Significance
Hyperpolarization by dissolution dynamic nuclear polarization can dramatically enhance signal intensities in MRI and NMR, notably for metabolic tracers for imaging and diagnosis. It is applicable to a variety of substrates for in vivo imaging and chemistry but requires the use of contaminants (glassing agents and free radicals) that may interact with cells and proteins and can have potential side effects. These contaminants can sometimes be eliminated by precipitation followed by filtration or solvent extraction, but these methods are substrate-specific, are usually time-consuming, and typically result in signal loss. Here, production of pure hyperpolarized liquids free of contaminants is shown by a simple wetting–polarization–filtration sequence for a solid silica matrix containing homogeneously distributed persistent radicals.
Keywords: D-DNP, NMR signal enhancement, molecular imaging, mesostructured hybrid silica, porous materials
Abstract
Hyperpolarization of substrates for magnetic resonance spectroscopy (MRS) and imaging (MRI) by dissolution dynamic nuclear polarization (D-DNP) usually involves saturating the ESR transitions of polarizing agents (PAs; e.g., persistent radicals embedded in frozen glassy matrices). This approach has shown enormous potential to achieve greatly enhanced nuclear spin polarization, but the presence of PAs and/or glassing agents in the sample after dissolution can raise concerns for in vivo MRI applications, such as perturbing molecular interactions, and may induce the erosion of hyperpolarization in spectroscopy and MRI. We show that D-DNP can be performed efficiently with hybrid polarizing solids (HYPSOs) with 2,2,6,6-tetramethyl-piperidine-1-oxyl radicals incorporated in a mesostructured silica material and homogeneously distributed along its pore channels. The powder is wetted with a solution containing molecules of interest (for example, metabolites for MRS or MRI) to fill the pore channels (incipient wetness impregnation), and DNP is performed at low temperatures in a very efficient manner. This approach allows high polarization without the need for glass-forming agents and is applicable to a broad range of substrates, including peptides and metabolites. During dissolution, HYPSO is physically retained by simple filtration in the cryostat of the DNP polarizer, and a pure hyperpolarized solution is collected within a few seconds. The resulting solution contains the pure substrate, is free from any paramagnetic or other pollutants, and is ready for in vivo infusion.
Dissolution dynamic nuclear polarization (D-DNP) (1, 2) usually requires freezing molecules of interest, such as metabolites, together with persistent free radicals often called polarizing agents (PA) in a glassy matrix at very low temperatures (1 < T < 4 K), so that their nuclear spin polarization can be enhanced by up to four to five orders of magnitude. Such enhancements are achieved by saturating the ESR transitions of the PAs. D-DNP is generally performed in moderate magnetic fields (B0 = 3.35 or in this study, 6.7 T) and followed by rapid dissolution of the frozen sample with a burst of superheated water to give highly polarized solutions. Applications include detection of intermediates in chemical reactions (3–5), protein folding in real time (6), and detection of cancer by monitoring abnormal rates of metabolic reactions in humans (7). PAs with narrow EPR lines, such as trityl radicals, are usually used for the direct polarization of 13C nuclei (2). In practice, polarizations P(13C) of 20% or higher can be obtained after dissolution. We have recently shown that DNP of 13C can be significantly accelerated by combining increased magnetic fields with polarization of 1H rather than 13C [using nitroxide radicals, such as 2,2,6,6-tetramethyl-piperidine-1-oxyl (TEMPO), with broader ESR lines than trityl radicals] followed by Hartmann–Hahn 1H→13C cross-polarization (CP) (8) to transfer the polarization from 1H to 13C. In this way, P(13C) = 40% after dissolution at 300 K was obtained in less than 20 min (9–13).
Longitudinal relaxation during heating, dissolution, transfer between magnets, and magnetic resonance spectroscopy or MRI measurements themselves erodes hyperpolarization. Relaxation losses are exacerbated by remaining paramagnetic PAs that no longer serve any function after dissolution; thus, one of the most effective ways to slow down the relaxation rate R1(13C) and hence, prolong the lifetime of the polarization P(13C) is to eliminate the radicals (14). For some radicals, such as trityls, separation can be achieved by solvent extraction (15) or precipitation by a jump in pH followed by mechanical filtration through a stack of polyethylene filters (16, 17). Lumata et al. (18, 19) have shown that precipitation can be used for 1,3 bisdiphenylene-2-phenylallyl (BDPA) and 2,2 diphenyl-1-picrylhydrazyl (DPPH). For TEMPO, with its derivatives including most currently used biradicals (20–26), we have shown that chemical PA quenching with sodium ascorbate (vitamin C) can convert the nitroxide radicals into diamagnetic species through reduction (14). However, for quantitative and rapid quenching, ascorbate must be used in excess, and the remaining ascorbate in solution may affect the analyte or sensitive components present in the NMR or MRI system, such as enzymes (5, 27, 28). Furthermore, the presence of potentially noninnocent additional products arising from the paramagnetic PAs is obviously undesirable for in vivo MRI experiments. In this light, methods to produce pure hyperpolarized solutions free of radicals, glassing, and reducing agents with a limited number of production steps could enable faster, safer, and more sensitive in vitro and in vivo applications.
