Significance
Recent theoretical approaches to understanding the evolution of cooperation point to a close link between spatial structure and cooperative tendencies and question the importance of kin relations. We here show that Guinea baboon males living in a multilevel society maintain strong male bonds, irrespective of relatedness, and exhibit low levels of overt aggression. Although our results are compatible with the idea that kin relations may have favored male tolerance and bond formation in the course of evolution, they also support the notion that these relations are not necessary to maintain cooperative relationships in a multilevel society. Guinea baboons thus may constitute a valuable model for understanding the conditions that played a role in the emergence of human social evolution.
Keywords: association index, fission–fusion, multilevel society, range expansion, social network analysis
Abstract
Male relationships in most species of mammals generally are characterized by intense intrasexual competition, with little bonding among unrelated individuals. In contrast, human societies are characterized by high levels of cooperation and strong bonds among both related and unrelated males. The emergence of cooperative male–male relationships has been linked to the multilevel structure of traditional human societies. Based on an analysis of the patterns of spatial and social interaction in combination with genetic relatedness data of wild Guinea baboons (Papio papio), we show that this species exhibits a multilevel social organization in which males maintain strong bonds and are highly tolerant of each other. Several “units” of males with their associated females form “parties,” which team up as “gangs.” Several gangs of the same “community” use the same home range. Males formed strong bonds predominantly within parties; however, these bonds were not correlated with genetic relatedness. Agonistic interactions were relatively rare and were restricted to a few dyads. Although the social organization of Guinea baboons resembles that of hamadryas baboons, we found stronger male–male affiliation and more elaborate greeting rituals among male Guinea baboons and less aggression toward females. Thus, the social relationships of male Guinea baboons differ markedly from those of other members of the genus, adding valuable comparative data to test hypotheses regarding social evolution. We suggest that this species constitutes an intriguing model to study the predictors and fitness benefits of male bonds, thus contributing to a better understanding of the evolution of this important facet of human social behavior.
Traditional human societies typically consist of stable communities comprising several conjugal family groups (1). Sexual relationships are predominantly monogamous, and individuals of both sexes may disperse from their family groups or stay, resulting in coresidence of both brothers and sisters (2). Strikingly, men from different family groups may form long-term alliances within the community, resulting in cooperative relationships among individuals who often are not genetic relatives (3). The advent of such exceptional cooperative relationships within human societies has been linked to their multilevel organization (4). However, what are the evolutionary dynamics that give rise to multilevel systems in the first place, and how do social organization and cooperative tendencies stabilize each other?
Evolutionary game theory has been used to model the conditions that favor cooperation among unrelated individuals (5, 6). Such analyses reveal that the evolutionary dynamics have a strong spatial component, in which cooperators prevail against defectors by forming clusters within the social network (7). This theoretical insight is bolstered by empirical studies of the Hadza, a population of hunter-gatherers in Tanzania. In this study, cooperators were found to cluster in physical space, i.e., in the same camp (8). Assortative processes thus may stabilize cooperation, and vice versa.
Further empirical evidence to explain key facets of human social evolution comes from comparative studies of nonhuman primates (9–11) and other mammalian species as well. Among mammals, cooperative relationships among unrelated individuals are considered generally rare (12), particularly among males, who—according to sexual selection theory—are predicted to compete with other males over access to females. However, there are notable exceptions, including cooperative hunting and territorial patrols by chimpanzees (Pan troglodytes) (13), joint foraging by male coastal river otters (Lontra canadensis) (14), and the defense of females by male dolphins, Tursiops spp. (15). Such instances of cooperative behavior among unrelated animals can be explained by mutualism, whereby individuals benefit immediately from cooperating, and by reciprocity, whereby one individual experiences a short-term cost by cooperating but obtains a future benefit greater than the initial investment (16, 17).
We here show that Guinea baboons (Papio papio) live in a multilevel society with extensive cooperation among unrelated males. Until now, comparatively little attention had been paid to this species, and its social organization was disputed. Although some previous studies, mainly from captivity or short field stints, suggested that Guinea baboon groups, like groups of hamadryas baboons (Papio hamadryas) (18), are comprised of one-male units that aggregate into larger parties (19, 20), other studies suggested a multimale/multifemale organization comparable to that of savanna baboons (21) or one that differs from both the savanna and the hamadryas baboon types (22, 23). However, quantitative data from observations of individually identified animals in the wild were lacking.
We used ranging data collected from animals equipped with Global Positioning System (GPS) collars, proximity, and behavioral measures recorded during focal observations from individually identified males, as well as population genetic analyses based on microsatellites to describe the association patterns and individual interactions of Guinea baboons in space and time. Our aim was to contribute to a deeper understanding of the link between social organization and the formation of male bonds and to provide critical empirical evidence for modeling the processes that gave rise to some of the hallmarks in human evolution.
Results
Grouping Patterns.
