Significance
SAMHD1 is a dNTPase that depletes the cellular dNTP pool to inhibit the replication of retroviruses, including HIV-1. The dNTPase activity of SAMHD1 also enables the enzyme to be a major regulator of cellular dNTP levels in mammalian cells, in addition to be implicated in the pathogenesis of chronic lymphocytic leukemia (CLL) and Aicardi Goutières syndrome (AGS). Here we present extensive structural and enzymatic data to reveal how SAMHD1 is activated and regulated via the combined actions of GTP and all cellular dNTPs. Our work establishes a complete spectrum of nucleotide binding and the exquisite regulatory mechanism of SAMHD1 in cellular dNTP metabolism, retrovirus restriction, and the pathogenesis of CLL and AGS.
Keywords: HIV restriction factor, dNTP metabolism, tetramerization, triphosphohydrolase, allosteric regulation
Abstract
The sterile alpha motif and HD domain-containing protein 1 (SAMHD1), a dNTPase, prevents the infection of nondividing cells by retroviruses, including HIV, by depleting the cellular dNTP pool available for viral reverse transcription. SAMHD1 is a major regulator of cellular dNTP levels in mammalian cells. Mutations in SAMHD1 are associated with chronic lymphocytic leukemia (CLL) and the autoimmune condition Aicardi Goutières syndrome (AGS). The dNTPase activity of SAMHD1 can be regulated by dGTP, with which SAMHD1 assembles into catalytically active tetramers. Here we present extensive biochemical and structural data that reveal an exquisite activation mechanism of SAMHD1 via combined action of both GTP and dNTPs. We obtained 26 crystal structures of SAMHD1 in complex with different combinations of GTP and dNTP mixtures, which depict the full spectrum of GTP/dNTP binding at the eight allosteric and four catalytic sites of the SAMHD1 tetramer. Our data demonstrate how SAMHD1 is activated by binding of GTP or dGTP at allosteric site 1 and a dNTP of any type at allosteric site 2. Our enzymatic assays further reveal a robust regulatory mechanism of SAMHD1 activity, which bares resemblance to that of the ribonuclease reductase responsible for cellular dNTP production. These results establish a complete framework for a mechanistic understanding of the important functions of SAMHD1 in the regulation of cellular dNTP levels, as well as in HIV restriction and the pathogenesis of CLL and AGS.
SAMHD1 is a dNTPase that hydrolyzes dNTPs into deoxyribonucleosides (dNs) and triphosphates (1). It has recently been identified as a restriction factor that blocks infection by a broad range of retroviruses, including HIV-1, in noncycling myeloid-lineage cells and quiescent CD4+ T lymphocytes (2–7). The dNTPase activity of SAMHD1 depletes the cellular dNTP pool, inhibiting the reverse transcription of the viral RNA genome (8–10). SAMHD1 was also found to inhibit infection by certain DNA viruses including herpes simplex virus type 1 (HSV-1) and vaccinia virus in nondividing macrophages (11, 12). Apart from viral restriction, SAMHD1 is ubiquitously expressed in both differentiated and undifferentiated cells of various human organs (2, 13), where it functions in the regulation of DNA damage signaling and proper activation of the innate immune response (14, 15). Mutations in SAMHD1, many of which result in deficiency in the dNTPase activity, are associated with chronic lymphocytic leukemia (CLL) (15, 16) and the autoimmune condition Aicardi-Goutieres syndrome (AGS) (17, 18).
It has been recognized that SAMHD1 may play an important role in the regulation of cellular dNTP levels, which are critical to the fidelity of DNA synthesis and the stability of the genome (13). Abnormal size and/or the imbalance of the dNTP pool may activate the S-phase checkpoint for cell cycle arrest (19, 20). In mammalian cells, the concentration of cellular dNTPs is tightly regulated and maintained during the cell cycle, notably by ribonucleotide reductase (RNR) during dNTP synthesis. RNR increases the dNTP pool by converting ribonucleoside diphosphates (NDPs) into dNDPs in a tightly controlled cell cycle-dependent manner (21). The activity of RNR is elegantly regulated by the binding of ATP, dATP, dTTP, and dGTP at two allosteric sites, which results in balancing of the production of all four dNDPs in the catalytic site of the enzyme. The ability of SAMHD1 to hydrolyze dNTPs allows it contribute to intracellular dNTP control naturally through dNTP degradation, thus influencing cell cycle progression and DNA replication (13).
SAMHD1 has an N-terminal sterile α motif (SAM) and a central histidine-aspartic (HD) domain. The SAM domain is a putative protein–protein and protein–nucleic acid interaction module, whereas the HD domain is responsible for dNTPase and viral restriction activities (1, 22–24). Structural and biochemical studies revealed that SAMHD1 was activated by allosteric dGTP-induced tetramerization, which shapes the substrate-binding pocket for dNTP binding and catalysis (25). Interestingly, recent enzymatic studies suggested that GTP is the major activator of SAMHD1 (26) and dNTPs substrates may also be involved in activating the enzyme (27), indicating a regulation mechanism more complex than previously thought.
To understand the complete mechanism of SAMHD1 activation and its potential role in cellular dNTP regulation, we carried out extensive structural and enzymatic studies on SAMHD1 interactions with nucleotides. Remarkably, the results reveal an exquisitely controlled enzymatic system, with eight allosteric binding sites and four catalytic sites in a functional tetramer, whose activity is regulated by the combined action of GTP and all four dNTPs. The regulation of SAMHD1 by cellular nucleotides is reminiscent of that of RNR, providing further mechanistic insight for the function of SAMHD1 in the enzymatic network of dNTP metabolism.
Results
GTP Together with Any of the Four dNTPs Induces the Formation of Active SAMHD1 Tetramer.
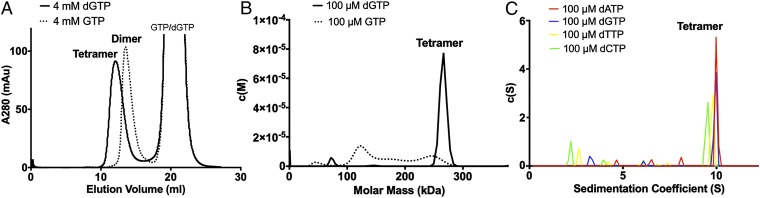
As GTP, instead of dGTP as previously reported (1), was recently suggested to be responsible for SAMHD1 activation (26), we investigated whether GTP binding alone can lead to the active tetrameric form of SAMHD1. Our results show that incubation of SAMHD1 with only GTP does not yield stable tetramers (Fig. 1). We used the inactive mutant of human SAMHD1 catalytic core SAMHD1c-RN (residues 113–626 with mutations of H206R/D207N), which has been shown previously to have identical nucleotide positioning and oligomerization properties as the active catalytic core (25). We first incubated SAMHD1c-RN with 4 mM dGTP or GTP and used size exclusion chromatography to examine the oligomeric state of the enzyme. The results show that SAMHD1c-RN incubated with GTP eluted from the column at a volume corresponding to a dimer, in contrast to the corresponding tetramer position for SAMHD1c-RN with dGTP (Fig. 1A). For independent verification, we measured the sedimentation rate of SAMHD1c-RN with 100 μM dGTP or GTP using sedimentation velocity analytical ultracentrifugation (SV-AUC). Indeed, SAMHD1c-RN with dGTP sedimented as a tetramer as reported previously (28), whereas little tetramer was detected in the sedimentation profile for SAMHD1c-RN with GTP (Fig. 1B). These experiments demonstrate that, unlike dGTP, GTP does not efficiently induce stable SAMHD1 tetramers.
Fig. 1.
GTP together with any dNTP, but not GTP alone, efficiently induces tetramerization of SAMHD1. (A) Size exclusion chromatograms of SAMHD1c-RN incubated with dGTP or GTP. Purified samples of SAMHD1c-RN (2 mg/mL, 200 μL) mixed with a final concentration of 4 mM dGTP or 4 mM GTP were applied to a Superdex 200 10/300 GL column. (B) Sedimentation velocity AUC results for SAMHD1c (1 mg/mL) with GTP (100 μM) or dGTP (100 μM). Little tetramer was formed with GTP alone. (C) Sedimentation velocity AUC results for SAMHD1c (1 mg/mL) with GTP (100 μM) and dNTP (100 μM) of any type.