Eichhorn et al. (29) have recently proposed a promising method for producing hyperpolarized pyruvate solutions without free radicals. This route consists in performing D-DNP on pure [1-13C]-pyruvic acid, in which photo-induced radicals have been generated by intense UV radiation. However, the scope of D-DNP is certainly not limited to pyruvate but should encompass a wide variety of substrates that can be used as metabolic tracers, protein ligands, or chemical reagents. In fact, the UV irradiation process degrades some of the pyruvate into [1-13C]-acetate, which may interfere in vivo, because it is also a metabolite in the Krebs cycle.
One approach to obtain highly polarized solutions free of radicals would be to use a solid polarizing matrix, which would be easily separable from the solution by filtration. Immobilization of radicals on solid materials, such as silica gels or thermoresponsive polymers (30–32), has been reported for room temperature Overhauser DNP of liquid water. More recently, low temperature DNP at T = 4.2 K was shown by exploiting electron spins at dangling bond sites near the surface of silicon nanoparticles, producing, however, only modest enhancements (∼2) (33). One critical problem in solid polarizing matrices is to avoid polarization loss through radical–radical interactions; an optimal polarization matrix, thus, requires control of both the radical density and distribution in the solid.
Here, we show the high polarizing efficiency at very low temperatures (1.2 and 4.2 K) of hybrid polarizing solids (HYPSOs), a family of hybrid organosilica materials, prepared by a Sol-Gel process using a templating route, in which PAs are covalently linked to the pore channels and homogeneously randomly distributed in the mesostructured silica matrix (34). Such control of distribution is not accessible through classical grafting postfunctionalization approaches (34, 35). We show that, after polarization, radical-free hyperpolarized solutions can be easily and rapidly (<11 s) obtained by physical retention during dissolution. This approach leads to large 13C polarization in the solid state, and we show that as much as P(13C) = 25.3% is obtained in the liquid state after dissolution, corresponding to an enhancement factor εDNP > 32,000 with respect to the Boltzmann distribution at 7 T and 300 K. The whole approach is illustrated in Fig. 1.
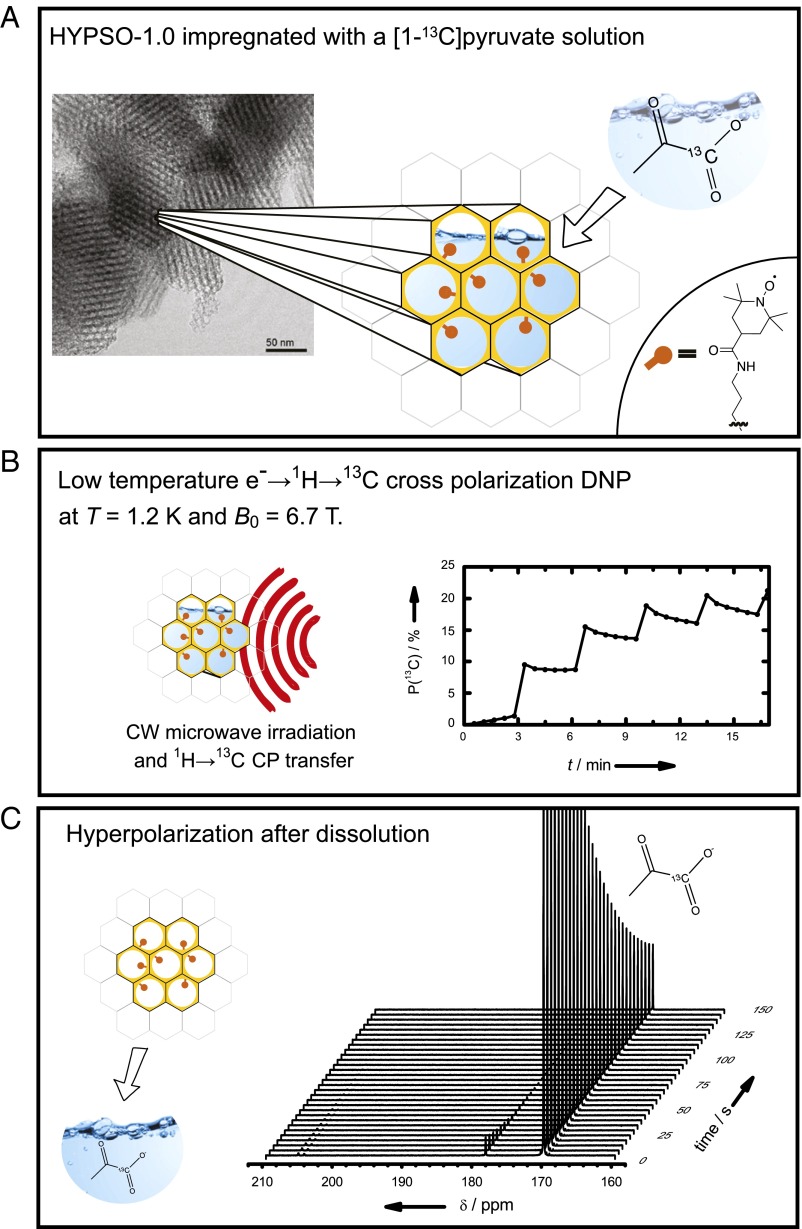
Fig. 1.
