Significance
Higher blood levels of vitamin D are associated with better health outcomes. Vitamin D deficiency, however, is common among the elderly. Despite targets in the brain, little is known about how vitamin D affects cognitive function. In aging rodents, we modeled human serum vitamin D levels ranging from deficient to sufficient and tested whether increasing dietary vitamin D could maintain or improve cognitive function. Treatment was initiated at middle age, when markers of aging emerge, and maintained for ∼6 mo. Compared with low- or normal-dietary vitamin D groups, only aging rats on higher vitamin D could perform a complex memory task and had blood levels considered in the optimal range. These results suggest that vitamin D may improve the likelihood of healthy cognitive aging.
Keywords: Vitamin D status, cholecalciferol, 25-hydroxyvitamin D
Abstract
Vitamin D is an important calcium-regulating hormone with diverse functions in numerous tissues, including the brain. Increasing evidence suggests that vitamin D may play a role in maintaining cognitive function and that vitamin D deficiency may accelerate age-related cognitive decline. Using aging rodents, we attempted to model the range of human serum vitamin D levels, from deficient to sufficient, to test whether vitamin D could preserve or improve cognitive function with aging. For 5–6 mo, middle-aged F344 rats were fed diets containing low, medium (typical amount), or high (100, 1,000, or 10,000 international units/kg diet, respectively) vitamin D3, and hippocampal-dependent learning and memory were then tested in the Morris water maze. Rats on high vitamin D achieved the highest blood levels (in the sufficient range) and significantly outperformed low and medium groups on maze reversal, a particularly challenging task that detects more subtle changes in memory. In addition to calcium-related processes, hippocampal gene expression microarrays identified pathways pertaining to synaptic transmission, cell communication, and G protein function as being up-regulated with high vitamin D. Basal synaptic transmission also was enhanced, corroborating observed effects on gene expression and learning and memory. Our studies demonstrate a causal relationship between vitamin D status and cognitive function, and they suggest that vitamin D-mediated changes in hippocampal gene expression may improve the likelihood of successful brain aging.
Vitamin D, a secosteroid hormone known for its role in bone and calcium homeostasis, is now well recognized for its many diverse functions and actions on a variety of tissues and cell types (1, 2). Vitamin D typically refers to the precursor forms of the hormone obtained through the skin’s exposure to sunlight [vitamin D3 (VitD3)] or from dietary sources (VitD3 or VitD2). A metabolite of vitamin D, 25-hydroxyvitamin D (25OHD), is a serum biomarker of vitamin D status or repletion. In recent years, there is particular concern that large segments of the population may have low levels of 25OHD, and therefore are vitamin D-deficient (3). Due to factors such as reduced intake, absorption, and decreased exposure to sunlight, aging adults (≥50 y of age) are especially susceptible (3–6). Notably, this predisposition for lower 25OHD levels in the elderly has been linked to higher risk for numerous age-related disorders, including cancer and metabolic and vascular diseases (7–10).
Inadequate vitamin D status also correlates with a greater risk for cognitive decline in the elderly (4, 11–15), suggesting that optimal levels may promote healthy brain aging (16, 17). Because the brain expresses vitamin D receptors (VDRs) and can synthesize the active form of the hormone, the possible cognitive enhancing effects of vitamin D may reflect a primary action in the brain rather than a result of secondary systemic effects (18–22). Indeed, we and others have shown that vitamin D, as well as the biologically active form of the hormone, 1,25-dihydroxyvitamin D, has direct neuroprotective actions and can reduce some biomarkers of brain aging (20, 23–28).
Given that the aging population is projected to increase dramatically in the near future (29), along with estimates that a significant proportion of the elderly are vitamin D-deficient (3), there is a critical need to determine whether efforts to improve vitamin D status can reduce age-related cognitive decline. Despite calls for more definitive research along these lines (30), few long-term intervention studies have examined the impact of manipulating vitamin D on cognitive function with advancing age. To test the hypothesis that higher vitamin D levels improve cognitive function in aging animals, middle-aged male F344 rats were placed on diets containing low, medium [National Research Council (NRC)-required], or high levels of VitD3 (or cholecalciferol) for 5–6 mo. The middle-age period was chosen because it increasingly appears to be an important window of time at which to initiate interventions designed to preserve cognitive function into the geriatric period. At midlife, subtle cognitive impairments begin to appear, along with structural and genomic changes associated with brain aging (31–34). Our results show that higher than normal dietary VitD3 may improve the chances of successful brain aging and that changes in neuronal synaptic function in the hippocampus may underlie its protective effects against age-related cognitive decline.
Results
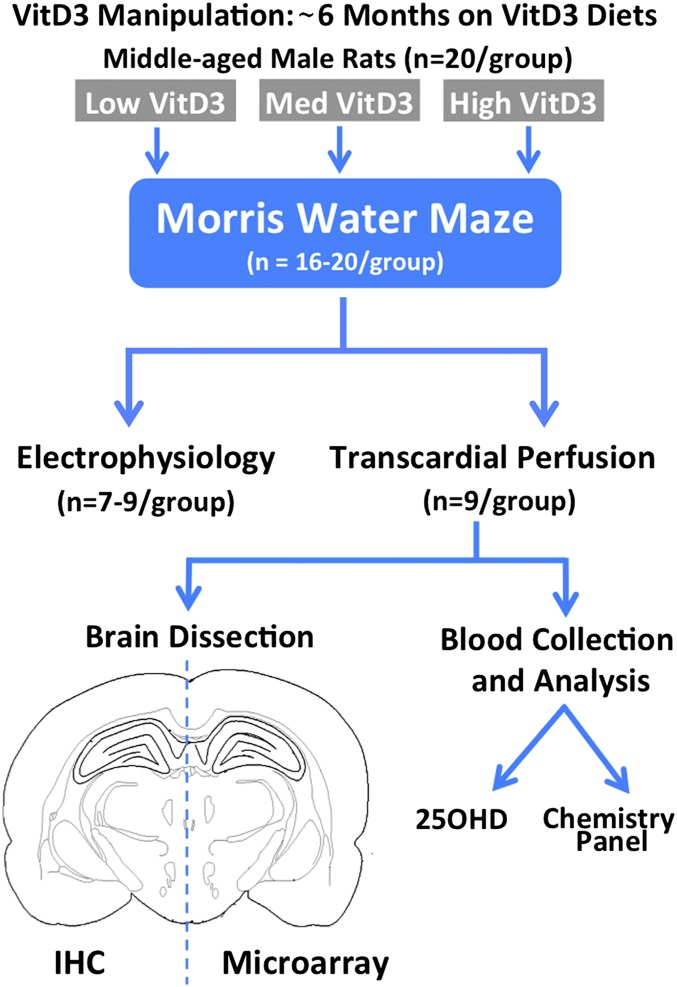
Fig. 1 provides an overview of the present study. Middle-aged rats on diets containing varying amounts of VitD3 (low, medium, or high) were trained and tested for cognitive performance. In a subset of the animals, one hemisphere was used to identify changes in hippocampal gene expression and the other was used for immunohistochemistry (IHC). The remaining rats were used for hippocampal slice electrophysiology.
Fig. 1.
Overview of study design. Middle-aged (11–13 mo old) rats were treated with the indicated diets (VitD3, cholecalciferol) for 6 mo and evaluated for learning and memory behavior and other outcome measures.
Physiological Parameters and Blood Analyses.
No differences in food intake (Table 1) or corresponding body weights (Fig. S1) were detected between groups throughout the course of the study. At the beginning, animals weighed ∼500 g, and they gained ∼30–60 g by the end of the study. Because vitamin D is an important regulator of bone and mineral metabolism, blood chemistry panels were used to assess the effects of dietary manipulation of VitD3 on calcium and phosphorus, among other common serum variables (Table 1 and Table S1). Serum calcium and phosphorus levels were unchanged with the dietary VitD3 manipulations in this study, as were multiple markers of kidney or liver function.