Because SAMHD1 has two allosteric nucleotide-binding sites for each of the four subunits in the tetramer, it is possible that GTP and dNTP act in a cooperative manner to induce the tetramers and activate the enzyme. In fact, in the work that reported GTP as the primary activator, dNTP substrates were always present, together with GTP, in the SAMHD1 activity assays (26). To test the hypothesis that a combination of GTP and dNTP induces tetramerization of SAMHD1, we mixed GTP and a dNTP (100 μM) with SAMHD1c-RN and analyzed its oligomer state by SV-AUC. Indeed, the presence of GTP and a dNTP caused SAMHD1 to sediment at a position corresponding to a tetramer (Fig. 1C). These results, together with the previous observations that dGTP, but not any other dNTP, alone can induce tetramerization, suggest that the formation of the active SAMHD1 tetramer requires a guanine nucleotide (GTP or dGTP) together with another dNTP of any type.
Crystal Structures of Tetrameric SAMHD1 in Complex with GTP and a dNTP of Any Type.
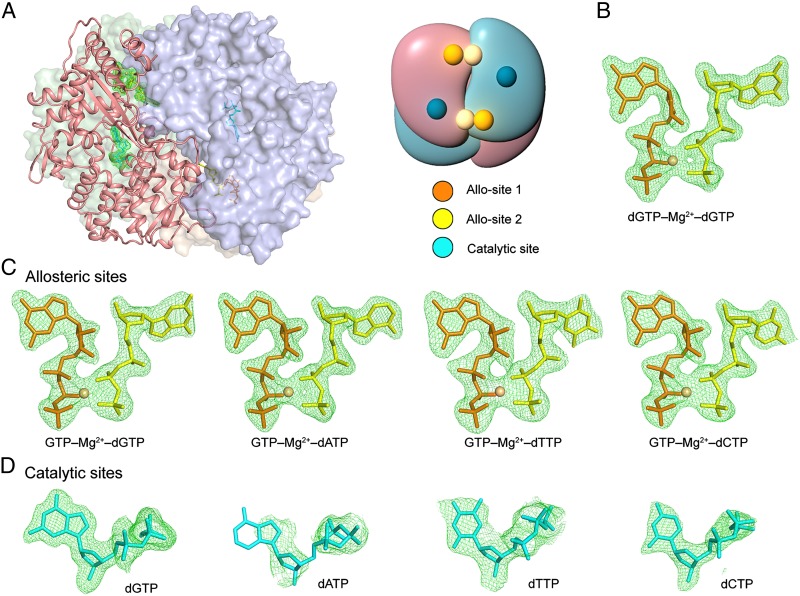
To understand the molecular basis of SAMHD1 activation by GTP and dNTPs, we cocrystallized SAMHD1c-RN with GTP and each of the four dNTPs at resolutions ranging from 2.2 to 2.9 Å. The structures were solved by molecular replacement using the dGTP-complex of tetrameric SAMHD1c-RN [Protein Data Bank (PDB) ID code 4BZB] (25), with the bound dGTP excluded, as a search model. Unbiased difference electron density (Fo-Fc) of high quality clearly revealed the identity of the bound nucleotides and allowed for successful model building (Fig. 2). Data collection and refinement statistics are listed in Table 1 and Table S1.
Fig. 2.
Crystal structures of SAMHD1 tetramer in complex with GTP and a dNTP of any type. (A) Structure of SAMHD1 tetramer bound to nucleotides (Left). The four subunits are shown as ribbon or surface presentation, colored in light blue, pale cyan, salmon, and wheat, respectively. Nucleotides are shown as sticks. The representative allosteric and catalytic nucleotides in one subunit (salmon) were highlighted with green meshes. (Right) The schematic of the tetramer. (B–D) Unbiased Fo-Fc difference electron density (σ = 3.0) of the nucleotides bound to the SAMHD1 tetramer. (B) The allosteric site dGTP-Mg2+-dGTP (PDB ID code 4BZB). (C) The four scenarios of allosteric site GTP-Mg2+-dNTPs. (D) The dNTP substrates at the catalytic site.
Table 1.
Summary of cocrystallization and ligand binding data
| Data | Cocrystallization nucleotides concentration (mM) ratio | Resolution (Å) | Rwork/Rfree | Allo-site 1 | Allo-site 2 | Catalytic site | |
| 1 | GTP:dATP | 4:1 | 2.6 | 0.22/0.24 | GTP | dATP | dATP* |
| 2 | GTP:dATP | 1:4 | 2.9 | 0.19/0.23 | GTP | dATP | dATP* |
| 3 | GTP:dATP | 1:10 | 2.8 | 0.22/0.24 | GTP | dATP | dATP* |
| 4 | GTP:dGTP | 4:0.4 | 2.3 | 0.17/0.21 | GTP | dGTP | dGTP* |
| 5 | GTP:dGTP | 2:2 | 2.6 | 0.21/0.25 | GTP | dGTP | dGTP* |
| 6 | GTP:dTTP | 4:1 | 2.4 | 0.19/0.24 | GTP | dTTP | dTTP* |
| 7 | GTP:dTTP | 1:4 | 2.4 | 0.20/0.24 | GTP | dTTP | dTTP |
| 8 | GTP:dTTP | 1:20 | 2.6 | 0.16/0.20 | GTP | dTTP | dTTP |
| 9 | GTP:dCTP | 1:4 | 2.4 | 0.18/0.20 | GTP | dCTP | dCTP |
| 10 | GTP:dATP:dGTP | 1:4:4 | 2.6 | 0.21/0.24 | dGTP | dATP | dGTP |
| 11 | GTP:dATP:dTTP | 1:4:4 | 2.4 | 0.21/0.24 | GTP | dATP | dTTP |
| 12 | GTP:dATP:dCTP | 1:4:4 | 2.8 | 0.21/0.23 | GTP | dATP | dCTP |
| 13 | GTP:dATP:dCTP | 1:1:4 | 1.9 | 0.19/0.21 | GTP | dATP | dCTP |
| 14 | GTP:dATP:dCTP | 1:0.1:5 | 2.3 | 0.19/0.22 | GTP | dATP | dCTP |
| 15 | GTP:dATP:dCTP | 1:0.1:10 | 2.6 | 0.22/0.24 | GTP | 2dATP:2dCTP | dCTP |
| 16 | GTP:dATP:dCTP | 1:0.1:20 | 2.0 | 0.20/0.22 | GTP | dCTP | dCTP |
| 17 | GTP:dATP:dCTP | 1:20:0.4 | 2.9 | 0.19/0.22 | GTP | dATP | † |
| 18 | GTP:dATP:dCTP | 1:20:0.1 | 2.5 | 0.17/0.21 | GTP | dATP | † |
| 19 | GTP:dGTP:dTTP | 1:4:4 | 2.3 | 0.19/0.22 | dGTP | dGTP | 3dGTP:1dTTP |
| 20 | GTP:dGTP:dCTP | 1:4:4 | 2.1 | 0.16/0.21 | dGTP | dGTP | dCTP |
| 21 | GTP:dGTP:dCTP | 1:1:10 | 2.1 | 0.20/0.23 | GTP | dGTP | dCTP |
| 22 | GTP:dTTP:dCTP | 1:4:4 | 2.8 | 0.23/0.26 | GTP | dTTP | dCTP |
| 23 | GTP:dTTP:dCTP | 1:1:10 | 2.9 | 0.21/0.24 | GTP | dTTP | dCTP |
| 24 | GTP:dGTP:dATP:dTTP | 1:4:4:4 | 2.4 | 0.22/0.24 | dGTP | dATP | 3dTTP:1dGTP |
| 25 | GTP:dATP:dTTP:dCTP | 1:4:4:4 | 2.1 | 0.17/0.20 | GTP | dATP | dCTP |
| 26 | GTP:dGTP:dCTP:dTTP | 1:4:4:4 | 2.4 | 0.21/0.24 | dGTP | dGTP | dCTP |
The base portion of the nucleotide is not fully observed.
Types of nucleotide could not be determined.