Hyperpolarization by D-DNP with HYPSO. (A) HYPSO 1.0 is impregnated with a solution of the analyte to be polarized without addition of any glass-forming agents. The transmission EM image (taken with a Philips CM30 TEM operated at 300 kV) shows the porous structure of the material. The red dots schematically represent the PAs. (B) Proton DNP is performed (Methods) on 20 mg HYPSO 1.0 material (88 µmol/g) impregnated with 36 µL 3 M solution of [1-13C]-pyruvate in D2O. The proton polarization rapidly builds up with a time constant τDNP(1H) = 119 ± 1.5 s, and by applying 1H→13C CP, a polarization of P(13C) > 20% is reached in 17 min. CW, continuous wave. (C) The DNP solution is dissolved and expulsed from HYPSO 1.0 by injecting 5 mL superheated D2O (more details in Methods) and transferring to a 300-MHz spectrometer; a series of 13C NMR spectra of [1-13C]-pyruvate is measured every 5 s. The liquid-state polarization obtained P(13C) = 25.3% corresponds to an enhancement εDNP > 32,000 compared with the Boltzmann equilibrium at 300 K and 7 T, and decays with T1(13C) = 49.4 ± 0.4 s are typical of a pure D2O solution of [1-13C]-pyruvate without any free radicals.
Results and Discussion
The HYPSO family of mesostructured hybrid organosilica materials was chosen, because enhancement factors of up to εon/off = 36 were reported (34) under magic angle spinning-DNP (MAS-DNP) conditions at T = 100 K and B0 = 9.4 T when impregnated with a water or 1,1,2,2-tetrachloroethane solution. The first generation of material, HYPSO 1.0, contains homogeneously distributed TEMPO moieties covalently bound to the silica surface through a propylamide linker [O1.5Si–(CH2)3–NHCO–TEMPO] with varying radical concentration. HYPSO 1.0 has a large pore volume, which allows the polarization of up to ∼1.8 mL solution per 1 g material through a complete filling of the pores (∼1.0 mL/g) and the intergrain volumes (∼0.8 mL/g). The effective filling factor amounts to η = Vsolution/Vtotal = 0.85 (illustrating that the use of such materials does not significantly reduce the volume of polarized solution through the addition of the solid PA). After HYPSO 1.0 is impregnated with a solution of interest, routine DNP can readily be performed in a standard manner by microwave irradiation of the ESR transition. In addition, the covalent linkage between the PA and the silica matrix allows the PAs to remain attached to the surface of the solid matrix during the combined dissolution and filtration step to produce a pure hyperpolarized solution (vide infra). The solution is easily expelled from HYPSO 1.0 by injecting hot water under pressure (Tdiss = 450 K, Pdiss = 1 MPa), which is usually done for regular frozen glassy DNP samples. The resulting slurry is then forced by pressurized hot water with a helium pressure of Ppush = 6 MPa through a home-built cellulose fiber filter mounted just above the DNP sample holder as near as possible to the center of the 6.7-T magnetic field of the polarizer.