Table 1.
Dietary vitamin D3 (cholecalciferol) treatment
| VitD3 treatment and serum electrolytes | Low VitD3 | Medium VitD3 | High VitD3 |
| VitD3, IU/kg of diet | 100 | 1,000 | 10,000 |
| Daily food intake,* g | 16.4 ± 0.4 | 16.5 ± 0.4 | 15.9 ± 0.5 |
| VitD3 dose,*,† IU per day | 1.7 ± 0.4 | 16.5 ± 0.4 | 159.0 ± 5.2 |
| Serum calcium, mg/dL | 11.4 ± 0.1 | 11.4 ± 0.01 | 11.5 ± 0.1 |
| Serum phosphorus, mg/dL | 5.7 ± 0.3 | 4.9 ± 0.2 | 5.5 ± 0.2 |
Previously reported in Keeney et al. (35).
Average daily dose was calculated by dividing the amount of VitD3 in the diet by the amount consumed in the chow.
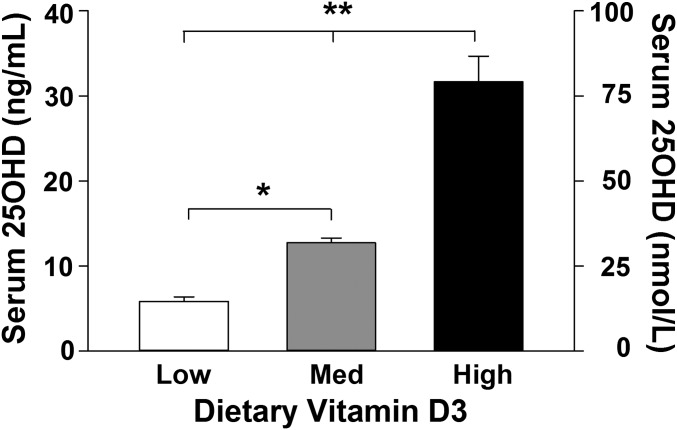
To determine if dietary manipulation of VitD3 affected vitamin D status, blood levels of 25OHD were determined in a subset of animals using a blood spot LC-tandem MS (MS/MS)–based method (36, 37). 25OHD is the most common indicator of vitamin D status because it is a stable and long-lived metabolite of vitamin D. Results showed that circulating 25OHD levels were proportional to the amount of VitD3 in the diet. For each 10-fold increase in dietary VitD3 content, circulating 25OHD levels approximately doubled, confirming that vitamin D status could be altered by dietary intake (Fig. 2).
Fig. 2.
Vitamin D status. Serum levels of 25OHD in response to low, medium (Med), or high dietary VitD3 intake for 6 mo. A 10-fold increase in dietary VitD3 corresponded (Table 1) to an approximate twofold increase in 25OHD. [F(2,24) = 55.3; n = 9 rats per group]. *P = 0.01; **P = 0.001. Reported in Table 1 of Keeney et al. (35).
Morris Water Maze.
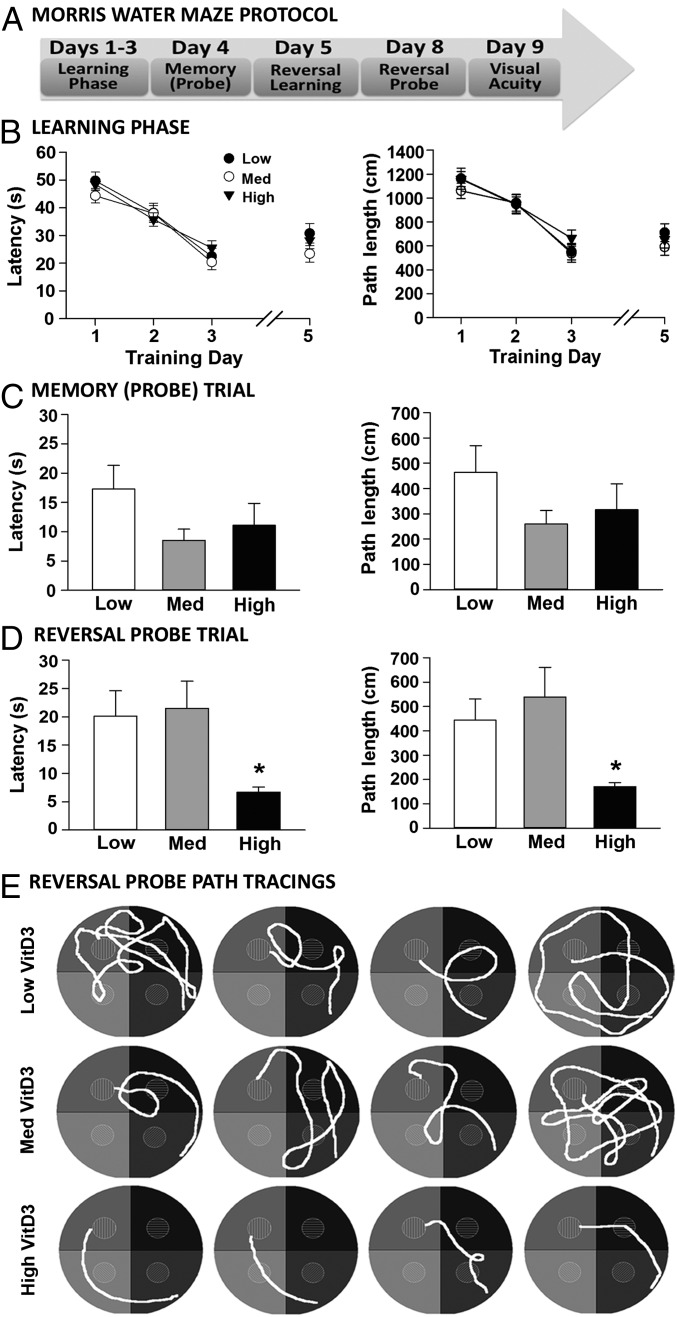
After 5–6 mo of dietary VitD3 manipulation, performance in the Morris water maze (MWM) was evaluated to determine effects on spatial learning and memory. All groups learned the task equally well, as demonstrated by similar path lengths and latencies to the hidden platform during the 3-d training period (Fig. 3B). Twenty-four hours later, memory was assessed during the probe trial (platform removed). There was no statistical difference between groups on either latency (P = 0.14) or path length (P = 0.22) (Fig. 3C), although animals on the low VitD3 diet showed trends toward higher latencies and path lengths.
Fig. 3.
High dietary VitD3 improves performance on a cognitive task. (A) MWM protocol. (B) Latencies and path lengths during training days 1–3. All groups showed a similar performance and improvement in reaching the platform. On day 5, all groups learned the new platform location during spatial reversal training (results are the average of the second and third trials. (C) Latency and path length to the goal during the probe trial (day 4) did not differ significantly between groups [latency: F(2,52) = 2.02, P = 0.14; path length: F(2,52) = 1.57, P = 0.22]. (D) On day 8 (reversal probe trial), the high-VitD3 group significantly outperformed the other groups on latency [F(2,52) = 3.8, P = 0.03] and path length [F(2,52) = 4.0, P = 0.02; n = 16–20 per group]. (E) Representative reversal probe path tracings.
We next assessed performance using a more challenging cognitive task requiring animals to learn a new platform location (spatial reversal) (Fig. 3A). All groups were able to learn the new location with only 1 d of training because latencies and path lengths (training day 5) were comparable to those seen during the prior task (training day 3); there was no difference between groups (Fig. 3B). The reversal probe, conducted 72 h later (day 8), showed that rats on the high VitD3 diet reached the goal in less time and traveled shorter distances, significantly outperforming rats in the other two groups (Fig. 3D, for latency and path length). Representative path length tracings (Fig. 3E) demonstrate the significantly better performance of the animals on the high-VitD3 diet.