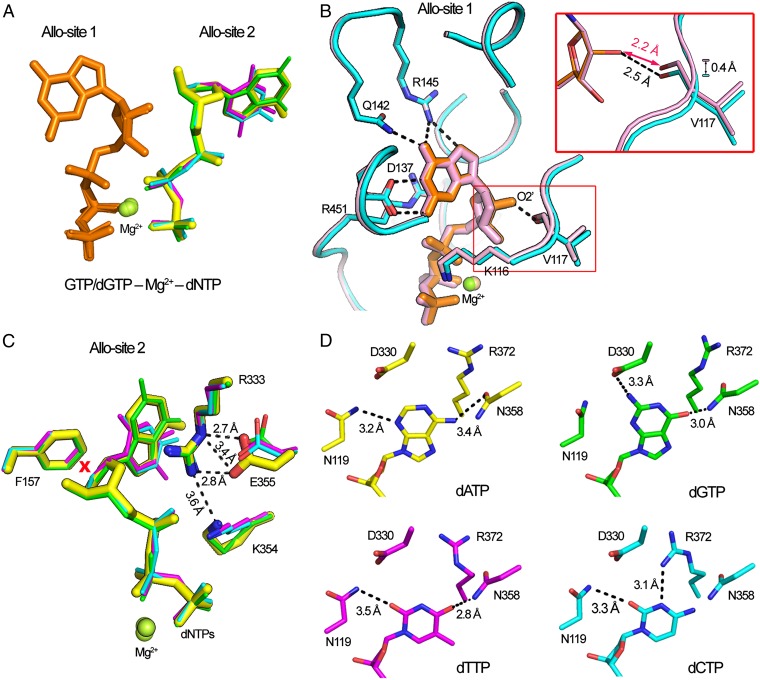
Consistent with our AUC results, SAMHD1c-RN in complex with GTP and various dNTPs crystallized as tetramers with virtually identical protein structures (RMSD 0.66–0.85 Å) to that of SAMHD1c tetramer observed previously (25). The four subunits in the SAMHD1 tetramer adopt the same conformation (RMSD < 0.25 Å), each containing two allosteric nucleotide-binding sites and a catalytic nucleotide-binding site. The two nucleotides at the allosteric sites are closely juxtaposed in an architecture that had been observed before, with a Mg2+ ion bridging their phosphate groups (Figs. 2 and 3A). Interestingly, one of the two allosteric sites (designated as Allo-site 1) can only be occupied by GTP or dGTP, whereas the other (designated as Allo-site 2) can bind all dNTPs. The two bound nucleotides have a GTP/dGTP–Mg2+–dNTP configuration (Figs. 2 and 3A), which includes the previous observed dGTP–Mg2+–dGTP configuration (25, 29).
Fig. 3.
The structures of the GTP-Mg2+-dNTPs at the allosteric sites. (A) Overlay of the structures of dGTP-Mg2+-dGTP and four dGTP-Mg2+-dNTPs. Nucleotides are shown as sticks and Mg2+ as spheres. Allo-site 1 nucleotides are colored in orange. Allo-site 2 nucleotides are colored individually according to dNTP types: dATP in yellow, dGTP in green, dTTP in magenta, and dCTP in cyan. (B) Superposition of Allo-site 1 structures of dGTP- or GTP-bound SAMHD1. SAMHD1 structures (pink: dGTP-bound, cyan: GTP-bound) are shown as ribbon with side chains of labeled residues shown as sticks. GTP and dGTP are shown in orange and pink, respectively. (Inset) Extra hydrogen bond (dashed line) between the 2’-hydroxyl group of GTP and main chain oxygen of V117. (C) Superposition of the structures of the four different dNTPs at Allo-site 2 of SAMHD1. dNTPs and labeled protein side chains are shown as sticks. The dATP-bound structure (yellow) is shown thicker than those of the other three. R333 stabilizes the bound dNTP through stacking interaction. The rigid side chain of F157 would cause steric clash (marked by a red X) with the 2′-OH group of an NTP. (D) The side chains of the surrounding residues adopt different conformations to adapt to the four different dNTP bases bound at Allo-site 2.
Allo-Site 1 Strictly Selects Guanine Nucleotides.
Guanine nucleotides, GTP or dGTP, are exclusively selected at Allo-site 1, where a network of five specific hydrogen bonds are formed between the base edge of guanine and the surrounding residues of D137, Q142, and R145. Nucleotides of other base identity, such as dATP, dTTP, or dCTP, cannot bind to Allo-site 1 even with the high concentrations (up to 20 mM) as used in the cocrystallization experiments. Superposition of the GTP- or dGTP-bound SAMHD1 at Allo-site 1 showed only minor structural changes for the protein on binding of the two nucleotides, which adopt nearly identical conformations including sugar puckering (Fig. 3 A and B). The only difference between the two structures is a small shift (∼0.4 Å) of residues adjacent to the GTP/dGTP-binding Allo-site 1 (residues 114–121). The observed shift is significant considering the data and model quality (30). This shift enables an extra hydrogen bond to be formed between the 2′-OH group of GTP and the main chain oxygen of V117. This additional interaction suggests a modestly higher affinity for GTP at this site. To verify this prediction, we cocrystallized SAMHD1c-RN with various concentrations of GTP and dGTP together and inspected the nucleotide species at Allo-site 1 in the resulting crystal structures. As expected, whenever equal or higher concentration of GTP was mixed with dGTP, GTP was found to occupy Allo-site 1. A structure with dGTP bound to Allo-site 1 was obtained with a dGTP/GTP ratio of 4. Considering that the physiological concentration of GTP is 1,000-fold higher than dGTP (31), we conclude that GTP occupies Allo-site 1 exclusively in cells.
Allo-Site 2 Can Accommodate a dNTP of Any Type, but Not NTPs.
Allo-site 2 precludes the binding of NTPs but is promiscuous for any dNTP. Cocrystallization of SAMHD1 with GTP and any dNTP, including with 10-fold excess of GTP over dGTP, did not yield structures with GTP bound to Allo-site 2 (Table 1). GTP is likely excluded because of the steric clash between its 2-OH′ group and the aromatic side chain of F157 in the tight binding pocket (25) (Fig. 3C). This exclusion explains why GTP alone is unable to form the allosteric nucleotide pair to induce the tetramerization of SAMHD1 (Fig. 1). In fact, we could not obtain any crystals of SAMHD1 with GTP alone or together with any other NTPs. In contrast, SAMHD1 crystals are readily grown in the presence of GTP and a dNTP, where different dNTPs can bind to Allo-site 2 with almost identical conformations (Fig. 3C). A flexible interaction network accommodates the binding of the four dNTPs, with the side chains of N119, D330, N358, and R372 changing conformations to maintain two hydrogen bonds with each of the different bases (Fig. 3D).
Base-stacking interactions also stabilize the nucleotides at the allosteric sites and can potentially lead to affinity variations for dNTP binding at Allo-site 2. In particular, the guanidinium group of R333 stacks with the base of the dNTP at Allo-site 2. Mutation of R333A has been shown to affect SAMHD1 tetramerization and abolish its function (25). The stacking interaction suggests a binding preference where the larger purine dNTPs are potentially favored at Allo-site 2. Interestingly, only when dATP binds to Allo-site 2 does the side chain of E355 move to further stabilize R333 by forming additional salt bridges (Fig. 3C). This local environment may subsequently increase the stability of the stacked dATP and result in a higher affinity for dATP over the other dNTPs at Allo-site 2.
The Catalytic Site Accommodates Any dNTPs.