Data in Fig. 1 were recorded with 20 mg HYPSO 1.0 material containing 88 µmol radical per gram (the optimal concentration to obtain the highest polarization and the best buildup rate; see below) impregnated with 36 µL 3 M solution of [1-13C]-pyruvate in D2O. After impregnation (Fig. 1A), the sample is inserted into our home-built DNP polarizer operating at 6.7 T (11, 12, 36–38); 20 min of polarization (more details in Methods) suffice for P(13C) to exceed 20% (Fig. 1B). The monoexponential DNP buildup (absence of long-range spin diffusion behavior) confirms that the solution has filled the pores of the material. Dissolution is subsequently performed by injecting 5 mL superheated water (working equally well with D2O or H2O) at T = 450 K and P = 1 MPa. During this process, the entire hyperpolarized solution is expelled from the pores of the material (as determined by quantitative 1H NMR), whereas the PAs remain attached to the surface of the solid. The solution is then transferred to a 7-T NMR spectrometer (300 MHz for protons), and the hyperpolarized [1-13C]-pyruvate signal is measured with an enhancement factor εDNP = 32,500 compared with its thermal equilibrium signal after complete relaxation corresponding to a polarization P(13C) = 25.3% (Fig. 1C). Note that the entire process—dissolution, separation, and polarization measurement at room temperature—requires only 10.2 s, clearly showing the efficiency of this approach. The corresponding grafted materials at similar concentrations gave poor polarization (εDNP = 2), because they do not meet the requirement of a homogeneous distribution of radicals. The fact that the solution is radical-free is illustrated by the fact that the measured 13C T1 is found to be ∼50 s.
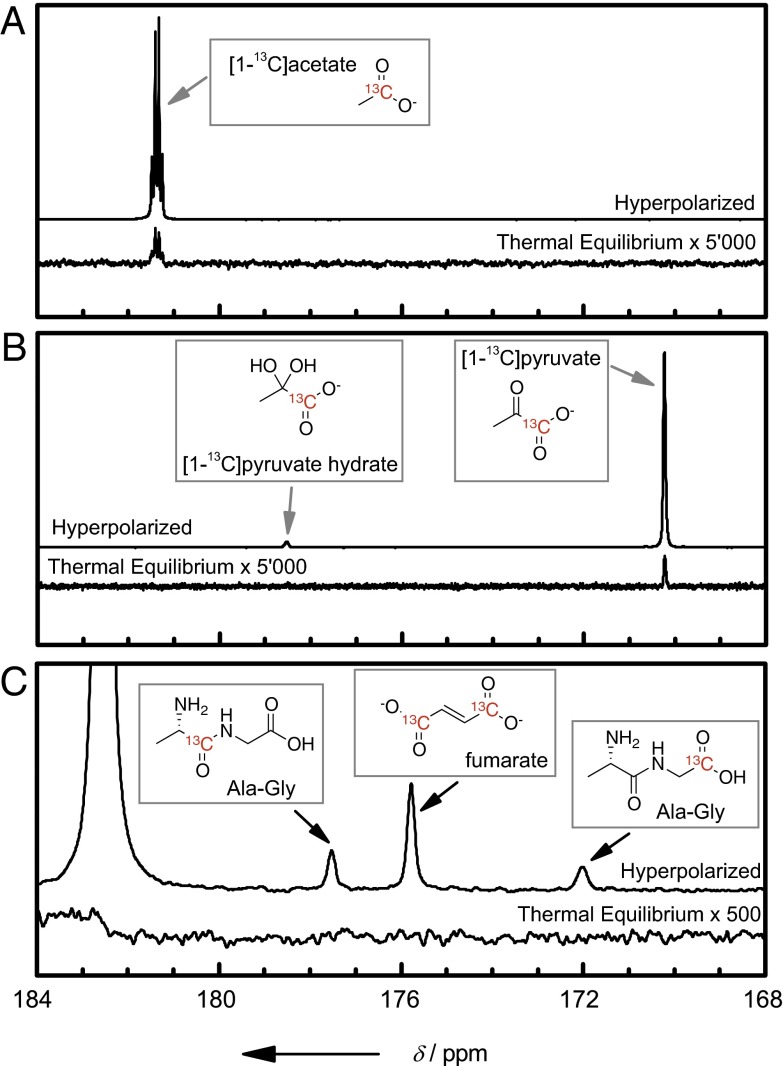
Fig. 2 allows one to compare the hyperpolarized [1-13C]-pyruvate and [1-13C]-acetate signals after dissolution with respect to their thermal equilibrium values, confirming nuclear spin polarizations as high as P(13C) = 25.3% and P(13C) = 16.5%, respectively. As proof of general applicability, the same experiment was also performed on fumarate [with P(13C) = 19.9% for both carbonyl carbons] and the dipeptide alanine-glycine [with P(13C) = 15.0% and P(13C) = 13.6% for the carbonyl carbons of alanine and glycine, respectively]. Note that the production of hyperpolarized solutions of folded proteins is still a challenge, mainly because of the rapid nuclear spin lattice relaxation rates at low magnetic field (leading to most of the hyperpolarization being lost during the dissolution process).
Fig. 2.