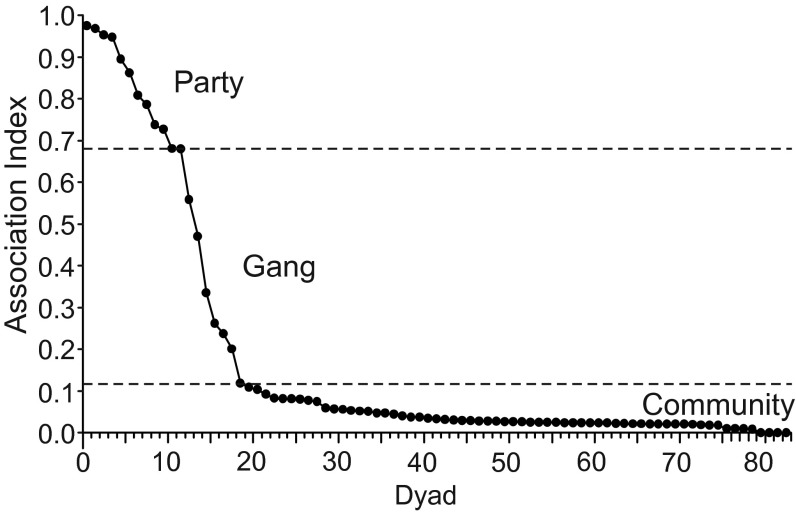
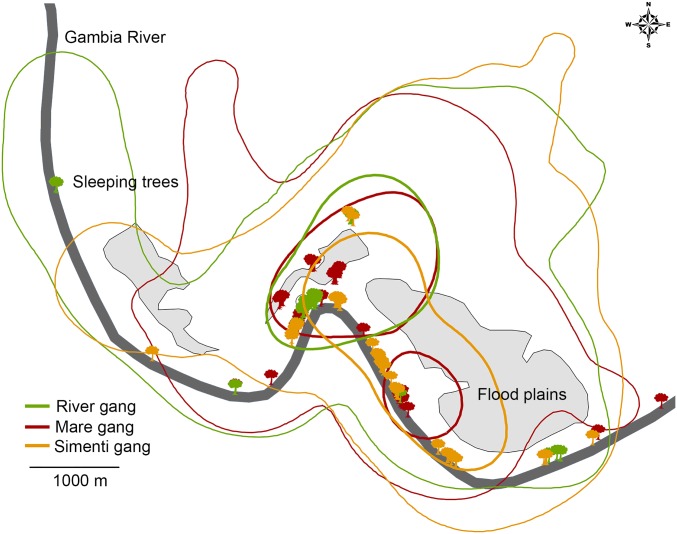
The results of the change-point analysis based on GPS data indicated that the Guinea baboon society consists of three structural levels. First, the algorithm detected a change point-reflecting dyads that associated more than 68% of the time (n = 12); these dyads were classified as belonging to the same “party,” The mean party size was 25 individuals (range, 16–35). Second, we found a significant split separating dyads that spent up to 12% of the time together. This division corresponds to dyads that belong to the same “gang” (Fig. 1). The remaining dyads (65 of 83) associated rarely and are referred to as belonging to the same “community.” The split between members of parties and gangs was less well supported than the division between gangs and the community (91% vs. 100% confidence level). The community–gang split was confirmed in 28 of 30 runs (93%), and the gang–party split was confirmed in 22 of 30 runs (73%). Individuals that belonged to the same party maintained a median distance of 143 m [interquartile range (IQR), 41–301]. Individuals from different parties but within the same gang maintained a median distance of 757 m (IQR, 327–1028), and individuals from different gangs within the community maintained a median distance of 2,246 m (IQR, 1930–2695). The differences between the different levels were significant (n = 83; degrees of freedom = 2; test statistic H = 41.52; Pcommunity vs. gang = 0.002; Pcommunity vs. party ≤ 0.001). Notably, the three observed gangs occupied almost identical home ranges (Fig. 2), although they did not spend much time in close proximity.
Fig. 1.
Distribution of dyadic spatial association indices of 83 dyads within the study community. The two dashed lines indicate change points in the distribution, suggesting three levels. The complete GPS dataset is represented.
Fig. 2.
Sketch of the study area including fixed kernel home ranges and sleeping trees of three gangs based on GPS data obtained from March to June 2011. Colored lines represent the home ranges of the different gangs; thin lines indicate the 95% kernel home range; thick lines indicate 50% kernel core area. The 50% data showed a somewhat higher differentiation, in which one area was used by the Mare and Simenti gangs but not by the River gang.
We conducted behavioral observations of males from the Mare gang in 2010 and from males of the Mare and the Simenti gang in 2011. The results of the hierarchical cluster analysis based on dyadic association indices (AIs) extracted from proximity measures taken during group scans (males within a 20-m distance) also supported the view of a multilevel organization. Fig. 3 shows three clusters consisting of three adult males each from the 2010 dataset. The identification of three clusters was confirmed by Tabu Search cluster analysis, resulting in a best fit for three clusters [Pearson’s correlation coefficient (r2) = 0.91]. In the 2011 dataset the first bifurcation reflects the split into the two gangs (eight adult males each). Within both gangs, we found two clusters (best fit four clusters; r2 = 0.74), each including four males, representing the different parties (Fig. 3). On average, the AIs among party dyads were 0.65 (±0.10 SD), and those among gang dyads were 0.24 (±0.10 SD). See SI Materials and Methods for methodological details.
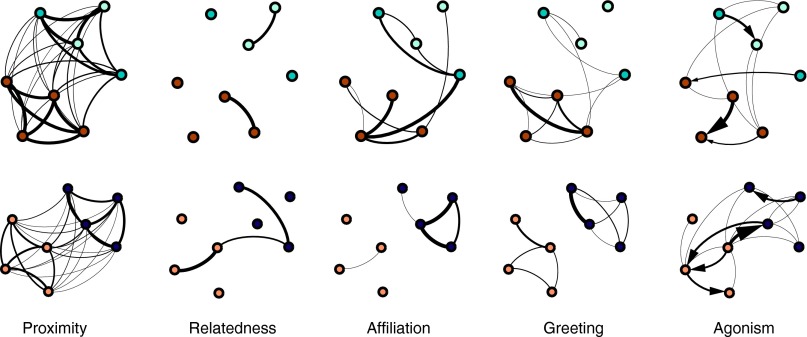
Fig. 3.