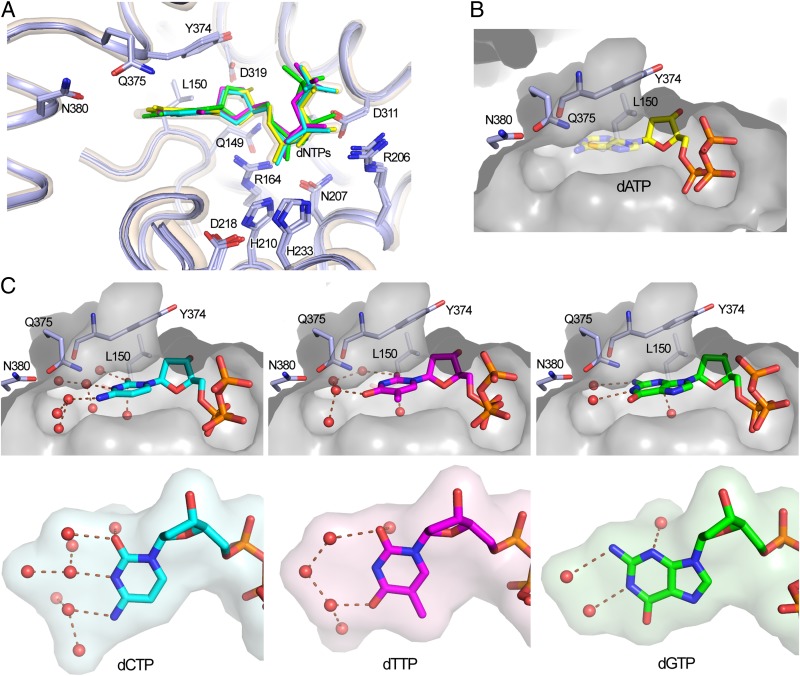
We obtained crystal structures of SAMHD1c-RN with each different dNTP bound to the catalytic site. The nucleotide-binding pocket and the bound dNTP adopt nearly identical conformations as seen in the previously reported SAMHD1-dGTP structure (25) (Fig. 4A). There is no specific contact between the enzyme and the base edge of any dNTPs, consistent with the ability of SAMHD1 to hydrolyze all four dNTPs. Instead water molecules mediate interactions between the base edge of the dNTPs and the enzyme nonspecifically. Interestingly, it appears that dNTPs with pyrimidine bases (dCTP and dTTP) have more extensive bridging-water networks than those of purine dNTPs (Fig. 4 B and C). These extensive water networks could potentially favor dCTP/dTTP binding at the catalytic site. We did not observe full occupancy of dATP at the catalytic site (Fig. 2D), even with the highest concentration (10 mM) among all dNTPs used for cocrystallization. This observation suggests that dATP may have the weakest binding affinity at the catalytic site. It should be noted that the RN mutation of SAMHD1 does not alter the positioning of the dNTP substrate in the native conformation (25). It is possible that the overall binding affinities are changed due to the mutation affecting the strength of interaction with the triphosphate group. However, the relative binding preference/selectivity to various dNTPs should be maintained, as this is dictated by SAMHD1 interaction with the bases and is not affected by the mutation.
Fig. 4.
The structures of the dNTP substrates at the catalytic site. (A) Superposition of the structures of different dNTPs bound to the catalytic site of SAMHD1. The color scheme of the dNTPs is the same as in Fig. 3. The structure of the SAMHD1-dGTP complex is in semitransparent ribbon presentation (wheat). dNTPs and critical side chains were shown as sticks. (B) dATP binds weakly to the substrate-binding pocket (surface) with no clear conformation observed for the adenine base (semitransparent sticks). (C) The substrate-binding pockets (surface) and the bound dCTP, dTTP, and dGTP (sticks). Extensive water molecules (red spheres) at the dNTP base edges bridge the nonspecific interactions with the substrate-binding pocket. Perpendicular views of the base and water surfaces are shown in the lower panels. The pyrimidine bases (dCTP and dTTP) appear to have more extensive water networks than that of the purine base of dGTP.
Relative Binding Affinities for Different dNTPs.
We used a structure-based approach to assess the binding affinity of dNTPs at the allosteric and catalytic sites, using an additional 18 crystal structures of SAMHD1 cocrystallized with various nucleotide mixtures. The structure-based approach was necessary because of the complexity of the large number (12) of nucleotide-binding sites in the SAMHD1 tetramer. The large number of binding sites, together with the monomer/dimer to tetramer transitions induced by GTP/dNTP binding, prohibits the analysis by standard solution biophysical techniques. We set up a series of simultaneous cocrystallization trials for SAMHD1c-RN in the presence of 1 mM GTP and different combinations of dNTPs. In each cocrystallization experiment, a mixture of dNTPs was used for either pairwise or three-dNTP binding competition at various ratios. All of the crystals used in the analysis diffracted to resolutions between 2.0 and 2.9 Å, which yielded clear unbiased electron density for the bound nucleotides (Fig. S1). The data and refinement statistics are summarized in Table 1 and Table S1.
Our comprehensive structure-based binding analysis shows that GTP always occupy Allo-site 1 under physiological conditions (as discussed above), and interestingly, the order of preference for dNTPs at Allo-site 2 tends to be the opposite of that at the catalytic site. As anticipated from the structural analysis, purine nucleotides showed higher affinity to bind Allo-site 2 than pyrimidine nucleotides, with the order of dATP > dGTP > dTTP > dCTP. Notably, dATP is the favored ligand at Allo-site 2 and cannot be competed off even with a 100-fold higher concentration of dCTP (Table 1). However, dATP displayed the weakest binding at the catalytic site, where the order of the binding preference is dCTP > dGTP/dTTP > dATP. We observed uniform binding of a dNTP at the four equivalent Allo-sites 2 or catalytic sites in a SAMHD1 tetramer in most of the cases. This observation indicates that under the experimental conditions, such as when equal concentrations of all four dNTPs are presented to the enzyme, the affinity differences between the nucleotides are large enough to ensure binding of the same dNTP at all equivalent sites. Interestingly, when the ratio of the nucleotides was varied, we observed mixed binding of different dNTPs at equivalent sites in the tetramer in three cases (Table 1). This finding demonstrates that SAMHD1 can tetramerize and be activated by binding of a mixture of dNTPs and can potentially hydrolyze different substrates simultaneously within the same tetramer complex.
GTP and Any of the Four dNTPs Can Allosterically Activate SAMHD1.
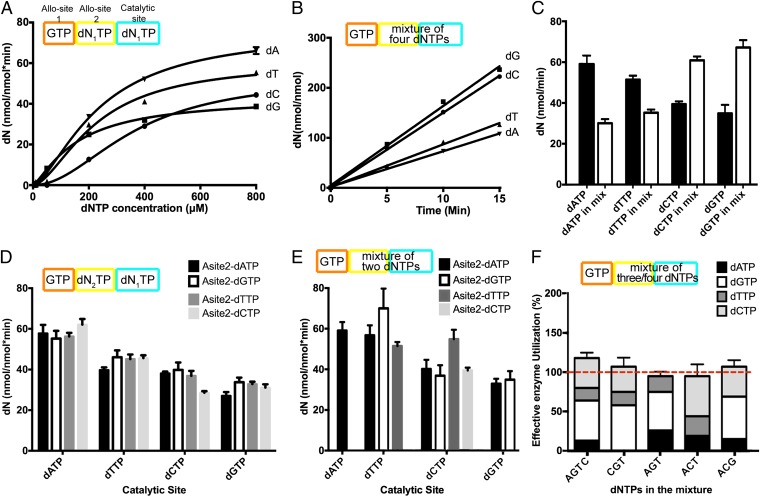
As GTP together with any dNTP can promote the formation of SAMHD1 tetramer required for the dNTPase activity, we next investigated whether GTP and dNTP act in a cooperative manner to activate SAMHD1. WT SAMHD1c was incubated with GTP and a dNTP at various equal concentrations (0–800 μM), and the reaction product dN was monitored by HPLC. This experimental design ensured that Allo-site 1 was occupied by GTP, and a specific dNTP was present in both Allo-site 2 and the catalytic site. The results confirmed that in the presence of GTP and dNTP, SAMHD1 was indeed activated to hydrolyze dNTPs rapidly. The apparent turnover rates vary up to twofold for different dNTP substrates in the order of dATP > dTTP > dCTP > dGTP (Fig. 5A). The reaction profiles were fitted to a sigmoidal kinetic function, which resulted in various degrees of sigmoidal shaped curves expected from cooperativity effect due to allosteric binding. The dGTP reaction showed the lowest cooperativity, which is consistent with that observed previously (27). In contrast, the dCTP reaction profile showed the most pronounced sigmoidal behavior of activation. This reaction profile agrees with our crystallographic result that dCTP has the lowest binding affinity at Allo-site 2 and thus has the least capacity to induce tetramerization.
Fig. 5.