Hyperpolarized [1-13C]-acetate, [1-13C]-pyruvate, l-alanine-glycine, and fumarate. Thermal equilibrium and hyperpolarized signals of (A) [1-13C]-acetate, (B) [1-13C]-pyruvate, and (C) l-alanine-glycine and fumarate. Hyperpolarization was performed as described in the text by 1H→13C CP-DNP with HYPSO 1.0 followed by dissolution. The hyperpolarized signals were acquired with single 5° nutation angle pulses, whereas the thermal equilibrium signals, scaled by a factor of 5,000, were measured with 27, 128, and 512 scans for [1-13C]-acetate, [1-13C]-pyruvate, and l-alanine-glycine and fumarate, respectively, using 90° nutation angle pulses applied every 300 s.
To optimize the materials, using optimal microwave frequency and power conditions (fμw = 188.3 GHz and Pμw = 100 mW), we investigated the influence of the PA density in HYPSO 1.0 on polarization efficiency. The proton polarization displays a broad optimum around 88 µmol⋅g−1 (Fig. S1A), which roughly corresponds to an electron concentration of 49 mM in the pores, close to the optimal value of 50 mM that is normally used in D2O:glycerol-d8 mixtures (12). Note that the DNP enhancement measured as a function of the applied microwave frequency depicts a curve (often called microwave spectrum) that is similar to that typically obtained for DNP in frozen glasses without porous materials: two DNP optima are reached for positive or negative polarization at microwave frequencies fµw = 187.85 and 188.3 GHz, respectively (Fig. S1B). The DNP obtained as a function of microwave power (often called a saturation curve) at T = 1.2 K indicates that 80% of the full saturation can be attained with a moderate power of Pµw = 100 mW (Fig. S1C). Although all of these parameters were carefully optimized, the polarization P(13C) = 25.3% in [1-13C]-pyruvate obtained with HYPSO 1.0 is somewhat lower than the record P(13C) = 40% previously reported (12).
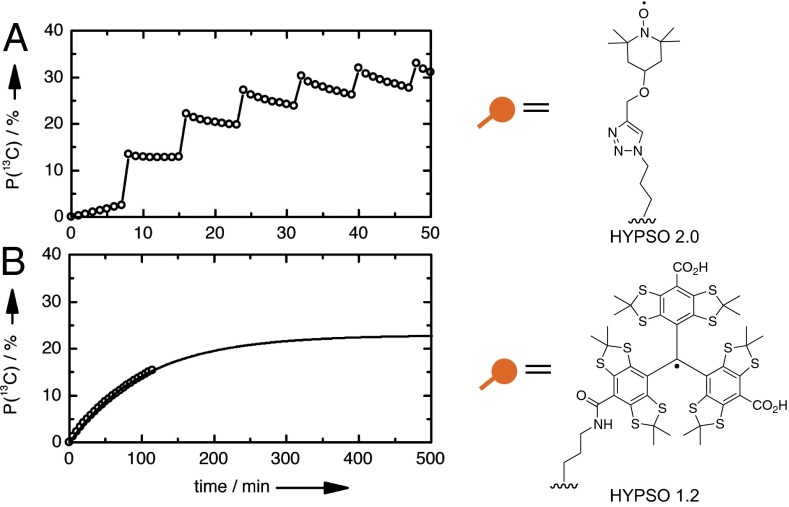
P(13C) can be increased to 36% in [1-13C]-pyruvate (Fig. 3A) (39) on additional tuning of the PA through the incorporation of the TEMPO functionalities in HYPSO 2.0 (shown schematically in Fig. 3A) using a different linker and Click chemistry, which increases the yield of incorporation (details in SI Methods, Fig. S2, and Tables S1 and S2) (40).
Fig. 3.
(A) 1H→13C CP-DNP performed on 20 mg HYPSO 2.0 material (41 µmol/g) impregnated with 36 µL 3 M solution of [1-13C]-pyruvate in D2O. P(13C) > 30% is reached in 32.5 min with 1H→13C CP applied at 7.5-min intervals. (B) Direct 13C DNP performed on 20 mg HYPSO 1.2 material (16 µmol/g) impregnated with 36 µL 3 M solution of [1-13C]-pyruvate in D2O. P(13C) = 15% is achieved after 2 h with a monoexponential buildup time τDNP(13C) = 104.6 ± 2.4 min, potentially toward a maximum P(13C)max = 22.9%. Note that the horizontal scale has been extended by a factor of 10 for B with respect to A. Schematic representations of the radicals in the materials are given in A, Right and B, Right.
Another important asset of HYPSO materials is that glassing agents, such as glycerol or DMSO, which may be proscribed in certain applications (e.g., in vivo imaging as well as the monitoring of chemical/enzymatic reactions, where the glassing agent may not be innocuous), are not required, because the matrix itself prevents crystallization. A clear demonstration of this feature is given with the example of the polarization of pure H2O:D2O [10:90 (vol:vol)] (Fig. S3).