Identification of Vitamin D-Sensitive Genes/Biological Pathways.
For a subset of the animals, the hippocampus of each rat was processed on an individual Affymetrix microarray (n = 9 rats for each treatment group) (Fig. 1). The hippocampus was analyzed because of its role in learning/memory and susceptibility to age-related changes in synaptic function. After filtering for present and well-annotated gene probes (31), 10,071 genes were retained for testing. Each of these genes was tested by one-way ANOVA across all three VitD3 treatment groups, and a total of 144 genes were found to differ at P ≤ 0.005, with a false discovery rate (FDR) of 0.31 (38); of these genes, 128 were regulated in a VitD3 dose-dependent manner (Dataset S1). We also analyzed microarray data using a more rigorous P value of P ≤ 0.0015 and an FDR of 0.17, which yielded a total of 52 genes (shown at the top of each treatment category in Dataset S1).
To determine whether significant genes were overrepresented in certain biological categories, we further analyzed the significant genes (P ≤ 0.005) using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) bioinformatics tool (39–42). Such an approach can confer biological insight into a list of statistically significant genes and can also provide a second tier of confidence against multiple testing. That is, genes incorrectly identified by multiple testing error would be highly unlikely to group within pathways. With high dietary VitD3, the overrepresented pathways identified by the DAVID analysis (Table 2) included the categories of G protein-coupled receptor activity, synaptic transmission, and cell communication. Interestingly, of the multiple cell types found in the brain (e.g., neurons, astrocytes, oligodendrocytes, microglia), the genes in the high-VitD3 up-regulated pathways appear to be primarily neuronal in nature. Categories up-regulated in a dose-dependent manner with VitD3 included cation homeostasis, cytoplasmic membrane part, and calcium binding, all of which have some component related to calcium regulation/homeostasis. These latter results suggest that in addition to its established role in regulating calcium-dependent functions in the periphery, vitamin D appears to play a similar role in the brain. It is notable that many of the more highly significant genes (P ≤ 0.0015) were included in the pathways identified by the DAVID analysis (indicated in Table 2).
Table 2.
DAVID functional pathway analysis of up-regulated VitD3-sensitive genes
| Biological pathway | No. of genes | Gene description and symbol | DAVID P value | |
| Up-regulated by high dietary VitD3 | ||||
| G protein-coupled receptor activity | 5 | Dopamine receptor 5 (Drd5); glutamate receptor, ionotropic, kainate 3 (Grik3); neuromedin B receptor (Nmbr)*; serotonin receptor 2C (Htr2c), thyroid-stimulating hormone releasing hormone receptor (Trhr) | 0.004 | |
| Synaptic transmission | 4 | Synaptojanin 1 (Synj1)*, synaptotagmin 2 (Syt2), Drd5, Grik3 | 0.03 | |
| Cell communication | 5 | Nuclear receptor 4A2 (Nr4a2)*, Drd5, Grik3, Synj1*, Syt2 | 0.05 | |
| Up-regulated by medium and high dietary VitD3 (dose-dependent) | ||||
| Cation homeostasis | 4 | Plasma membrane Ca2+ ATPase 4 (Atp2b4)*, Ca2+/calmodulin-dependent protein kinase IIδ (Camk2d)*, Na+/H+ exchanger 7 (Slc9a7), synaptophysin-like 2 (Sypl2) | 0.003 | |
| Cytoplasmic vesicle membrane part | 5 | Vesicular glutamate transporter 2 (Slc17a6)*, Atp2b4*, Camk2d*, Slc9a7, Sypl2 | 0.03 | |
| Calcium ion binding | 4 | Delta/notch-like EGF repeat containing (Dner)*; neurocalcin-delta (Ncald); sparc/osteonectin, cwcv, and kazal-like 3 (Spock3)*; Atb2b4* | 0.05 | |
Specific functional pathways altered in the hippocampus by dietary VitD3 dose are indicated underneath the bold headings.
Highly significant genes identified in microarrays.
To identify genes that might be directly targeted by the VDR, we searched our list of significant genes (Dataset S1) for those genes that may contain classical VDR element (VDRE) motifs (43). Table 3 shows the hippocampal genes that were significantly up-regulated with high VitD3 and contained putative VDREs. The corresponding consensus sequences, the highly conserved nucleotides, and the quality score relating homology to the classical motif are also indicated. Several of these genes (i.e., synaptojanin 1, synaptotagmin 2, contactin 4, beta-1–syntrophin) are mostly neuronal. More definitive studies, using techniques such as ChIP, will be required to confirm whether these hippocampal genes with putative VDREs and identified using an in silico approach, do indeed contain functional VDREs.
Table 3.
Hippocampal genes up-regulated with high VitD3 that contain putative VDREs
| Gene symbol | Corresponding protein | P value* | RXR-VDR† | Quality score‡ |
| Cntn4 | Contactin 4 | 0.001 | AGTTCA TGG AGTTCA | 0.74 |
| Ctps2 | CTP synthase II | 0.003 | GGGTCC TAG GGTTCA | 0.73 |
| Ppp2r2b | Protein phosphatase 2, regulatory subunit B, beta isoform | 0.003 | GGGTCA AAG GGTTTA | 0.80 |
| Sntb1 | Syntrophin, beta-1 | 0.0002 | GGGTCA TCC AGTTCA | 0.76 |
| Synj1 | Synaptojanin 1 | 0.0006 | GGGTCA TGG GGATCA | 0.75 |
| Syt2 | Synaptotagmin 2 | 0.004 | GGGTCA AAG GGTCCA | 0.73 |
| Tusc3 | Tumor suppressor candidate 3 | 0.001 | GGGTCA TTG GGGTCA | 0.73 |
RXR, retinoid-X receptor.
P value: Significance of up-regulation from microarray.
VDRE consensus sequence from Jaspar motif database: Pu-G-G/T-T-C/G-A-nnn-Pu-G-G/T-G/T-C-A. Underlined T's represent highly conserved nucleotides in the classic consensus VDRE (43).
Sequence quality score according to analysis with Partek Genomics software.
Visualization of Vitamin D-Sensitive Targets.
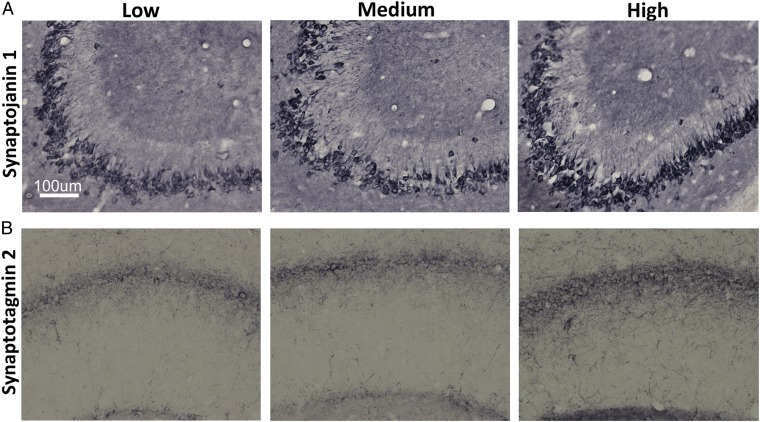
Several proteins (VDR, synaptojanin 1, and synaptotagmin 2) were selected for analysis by IHC. Consistent with prior studies, the presence of VDR was confirmed in hippocampal neurons (18, 19). No difference in VDR staining was detected between groups (Fig. S2). Synaptojanin 1 and synaptotagmin 2, which are highly representative of synaptic plasticity pathways and contain a putative VDRE, were selected to extend the gene microarray data. The IHC data validated the gene microarray results by showing a strong effect in the predicted direction for both proteins [synaptojanin 1: P = 0.05, Student t test (Fig. 4A); synaptotagmin 2: P = 0.03, Student t test (Fig. 4B)].