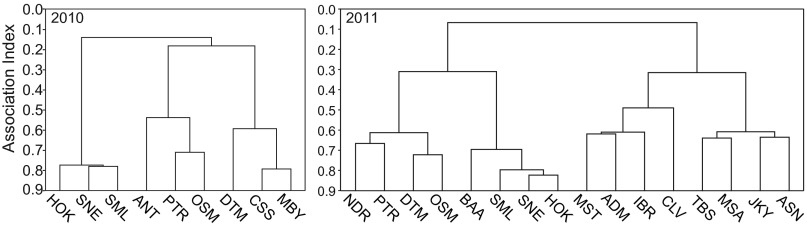
Dendrograms resulting from hierarchical cluster analysis based on association indices among nine adult males during the 2010 observation period (n = 36 dyads) and among 16 adult males during the 2011 observation period (n = 120 dyads). Letter codes represent individual males.
Males spent considerable time (17%, 252/1,480 instances) within 1 m of other individuals. The other individual was an adult female in 58.7% of these 252 instances and an adult male in 29.4%. In the remaining 11.9% instances, the male was within 1 m of both a female and a male. These proportions largely reflected the sex ratio in the study population (Table S1). In 22.1% of the instances when a male was sitting near another male, the two males belonged to different parties of the same gang.
Relatedness.
Males that belonged to the same gang were significantly more closely related than males belonging to different gangs, but there was no significant difference in relatedness for males that belonged either to the same or to different parties within the same gang (Table 1 and SI Materials and Methods). Overall, we detected 17 dyads that appeared to be highly related (pairwise r values ranged from 0.25–0.51) (Tables S2 and S3). Three of the five parties we observed appeared to comprise one closely related dyad and additional males that were not highly related. One highly related dyad was found in the same gang but not the same party. Members of five highly related dyads belonged to different gangs. The remaining eight highly related dyads included males that we were unable to assign to any party or gang because we did not observe them again after taking the samples for genetic analyses.
Table 1.
Mean pairwise relatedness (r ± SD) of adult males at different social levels within the community for both study periods
| Level | 2010 | No. of dyads | 2011 | No. of dyads |
| Within party | 0.02 ± 0.22 | 29 | 0.01 ± 0.21 | 34 |
| Between parties | 0.00 ± 0.15 | 43 | 0.01 ± 0.16 | 30 |
| Within gang* | 0.01 ± 0 0.18 | 72 | 0.01 ± 0.19 | 64 |
| Between gangs | −0.05 ± 0.16 | 456 | −0.05 ± 0.15 | 464 |
Within-gang dyads were significantly more related than between-gang dyads at P < 0.01 in both years. The number of dyads varies between years because of demographic changes.
Male Relationships.
The majority of male–male social interactions in which both partners were individually identified took place within a male’s gang (78% of 580 interactions). All social interactions except severe aggression were observed significantly more frequently within, rather than between, parties of the same gang (exact Wilcoxon test, n = 14; affiliative: W = –91.0, P < 0.001; agonistic: W = −81.0, P = 0.009; greetings: W = −105.0, P < 0.001; support: W = −91.0, P = 0.006; Table 2). Severe aggression, in contrast, was observed more often between rather than within parties of the same gang (W = 47.0, P = 0.014).
Table 2.
Association index, interaction frequencies, and proportion of possible partners interacted with for different social levels (mean ± SD)
| Level | AI | Affiliation | Agonism | Greetings | |||
| IF | PP | IF | PP | IF | PP | ||
| Party | 0.65 ± 0.10 | 0.08 ± 0.11 | 0.47 ± 0.21 | 0.04 ± 0.03 | 0.58 ± 0.16 | 0.24 ± 0.16 | 0.70 ± 0.07 |
| Gang | 0.24 ± 0.10 | 0.01 ± 0.02 | 0.13 ± 0.13 | 0.01 ± 0.02 | 0.46 ± 0.22 | 0.04 ± 0.04 | 0.71 ± 0.21 |
| Community* | 0.07 ± 0.02 | ||||||
Values refer to mutually exclusive categories (i.e., “Party” refers to dyads within parties, “Gang” refers to dyads in different parties of the same gang; “Community” refers to dyads in different gangs). We observed no interactions between the individually identified members of the two focal gangs. AI, association index (i.e., the proportion of time spent in 20 m proximity); IF, dyadic hourly interaction frequency; PP, average proportion of possible partners with whom individuals effectively interact.
2011 only.
Males exchanged a sizable share of affiliative behavior with other males: In 18.6% of 591 male affiliative interactions, the partner was another adult male. Affiliative interactions typically were restricted to an average of only 2.4 partners (Fig. 4); overall, one-third of all possible male–male dyads exchanged affiliative interactions. Except for two pairs of males, all dyads that maintained a strong bond, i.e., who interacted affiliatively with one another more frequently than average, consisted of males of the same party. Moreover, the majority of coalitions (28 of 30) were formed between males of the same party, and all of these occurred between males that maintained a strong bond. There was a significant positive correlation between the support (coalition) and the affiliation network [quadratic assignment procedure (QAP) correlation; Mare gang 2010: r = 0.88, P = 0.001; Mare gang 2011: r = 0.66; P = 0.002; Simenti gang 2011: r = 0.52, P = 0.016]. Furthermore, the same males maintained close relationships across both years.
