Significance
This study uncovers a critical role for a general translation factor in specific developmental stages, including immature oocytes and ES cells, and during growth-factor deprivation of mammalian cells, which induces the transition to cell-cycle arrest. These conditions alter and decrease general translation yet maintain ongoing translation. We reveal upregulation of the eukaryotic translation factor 5B (eIF5B), which becomes essential for general translation, specifically in these conditions. Importantly, our data demonstrate that eIF5B controls these cell-cycle transition and developmental stages, promoting oocyte maturation and inhibiting cell-cycle arrest. These findings underscore the importance of translational regulation in cell-cycle transitions and development.
Keywords: embryonic stem cells, early serum starvation, eIF2α phosphorylation
Abstract
Proliferation arrest and distinct developmental stages alter and decrease general translation yet maintain ongoing translation. The factors that support translation in these conditions remain to be characterized. We investigated an altered translation factor in three cell states considered to have reduced general translation: immature Xenopus laevis oocytes, mouse ES cells, and the transition state of proliferating mammalian cells to quiescence (G0) upon growth-factor deprivation. Our data reveal a transient increase of eukaryotic translation initiation factor 5B (eIF5B), the eukaryotic ortholog of bacterial initiation factor IF2, in these conditions. eIF5B promotes 60S ribosome subunit joining and pre-40S subunit proofreading. eIF5B has also been shown to promote the translation of viral and stress-related mRNAs and can contribute indirectly to supporting or stabilizing initiator methionyl tRNA (tRNA-Meti) association with the ribosome. We find that eIF5B is a limiting factor for translation in these three conditions. The increased eIF5B levels lead to increased eIF5B complexes with tRNA-Meti upon serum starvation of THP1 mammalian cells. In addition, increased phosphorylation of eukaryotic initiation factor 2α, the translation factor that recruits initiator tRNA-Meti for general translation, is observed in these conditions. Importantly, we find that eIF5B is an antagonist of G0 and G0-like states, as eIF5B depletion reduces maturation of G0-like, immature oocytes and hastens early G0 arrest in serum-starved THP1 cells. Consistently, eIF5B overexpression promotes maturation of G0-like immature oocytes and causes cell death, an alternative to G0, in serum-starved THP1 cells. These data reveal a critical role for a translation factor that regulates specific cell-cycle transition and developmental stages.
Specific cell states and transitions, including distinct developmental stages and cell-cycle arrest, alter and decrease general translation (1, 2) yet exhibit ongoing translation (3). In immature Xenopus laevis oocytes, translation of mRNAs is regulated and active after maturation (3, 4); however, mRNAs are translated during immature stages preceding maturation (5). Similarly, canonical translation is altered in mouse ES cells until differentiation (6), but translation ensues in ES cells (7), indicating that uncharacterized factors operate to support general translation in these cell states.
The transition from immature to mature oocytes shows some features similar to the entry into mammalian G1/cell cycle from quiescence (G0) (8), an assortment of reversible, cell-cycle–arrested states that can withstand unfavorable environments (9). Serum deprivation of proliferating mammalian cells induces an early stage of transient stress that alters gene expression; cells subjected to such stress either adapt to these nonproliferative conditions and proceed further into G0 or alternatively undergo cell death (9). Early (1 day) serum starvation represents a transient and heterogeneous state and is distinct from prolonged serum starvation in cells that have entered G0 and late G0 (9). The reprogramming of gene expression during this transition involves a decrease in overall translation, yet ongoing translation is observed (10), indicating that undiscovered factors support translation in these conditions.