Steady state kinetics for SAMHD1 dNTPase activity. All experiments were performed in triplicate. Schematic of the experimental setup for each assay is shown above the plots. (A) Single-dNTP substrate dNTPase assays. The dNTPase activity of SAMHD1c (0.5 μM) was assayed with indicated concentrations of dNTP, together with the same concentrations of GTP, for 5–15 min. The amount of dN product generated in the reactions was quantified by HPLC. (B) dNTPase assays with a mixture of all four dNTP substrates. Experiments were performed with a mixture of four dNTPs (500 μM each), together with SAMHD1c (0.5 μM) and GTP (500 μM). The linear initial rates of dN production were plotted. (C) Comparison of the dNTP hydrolysis rates in the single-substrate (black columns) and the four-substrate (white columns) assays at the dNTP(s) concentration of 500 μM. Each rate was normalized to that at the same concentration ratio of enzyme to total dNTPs. (D) dNTPase assays with preassembled SAMHD1 tetramers. SAMHD1c tetramers were preassembled by incubation with GTP and a particular Allo-site 2 dN2TP as indicated. After 100-fold dilution with reaction buffers containing a specific dN1TP substrate, the product was quantified and plotted as indicated. (E) dNTPase assays with pair-wise dNTP substrates. SAMHD1c (0.5 μM) activity assay were performed with GTP (500 μM) and a pair of dNTPs (500 μM each). The production rates of dNs were quantified and kappdN1-dN2 values were calculated as described in Materials and Methods. The rates are displayed as indicated. (F) Validation of the calculated kappdN2-dN1 values by the predicted effective enzyme utilization in the independent dNTPase assays with 3-dNTP or 4-dNTP mixtures. The kappdN2-dN1 values obtained in E lead to a calculated total effective enzyme utilization of 100% in each experiment, indicating the rates were correctly obtained.
GTP and All dNTPs Together Control the Rates of Substrate Hydrolysis.
To examine how GTP and all four dNTPs together control SAMHD1 activity, as is the situation in vivo, we mixed an equal amount of all four dNTPs (500 μM each) with SAMHD1c in the presence of 500 μM GTP and analyzed the reaction products (Fig. 5B). The results show that in the presence of all four dNTP substrates, the hydrolysis rates are in the order of dGTP > dCTP > dTTP > dATP, which is the opposite of that obtained in the single dNTP substrate experiments (dATP > dTTP > dCTP > dGTP) (Fig. 5C). The order of rates obtained with the mixture of all dNTPs agrees with the previous observations in vitro with Allo-site 1 of SAMHD1 occupied by dGTP (1, 24, 28) and the siRNA silencing experiment in vivo (13). The rate changes between the single dNTP and the dNTP mixture assays may reflect potential cross-talk between the allosteric sites and the catalytic site, i.e., different apparent turnover rates for the same dNTP substrate when Allo-site 2 was occupied by different dNTP cofactors. The overall rate change may also be a result of the competition of various dNTPs for the same catalytic sites, even though the individual rates do not differ by very much.
Different Allosteric dNTP Cofactors Activate SAMHD1 to a Similar Level.
We used multiple approaches to investigate whether different allosteric dNTPs affect the catalysis rate for any given substrates. We analyzed the apparent turnover rate of each dNTP with various dNTP cofactors at Allo-site 2, kappdN1-dN2 (dN1: substrate dNTP; dN2: Allo-site 2 dNTP). Our first approach uses SAMHD1 tetramers preassembled with a specific dNTP and measures the rate of hydrolysis of other dNTPs. This approach is possible because our previous biochemical data indicate that SAMHD1 tetramers are stable and may perform multiple catalytic cycles without having to go through the dimer–tetramer transition (28). This approach is also supported by the recent report that the activated state of SAMHD1 is long-lived even when dNTP levels are subsequently reduced to very low levels (27). We preincubated SAMHD1c with GTP (500 μM) and a specific dNTP (dN2, 500 μM) to form the active tetramer. The solution was subsequently diluted 100-fold rapidly by adding the assay buffer containing the desired dNTP substrate (dN1, 500 μM) to initiate the reaction. The results show that the apparent turnover rate for a substrate barely changed when Allo-site 2 was occupied by different dNTPs (Fig. 5D). These experiments demonstrate that different allosteric dNTPs activate SAMHD1 to a similar level. A potential caveat of this assay is that the preassembled SAMHD1 tetramer is not stable and allows for the exchange of substrate dNTPs into Allo-site 2. Consequently, the measured rate would be a mixture of kappdN1-dN2 and kappdN1-dN1 and may even be dominated by kappdN1-dN1 due to the 100-fold higher concentration of dN1 vs. the concentration of dN2.
We used a second approach to confirm the measured apparent turnover rates kappdN1-dN2 while avoiding the potential dissociation problem of the preassembled SAMHD1 tetramers. This assay is based on measurements of individual dN products within different pairwise combinations of dNTPs that were presented to SAMHD1 with GTP (Table S2). A key to this approach of rate analysis is to delineate the effective concentration of the SAMHD1 enzyme, with a particular dNTP cofactor at Allo-site 2, used by each dNTP substrate in the mixture, [SAMHD1dN1-dN2]. The analysis was made possible by our knowledge of the hydrolysis rates determined in the single-substrate experiments (kappdNi-dNi) and the relative ranking of dNTP affinities at Allo-site 2 obtained from our structure based affinity assays. For example, when equal concentrations of dATP and dCTP are mixed with SAMHD1/GTP, dATP will occupy Allo-site 2 because of its much higher affinity (Table 1). We can then calculate [SAMHD1dA-dA] by comparing the dA production rate measured in the dATP/dCTP mixture experiment with that obtained with dATP alone. This calculation in turn gives the remaining fraction of SAMHD1 used by the dCTP substrate, [SAMHD1dC-dA]. kappdC-dA can finally be calculated by dividing the measured production rate of dC by [SAMHD1dC-dA].
We obtained 10 possible kappdN1-dN2 values from experiments performed with all combinations of dNTP pairs at equal concentrations (Fig. 5E and Table S2), which reflect the cellular condition of balanced dNTP pools in the cell. Under these conditions, some turnover events are not preferred as our structure-based affinity assays showed that the dNTP with higher affinity dominates Allo-site 2. For example, little dCTP will occupy Allo-site 2 in the presence of equal concentration of dATP, i.e., only kappdA-dA or kappdC-dA, but not kappdA-dC, is relevant and measurable. In fact, in our experiment with a mixture of all four dNTPs described above, dATP (highest affinity) is most likely incorporated into the majority of Allo-site 2 in each enzyme, which then acts on four dNTP substrates with individual apparent turnover rates of kappdN-dA (Fig. 5C). The results from the dNTP-pair experiments again show the hydrolysis rates of a dNTP do not differ significantly with different allosteric dNTPs (Fig. 5E), confirming the observation made from the preassembled SAMHD1.
To further verify our experimental design and calculations, we also performed activity assays with all combinations of three dNTPs as substrates. The procedure is similar to that described for the pairwise dNTP experiments with a variation in analysis. In this case the measured dN production rates, together with the kapp values obtained earlier, were used to predict the amount of effective SAMHD1 used by each dNTP substrate in the 3-dNTP mixtures. Only when the kapp values were accurately obtained in the 2-dNTP experiments can we use them to predict the correct effective enzyme utilization by different substrates in the 3-dNTP mixtures, which should sum up to 100% in each experiment. We also performed the same analysis for the experiment with the mixture of all four dNTPs. Indeed, the total effective enzyme used by all dNTPs in each experiment converged to ∼100%, which validates our calculations and supports the result that the apparent turnover rates for a given dNTP substrate are basically unaltered when different dNTP cofactors are bound at the allosteric site of SAMHD1 (Fig. 5F).