Fig. 4.
Male spatial, genetic relatedness, and social interaction (affiliation, greeting, and agonism) networks based on data from 2011 for the Mare gang (Upper) and the Simenti gang (Lower). Different colors of the nodes reflect party membership. Light blue nodes represent individuals that were not sampled in the focal observations. Networks were produced using Gephi (https://gephi.github.io) with a Force Atlas layout, using the 20-m proximity data to depict spatial relationships among individuals. The other types of relationships then were projected as edges of different weight (strength) onto the nodes. Only relatedness values >0.125 (51) are indicated.
Greeting networks mainly encompassed males of the same party, but this pattern was more clearcut in the Mare gang than in the Simenti gang (Fig. 4). Overall, about 80% of the possible dyads were observed to exchange greetings. On average, each male exchanged greetings with 5.8 different male partners. Of the 93 recorded agonistic interactions (including supplants, threats, chases, and fights), only 64 had a clear winner and loser. Forty-two of these agonistic interactions occurred between males belonging to the same party, and 22 took place between members of different parties. We were not able to detect a significantly linear dominance hierarchy among males (MatMan: all h′ = 0.3–0.4; all P > 0.386) either within or between parties. This absence of a dominance hierarchy likely resulted from a substantial amount of empty cells, because agonistic interactions mostly occurred between a few specific males (Fig. 4). However, agonistic interactions generally were unidirectional (all directional consistency indices 0.7–0.8), and there were no intransitive dominance relationships. Overall, males exchanged agonistic interactions with 4.1 partners on average. Generally, males directed agonistic behavior toward males and females at comparably low rates: 0.23 ± 0.10 aggressive events per hour per subject (mean ± SD) toward males, and 0.24 ± 0.15 aggressive events per hour per subject toward females.
Kinship did not predict social interaction patterns, beause there was no correlation between the genetic and any of the social interaction networks (Table 3). Thus, kin were neither more likely to affiliate nor more likely to engage in agonistic interaction patterns than nonkin.
Table 3.
Correlations between matrices of genetic relatedness and interaction networks
| Gang | Affiliation | Agonism | Greetings | |||
| r | P | r | P | r | P | |
| Mare 2010 | 0.02 | 0.44 | 0.15 | 0.19 | 0.01 | 0.48 |
| Mare 2011 | 0.11 | 0.29 | 0.24 | 0.14 | 0.07 | 0.35 |
| Simenti 2011 | −0.34 | 0.04 | −0.47 | 0.01 | 0.02 | 0.46 |
Social interaction networks are based on weighted matrices (according to rates of interactions). Pearson correlation coefficients (r) derive from a QAP. We ran 10,000 permutations to obtain P values.
Discussion
Based on the ranging patterns derived from GPS data and the proximity data collected during focal observations, we found support for a multilevel social system in Guinea baboons consisting of the party, the gang, and the community. Ongoing observations corroborate the notion that females are closely associated with one specific male (19), suggesting that parties encompass another level, namely that of reproductive units, consisting of the primary male, his females, and the females' offspring. Accordingly, the Guinea baboon party likely is equivalent to P. hamadryas clans (comprising several one-male units), whereas the Guinea baboon gang corresponds to the hamadryas band, which consists of several clans. Our results also are in line with previous notions that the Guinea baboon society reveals second- and third level-groupings (20). Overall, the social layers were relatively predictable, unlike individualistic fission–fusion societies such as in chimpanzees, in which high variability in association patterns is observed (24). Instead, the Guinea baboons conformed to a “molecular” type of multilevel society (10).
Males on average were more closely related within than between gangs, but within gangs we found close associations between both related and unrelated males. This pattern is similar to that in chimpanzees, in which affiliation and cooperation do not always map onto relatedness (13). In addition, a broader analysis of the population genetic structure supports the view that Guinea baboon males are predominantly philopatric and that females disperse (25, 26). Overall, however, the genetic relatedness in the present study was rather low, and close genetic relationships also existed between males from different gangs. The low average relatedness might indicate a low reproductive skew in this species and is in line with other studies that show no higher mean average relatedness for the philopatric sex in large groups (27).
Agonistic interactions were generally rare and largely restricted to few dyads. Male Guinea baboons also show relatively low levels of aggression toward females (28). Males within parties and gangs frequently engaged in ritualized greetings, which have been suggested as testing bonds among males (29). The high degree of home range overlap and the lack of overt aggression during encounters between gangs indicate a high degree of spatial tolerance at the group level. This tolerance is further corroborated by a playback study with our subjects, which showed that males responded strongly only to grunts from males of their own gang but largely ignored grunts recorded from either neighboring gangs or unknown animals (30).
The combined results of the present and previous studies justify the assumption that the Guinea baboon society differs substantially from that of other members of the genus. Although their multilevel organization is superficially similar to that of hamadryas baboons, adult male Guinea baboons are highly tolerant of one another and maintain strong affiliative relationships, whereas hamadryas baboon males mainly restrict their affiliative social interactions to a small number of females (18). Geladas (Theropithecus gelada) also reveal different layers in their social organization, but the species is female-bonded (31), and members of different units never interact affiliatively. More detailed observations on social relationships, including data on female social relationships and mating patterns, will be needed to characterize the social system of Guinea baboons fully.