The mechanism of translation involves several steps, including recruitment of the initiator methionyl tRNA (tRNA-Meti) to the small 40S ribosome subunit by eukaryotic initiation factor 2 (eIF2) and joining of the large 60S ribosome subunit by eukaryotic initiation factor 5B (eIF5B) (1, 2, 11). eIF5B is the mammalian ortholog of bacterial IF2 that is important in the formation of the 30S initiation complex, stimulates 50S association to form 70S complexes, and can contribute to stabilizing tRNA-Meti association with the ribosome (2, 11–13). eIF5B functions in 60S ribosome subunit joining during canonical translation (2, 13, 14) and is involved in pre-40S ribosome subunit proofing (15, 16). eIF5B is also required for the translation of a few viral and specialized mRNAs (12, 17–20) and can contribute to supporting or stabilizing tRNA-Meti association, including in specific conditions where phosphorylation of the eIF2 subunit, eIF2α, is also observed (12, 17–19). In some cases, phosphorylation of eIF2α is sufficiently increased relative to the levels of its guanine nucleotide exchange factor (eIF2B), which leads to decreased release of phosphorylated eIF2α from eIF2B. This decrease prevents the recycling of inactive eIF2α-GDP to the active eIF2α-GTP form and can cause altered translation (2, 21, 22).
Here we investigated an altered translation factor in cell states considered to have reduced general translation: in late immature oocytes, in ES cells, and during the transient stress induced in the early stages of growth-factor deprivation of proliferating mammalian cells (3, 4, 6, 7, 10). We find that eIF5B is transiently increased in these three conditions. The role of eIF5B in 60S subunit joining and other functions (2, 13–16) may be important for translation in these conditions and may indirectly enable tRNA-Meti association. Accordingly, depletion of eIF5B reduces translation, indicating that eIF5B is limiting, in part, for translation in these three conditions. In serum-starved cells, the increased levels of eIF5B lead to increased formation of eIF5B complexes with tRNA-Meti, consistent with its previously described role with viral and stress mRNAs (12, 17–19). Additionally, increased phosphorylation of eIF2α is observed in these three conditions. Importantly, we find that eIF5B overexpression promotes the transition away from G0 or G0-like states (immature oocytes; ref. 8), enhancing the maturation of late G0-like immature oocytes and causing cell death, the alternative to G0, upon serum deprivation of proliferating mammalian cells. These data suggest that eIF5B promotes translation in these specific conditions and is an antagonist of G0 and G0-like states; consistently, eIF5B depletion decreases translation, promotes the immature state of oocytes, and enables earlier G0 arrest upon serum starvation of proliferating mammalian cells. These studies reveal a critical role for a translation factor that is important for general translation under specific conditions and regulates distinct cell and developmental stages.
Results
eIF5B Levels Are Increased in Late X. laevis Immature Oocytes.
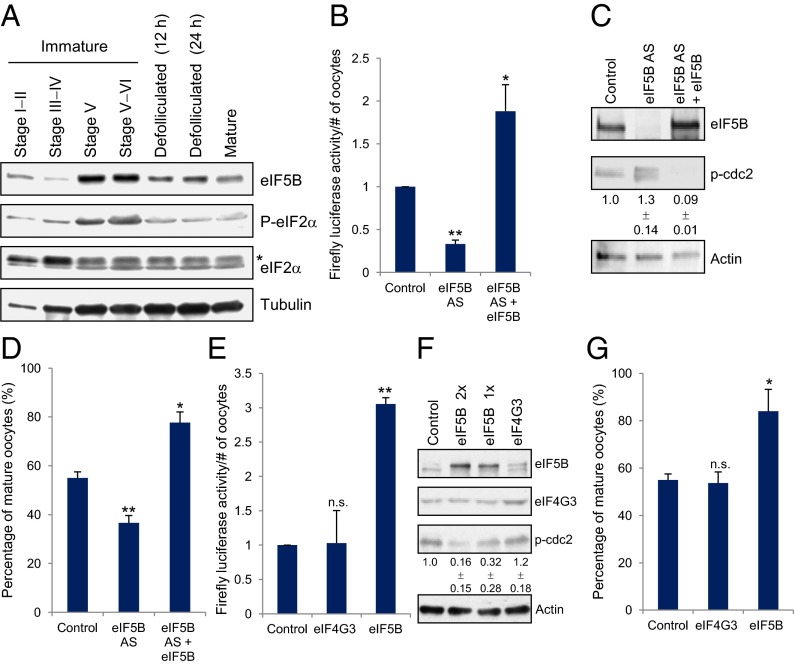
Translation is regulated and active in X. laevis oocytes after maturation (3, 4); however, a subset of mRNAs is translated in immature oocytes (3, 5), indicating that uncharacterized factors support translation in these conditions. eIF5B is known to recruit 60S ribosome subunits (2, 11, 13) and to support the translation of viral, stress-related, and other specific mRNAs in embryos and in stress conditions in which translation is regulated (12, 17–20). Therefore, we compared eIF5B levels in folliculated immature, defolliculated, and mature oocytes (5). We found that eIF5B is increased in the late immature stages V and VI that precede maturation (Western blot; Fig. 1A). These data potentially indicate that eIF5B may be important for supporting general translation in late immature oocytes.