Fig. 4.
High VitD3 enhances expression of synaptic proteins in hippocampal neurons. Representative micrographs of hippocampal sections demonstrate increased immunoreactivity for synaptojanin 1 (A) and synaptotagmin 2 (B) staining in the CA2/3 and CA1 cell layers, respectively, from high-VitD3 animals compared with the low- and medium-VitD3 groups. Synaptojanin 1: optical density of low and medium VitD3 = 0.465 ± 0.0.03, optical density of high VitD3 = 0.54 ± 0.0.02 (P ≤ 0.05). Synaptotagmin 2: optical density of low and medium VitD3 = 0.425 ± 0.0.01, optical density of high VitD3 = 0.442 ± 0.0.008 (P ≤ 0.05).
Hippocampal Slice Electrophysiology.
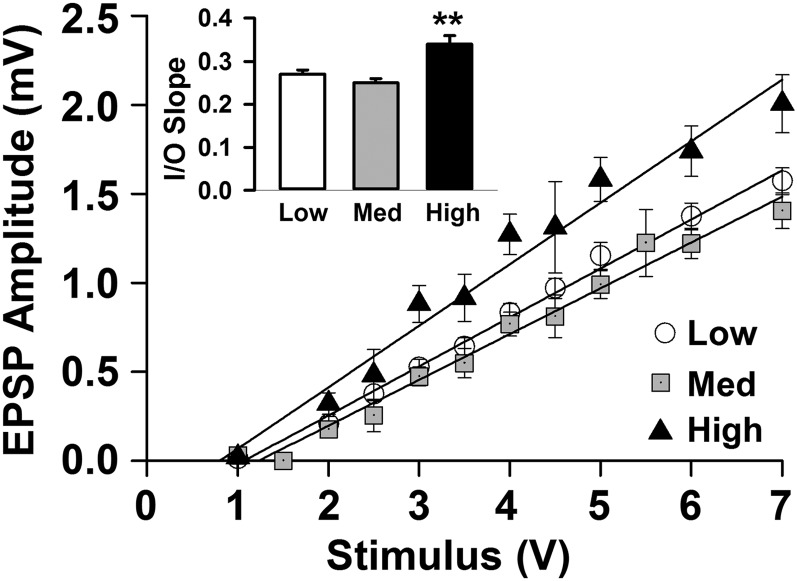
To determine if manipulating VitD3 status affected physiological functions related to cognition, long-term potentiation (LTP) was examined in the CA1 region of the hippocampus. The field EPSP slopes were compared before and after theta burst stimulation, and no difference in LTP between groups was observed (Fig. S3). However, an input/output (I/O) curve, obtained by plotting progressive increases in the input stimulation against the measured evoked response, revealed a significant difference between groups (Fig. 5). Compared with the low- and medium-VitD3 groups, the high-VitD3 group had a significantly steeper I/O slope. These results suggest that high VitD3 may improve neuronal excitability, which is known to decrease with age (44). However, this increased responsiveness with high VitD3 did not appear to be due to hyperexcitability, because slice survival and integrity were comparable across groups.
Fig. 5.
High dietary VitD3 increases excitability of hippocampal neurons. The I/O curves show evoked fEPSPs in response to increasing stimulus intensities in hippocampal slices from low-, medium-, and high-vitD3 groups. The steeper slope with high vitD3 demonstrates a greater response for a given stimulus [low = 0.27 ± 0.01, medium = 0.25 ± 0.01, and high = 0.34 ± 0.02; F(2,462) = 11.3, P ≤ 0.001]. Results are from 16 to 22 hippocampal slices from seven to nine animals per group.
Discussion
Despite growing concerns that vitamin D deficiency is a risk factor associated with unhealthy cognitive aging, few studies have investigated the effects of chronic vitamin D (VitD3) supplementation. It is therefore unclear whether a potential causal relationship exists. Here, we examined the effects of long-term dietary manipulation of serum vitamin D (25OHD) and tested the hypothesis that cognitive decline with aging can be slowed or prevented by higher vitamin D levels. Middle-aged male F344 rats were fed diets containing low, medium (NRC-required), or high VitD3 for 5–6 mo, followed by assessments of cognitive function, hippocampal electrophysiology, and gene expression. The results of our study provide evidence of a potential cause-and-effect relationship because raising 25OHD levels prevented age-related cognitive decline. In addition, these studies identify effects on synaptic function as a mechanism by which vitamin D may promote healthy brain aging (Fig. 6). There are relatively few risks associated with increased vitamin D intake, especially when taken in the inactive form (VitD3 or cholecalciferol) (45). The most concerning potential side effect is hypercalcemia; however, hypercalcemia is rarely seen except at serum 25OHD levels far exceeding those levels recommended for optimum health (45). The animals on the high-VitD3 diet in this study had 25OHD levels of ∼30 ng/mL with no changes in serum calcium (Fig. 2 and Table 1).
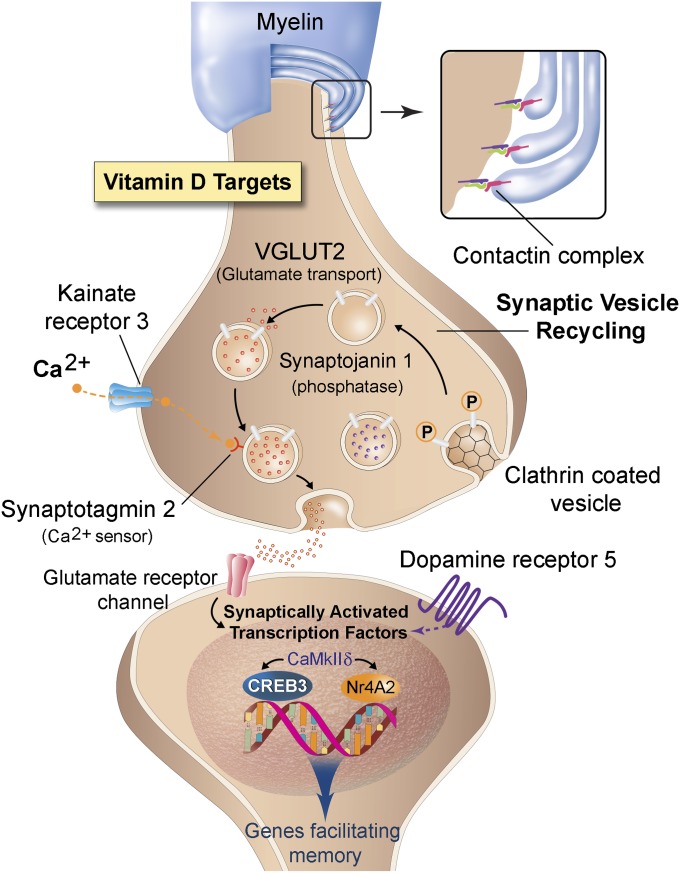
Fig. 6.
Proposed model of synaptic changes with VitD3. Optimal levels of VitD3 stabilize myelin structure and enhance synaptic vesicle recycling and transcription factors facilitating cognitive processes. Together, these changes preserve memory in aging. (Inset) Anchoring of the myelin to the axon facilitated by contactin. (Presynaptic targets) Empty clathrin-coated vesicles undergo endocytosis or recycling driven, in part, by the phosphatase synaptojanin 1. Vesicle refilling of glutamate (red molecules) occurs via vesicular glutamate transporter 2 (VGLUT2). Ca2+ enters the synapse via kainate receptor 3 (Grik3), and docking of the filled vesicle is facilitated by Ca2+ binding to synaptotagmin 2. The synapse also contains a dopamine-filled synaptic vesicle (purple molecules), which can colocalize with kainate receptor 3 terminals. (Postsynaptic targets) Stimulation of glutamate (or dopamine 5) receptors initiates a CaMKIIδ signaling cascade resulting in activation of transcription factors (CREB3 and NR4A2) and subsequent expression of genes promoting memory-related processes. (Illustration by Tom Dolan, University of Kentucky.)