Implications for the Evolution of Social Systems.
The observed relatedness pattern among male Guinea baboons may indicate that kin dyads form the nuclei in the formation of parties, whereas long-term bonds among related as well as unrelated individuals may become more important than kinship alone over time. Previous observations from a colony of captive animals suggested that male bonds formed among adolescent males persisted into adulthood (32), but whether these pairs tended to be brothers was unclear. In chimpanzees, maternal brothers affiliate and cooperate, but there also are a large number of affiliative and cooperative dyads that are unrelated or only distantly related, suggesting that cooperation provides direct benefits to the individuals (13). Fitness benefits associated with unrelated male coalitions also have been shown in Assamese macaques (33) and in several nonprimate species, including lions (Panthera leo) (34), and manakins (Pipra filicaudata) (35). Indirect fitness benefits also may play a role, however. A study of fruit flies (Drosophila melanogaster) showed that females which were kept with males that were related to each other experienced less aggression than females kept with unrelated males (36). Apparently, reduced competition between related males also reduced aggression against females who might be bearing the offspring of related males. Notably, reduced aggression also was observed in situations where not all males were related (36), indicating that the presence of related males may benefit both unrelated males and females across species as diverse as fruit flies and baboons.
Guinea baboon males with strong bonds benefit from mutual support in agonistic interactions with other males, perhaps most likely in the defense of females. Cooperation against the “bachelor threat” has been invoked in geladas (37), snub-nosed monkeys (Rhinopithecus roxellana) (38), Asian colobines (39), humans (3), and zebras (40). Recent analyses have stressed the importance of threats or potential risks in stabilizing cooperation (41), and this effect might explain the lack of overt intergroup hostility in Guinea baboons. Further data will be needed to assess whether some parties prove to be more successful than others in attracting and defending females, thereby contributing to the reproductive success of (most of) its male members.
Dynamics During Range Expansion.
Ultimately, the question arises: Which processes give rise to the variation in social systems. Evidence is accumulating that current variation in the key factors considered in socioecological models, such as resource distribution, predation pressure, and infanticide risk, is not sufficient to explain the grouping patterns observed in extant nonhuman primates. Attempts to explain primate social evolution therefore must take phylogenetic relationships into consideration (42). It is becoming increasingly clear that the diversity in social organization among populations may arise not only from past or current selection pressures but also from the dynamics and stochasticity of spatial processes during range expansion. Range expansions have played an important role in human evolution and are assumed to influence genetic diversity (43). More specifically, mutations that occur at the edge of an expanding population can reach much higher allele frequencies than seen in stationary populations (44). This effect, known as “gene surfing” (45), has been documented in microbial communities (46, 47), tortoises (48), and humans (49). Therefore an intriguing question is whether some of the variation among baboons may be related to demographic factors acting during the range expansion of the genus during the Pleistocene. More specifically, genes predisposing for male philopatry in frontier populations might have accumulated and eventually become fixed, facilitating the emergence of high tolerance and cooperation (50).
Summary and Outlook.
Our findings are in line with the view that a multilevel social organization is associated with the emergence and maintenance of cooperation, irrespective of kin relations. Although kin relations may play a role in initiating cooperative relationships over evolutionary and ontogenetic time scales, they are not necessary to maintain cooperative relations in a multilevel society. Future studies will investigate the formation of bonds between males and aim to clarify their immediate and long-term benefits in a society with little overt aggression between groups. Of particular interest in this context is the role of females, who in this species appear to have considerable leverage in the formation of male–female associations. A hypothesis to be tested is not only whether males with strong bonds are able to defend their females more efficiently but also whether females actively prefer these males. We believe that Guinea baboons will provide important empirical data to deepen our understanding of the conditions that favor the emergence of cooperation among unrelated individuals.
Materials and Methods
Field Site and Study Subjects.
All observational methods and capturing and handling procedures complied with the current law of Senegal and Germany and were conducted under permits issued by the Diréction des Parcs Nationaux du Senegal.
The study site lies close to the field station of the German Primate Center, the Centre de Recherche de Primatologie Simenti in the Niokolo-Koba National Park in southeastern Senegal (for further details, see ref. 23). Observations were conducted on two gangs of Guinea baboons, consisting of 55–60 subjects each. Details regarding group composition are given in Table S1.
GPS Data.
Initially, we captured subjects opportunistically without knowledge about their specific association patterns. In total, we fitted 18 baboons (11 males, 7 females) from three gangs with GPS collars (Tellus GPS; Televilt) to obtain data on their ranging patterns. Collars were programmed to take synchronous fixes every other hour between 06:00 and 18:00 and at 21:00, midnight, and 03:00. From these fixes, we obtained 110,426 dyadic distance measures for 83 dyads from November 2009 to January 2012 (mean, 1,330 per dyad; range, 13–3,997) (see SI Materials and Methods for details). We calculated dyadic AIs as the proportion of the number of fixes in which two animals were found within 100 m of each other (a reasonable distance within which animals potentially could interact) divided by the number of fixes available for the respective dyad.
