 mice are described in SI Materials and Methods.
Cell Culture, Survival Assay, and Immunofluorescence Staining.
Detailed descriptions of cell culture, the survival assay, and immunofluorescence staining procedures can be found in SI Materials and Methods. Cell culture, survival assays, and immunofluorescence staining were performed as described in refs. 9, 19, 21, 22, and 25. All cell-culture experiments were performed in triplicate and were repeated twice.
Receptor Activation, Western Blot, and Real-Time PCR.
Detailed descriptions of receptor activation, Western blot, and real-time PCR procedures can be found in SI Materials and Methods and Table S1. Receptor activation, Western blot, and real-time PCR were performed as described in refs. 9, 19, and 22.
Statistics.
Two-tailed Student t test and one-way ANOVA were used for statistical analysis. Differences were considered statistically significant when P < 0.05. All values are presented as mean ± SEM of the number of determinations.
Supplementary Material
Acknowledgments
This research was supported by Key Program of the National Natural Science Foundation of China (NSFC) Grant 81330021 (to X. Li), by NSFC Grant 81271010 (to X. Liu), by the Taishan Scholar’s Program (X. Li and B.W.), by the Innovation Team Program of the Jiangsu Province (C.Z. and X. Li), and by the State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou, People’s Republic of China.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1404140111/-/DCSupplemental.
References
- 1.Ferrara N. Vascular endothelial growth factor and age-related macular degeneration: From basic science to therapy. Nat Med. 2010;16(10):1107–1111. doi: 10.1038/nm1010-1107. [DOI] [PubMed] [Google Scholar]
- 2.Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci. 2008;9(3):169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- 3.Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS. Photoreceptor degeneration: Genetic and mechanistic dissection of a complex trait. Nat Rev Genet. 2010;11(4):273–284. doi: 10.1038/nrg2717. [DOI] [PubMed] [Google Scholar]
- 4.Grunwald JE, et al. CATT Research Group Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121(1):150–161. doi: 10.1016/j.ophtha.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jalali S, Balakrishnan D, Zeynalova Z, Padhi TR, Rani PK. Serious adverse events and visual outcomes of rescue therapy using adjunct bevacizumab to laser and surgery for retinopathy of prematurity. The Indian Twin Cities Retinopathy of Prematurity Screening database Report number 5. Arch Dis Child Fetal Neonatal Ed. 2013;98(4):F327–F333. doi: 10.1136/archdischild-2012-302365. [DOI] [PubMed] [Google Scholar]
- 6.Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K. SEVEN-UP Study Group Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: A multicenter cohort study (SEVEN-UP) Ophthalmology. 2013;120(11):2292–2299. doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 7.Bartus C, Brown LF, Bonner MY, Arbiser JL. High level expression of angiopoietin-2 in human abscesses. J Am Acad Dermatol. 2011;64(1):200–201. doi: 10.1016/j.jaad.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee C, Zhang F, Tang Z, Liu Y, Li X. PDGF-C: A new performer in the neurovascular interplay. Trends Mol Med. 2013;19(8):474–486. doi: 10.1016/j.molmed.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Tang Z, et al. Survival effect of PDGF-CC rescues neurons from apoptosis in both brain and retina by regulating GSK3beta phosphorylation. J Exp Med. 2010;207(4):867–880. doi: 10.1084/jem.20091704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moriya J, et al. PDGF-C promotes revascularization in ischemic limbs of diabetic mice. J Vasc Surg. 2014 doi: 10.1016/j.jvs.2013.04.053. 59(5):1402-9.e1-4. [DOI] [PubMed] [Google Scholar]
- 11.Crawford Y, et al. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15(1):21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Li X, et al. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol. 2000;2(5):302–309. doi: 10.1038/35010579. [DOI] [PubMed] [Google Scholar]
- 13.Kazlauskas A. A new member of an old family. Nat Cell Biol. 2000;2(5):E78–E79. doi: 10.1038/35010508. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Eriksson U. Novel PDGF family members: PDGF-C and PDGF-D. Cytokine Growth Factor Rev. 2003;14(2):91–98. doi: 10.1016/s1359-6101(02)00090-4. [DOI] [PubMed] [Google Scholar]
- 15.Cao R, et al. Angiogenesis stimulated by PDGF-CC, a novel member in the PDGF family, involves activation of PDGFR-alphaalpha and -alphabeta receptors. FASEB J. 2002;16(12):1575–1583. doi: 10.1096/fj.02-0319com. [DOI] [PubMed] [Google Scholar]
- 16.Peng F, Yao H, Akturk HK, Buch S. Platelet-derived growth factor CC-mediated neuroprotection against HIV Tat involves TRPC-mediated inactivation of GSK 3beta. PLoS ONE. 2012;7(10):e47572. doi: 10.1371/journal.pone.0047572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Son D, Na YR, Hwang ES, Seok SH. Platelet-derived growth factor-C (PDGF-C) induces anti-apoptotic effects on macrophages through Akt and Bad phosphorylation. J Biol Chem. 2014;289(9):6225–6235. doi: 10.1074/jbc.M113.508994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, et al. Revascularization of ischemic tissues by PDGF-CC via effects on endothelial cells and their progenitors. J Clin Invest. 2005;115(1):118–127. doi: 10.1172/JCI19189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou X, et al. PDGF-CC blockade inhibits pathological angiogenesis by acting on multiple cellular and molecular targets. Proc Natl Acad Sci USA. 2010;107(27):12216–12221. doi: 10.1073/pnas.1004143107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, et al. VEGF-independent angiogenic pathways induced by PDGF-C. Oncotarget. 2010;1(4):309–314. doi: 10.18632/oncotarget.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang F, et al. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci USA. 2009;106(15):6152–6157. doi: 10.1073/pnas.0813061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, et al. VEGF-B inhibits apoptosis via VEGFR-1-mediated suppression of the expression of BH3-only protein genes in mice and rats. J Clin Invest. 2008;118(3):913–923. doi: 10.1172/JCI33673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson-Berka JL, et al. Inhibition of platelet-derived growth factor promotes pericyte loss and angiogenesis in ischemic retinopathy. Am J Pathol. 2004;164(4):1263–1273. doi: 10.1016/s0002-9440(10)63214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang F, et al. Proliferative and survival effects of PUMA promote angiogenesis. Cell Reports. 2012;2(5):1272–1285. doi: 10.1016/j.celrep.2012.09.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.