Fig. 1.
eIF5B levels and eIF2α phosphorylation are increased in late immature oocytes (stages V and VI), in which eIF5B promotes translation and maturation. (A) eIF5B and total and phosphorylated eIF2α levels are shown by Western blot analysis comparing 20 oocytes from each stage. In the eIF2α blot the asterisk indicates that the top band is a nonspecific band. (B) Control or eIF5B antisense (AS)-treated stage V immature oocytes were injected with a Firefly luciferase reporter plasmid after 6 h of incubation to measure the effects of eIF5B levels on general translation. In parallel samples, human eIF5B (eIF5B) or vector control mRNA was coinjected with the antisense to observe rescue of the loss of eIF5B function. (C) Western blot of eIF5B and the marker for immaturity, phospho-Cdc2 (p-Cdc2) (5), and its quantitation (given below the blots) from three replicates in oocytes injected with control antisense+GFP (Control), eIF5B antisense+GFP (eIF5B AS), or eIF5B antisense+human eIF5B (eIF5B AS+eIF5B). Phospho-Cdc2 runs variably as multiple bands due to modifications that include multiple-site phosphorylated forms in these extracts to which phosphatase inhibitors were not added (23) and due to a variably observed truncated form that migrates as a lower band and was observed previously (figure S5 in ref. 5). The lack of phosphatase inhibitors in the extracts and the set amount of Cdc2 in these oocytes would limit further increases in phospho-Cdc2 levels upon eIF5B depletion. All bands were quantitated, and the average from multiple experiments is shown below the blots. (D) Control or eIF5B antisense-treated and rescued immature oocytes were scored for germinal vesicle breakdown to measure the effects of eIF5B levels on maturation (Table S1) (5). (E) Ten nanograms of vector control, eIF4G3, or eIF5B mRNAs were injected into immature oocytes; after 6 h, these oocytes were injected with Firefly luciferase reporter plasmid to measure the effects of overexpression of eIF5B or the control translation factor, eIF4G3, on general translation by luciferase analyses. (F) Western blot of eIF5B and the immaturity marker p-Cdc2 and its quantitation from three replicates (shown below the blots), in oocytes with control, eIF4G3, or eIF5B mRNA overexpression (1× = 5 ng; 2× = 10 ng). Multiple bands are observed variably with eIF5B due to dephosphorylation in the absence of phosphatase inhibitors. (G) Control, eIF4G3, or eIF5B overexpression in immature oocytes scored for germinal vesicle breakdown to measure the effects of eIF5B overexpression on maturation (Table S1). Actin (a stable, abundant protein) was used as a loading control. The graphs show the average of three independent experiments; error bars indicate SEM. n.s., not significant. *P < 0.05; **P < 0.01.
eIF5B Promotes Translation in Late X. laevis Immature Oocytes.