To detect different levels in the social organization based on variation in interindividual distances, we conducted a change-point analysis (Change Point Analyzer 2.3; Taylor Enterprises, Inc.) to uncover significant changes in the mean squared error distribution of the data (51). We ran 10,000 bootstraps without replacements and set the confidence interval at 90%. We repeated the procedure 30 times until the solution converged. We derived home range estimates using the fixed kernel density estimation from Hawth’s Tools implemented in ArcGIS 9.3 (Environmental Systems Research Institute, Inc.). We calculated 95% and 50% kernel density plots [hereafter referred to as “kernel home ranges” (52)] using the GPS data from one representative for each of the three gangs in which data were collected in 2011 (Table S4). Visual inspection of data points showed that data for members of the same gang were highly correlated with each other. To compare mean dyadic distances according to the social level, we conducted a Kruskal–Wallis ANOVA in Statistica 10 (StatSoft).
Behavioral Observations.
A.P. conducted behavioral observations from March to July 2010 (75 observation days) and from January to June 2011 (106 observation days), between 06:00 and 12:00 and occasionally between 16:00 and 19:00. First, we assessed spatial association patterns from 239 group scans in 2010 and 318 group scans in 2011 in which the identities of all adult males who were seen within 20 m distance of each other was noted. Group scans were conducted before focal observations, approximately at hourly intervals. Data were collected using an HP Tungsten Palm E2 with custom forms created with Pendragon 5 software (Pendragon Software Cooperation). We calculated dyadic association indices as (AB+BA)/2 with AB being the proportion of scans for male A in which male A was seen with male B, and BA being the proportion of scans for male B in which male B as seen with male A. We analyzed the datasets of 2010 and 2011 separately, applying hierarchical clustering based on Euclidean distances in Statistica 10 (StatSoft) (see SI Materials and Methods for details).
Additionally, A.P. recorded the identities of all adult individuals within a 1-m radius of the focal male at 10-min intervals and calculated the percentage of scans in which the focal males were in close proximity to an adult female, an adult male, or both simultaneously. We collected 1,480 such proximity estimates for 11 focal males (five in the Mare gang and six in the Simenti gang) in 2011 (mean, 135 scans per male; range, 127–140).
Finally, A.P. collected 466.1 h of continuous focal observations of 14 males, noting spatial proximity, affiliative interactions, greetings, and agonistic interactions, including the outcome of the agonistic interaction (decided/undecided) (SI Materials and Methods). Coalitionary support among adult males was recorded ad libitum. We defined “bond strength” based on the frequency with which two individuals sat in body contact (i.e., <10 cm), embraced, or groomed each other. Dyads that affiliated more frequently than the gang average [i.e., whose sociality index (the number of affiliative interactions per hour)/average number of affiliative interactions per hour in the subject’s gang) was >1] were considered to have a strong bond (7). Similarly, we identified preferred greeting partners and strong agonistic relationships by identifying those dyads that greeted each other or engaged in agonistic interactions more frequently than the gang average. However, correlations among networks were based on all interactions. Social interaction networks were based on weighted matrices (according to rates of interactions). Pearson correlation coefficients (r) were derived from a QAP. We ran 10,000 permutations to obtain P values. The dominance rank order was calculated in MatMan for Microsoft Excel Version 1.1 [Noldus Information Technology BV (53)], based on a giver–receiver matrix of decided agonistic interactions. We tested the presence of a linear dominance hierarchy separately for each period and gang. We used the improved linearity index (h’) because not all relationships between dyads were known. To assess the statistical significance of the degree of linearity, a two-step randomization test (10,000 randomizations) was performed. This test was one-tailed, and the significance level was set at 0.05.
Genetic Data.
Genetic relatedness was determined based on 25 autosomal microsatellite markers (Table S5). Dyadic relatedness coefficients were estimated in COANCESTRY v 1.0 (54). Correlations between the genetic and interaction networks and between the coalition and affiliation networks were calculated in UCInet (55).
Supplementary Material
Acknowledgments
We thank the Diréction des Parcs Nationaux and Ministère de l'Environnement et de la Protéction de la Nature de la République du Sénégal for permission to work in the Niokolo-Koba National Park (Permits 0383/24/03/2009 and 0373/10/3/2012); the conservator of the park, Mamadou Sidibe, for his support and cooperation; all the field assistants of the Centre de Recherche de Primatologie Simenti, especially Cheikh Sane, Samba Ciss, and Jacky Bassene; Noah Snyder-Mackler and Jinliang Wang for advice regarding the genetic analysis; Kurt Hammerschmidt for statistical support; and Adeelia Goffe, Thore Bergman, Brandon Wheeler, Peter Henzi, and Dorothy Cheney for valuable comments on earlier versions of the manuscript. The study was supported by the Deutsche Forschungsgemeinschaft Grant Fi707/9-1, the German Initiative of Excellence, and the Leibniz Graduate School for the Foundations of Primate Social Behaviour (Göttingen, Germany).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.C.A. is a Guest Editor invited by the Editorial Board.
See Commentary on page 14645.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1405811111/-/DCSupplemental.