Furthermore, these platform materials allow access to a broad range of solid polarizing matrices (for instance, with silica materials containing trityl radical functionalities). For example, direct 13C polarization with trityl radicals might be preferred over indirect 1H→13C CP DNP with TEMPO (because the recently introduced 1H→13C CP technology is not yet commercially available). Preliminary results with a first generation of HYPSO 1.2 materials (Fig. 3B, SI Methods, Fig. S2, and Tables S1 and S2) with 16 µmol trityl functionalities per gram yield 15% 13C polarization after 2 h, with potential for 25% at saturation (Fig. 3B).
In conclusion, D-DNP can be performed very efficiently using the HYPSO family of PAs. The sample preparation is carried out without glassing agents to provide pure hyperpolarized solutions (no radical contamination), which can easily be separated from the polarizing solids using standard filters. The efficiency of these solid polarization matrices is the result of the controlled incorporation of a homogeneous distribution of radicals along the pore channels of a highly porous mesostructured material. Although already shown here with pyruvate, acetate, fumarate, pure water, and a dipeptide (alanine-glycine), the approach should be applicable to a broad range of molecules that can be hyperpolarized by D-DNP (41).
Methods
Low-Temperature DNP with 1H→13C CP.
The DNP apparatus is equipped with a DNP insert that comprises a microwave shield coupled to an oversized circular waveguide for microwave irradiation and a doubly resonant NMR Helmholtz coil of 0.5 cm3 inner volume (13C and 1H at 71.73 and 285.23 MHz, respectively). The main axis of the radiofrequency (rf) coil is parallel to the static field to enable rapid dissolution. An ELVA (VCOM 10/94/400) microwave source operating at fµw = 94 GHz ± 250 MHz up to Pµw = 400 mW is coupled to a VDI doubler (D200) with ∼30% power conversion efficiency. The 1H spins are polarized by microwave irradiation (fµw = 188.3 GHz and Pµw = 100 mW) at T = 1.2 K. The proton polarization P(1H) is boosted by DNP and subsequently transferred by CP from 1H to 13C. The 13C polarization builds up an iterative CP scheme, comprising two pairs of chirp pulses applied to both 1H and 13C channels with 1-ms duration, 100-kHz sweep width, and B1 = 30 kHz rf amplitude. The 13C polarization is monitored by application of 5° nutation pulses every 30 s. Because the rf fields are currently not sufficiently intense to compete with the dipolar interactions among the protons, the CP transfer is not 100% efficient, and therefore, only a fraction of P(1H) is transferred to 13C. To further enhance P(13C), we reiterate the CP step 5 < n < 20 times at intervals of 180 s.
Dissolution Experiments.
Dissolution is performed with 5 mL superheated D2O (T = 450 K and P = 1 MPa). The time sequence for the whole experiment is as follows: dissolution in tdiss = 700 ms, transfer in ttransfer = 5 s, injection in tinject = 3.5 s, and settling in the NMR tube during tsettle = 1 s. Overall, the liquid-state NMR experiment can start 10.2 s after dissolution.
Liquid-State NMR Experiments.
After DNP and dissolution, a series of 13C NMR spectra of [1-13C]-pyruvate is measured in a 7-T NMR spectrometer (300 MHz for protons) every 5 s with 5° pulses. After the hyperpolarization has completely relaxed to Boltzmann equilibrium, a thermal equilibrium spectrum is measured for comparison with a train of 90° nutation angle pulses (for example, 128 scans) and a long recycle delay (here, 350 s) to allow full relaxation.
Supplementary Material
Acknowledgments
The authors thank Martial Rey and Dr. Pascal Miéville for valuable assistance. Transmission EM pictures were recorded by Dr. Frank Krumeich at the ScopeM. This work was supported by the Swiss National Science Foundation (SNF), the Ecole Polytechnique Fédérale de Lausanne, Eidgenössiche Technische Hochschule, the Swiss Commission for Technology and Innovation, Bruker BioSpin Switzerland AG, the Centre National de la Recherche Scientifique, Lyon Science Transfert Grant LST-1065, Equipements d'Excellence (EQUIPEX) Contract ANR-10-EQPX-47-01, European Research Council (ERC) Advanced Grants 320860 and 339754, and SNF grants.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1407730111/-/DCSupplemental.