Higher Vitamin D Levels Reduce Cognitive Deficits at Middle Age.
The MWM task is used to test spatial reference memory and is widely thought to have relevance for human hippocampal-dependent memory (46–50). Although not as commonly used, reversal learning in the MWM is more difficult than the standard task, and therefore can be used to detect subtle memory deficits (46). Further, because of its more complex nature, reversal learning may be viewed as a task of executive function (51, 52). The middle-age period is often characterized by the onset of such subtle changes in cognitive performance, most notably in executive function and processing speed (32, 53, 54). We initially assessed spatial reference memory, where rats were trained to locate a hidden platform in a specific location, and found no significant differences among groups of middle-aged animals treated with varying levels of vitamin D. During spatial reversal, however, the animals were required to learn a new platform location in a single day and then to remember the new location after several days. This more difficult task required differentiating an old, now irrelevant, memory from a new one, and it may involve both the hippocampus and higher cortical regions. The low- and medium-VitD3 groups demonstrated deficits and struggled to recall the new platform location, appearing lost or confused during the reversal probe. The high-VitD3 group, on the other hand, performed this task extremely well, reaching the goal in half of the time and distance compared with the other two groups. Therefore, these results suggest that higher serum levels of 25OHD may be protective against some of the early, subtle changes in cognitive function.
Vitamin D Targets the Molecular Machinery of the Synapse.
A common feature of brain aging is a decrease in synaptic strength or impaired communication between neurons (32, 44, 55–58). Synaptic connections become dysfunctional, perhaps in part as a result of reduced vesicle trafficking and neurotransmitter release, causing diminished plasticity (56, 59–61). These changes may contribute to age-related cognitive decline and appear to be among the earliest age-related changes that occur in the brain. In aging animals, high VitD3 up-regulated multiple genes/functional pathways involved in synaptic vesicle trafficking and neurotransmission (Table 2). Several genes, including synaptojanin 1 and synaptotagmin 2, contain a potential VDRE in their promoter region and may be subject to direct modulation by vitamin D (Table 3). Up-regulation of both of these genes was also confirmed by an increase in the immunoreactivity of the corresponding protein in the hippocampus (Fig. 4). At presynaptic terminals, synaptojanin 1 aids in the recycling of synaptic vesicles through its phosphatase activity, whereas synaptotagmin 2 initiates vesicle docking and fusion to the presynaptic membrane through its calcium-sensing function, ultimately resulting in the release of neurotransmitter (62, 63). Another component of the presynaptic machinery increased with high VitD3 was vesicular glutamate transporter 2, a vesicular transporter that packages glutamate into presynaptic vesicles and makes it available for release at the synapse (64, 65).
Other genes important for pre/postsynaptic function were also up-regulated by VitD3 (Table 2 and Dataset S1). These genes included calcium/calmodulin-dependent protein kinase IIδ (CaMKIIδ) and receptors for several major neurotransmitters (dopamine, glutamate, and serotonin). The CaMKIIs are calcium-activated enzymes enriched at postsynaptic sites, and abundant evidence suggests that they enhance synaptic strength and memory formation (66). CaMKIIδ is localized to the nucleus and plays a key role in modulating activity-dependent gene transcription via the cAMP response element binding protein (CREB), a major transcription factor. CaMKIIδ phosphorylates CREB, leading to the expression of BDNF, a CNS growth factor that enhances neurogenesis, dendritic outgrowth, and synaptic plasticity, all of which are believed ultimately to facilitate memory (32, 66–68). Another transcription factor that targets BDNF and is up-regulated by high VitD3 was nuclear receptor 4A2 (Nr4a2), which belongs to a family of orphan nuclear receptors. Nr4a2 is regarded as a promising target for enhancing cognition because it appears to have a role in selectively promoting the consolidation of long-term memories (69), such as demonstrated here by high-VitD3 animals on the spatial reversal task (Fig. 3 D and E).
Additional Pathways Potentially Related to Cognitive Function.
Consistent with its role as a calcium-regulating hormone in the periphery (1–3, 5, 7, 70), we found that vitamin D also targeted calcium-regulatory pathways in the hippocampus (Table 2). These results complement the effects on synaptic function, because synaptic processes are also calcium-dependent. We and others have shown that calcium dyshomeostasis plays a key role in brain-aging processes (44, 56, 71) and have demonstrated that vitamin D reverses calcium-related electrophysiological markers of brain aging (24, 27). Together, these results suggest that the effects of vitamin D on calcium regulation and synaptic function may interact to counter cognitive decline with aging.
Other studies have found related effects of vitamin D on select genes involved in neuronal function, including genes for neurotrophic factors, calcium-binding proteins, and proteins involved in neurotransmitter synthesis (20, 30). Some studies have also implicated compounds containing vitamin D in countering glial reactivity (28). We and others have previously shown that genes involved in glial reactivity and inflammation, which may alter neuronal energy metabolism, are among the earliest affected by brain aging and are correlated with cognitive impairment (28, 34, 41, 72, 73). The observation here that vitamin D is to able maintain and enhance synaptic function, apparently by increasing expression of key synaptic molecules, raises the possibility that vitamin D acts directly on neuronal pathways and does not interact directly with glial responses in aging.
There is also a well-documented association of vitamin D deficiency with demyelinating disorders, such as multiple sclerosis (1–3, 74). Although the DAVID analysis (Table 2) did not identify a functional pathway specific for myelin-related processes, genes for contactin 4 and beta-1–syntrophin were up-regulated with high VitD3 (Dataset S1). These genes contain a putative VDRE (Table 3) and are known to play key roles in myelin structure and function. Contactins, in particular, are critical for anchoring myelin to the axon, and their deletion results in impaired nerve conduction (75). Moreover, contactin and its anchoring complex decrease in the CNS with age (34, 76, 77). Given that alterations in myelin and the resultant slowing of conduction are linked to impaired brain function (78, 79), the up-regulation of contactin and syntrophin by vitamin D may reflect another mechanistic pathway by which vitamin D enhances cognitive function in aging (80). Fig. 6 integrates the myelin-related changes, along with changes at the synapse, into a proposed model showing how VitD3 may facilitate synaptic transmission and influence cognitive processes such as memory.
Conclusions
Our studies raise the question of their relevance for human cognitive aging. Notably, several aspects of brain aging in this animal model mirror brain aging in humans (81). First, the initial signs of cognitive aging, characterized by subtle deficits, occur at approximately the same time in the lifespan of humans and rats, during middle age (32–34, 82). Second, the behavioral task used here, assessing hippocampal-dependent spatial memory, also has relevance for human memory because patients with hippocampal lesions perform poorly in a virtual maze test (47, 48). Finally, the vitamin D levels achieved here model clinically relevant levels found in humans, ranging from deficient to sufficient (70, 83, 84), and animals with the highest 25OHD levels, considered “optimal” by some recommendations (5, 85–88), outperformed animals with lower levels on a challenging cognitive task.