These experiments demonstrate that SAMHD1 has an overall regulation mechanism where any allosteric dNTP cofactor likely activates the enzyme to a similar extent. The final reaction rates for the dNTP substrates are then controlled by their competition for the catalytic sites. This property is exemplified in the case of dATP, where there is only a single, relatively large rate kappdA-dA in experiments with either dATP alone or the mixture of all four dNTPs. However, the rate of dA production in the 4-dNTP mixture dropped to below half of that of dATP alone, which can be explained by the weak ability of dATP to compete for SAMHD1 active sites. This explanation is consistent with our structure-based affinity analysis, where dATP has the lowest affinity at the catalytic site. Small variations of kapp values are observed for the same dNTP substrate with SAMHD1 bound with different dNTP cofactors, which can be a result of a subtle regulation of SAMHD1 activity by different dNTPs at Allo-site 2. Our steady-state kinetic analysis offers an initial evaluation of the overall rate-limiting step of the complex reactions of dNTP hydrolysis by SAMHD1. More comprehensive analysis is required to dissect the detailed kinetic reaction pathway.
Discussion
SAMHD1 has been proposed to play an important role in the control of cellular dNTP pools, in addition to its functions in preventing viral infection and autoimmunity (13, 32). A central question with respect to these SAMHD1 functions is how the dNTPase activity of the enzyme is regulated by dNTP metabolism. It has been demonstrated previously that SAMHD1 is activated by allosteric dGTP- and/or GTP-induced tetramerization. Although the cellular dGTP concentration may provide a convenient signal for activity control, sensing the level of a single nucleotide only (dGTP) does not appear to be a robust regulatory mechanism. Based on SAMHD1 activity on dGTP and dUTP, it has been recently proposed that GTP and dNTP substrates activate SAMHD1 together (27). However, fundamental questions about whether and how GTP collaborates with each of the four dNTPs to active SAMHD1 and the regulation of SAMHD1 activity by different cellular dNTPs have not been elucidated.
The study presented herein establishes a comprehensive structural and biochemical framework for understanding the important functions of SAMHD1 in cellular dNTP regulation. Our crystal structures and enzymatic assays illustrate a robust regulatory mechanism by which GTP and all four dNTPs act together to control the activity of SAMHD1. Although both GTP and dGTP can occupy Allo-site 1, GTP should be the primary activator given its intrinsic higher affinity to the site and its much higher cellular concentration. It appears that all four dNTPs can activate SAMHD1 to a similar level, making the enzyme a robust, general sensor of cellular dNTP pools. Interestingly, under conditions of a balanced cellular pool where all dNTPs are at a similar concentrations, it is most likely that dATP occupies Allo-site 2 due to its highest affinity for this site (Table 1). Therefore, the most probable activator configuration at the two juxtaposed allosteric sites is GTP–Mg2+–dATP, which should occur under normal cellular conditions in nondividing cells and the G1 phase of dividing cells. Nonetheless, the ability of SAMHD1 to sense all dNTPs enables a general feedback system to achieve a balanced cellular dNTP pool in uncommon or extreme conditions where cellular dNTPs are unbalanced, such as during malfunction in cellular dNTP production or during the invasion of certain dsDNA viruses that manipulate the dNTP pool to favor viral reproduction (12).
Our study provides a structural basis and complementary analysis to the reported study on dGTP/dUTP catalysis by SAMHD1 (27). During the preparation of this manuscript, Hansen et al. (27) reported the combined activation of SAMHD1 tetramer by GTP and dUTP. Based on enzymatic activity assays for dUTP/dGTP hydrolysis and structural modeling, the report predicted that any type of dNTP could bind to Allo-site 2 and activate SAMHD1 in a similar way. Our crystal structures confirm the proposal and provide a structural explanation to the reported experimental data. Furthermore, our kinetic analysis extends to all cellular dNTPs, including analysis of double- and triple-nucleotide combinations, which result in reaction profiles and range of reaction rates consistent with the published data from single-nucleotide experiments on dUTP or dGTP. These complementary results further illuminate the dNTPase activity of SAMHD1.
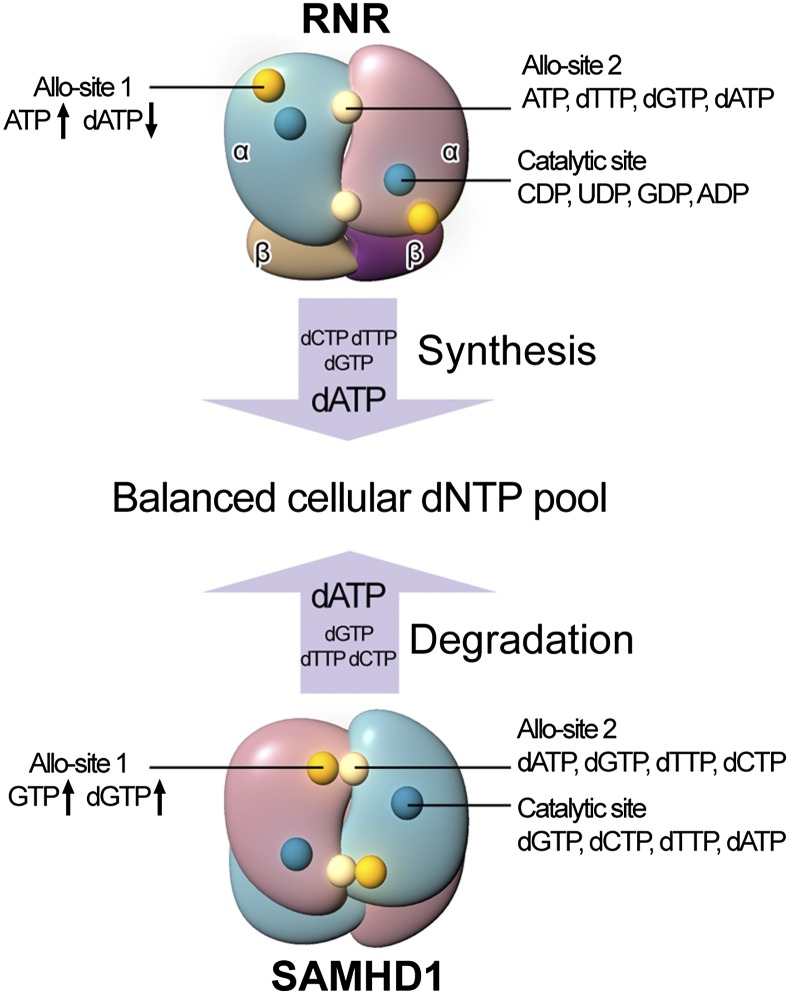
Striking connections exist between the enzymatic properties of SAMHD1 and RNR (Fig. 6), which are responsible for cellular dNTP degradation and production, respectively. First, both of the enzymes are active oligomers with two types of allosteric sites that sense the cellular nucleotide concentrations and control the activity at a specific catalytic site. Second, the activity of both enzymes is finely regulated by binding of nucleotides at the respective allosteric sites, which influences the conformations of the catalytic sites to either activate or inactivate the enzymes. Third, in each case Allo-site 1 is nucleotide base specific and Allo-site 2 has binding promiscuity toward multiple dNTPs. Specific NTP/dNTP binding at the Allo-site 1 of either enzyme controls the respective catalytic activity, although in different ways. Human RNR is activated as a α2β2 heterotetramer when ATP is bound to Allo-site 1, whereas binding of dATP at the same site inhibits the activity. In contrast, the SAMHD1 homotetramer is activated by binding of either dGTP or GTP at the Allo-site 1. Another major difference between the two enzymes is that ATP and dNTPs bound to the allosteric sites of RNR control its activity on nucleoside diphosphates (rNDPs) substrates bound to the catalytic sites, whereas dNTPs are used as both allosteric activators and substrates for SAMHD1.
Fig. 6.
SAMHD1 and RNR regulate cellular dNTP pool together. RNR and SAMHD1 are responsible for the production and degradation of dNTPs, respectively. Both of them have three specific nucleotides binding sites as indicated, with the types of the nucleotides that can occupy the sites marked on the side. The concentrations of cellular dNTPs are sensed and regulated by both of the enzymes. dATP is produced last by RNR and degraded last by SAMHD1.