References
- 1.Rodseth L, Wrangham R, Harrigan A, Smuts BB. The human community as a primate society. Curr Anthropol. 1991;32(3):221–254. [Google Scholar]
- 2.Hill KR, et al. Co-residence patterns in hunter-gatherer societies show unique human social structure. Science. 2011;331(6022):1286–1289. doi: 10.1126/science.1199071. [DOI] [PubMed] [Google Scholar]
- 3.Rodseth L. From bachelor threat to fraternal security: Male associations and modular organization in human societies. Int J Primatol. 2012;33(5):1194–1214. [Google Scholar]
- 4.Foley R, Gamble C. The ecology of social transitions in human evolution. Philos Trans R Soc Lond B Biol Sci. 2009;364(1533):3267–3279. doi: 10.1098/rstb.2009.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Traulsen A, Nowak MA. Evolution of cooperation by multilevel selection. Proc Natl Acad Sci USA. 2006;103(29):10952–10955. doi: 10.1073/pnas.0602530103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nowak MA. Five rules for the evolution of cooperation. Science. 2006;314(5805):1560–1563. doi: 10.1126/science.1133755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nowak MA, Tarnita CE, Antal T. Evolutionary dynamics in structured populations. Philos Trans R Soc Lond B Biol Sci. 2010;365(1537):19–30. doi: 10.1098/rstb.2009.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apicella CL, Marlowe FW, Fowler JH, Christakis NA. Social networks and cooperation in hunter-gatherers. Nature. 2012;481(7382):497–501. doi: 10.1038/nature10736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kappeler PM, van Schaik CP. Evolution of primate social systems. Int J Primatol. 2002;23(4):707–740. [Google Scholar]
- 10.Grueter CC, Chapais B, Zinner D. Evolution of multilevel social systems in nonhuman primates and humans. Int J Primatol. 2012;33(5):1002–1037. doi: 10.1007/s10764-012-9618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swedell L, Plummer T. A papionin multilevel society as a model for hominin social evolution. Int J Primatol. 2012;33(5):1165–1193. [Google Scholar]
- 12.Clutton-Brock T. Cooperation between non-kin in animal societies. Nature. 2009;462(7269):51–57. doi: 10.1038/nature08366. [DOI] [PubMed] [Google Scholar]
- 13.Langergraber KE, Mitani JC, Vigilant L. The limited impact of kinship on cooperation in wild chimpanzees. Proc Natl Acad Sci USA. 2007;104(19):7786–7790. doi: 10.1073/pnas.0611449104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blundell GM. Kinship and sociality in coastal river otters: Are they related? Behav Ecol. 2004;15(5):705–714. [Google Scholar]
- 15.Randić S, Connor RC, Sherwin WB, Krützen M. 2012. A novel mammalian social structure in Indo-Pacific bottlenose dolphins (Tursiops sp.): Complex male alliances in an open social network. Proc R Soc B Biol Sci 279(1740):3083–3090. [DOI] [PMC free article] [PubMed]
- 16.Clutton-Brock T. Breeding together: Kin selection and mutualism in cooperative vertebrates. Science. 2002;296(5565):69–72. doi: 10.1126/science.296.5565.69. [DOI] [PubMed] [Google Scholar]
- 17.Trivers RL. The evolution of reciprocal altruism. Q Rev Biol. 1971;46(1):35–57. [Google Scholar]
- 18.Kummer H. Social Organization of Hamadryas Baboons: A Field Study. Univ of Chicago Press; Chicago: 1968. [Google Scholar]
- 19.Boese GK. 1973. Behavior and Social Organization of the Guinea Baboon (Papio papio). PhD dissertation (The Johns Hopkins University, Baltimore, MD)
- 20.Galat-Luong A, Galat G, Hagell S, Tuttle RH. In: Reproduction and Fitness in Baboons. Behavioral, Ecological, and Life History Perspectives. Swedell L, Leigh SR, editors. Springer; New York: 2006. pp. 105–121. [Google Scholar]
- 21.Sharman M. 1981. Feeding, Ranging and the Social Organisation of the Guinea Baboon. PhD dissertation (University of St. Andrews, St. Andrews, UK))
- 22.Dunbar RIM, Nathan MF. Social organization of the Guinea baboon, Papio papio. Folia Primatol (Basel) 1972;17(5):321–334. doi: 10.1159/000155453. [DOI] [PubMed] [Google Scholar]
- 23.Patzelt A, et al. Group composition of Guinea baboons (Papio papio) at a water place suggests a fluid fission-fusion social organisation. Int J Primatol. 2011;32(3):652–668. doi: 10.1007/s10764-011-9493-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehmann J, Boesch C. To fission or to fusion: Effects of community size on wild chimpanzee (Pan troglodytes verus) social organisation. Behav Ecol Sociobiol. 2004;56(3):207–216. [Google Scholar]
- 25.Kopp GH, et al. The influence of social systems on patterns of mitochondrial DNA variation in baboons. Int J Primatol. 2014;35(1):210–225. doi: 10.1007/s10764-013-9725-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fickenscher G, et al. Genetic evidence for male philopatry in Guinea baboons. Folia Primatol (Basel) 2011;82(6):365. [Google Scholar]
- 27.Lukas D, Reynolds V, Boesch C, Vigilant L. To what extent does living in a group mean living with kin? Mol Ecol. 2005;14(7):2181–2196. doi: 10.1111/j.1365-294X.2005.02560.x. [DOI] [PubMed] [Google Scholar]
- 28.Kalbitzer U. 2014. Foundations of Variation in Male Aggressiveness and Tolerance Between Chacma Baboons (Papio ursinus) in Botswana and Guinea Baboons (P. papio) in Senegal. PhD dissertation (Georg-August-Universität Göttingen, Göttingen, Germany)
- 29.Whitham JC, Maestripieri D. Primate rituals: The function of greetings between male guinea baboons. Ethology. 2003;109(10):847–859. [Google Scholar]
- 30.Maciej P, Patzelt A, Ndao I, Hammerschmidt K, Fischer J. Social monitoring in a multilevel society: A playback study with male Guinea baboons. Behav Ecol Sociobiol. 2013;67(1):61–68. doi: 10.1007/s00265-012-1425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.le Roux A, Beehner JC, Bergman TJ. Female philopatry and dominance patterns in wild geladas. Am J Primatol. 2011;73(5):422–430. doi: 10.1002/ajp.20916. [DOI] [PubMed] [Google Scholar]
- 32.Boese G. In: Socioecology and Psychology of Primates. Tuttle RH, editor. Mouton Publishers; The Hague: 1975. pp. 205–230. [Google Scholar]
- 33.Schülke O, Bhagavatula J, Vigilant L, Ostner J. Social bonds enhance reproductive success in male macaques. Curr Biol. 2010;20(24):2207–2210. doi: 10.1016/j.cub.2010.10.058. [DOI] [PubMed] [Google Scholar]
- 34.Packer C, Gilbert DA, Pusey AE, O’Brien SJ. A molecular genetic analysis of kinship and cooperation in African lions. Nature. 1991;351(6327):563–565. [Google Scholar]
- 35.Ryder TB, Parker PG, Blake JG, Loiselle BA. 2009. It takes two to tango: Reproductive skew and social correlates of male mating success in a lek-breeding bird. Proc R Soc B Biol Sci 276(1666):2377–2384.