Because there are few dose–response studies with vitamin D (89), it has been difficult to determine what levels are most favorable for achieving benefits beyond the known effects on bone (88). Lower levels, however, are increasingly associated with negative effects for the brain in human and animal studies (1–8, 11–14, 16, 17, 30, 74, 90–92). Additional studies are required to confirm the present findings and to better understand the underlying mechanisms, especially as they relate to cognitive health. Importantly, clinical intervention trials are currently underway, which will assess the effects of supplementation [2,000 international units (IU) of VitD3 daily] on cognitive decline in older adults (VITAL-Cog, DO-HEALTH) (93). Given that the initial signs of cognitive aging emerge at middle age and that age-related brain disorders, such as Alzheimer’s disease, are believed to have a long preclinical phase (94), our results raise the issue of the timing of the intervention. Thus, intervention with vitamin D at earlier stages of aging may be more efficacious in promoting healthy cognitive aging and slowing the appearance of multiple markers of the brain-aging process (95).
Materials and Methods
More detailed methods are provided in SI Materials and Methods.
Animals and Vitamin D Diets.
All protocols were approved by the Institutional Animal Care and Use Committee of the University of Kentucky. Sixty middle-aged male F344 rats (11–13 mo old) were divided into three groups and fed different dietary amounts of cholecalciferol (VitD3) (70) for 5–6 mo. The purified AIN-93 (Harlan–Teklad) diet was modified such that each kilogram of diet contained 100 IU (low), 1,000 IU (medium, typical amount) or 10,000 IU (high) VitD3. Animal weights (Fig. S1) and food intake (Table 1) were measured two to three times per week.
MWM.
MWM procedures were similar to the procedures previously described (96). Briefly, animals were trained to find a submerged platform in a pool of water (days 1–3). After learning and memory of this task were assessed (day 4), the platform was moved and rats were trained to learn a new platform location (day 5). Memory for the new location was assessed on day 8 (Fig. 3A). Maze performance was evaluated by measuring path length and latency to the platform.
Tissue Isolation/Blood Analyses.
Rats were deeply anesthetized and perfused with ice-cold saline, and brains were removed following decapitation. The right hemisphere was placed in 4% (wt/vol) paraformaldehyde and used for IHC. The hippocampus of the left hemisphere was dissected for microarray analyses. Blood spots were collected, and 25OHD levels were measured by ZRT Laboratory using LC-MS/MS methods (37) (Fig. 2). The 25OHD levels detected by LC-MS/MS correlate well with 25OHD levels obtained by radioimmunoassay (36, 37); however, they are somewhat lower because LC-MS/MS can distinguish 25OHD from other vitamin D metabolites (e.g., 24,25OHD, lactone form) (97). A chemistry panel was also performed on isolated serum (IDEXX RADIL, Research Animal Diagnostic Laboratory at the University of Missouri) (Table 1 and Table S1).
Microarrays.
Microarray procedures and analyses were performed as in prior work (34). Briefly, hippocampal RNA was isolated, quantified, and checked for RNA integrity. One low-VitD3 sample failed RNA quality control. The remaining RNA samples were applied to Affymetrix Rat Gene 1.0 ST arrays (one array per subject). Prestatistical filtering removed poorly annotated probe sets, low-intensity signals, and outlier values (>2 SD of the group mean). Filtered data were analyzed by one-way ANOVA to identify significant differences, and the FDR procedure (31, 38) was used to estimate the error of multiple testing. Significant genes were assigned to one of four idealized expression patterns using Pearson’s test and were separated by the sign of their correlation; relative gene expression values are provided on the log-2 scale (Dataset S1). Functional categorization for significant genes was determined using DAVID bioinformatic tools (39, 42) (Table 2). Results have been uploaded to the Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo/).
Identification of Putative VDREs.
Partek Genomics software was used to identify significant genes containing a motif that shared at least 70% homology with the classic VDRE (Pu-G-G/T-T-C/G-A-nnn-Pu-G-G/T-G/T-C-A) (43).
IHC.
VDR and the microarray-identified synaptojanin 1 and synaptotagmin 2 were selected for IHC analysis on 30-μm hippocampal sections. Immunoperoxidase staining was performed following incubation in primary antibody solutions for 2–3 d and appropriate secondary antibody for 1 h. Because expression of synaptojanin and synaptotagmin did not differ between the low- and medium-VitD3 groups, they were combined and compared with high VitD3.
Electrophysiology.
Analyses were performed on isolated hippocampal slices from rats not used for microarrays or IHC (Fig. 1). Hippocampal slices were obtained, and LTP was recorded as previously described (96). I/O relationships were examined by recording fEPSPs in the CA1 region resulting from stimuli of increasing intensity.
Statistics.
Food intake, blood chemistry, and behavior and electrophysiology data were analyzed by one-way ANOVA with the Newman–Keuls multiple comparison post hoc test. The Student t test was used for IHC, and a paired t test was used for body weight comparisons. Statistical significance was defined as P ≤ 0.05. Results are expressed as the average ± SEM.
Supplementary Material
Acknowledgments
We thank Dr. Nicholas J. Koszewski (College of Veterinary Medicine, Iowa State University) for the VDR antibody and Dr. Donna M. Wilcock (Sanders–Brown Center on Aging, University of Kentucky) for use of the imaging equipment. Dr. John C. Gant’s assistance with tissue isolation and the critical reading of the manuscript by Drs. Robert Hadley and Todd Porter were also greatly appreciated. This research was jointly funded by the National Institute on Aging (NIA) and the McKnight Brain Research Foundation Grant AG034605 (to P.W.L.); other grants from the NIA include AG004542 (to P.W.L.), AG033649 (to O.T.), AG037868 (to E.M.B.), AG010836 (to N.M.P.), and T32 AG000242 (to Greg Gerhardt).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a Prearranged Editor.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE61326).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1404477111/-/DCSupplemental.