SAMHD1 and RNR may have evolved to use cellular dATP levels as an overall control switch for their activities, albeit in opposite ways as dictated by the reciprocal function of the two enzymes. dATP is the last to be synthesized by RNR in a synthetic cycle and is used as a feed-back inhibitor for switching off the production of cellular dNTPs by RNR (33). In contrast, our data show that dATP has the highest affinity for the allosteric site of SAMHD1, enabling it to be the most sensitive activator for dNTP degradation. In addition, dATP has the weakest affinity for the catalytic site of SAMHD1 and has the slowest hydrolysis rate when all dNTPs are present (Fig. 5B); therefore, it is likely the last dNTP to be depleted in the cell. In summary, the coordinated uses of dATP facilitate the coordination of the activities of both RNA and SAMHD1. The last nucleotide made in the dNTP production pipeline is dATP and a high level of which will shut down the production enzyme RNR. A high level of dATP simultaneously activates the degradation enzyme SAMHD1, whose dNTPase activity only stops after all dNTPs are brought to low steady state levels, with dATP being the last to be degraded (Fig. 6).
Maintaining a balanced cellular dNTP pool at an appropriate level is of paramount importance for cell cycle progression, genome integrity, proper immune activation, and viral restriction of viruses (19, 34, 35). The opposite, cell cycle-dependent manner of regulation of RNR and SAMHD1 expression is the major control mechanism for maintaining the desired dNTP concentrations in the dividing cells. The expression of SAMHD1 is minimized, whereas the production of RNR is maximized during S-phase when large amount of dNTPs are essential for optimal rates of DNA replication (13). The work presented here provides a structural and enzymatic basis for understanding how SAMHD1 joins RNR for dNTP regulation in nondividing cells and G1 phase of dividing cells, where the coordinated actions between the two enzymes helps to keep the cellular dNTP pool low and balanced. This insight also forms a foundation for deciphering the mechanisms of SAMHD1 functions in HIV restriction and the pathogenesis of chronic lymphocytic leukemia and Aicardi Goutières syndrome.
Materials and Methods
Protein Expression and Purification.
N-terminal 6× His-tagged SAMHD1c (residues 113–626) and its RN mutant (H206R D207N) were expressed in Escherichia coli and purified using nickel-nitrilotriacetic acid (Ni-NTA) affinity and size-exclusion chromatography as previously described (25).
Analytical Size Exclusion Chromatography.
Purified samples of SAMHD1c-RN (2 mg/mL, 200 μL) mixed with a final concentration of 4 mM dGTP or 4 mM GTP were applied to a Superdex 200 10/300 GL column (GE Healthcare) preequilibrated in 50 mM Tris⋅HCl, pH 8.0, 150 mM NaCl, 5 mM MgCl2, and 0.5 mM Tris(2-carboxyethyl)phosphine hydrochloride (TCEP). The UV absorbance at 280 nm was recorded for the elution of SAMHD1 oligomers.
AUC.
Sedimentation velocity experiments were performed with a Beckman XL-I analytical ultracentrifuge. Samples were prepared with protein concentration of 1 mg/mL in the buffer containing 50 mM Tris⋅HCl, pH 8.0, 150 mM NaCl, 5 mM MgCl2, and 0.5 mM TCEP and equilibrated with a final concentration of 100 μM nucleotide. AUC was performed at 42,000 rpm and 20 °C with an An60-Ti rotor. The experimental parameters including sample partial specific volume, buffer density, and viscosity were calculated with SEDNTERP (http://sednterp.unh.edu/). Velocity data were analyzed using the program SEDFIT (36).
Crystallization and Data Collection.
SAMHD1c-RN in buffer (20 mM Tris⋅HCl, pH 7.8, 50 mM NaCl, 5 mM MgCl2, and 2 mM DTT) was mixed with various combinations of nucleotides (Table 1) and incubated at 4 °C for 30 min before crystallization. Crystals were grown at 25 °C using the microbatch under-oil method by mixing 1 μL protein (5 mg/mL) with 1 μL crystallization buffer [100 mM SPG (Qiagen) buffer, pH 7.4, 25% polyethylene glycol 1500 (PEG 1500)]. Crystals were cryoprotected by crystallization buffer supplemented with 25% (vol/vol) glycerol before frozen in liquid nitrogen. Diffraction data were collected at the Advanced Photon Source beamline 24-ID. The data statistics are summarized in Table S1.
Structure Determination and Refinement.
The structures were solved by molecular replacement using PHASER (37). The previously published SAMHD1 tetramer structure, with all bound nucleotides removed, was used as the search model (PDB ID code 4BZB). The model was refined with iterative rounds of TLS (translation/libration/screw) and restrained refinement using Refmac5 (38) followed by rebuilding the model to the 2Fo-Fc and the Fo-Fc maps using Coot (39). Refinement statistics are summarized in Table S1.
Coordinates and structure factors have been deposited in the Protein Data Bank, with accession codes listed in Table S1.
SAMHD1 dNTPase Activity Assays.
All SAMHD1 dNTPase activity assays were performed in a reaction buffer containing 50 mM Tris⋅HCl, pH 8.0, 150 mM NaCl, 5 mM MgCl2 and 0.5 mM TCEP. GTP, dNTP(s), and purified SAMHD1c were mixed at the indicated concentrations and incubated at 25 °C. Aliquots of reaction samples were collected at various times points with the reaction terminated by 5× dilution into ice cold buffer containing 10 mM EDTA, followed by spinning through an Amicon Ultra 0.5 mL 10 kDa filter (Millipore) at 16000 ×g for 20 min. Deproteinized samples were analyzed by HPLC using a Synergi C18 Column 150 × 4.6 mm (Phenomenex). The column was pre-equilibrated in 20 mM ammonium acetate, pH 4.5 (buffer A). Injected samples were eluted with a linear methanol gradient over 14 min at a flow rate of 1 mL/min. The yields were quantified by integration of the calibrated UV absorption peak at 260 nm.
In the single-dNTP substrate assay, the initial rates of product formation catalyzed by 0.5 μM SAMHD1c were measured up to no more than 20% completion and plotted against the substrate concentration of each dNTP. The reaction profiles were fitted to a sigmoidal kinetic function (Eq.1),
| [1] |
where Vmax is the maximum dNTP production rate, Khalf is the concentration of substrate that produces a half-maximal enzyme velocity and h is the Hill coefficient.
In the preassembled tetramer assay, samples containing of purified SAMHD1c (50 μM) were preincubated with GTP (500 μM) and a particular dNTP (500 μM) for 1 min before diluted 100-fold rapidly with the assay buffer containing 500 μM a desired substrate dNTP to initiate reactions, and the time course of product formation was measured by HPLC as described for the single-dNTP assays.
The pair-wise dNTP mixture assays were performed with equal amount of two dNTPs to obtain kappdN1-dN2, the apparent turnover rates for the dN1TP substrate with the dN2TP cofactor at Allo-site 2. The VdNimixture, the dNi (i = 1,2) production rate in the dNTPs mixture, were measured. The identity of the nucleotide (dN2TP) that occupies Allo-site 2 was determined from the results from our structure-based affinity assays. VdN2mixture and the apparent turnover rate kappdN2-dN2, obtained in the single-dNTP activity assays, was used to calculate [SAMHD1dN1-dN2], the fraction of SAMHD1 that hydrolyzes dN1TP with dN2TP at Allo-site 2 (Eq. 2–3). The kappdN1-dN2 parameter was then calculated as follows (Eq. 4):
| [2] |
| [3] |
| [4] |
Similar analyses were performed for three- or four-dNTP (500 μM each) mixture assays. The production rates of dNi were quantified and the dN2TP bound to Allo-site 2 was determined as above. The fractions of effective SAMHD1 used by dNiTP substrates, [SAMHD1dNi-dN2] (i = 1,2,3,4), were then calculated using VdNimixture and the kapp values determined in the pair-wise dNTP mixture assays (Eq.5). If each kapp rate remained unchanged in the various experiments and were correctly calculated, the sum of all [SAMHD1dNi-dN2] should give 100% effective enzyme utilization, which can therefore serve as a validation to the rates obtained in the assays.
| [5] |
Supplementary Material
Acknowledgments
We thank X. Jia, J. Fribourgh, H. Nguyen, and E. Weber for assistance and discussion, K. S. Anderson and W. H. Konigsberg for comments, and M. Alonso, J. Deacon, R. Vithayathil, and S. Wojcik for technical assistance. We also thank the staff at the Advanced Photon Source beamlines 24ID-C and E and the National Synchrotron Light Source beamlines X25. This work was supported by National Institutes of Health Grant AI097064 (to Y.X.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 4TNP, 4TNQ, 4TNR, 4TNX, 4TNY, 4TNZ, 4TO0, 4TO1, 4TO2, 4TO3, 4TO4, 4TO5, and 4TO6).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1412289111/-/DCSupplemental.