References
- 1.Abragam A, Goldman M. Principles of dynamic nuclear-polarization. Rep Prog Phys. 1978;41(3):395–467. [Google Scholar]
- 2.Ardenkjaer-Larsen JH, et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci USA. 2003;100(18):10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowen S, Sekar G, Hilty C. Rapid determination of biosynthetic pathways using fractional isotope enrichment and high-resolution dynamic nuclear polarization enhanced NMR. NMR Biomed. 2011;24(8):1016–1022. doi: 10.1002/nbm.1679. [DOI] [PubMed] [Google Scholar]
- 4.Hilty C, Bowen S. Applications of dynamic nuclear polarization to the study of reactions and reagents in organic and biomolecular chemistry. Org Biomol Chem. 2010;8(15):3361–3365. doi: 10.1039/c004420m. [DOI] [PubMed] [Google Scholar]
- 5.Lee Y, Heo GS, Zeng H, Wooley KL, Hilty C. Detection of living anionic species in polymerization reactions using hyperpolarized NMR. J Am Chem Soc. 2013;135(12):4636–4639. doi: 10.1021/ja4001008. [DOI] [PubMed] [Google Scholar]
- 6.Chen HY, Ragavan M, Hilty C. Protein folding studied by dissolution dynamic nuclear polarization. Angew Chem Int Ed Engl. 2013;52(35):9192–9195. doi: 10.1002/anie.201301851. [DOI] [PubMed] [Google Scholar]
- 7.Nelson SJ, et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-C-13]pyruvate. Sci Transl Med. 2013;5(198):198ra108. doi: 10.1126/scitranslmed.3006070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartmann SR, Hahn EL. Nuclear double resonance in the rotating frame. Phys Rev. 1962;128(5):2042–2053. [Google Scholar]
- 9.Jannin S, Bornet A, Colombo S, Bodenhausen G. Low-temperature cross polarization in view of enhancing dissolution dynamic nuclear polarization in NMR. Chem Phys Lett. 2011;517(4-6):234–236. [Google Scholar]
- 10.Bornet A, Melzi R, Jannin S, Bodenhausen G. Cross polarization for dissolution dynamic nuclear polarization experiments at readily accessible temperatures 1.2 < T < 4.2 K. Appl Magn Reson. 2012;43(1-2):107–117. [Google Scholar]
- 11.Jannin S, Bornet A, Melzi R, Bodenhausen G. High field dynamic nuclear polarization at 6.7 T: Carbon-13 polarization above 70% within 20 min. Chem Phys Lett. 2012;549:99–102. [Google Scholar]
- 12.Bornet A, et al. Boosting dissolution dynamic nuclear polarization by cross polarization. J Phys Chem Lett. 2013;4(1):111–114. doi: 10.1021/jz301781t. [DOI] [PubMed] [Google Scholar]
- 13.Batel M, et al. Dissolution dynamic nuclear polarization efficiency enhanced by Hartmann-Hahn cross polarization. Chem Phys Lett. 2012;554:72–76. [Google Scholar]
- 14.Miéville P, et al. Scavenging free radicals to preserve enhancement and extend relaxation times in NMR using dynamic nuclear polarization. Angew Chem Int Ed Engl. 2010;49(35):6182–6185. doi: 10.1002/anie.201000934. [DOI] [PubMed] [Google Scholar]
- 15.Harris T, Bretschneider C, Frydman L. Dissolution DNP NMR with solvent mixtures: Substrate concentration and radical extraction. J Magn Reson. 2011;211(1):96–100. doi: 10.1016/j.jmr.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ardenkjaer-Larsen JH, et al. Dynamic nuclear polarization polarizer for sterile use intent. NMR Biomed. 2011;24(8):927–932. doi: 10.1002/nbm.1682. [DOI] [PubMed] [Google Scholar]
- 17.Leach AM, Miller P, Telfeyan E, Whitt DB ; General Electric Co. (2009) Method and apparatus for the dissolution and filtration of a hyperpolarized agent with a neutral dissolution media. US Patent US2009263325-A1.