References
- 1.Plum LA, DeLuca HF. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov. 2010;9(12):941–955. doi: 10.1038/nrd3318. [DOI] [PubMed] [Google Scholar]
- 2.Christakos S, DeLuca HF. Minireview: Vitamin D: Is there a role in extraskeletal health? Endocrinology. 2011;152(8):2930–2936. doi: 10.1210/en.2011-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 4.Buell JS, et al. 25-Hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology. 2010;74(1):18–26. doi: 10.1212/WNL.0b013e3181beecb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hossein-nezhad A, Holick MF. Vitamin D for health: A global perspective. Mayo Clin Proc. 2013;88(7):720–755. doi: 10.1016/j.mayocp.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Timpini A, Pini L, Tantucci C, Cossi S, Grassi V. Vitamin D and health status in elderly. Intern Emerg Med. 2011;6(1):11–21. doi: 10.1007/s11739-010-0407-4. [DOI] [PubMed] [Google Scholar]
- 7.Heaney RP. Vitamin D in health and disease. Clin J Am Soc Nephrol. 2008;3(5):1535–1541. doi: 10.2215/CJN.01160308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kojima G, et al. Low dietary vitamin D predicts 34-year incident stroke: The Honolulu Heart Program. Stroke. 2012;43(8):2163–2167. doi: 10.1161/STROKEAHA.112.651752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouillon R, et al. Vitamin D and energy homeostasis—Of mice and men. Nat Rev Endocrinol. 2014;10(2):79–87. doi: 10.1038/nrendo.2013.226. [DOI] [PubMed] [Google Scholar]
- 10.Fleet JC, DeSmet M, Johnson R, Li Y. Vitamin D and cancer: A review of molecular mechanisms. Biochem J. 2012;441(1):61–76. doi: 10.1042/BJ20110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llewellyn DJ, Langa KM, Lang IA. Serum 25-hydroxyvitamin D concentration and cognitive impairment. J Geriatr Psychiatry Neurol. 2009;22(3):188–195. doi: 10.1177/0891988708327888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Annweiler C, et al. Association of vitamin D deficiency with cognitive impairment in older women: Cross-sectional study. Neurology. 2010;74(1):27–32. doi: 10.1212/WNL.0b013e3181beecd3. [DOI] [PubMed] [Google Scholar]
- 13.Llewellyn DJ, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170(13):1135–1141. doi: 10.1001/archinternmed.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slinin Y, et al. Study of Osteoporotic Fractures Research Group Association between serum 25(OH) vitamin D and the risk of cognitive decline in older women. J Gerontol A Biol Sci Med Sci. 2012;67(10):1092–1098. doi: 10.1093/gerona/gls075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui X, Groves NJ, Burne TH, Eyles DW, McGrath JJ. Low vitamin D concentration exacerbates adult brain dysfunction. Am J Clin Nutr. 2013;97(5):907–908. doi: 10.3945/ajcn.113.061176. [DOI] [PubMed] [Google Scholar]
- 16.Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460(2):202–205. doi: 10.1016/j.abb.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Annweiler C, Herrmann FR, Fantino B, Brugg B, Beauchet O. Effectiveness of the combination of memantine plus vitamin D on cognition in patients with Alzheimer disease: A pre-post pilot study. Cogn Behav Neurol. 2012;25(3):121–127. doi: 10.1097/WNN.0b013e31826df647. [DOI] [PubMed] [Google Scholar]
- 18.Stumpf WE, O’Brien LP. 1,25 (OH)2 vitamin D3 sites of action in the brain. An autoradiographic study. Histochemistry. 1987;87(5):393–406. doi: 10.1007/BF00496810. [DOI] [PubMed] [Google Scholar]
- 19.Langub MC, Herman JP, Malluche HH, Koszewski NJ. Evidence of functional vitamin D receptors in rat hippocampus. Neuroscience. 2001;104(1):49–56. doi: 10.1016/s0306-4522(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 20.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13(3):100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 21.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Eyles DW, Liu PY, Josh P, Cui X. Intracellular distribution of the vitamin D receptor in the brain: Comparison with classic target tissues and redistribution with development. Neuroscience. 2014;268:1–9. doi: 10.1016/j.neuroscience.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 23.Landfield PW, Cadwallader-Neal L. Long-term treatment with calcitriol (1,25(OH)2 vit D3) retards a biomarker of hippocampal aging in rats. Neurobiol Aging. 1998;19(5):469–477. doi: 10.1016/s0197-4580(98)00079-7. [DOI] [PubMed] [Google Scholar]
- 24.Brewer LD, et al. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci. 2001;21(1):98–108. doi: 10.1523/JNEUROSCI.21-01-00098.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang JY, et al. Vitamin D(3) attenuates 6-hydroxydopamine-induced neurotoxicity in rats. Brain Res. 2001;904(1):67–75. doi: 10.1016/s0006-8993(01)02450-7. [DOI] [PubMed] [Google Scholar]
- 26.Moore ME, Piazza A, McCartney Y, Lynch MA. Evidence that vitamin D3 reverses age-related inflammatory changes in the rat hippocampus. Biochem Soc Trans. 2005;33(Pt 4):573–577. doi: 10.1042/BST0330573. [DOI] [PubMed] [Google Scholar]
- 27.Brewer LD, Porter NM, Kerr DS, Landfield PW, Thibault O. Chronic 1alpha,25-(OH)2 vitamin D3 treatment reduces Ca2+-mediated hippocampal biomarkers of aging. Cell Calcium. 2006;40(3):277–286. doi: 10.1016/j.ceca.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Acosta S, et al. NT-020, a natural therapeutic approach to optimize spatial memory performance and increase neural progenitor cell proliferation and decrease inflammation in the aged rat. Rejuvenation Res. 2010;13(5):581–588. doi: 10.1089/rej.2009.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vincent GK, Velkoff VA. US Census Bureau . The Next Four Decades: The Older Population in the United States: 2010 to 2050. Washington, DC: US Department of Commerce, Economics, and Statistics Administration, US Census Bureau; 2010. pp. 1–14. [Google Scholar]
- 30.McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22(4):982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- 31.Blalock EM, et al. Gene microarrays in hippocampal aging: Statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23(9):3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch G, Rex CS, Gall CM. Synaptic plasticity in early aging. Ageing Res Rev. 2006;5(3):255–280. doi: 10.1016/j.arr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Finch CE. The neurobiology of middle-age has arrived. Neurobiol Aging. 2009;30(4):515–520, discussion 530–533. doi: 10.1016/j.neurobiolaging.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Kadish I, et al. Hippocampal and cognitive aging across the lifespan: A bioenergetic shift precedes and increased cholesterol trafficking parallels memory impairment. J Neurosci. 2009;29(6):1805–1816. doi: 10.1523/JNEUROSCI.4599-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keeney JT, et al. Dietary vitamin D deficiency in rats from middle to old age leads to elevated tyrosine nitration and proteomics changes in levels of key proteins in brain: Implications for low vitamin D-dependent age-related cognitive decline. Free Radic Biol Med. 2013;65:324–334. doi: 10.1016/j.freeradbiomed.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eyles D, et al. A sensitive LC/MS/MS assay of 25OH vitamin D3 and 25OH vitamin D2 in dried blood spots. Clin Chim Acta. 2009;403(1-2):145–151. doi: 10.1016/j.cca.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Newman MS, et al. A liquid chromatography/tandem mass spectrometry method for determination of 25-hydroxy vitamin D2 and 25-hydroxy vitamin D3 in dried blood spots: A potential adjunct to diabetes and cardiometabolic risk screening. J Diabetes Sci Tech. 2009;3(1):156–162. doi: 10.1177/193229680900300118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9(7):811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 39.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 40.Mirnics K, Pevsner J. Progress in the use of microarray technology to study the neurobiology of disease. Nat Neurosci. 2004;7(5):434–439. doi: 10.1038/nn1230. [DOI] [PubMed] [Google Scholar]
- 41.Rowe WB, et al. Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J Neurosci. 2007;27(12):3098–3110. doi: 10.1523/JNEUROSCI.4163-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gene Ontology Consortium The Gene Ontology in 2010: Extensions and refinements. Nucleic Acids Res. 2010;38(Database issue):D331–D335. doi: 10.1093/nar/gkp1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pike JW, Meyer MB. Fundamentals of vitamin D hormone-regulated gene expression. J Steroid Biochem Mol Biol. 2014;144PA:5–11. doi: 10.1016/j.jsbmb.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burke SN, Barnes CA. Senescent synapses and hippocampal circuit dynamics. Trends Neurosci. 2010;33(3):153–161. doi: 10.1016/j.tins.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85(1):6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- 46.Vorhees CV, Williams MT. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bartsch T, et al. Focal lesions of human hippocampal CA1 neurons in transient global amnesia impair place memory. Science. 2010;328(5984):1412–1415. doi: 10.1126/science.1188160. [DOI] [PubMed] [Google Scholar]