References
- 1.Goldstone DC, et al. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature. 2011;480(7377):379–382. doi: 10.1038/nature10623. [DOI] [PubMed] [Google Scholar]
- 2.White TE, et al. The retroviral restriction ability of SAMHD1, but not its deoxynucleotide triphosphohydrolase activity, is regulated by phosphorylation. Cell Host Microbe. 2013;13(4):441–451. doi: 10.1016/j.chom.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Descours B, et al. SAMHD1 restricts HIV-1 reverse transcription in quiescent CD4(+) T-cells. Retrovirology. 2012;9:87. doi: 10.1186/1742-4690-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldauf HM, et al. SAMHD1 restricts HIV-1 infection in resting CD4(+) T cells. Nat Med. 2012;18(11):1682–1687. doi: 10.1038/nm.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laguette N, et al. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474(7353):654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hrecka K, et al. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011;474(7353):658–661. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger A, et al. SAMHD1-deficient CD14+ cells from individuals with Aicardi-Goutières syndrome are highly susceptible to HIV-1 infection. PLoS Pathog. 2011;7(12):e1002425. doi: 10.1371/journal.ppat.1002425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St Gelais C, et al. SAMHD1 restricts HIV-1 infection in dendritic cells (DCs) by dNTP depletion, but its expression in DCs and primary CD4+ T-lymphocytes cannot be upregulated by interferons. Retrovirology. 2012;9:105. doi: 10.1186/1742-4690-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lahouassa H, et al. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol. 2012;13(3):223–228. doi: 10.1038/ni.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim B, Nguyen LA, Daddacha W, Hollenbaugh JA. Tight interplay among SAMHD1 protein level, cellular dNTP levels, and HIV-1 proviral DNA synthesis kinetics in human primary monocyte-derived macrophages. J Biol Chem. 2012;287(26):21570–21574. doi: 10.1074/jbc.C112.374843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim ET, White TE, Brandariz-Núñez A, Diaz-Griffero F, Weitzman MD. SAMHD1 restricts herpes simplex virus 1 in macrophages by limiting DNA replication. J Virol. 2013;87(23):12949–12956. doi: 10.1128/JVI.02291-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollenbaugh JA, et al. Host factor SAMHD1 restricts DNA viruses in non-dividing myeloid cells. PLoS Pathog. 2013;9(6):e1003481. doi: 10.1371/journal.ppat.1003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franzolin E, et al. The deoxynucleotide triphosphohydrolase SAMHD1 is a major regulator of DNA precursor pools in mammalian cells. Proc Natl Acad Sci USA. 2013;110(35):14272–14277. doi: 10.1073/pnas.1312033110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kretschmer S, et al. SAMHD1 prevents autoimmunity by maintaining genome stability [published online ahead of print January 29, 2014] Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clifford R, et al. SAMHD1 is mutated recurrently in chronic lymphocytic leukemia and is involved in response to DNA damage. Blood. 2014;123(7):1021–1031. doi: 10.1182/blood-2013-04-490847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi D. SAMHD1: A new gene for CLL. Blood. 2014;123(7):951–952. doi: 10.1182/blood-2013-12-545384. [DOI] [PubMed] [Google Scholar]
- 17.Xin B, et al. Homozygous mutation in SAMHD1 gene causes cerebral vasculopathy and early onset stroke. Proc Natl Acad Sci USA. 2011;108(13):5372–5377. doi: 10.1073/pnas.1014265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rice GI, et al. Mutations involved in Aicardi-Goutières syndrome implicate SAMHD1 as regulator of the innate immune response. Nat Genet. 2009;41(7):829–832. doi: 10.1038/ng.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar D, et al. Mechanisms of mutagenesis in vivo due to imbalanced dNTP pools. Nucleic Acids Res. 2011;39(4):1360–1371. doi: 10.1093/nar/gkq829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chabes A, Stillman B. Constitutively high dNTP concentration inhibits cell cycle progression and the DNA damage checkpoint in yeast Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2007;104(4):1183–1188. doi: 10.1073/pnas.0610585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nordlund P, Reichard P. Ribonucleotide reductases. Annu Rev Biochem. 2006;75:681–706. doi: 10.1146/annurev.biochem.75.103004.142443. [DOI] [PubMed] [Google Scholar]
- 22.White TE, et al. Contribution of SAM and HD domains to retroviral restriction mediated by human SAMHD1. Virology. 2012;436(1):81–90. doi: 10.1016/j.virol.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brandariz-Nuñez A, et al. Role of SAMHD1 nuclear localization in restriction of HIV-1 and SIVmac. Retrovirology. 2012;9:49. doi: 10.1186/1742-4690-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powell RD, Holland PJ, Hollis T, Perrino FW. Aicardi-Goutieres syndrome gene and HIV-1 restriction factor SAMHD1 is a dGTP-regulated deoxynucleotide triphosphohydrolase. J Biol Chem. 2011;286(51):43596–43600. doi: 10.1074/jbc.C111.317628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji X, et al. Mechanism of allosteric activation of SAMHD1 by dGTP. Nat Struct Mol Biol. 2013;20(11):1304–1309. doi: 10.1038/nsmb.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amie SM, Bambara RA, Kim B. GTP is the primary activator of the anti-HIV restriction factor SAMHD1. J Biol Chem. 2013;288(35):25001–25006. doi: 10.1074/jbc.C113.493619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen EC, Seamon KJ, Cravens SL, Stivers JT. GTP activator and dNTP substrates of HIV-1 restriction factor SAMHD1 generate a long-lived activated state. Proc Natl Acad Sci USA. 2014;111(18):E1843–E1851. doi: 10.1073/pnas.1401706111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan J, et al. Tetramerization of SAMHD1 is required for biological activity and inhibition of HIV infection. J Biol Chem. 2013;288(15):10406–10417. doi: 10.1074/jbc.M112.443796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu C, et al. Structural insight into dGTP-dependent activation of tetrameric SAMHD1 deoxynucleoside triphosphate triphosphohydrolase. Nat Commun. 2013;4:2722. doi: 10.1038/ncomms3722. [DOI] [PubMed] [Google Scholar]
- 30.Schneider TR. Objective comparison of protein structures: Error-scaled difference distance matrices. Acta Crystallogr D Biol Crystallogr. 2000;56(Pt 6):714–721. doi: 10.1107/s0907444900003723. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy EM, et al. Ribonucleoside triphosphates as substrate of human immunodeficiency virus type 1 reverse transcriptase in human macrophages. J Biol Chem. 2010;285(50):39380–39391. doi: 10.1074/jbc.M110.178582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu L. Cellular and biochemical mechanisms of the retroviral restriction factor SAMHD1. ISRN Biochemistry. 2013;2013:728392. doi: 10.1155/2013/728392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofer A, Crona M, Logan DT, Sjöberg BM. DNA building blocks: Keeping control of manufacture. Crit Rev Biochem Mol Biol. 2012;47(1):50–63. doi: 10.3109/10409238.2011.630372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gon S, Napolitano R, Rocha W, Coulon S, Fuchs RP. Increase in dNTP pool size during the DNA damage response plays a key role in spontaneous and induced-mutagenesis in Escherichia coli. Proc Natl Acad Sci USA. 2011;108(48):19311–19316. doi: 10.1073/pnas.1113664108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rampazzo C, et al. Regulation by degradation, a cellular defense against deoxyribonucleotide pool imbalances. Mutat Res. 2010;703(1):2–10. doi: 10.1016/j.mrgentox.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Brown PH, Schuck P. Macromolecular size-and-shape distributions by sedimentation velocity analytical ultracentrifugation. Biophys J. 2006;90(12):4651–4661. doi: 10.1529/biophysj.106.081372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCoy AJ, et al. Phaser crystallographic software. J Appl Cryst. 2007;40(Pt 4):658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vagin AA, et al. REFMAC5 dictionary: Organization of prior chemical knowledge and guidelines for its use. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2184–2195. doi: 10.1107/S0907444904023510. [DOI] [PubMed] [Google Scholar]
- 39.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.