- 36.Carazo P, Tan CKW, Allen F, Wigby S, Pizzari T. Within-group male relatedness reduces harm to females in Drosophila. Nature. 2014;505(7485):672–675. doi: 10.1038/nature12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pappano DJ, Snyder-Mackler N, Bergman TJ, Beehner JC. Social “predators” within a multilevel primate society. Anim Behav. 2012;84(3):653–658. [Google Scholar]
- 38.Xiang Z-F, et al. Males collectively defend their one-male units against bachelor males in a multi-level primate society. Am J Primatol. 2014;76(7):609–617. doi: 10.1002/ajp.22254. [DOI] [PubMed] [Google Scholar]
- 39.Grueter CC, van Schaik CP. Evolutionary determinants of modular societies in colobines. Behav Ecol. 2009;21(1):63–71. [Google Scholar]
- 40.Rubenstein D, Hack M. In: Sexual Selection in Primates. Kappeler PM, van Schaik CP, editors. Cambridge Univ Press; New York: 2004. pp. 266–279. [Google Scholar]
- 41.Cant MA. 2011. The role of threats in animal cooperation. Proc R Soc B Biol Sci 278(1703):170–178.
- 42.Koenig A, Scarry CJ, Wheeler BC, Borries C. Variation in grouping patterns, mating systems and social structure: What socio-ecological models attempt to explain. Philos Trans R Soc B Biol Sci. 2013;368(1618):20120348. doi: 10.1098/rstb.2012.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Excoffier L, Foll M, Petit RJ. Genetic consequences of range expansions. Annu Rev Ecol Evol Syst. 2009;40:481–501. [Google Scholar]
- 44.Klopfstein S, Currat M, Excoffier L. The fate of mutations surfing on the wave of a range expansion. Mol Biol Evol. 2006;23(3):482–490. doi: 10.1093/molbev/msj057. [DOI] [PubMed] [Google Scholar]
- 45.Excoffier L, Ray N. Surfing during population expansions promotes genetic revolutions and structuration. Trends Ecol Evol. 2008;23(7):347–351. doi: 10.1016/j.tree.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Datta MS, Korolev KS, Cvijovic I, Dudley C, Gore J. Range expansion promotes cooperation in an experimental microbial metapopulation. Proc Natl Acad Sci USA. 2013;110(18):7354–7359. doi: 10.1073/pnas.1217517110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hallatschek O, Hersen P, Ramanathan S, Nelson DR. Genetic drift at expanding frontiers promotes gene segregation. Proc Natl Acad Sci USA. 2007;104(50):19926–19930. doi: 10.1073/pnas.0710150104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graciá E, et al. Surfing in tortoises? Empirical signs of genetic structuring owing to range expansion. Biol Lett. 2013;9(3):20121091. doi: 10.1098/rsbl.2012.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moreau C, et al. Deep human genealogies reveal a selective advantage to be on an expanding wave front. Science. 2011;334(6059):1148–1150. doi: 10.1126/science.1212880. [DOI] [PubMed] [Google Scholar]
- 50.Jolly CJ. Fifty years of looking at human evolution: Backward, forward, and sideways. Curr Anthropol. 2009;50(2):187–199. doi: 10.1086/597196. [DOI] [PubMed] [Google Scholar]
- 51.Taylor WA. Change-point analysis: A powerful new tool for detecting changes. 2000 Available at: www.variation.com/cpa/tech/changepoint.html. Accessed August 26, 2014. [Google Scholar]
- 52.Worton BJ. Kernel methods for estimating the utilization distribution in home-range studies. Ecology. 1989;70(1):164–168. [Google Scholar]
- 53.de VRIES. Finding a dominance order most consistent with a linear hierarchy: A new procedure and review. Anim Behav. 1998;55(4):827–843. doi: 10.1006/anbe.1997.0708. [DOI] [PubMed] [Google Scholar]
- 54.Wang J. COANCESTRY: A program for simulating, estimating and analysing relatedness and inbreeding coefficients. Mol Ecol Resour. 2011;11(1):141–145. doi: 10.1111/j.1755-0998.2010.02885.x. [DOI] [PubMed] [Google Scholar]
- 55.Borgatti SP, Everett MG, Freeman LC. 2002. Ucinet for Windows: Software for social network analysis. Available at https://sites.google.com/site/ucinetsoftware/home. Accessed August 26, 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.