- 48.Foster TC, Defazio RA, Bizon JL. Characterizing cognitive aging of spatial and contextual memory in animal models. Front Aging Neurosci. 2012;4:12. doi: 10.3389/fnagi.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16(2):130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci USA. 1995;92(21):9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36(1):60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 52.Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: Monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 54.Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7(1):30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- 55.Landfield PW, McGaugh JL, Lynch G. Impaired synaptic potentiation processes in the hippocampus of aged, memory-deficient rats. Brain Res. 1978;150(1):85–101. doi: 10.1016/0006-8993(78)90655-8. [DOI] [PubMed] [Google Scholar]
- 56.Norris CM, Korol DL, Foster TC. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J Neurosci. 1996;16(17):5382–5392. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Disterhoft JF, Oh MM. Alterations in intrinsic neuronal excitability during normal aging. Aging Cell. 2007;6(3):327–336. doi: 10.1111/j.1474-9726.2007.00297.x. [DOI] [PubMed] [Google Scholar]
- 58.Kumar A, Foster TC. 2007. Neurophysiology of old neurons and synapses. Brain Aging: Models, Methods, and Mechanisms, Frontiers in Neuroscience, ed Riddle DR (CRC Press, Boca Raton, FL)
- 59.Applegate MD, Landfield PW. Synaptic vesicle redistribution during hippocampal frequency potentiation and depression in young and aged rats. J Neurosci. 1988;8(4):1096–1111. doi: 10.1523/JNEUROSCI.08-04-01096.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.deToledo-Morrell L, Geinisman Y, Morrell F. Age-dependent alterations in hippocampal synaptic plasticity: Relation to memory disorders. Neurobiol Aging. 1988;9(5-6):581–590. doi: 10.1016/s0197-4580(88)80117-9. [DOI] [PubMed] [Google Scholar]
- 61.Berchtold NC, et al. Synaptic genes are extensively downregulated across multiple brain regions in normal human aging and Alzheimer’s disease. Neurobiol Aging. 2013;34(6):1653–1661. doi: 10.1016/j.neurobiolaging.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wenk MR, De Camilli P. Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: Insights from vesicle recycling in nerve terminals. Proc Natl Acad Sci USA. 2004;101(22):8262–8269. doi: 10.1073/pnas.0401874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Südhof TC. A molecular machine for neurotransmitter release: Synaptotagmin and beyond. Nat Med. 2013;19(10):1227–1231. doi: 10.1038/nm.3338. [DOI] [PubMed] [Google Scholar]
- 64.Fremeau RT, Jr, et al. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31(2):247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- 65.Takamori S, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127(4):831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 66.Lee YS, Silva AJ. The molecular and cellular biology of enhanced cognition. Nat Rev Neurosci. 2009;10(2):126–140. doi: 10.1038/nrn2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mouravlev A, Dunning J, Young D, During MJ. Somatic gene transfer of cAMP response element-binding protein attenuates memory impairment in aging rats. Proc Natl Acad Sci USA. 2006;103(12):4705–4710. doi: 10.1073/pnas.0506137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morris KA, Gold PE. Age-related impairments in memory and in CREB and pCREB expression in hippocampus and amygdala following inhibitory avoidance training. Mech Ageing Dev. 2012;133(5):291–299. doi: 10.1016/j.mad.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hawk JD, et al. NR4A nuclear receptors support memory enhancement by histone deacetylase inhibitors. J Clin Invest. 2012;122(10):3593–3602. doi: 10.1172/JCI64145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fleet JC, et al. Serum metabolite profiles and target tissue gene expression define the effect of cholecalciferol intake on calcium metabolism in rats and mice. J Nutr. 2008;138(6):1114–1120. doi: 10.1093/jn/138.6.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer’s disease: Minding the store. Aging Cell. 2007;6(3):307–317. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gemma C, Vila J, Bachstetter A, Bickford PC. 2007. Oxidative stress and the aging brain: From theory to prevention. Brain Aging: Models, Methods, and Mechanisms, Frontiers in Neuroscience, ed Riddle DR (CRC Press, Boca Raton, FL)
- 73.Brinton RD. The healthy cell bias of estrogen action: Mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31(10):529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood) 2004;229(11):1136–1142. doi: 10.1177/153537020422901108. [DOI] [PubMed] [Google Scholar]
- 75.Boyle MET, et al. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 2001;30(2):385–397. doi: 10.1016/s0896-6273(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 76.Shimazaki K, Hosoya H, Takeda Y, Kobayashi S, Watanabe K. Age-related decline of F3/contactin in rat hippocampus. Neurosci Lett. 1998;245(2):117–120. doi: 10.1016/s0304-3940(98)00179-7. [DOI] [PubMed] [Google Scholar]
- 77.Shepherd MN, Pomicter AD, Velazco CS, Henderson SC, Dupree JL. Paranodal reorganization results in the depletion of transverse bands in the aged central nervous system. Neurobiol Aging. 2012;33(1):e13–e24. doi: 10.1016/j.neurobiolaging.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu PH, et al. Age-related slowing in cognitive processing speed is associated with myelin integrity in a very healthy elderly sample. J Clin Exp Neuropsychol. 2011;33(10):1059–1068. doi: 10.1080/13803395.2011.595397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peters A, Kemper T. A review of the structural alterations in the cerebral hemispheres of the aging rhesus monkey. Neurobiol Aging. 2012;33(10):2357–2372. doi: 10.1016/j.neurobiolaging.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Puzzo D, et al. F3/Contactin promotes hippocampal neurogenesis, synaptic plasticity, and memory in adult mice. Hippocampus. 2013;23(12):1367–1382. doi: 10.1002/hipo.22186. [DOI] [PubMed] [Google Scholar]
- 81.Henderson VC, Kimmelman J, Fergusson D, Grimshaw JM, Hackam DG. Threats to validity in the design and conduct of preclinical efficacy studies: A systematic review of guidelines for in vivo animal experiments. PLoS Med. 2013;10(7):e1001489. doi: 10.1371/journal.pmed.1001489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salthouse TA. When does age-related cognitive decline begin? Neurobiol Aging. 2009;30(4):507–514. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosen CJ, Gallagher JC. The 2011 IOM report on vitamin D and calcium requirements for north america: Clinical implications for providers treating patients with low bone mineral density. J Clin Densitom. 2011;14(2):79–84. doi: 10.1016/j.jocd.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 84.Rosen CJ, et al. IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab. 2012;97(4):1146–1152. doi: 10.1210/jc.2011-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dawson-Hughes B, et al. IOF position statement: Vitamin D recommendations for older adults. Osteoporos Int. 2010;21(7):1151–1154. doi: 10.1007/s00198-010-1285-3. [DOI] [PubMed] [Google Scholar]
- 86.Norman AW, Bouillon R. Vitamin D nutritional policy needs a vision for the future. Exp Biol Med (Maywood) 2010;235(9):1034–1045. doi: 10.1258/ebm.2010.010014. [DOI] [PubMed] [Google Scholar]
- 87.Holick MF, et al. Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 88.Heaney RP. Health is better at serum 25(OH)D above 30ng/mL. J Steroid Biochem Mol Biol. 2013;136:224–228. doi: 10.1016/j.jsbmb.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 89.Ross AC, et al. The 2011 Dietary Reference Intakes for Calcium and Vitamin D: What dietetics practitioners need to know. J Am Diet Assoc. 2011;111(4):524–527. doi: 10.1016/j.jada.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 90.Balden R, Selvamani A, Sohrabji F. Vitamin D deficiency exacerbates experimental stroke injury and dysregulates ischemia-induced inflammation in adult rats. Endocrinology. 2012;153(5):2420–2435. doi: 10.1210/en.2011-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34(1):47–64. doi: 10.1016/j.yfrne.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 92.Berridge MJ. Dysregulation of neural calcium signaling in Alzheimer disease, bipolar disorder and schizophrenia. Prion. 2013;7(1):2–13. doi: 10.4161/pri.21767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kupferschmidt K. Uncertain verdict as vitamin D goes on trial. Science. 2012;337(6101):1476–1478. doi: 10.1126/science.337.6101.1476. [DOI] [PubMed] [Google Scholar]
- 94.Sperling RA, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goldman DP, et al. Substantial health and economic returns from delayed aging may warrant a new focus for medical research. Health Aff (Millwood) 2013;32(10):1698–1705. doi: 10.1377/hlthaff.2013.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Blalock EM, et al. Effects of long-term pioglitazone treatment on peripheral and central markers of aging. PLoS ONE. 2010;5(4):e10405. doi: 10.1371/journal.pone.0010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu JG, Ochalek JT, Kaufmann M, Jones G, Deluca HF. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc Natl Acad Sci USA. 2013;110(39):15650–15655. doi: 10.1073/pnas.1315006110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.