- 18.Lumata L, et al. BDPA: An efficient polarizing agent for fast dissolution dynamic nuclear polarization NMR spectroscopy. Chemistry. 2011;17(39):10825–10827. doi: 10.1002/chem.201102037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lumata L, et al. The efficiency of DPPH as a polarising agent for DNP-NMR spectroscopy. RSC Adv. 2012;2(33):12812–12817. doi: 10.1039/C2RA21853D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song C, Hu KN, Joo CG, Swager TM, Griffin RG. TOTAPOL: A biradical polarizing agent for dynamic nuclear polarization experiments in aqueous media. J Am Chem Soc. 2006;128(35):11385–11390. doi: 10.1021/ja061284b. [DOI] [PubMed] [Google Scholar]
- 21.Hu KN, Song C, Yu HH, Swager TM, Griffin RG. High-frequency dynamic nuclear polarization using biradicals: A multifrequency EPR lineshape analysis. J Chem Phys. 2008;128(5):052302. doi: 10.1063/1.2816783. [DOI] [PubMed] [Google Scholar]
- 22.Dane EL, et al. Rigid orthogonal bis-TEMPO biradicals with improved solubility for dynamic nuclear polarization. J Org Chem. 2012;77(4):1789–1797. doi: 10.1021/jo202349j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zagdoun A, et al. A slowly relaxing rigid biradical for efficient dynamic nuclear polarization surface-enhanced NMR spectroscopy: Expeditious characterization of functional group manipulation in hybrid materials. J Am Chem Soc. 2012;134(4):2284–2291. doi: 10.1021/ja210177v. [DOI] [PubMed] [Google Scholar]
- 24.Kiesewetter MK, Corzilius B, Smith AA, Griffin RG, Swager TM. Dynamic nuclear polarization with a water-soluble rigid biradical. J Am Chem Soc. 2012;134(10):4537–4540. doi: 10.1021/ja212054e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zagdoun A, et al. Large molecular weight nitroxide biradicals providing efficient dynamic nuclear polarization at temperatures up to 200 K. J Am Chem Soc. 2013;135(34):12790–12797. doi: 10.1021/ja405813t. [DOI] [PubMed] [Google Scholar]
- 26.Sauvée C, et al. Highly efficient, water-soluble polarizing agents for dynamic nuclear polarization at high frequency. Angew Chem Int Ed Engl. 2013;52(41):10858–10861. doi: 10.1002/anie.201304657. [DOI] [PubMed] [Google Scholar]
- 27.Lee Y, Zeng H, Ruedisser S, Gossert AD, Hilty C. Nuclear magnetic resonance of hyperpolarized fluorine for characterization of protein-ligand interactions. J Am Chem Soc. 2012;134(42):17448–17451. doi: 10.1021/ja308437h. [DOI] [PubMed] [Google Scholar]
- 28.Lee Y, et al. Hyperpolarized binding pocket nuclear Overhauser effect for determination of competitive ligand binding. Angew Chem Int Ed Engl. 2012;51(21):5179–5182. doi: 10.1002/anie.201201003. [DOI] [PubMed] [Google Scholar]
- 29.Eichhorn TR, et al. Hyperpolarization without persistent radicals for in vivo real-time metabolic imaging. Proc Natl Acad Sci USA. 2013;110(45):18064–18069. doi: 10.1073/pnas.1314928110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gitti R, et al. Solid liquid intermolecular transfer of dynamic nuclear-polarization-enhanced flowing fluid h-1-nmr signals via immobilized spin labels. J Am Chem Soc. 1988;110(7):2294–2296. [Google Scholar]
- 31.Dollmann BC, et al. Thermoresponsive, spin-labeled hydrogels as separable DNP polarizing agents. Phys Chem Phys. 2010;12(22):5879–5882. doi: 10.1039/c003349a. [DOI] [PubMed] [Google Scholar]
- 32.McCarney ER, Han S. Spin-labeled gel for the production of radical-free dynamic nuclear polarization enhanced molecules for NMR spectroscopy and imaging. J Magn Reson. 2008;190(2):307–315. doi: 10.1016/j.jmr.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Cassidy MC, Ramanathan C, Cory DG, Ager JW, Marcus CM. Radical-free dynamic nuclear polarization using electronic defects in silicon. Phys Rev B. 2013;87(16):161306. [Google Scholar]
- 34.Gajan D, et al. Solid-phase polarization matrixes for dynamic nuclear polarization from homogeneously distributed radicals in mesostructured hybrid silica materials. J Am Chem Soc. 2013;135(41):15459–15466. doi: 10.1021/ja405822h. [DOI] [PubMed] [Google Scholar]
- 35.Conley MP, Copéret C, Thieuleux C. Mesostructured hybrid organic-silica materials: Ideal supports for well-defined heterogeneous organometallic catalysts. ACS Catal. 2014;4(5):1458–1469. [Google Scholar]
- 36.Comment A, et al. Design and performance of a DNP prepolarizer coupled to a rodent MRI scanner. Concepts Magn Reso B. 2007;31B(4):255–269. [Google Scholar]
- 37.Comment A, et al. Principles of operation of a DNP prepolarizer coupled to a rodent MRI scanner. Appl Magn Reson. 2008;34(3-4):313–319. [Google Scholar]
- 38.Jannin S, et al. A 140 GHz prepolarizer for dissolution dynamic nuclear polarization. J Chem Phys. 2008;128(24):241102. doi: 10.1063/1.2951994. [DOI] [PubMed] [Google Scholar]
- 39.Bornet A, et al. Microwave frequency modulation to enhance dissolution dynamic nuclear polarization. Chem Phys Lett. 2014;602:63–67. [Google Scholar]
- 40.Kolb HC, Finn MG, Sharpless KB. Click chemistry: Diverse chemical function from a few good reactions. Angew Chem Int Ed Engl. 2001;40(11):2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 41.Vuichoud B, et al. Hyperpolarization of deuterated metabolites via remote cross-polarization and dissolution dynamic nuclear polarization. J Phys Chem B. 2014;118(5):1411–1415. doi: 10.1021/jp4118776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.