To explore the requirement for eIF5B in regulating translation in late immature oocytes, eIF5B was depleted in immature stage V oocytes and in matured oocytes, using an antisense oligonucleotide and, in parallel, was rescued with human eIF5B mRNA. These oocytes then were injected with a Firefly luciferase reporter to measure effects on general mRNA translation (5). Human eIF5B mRNA is not complementary to, and therefore is not depleted by the antisense oligonucleotide. When endogenous eIF5B was depleted, luciferase reporter activity decreased as compared with the control antisense treatment (Fig. 1 B and C). When oocytes injected with eIF5B antisense oligonucleotide were rescued with human eIF5B mRNA, luciferase reporter translation increased significantly as compared with oocytes injected with the control (GFP) (Fig. 1 B and C), indicating the ability of eIF5B to promote general translation. Luciferase RNA levels did not change significantly, indicating that these effects occurred at the translation level (Fig. S1A). Interestingly, no significant changes were observed with eIF5B depletion or rescue in matured oocytes (Fig. S1B), conditions in which eIF5B is not increased (Fig. 1A). These data indicate that eIF5B promotes general translation specifically in late immature oocytes.
EIF2α Is Phosphorylated in Late X. laevis Immature Oocytes.
Since it has been observed that eIF5B is important for translation of viral and stress-related mRNAs in conditions that also exhibit increased eIF2α phosphorylation (12, 17–19), we examined eIF2α (2, 21) in folliculated immature, defolliculated, and mature oocytes (5). We found that eIF2α phosphorylation is increased in late immature oocytes stages V and VI that precede maturation (Western blot, Fig. 1A).
EIF2α levels are also reduced in late immature oocyte stages as compared with mature oocytes (Fig. S1C, Right for quantitation). To test whether eIF2 is required for translation in these conditions, the critical subunit of eIF2, eIF2γ (2, 21, 22), was depleted with an antisense oligonucleotide in immature stage V and mature oocytes, which were then tested for translation. Oocytes treated with control and eIF2γ antisense were subsequently injected with a Firefly luciferase reporter to measure effects on general mRNA translation (5). Unlike eIF5B (Fig. 1 B and C), depletion of eIF2γ by eIF2γ antisense did not decrease translation in immature oocytes as compared with control antisense treatment (Fig. S1 D and E); rather, translation was slightly improved, indicating that eIF2 is not obligatory for translation in these conditions (Fig. S1D). In contrast, in oocytes that matured, translation was reduced significantly upon eIF2γ depletion as compared with control antisense treatment, indicating that eIF2-dependent translation occurs in mature oocytes (Fig. S1 D and E). These results indicate that the translation factor eIF2 is not limiting for translation in immature oocytes.
eIF5B Promotes Maturation.
Since maturation involves translation (3, 4), and these late immature oocyte stages [in which eIF5B is up-regulated and promotes translation (Fig. 1 A and B)] precede maturation, we hypothesized that eIF5B may be increased specifically to promote the transition from late immature stages to maturation. If so, eIF5B expression would promote maturation, inhibiting the G0-like, immature state (8), while eIF5B depletion would promote the immature state. Consistently, Western blot analysis demonstrated that phospho-Cdc2, an immaturity marker, increases upon eIF5B depletion, indicating increased immaturity (i.e., a decreased number of mature oocytes), and decreases upon rescue with eIF5B, indicating increased numbers of mature oocytes (Fig. 1C). Phospho-Cdc2 runs variably as multiple bands because of modifications that include multiple-site phosphorylated forms in these extracts to which phosphatase inhibitors were not added (23) and a variably observed truncated form that migrates as a lower band and had been observed previously (5). The lack of phosphatase inhibitors in the extracts and the set amount of Cdc2 in these oocytes would limit further increase in phospho-Cdc2 levels upon eIF5B depletion. These results were validated by scoring for germinal vesicle breakdown that marks the onset of maturation (5), 6–8 h after injection (Table S1). Depletion of eIF5B demonstrated increased immaturity (37% maturity/63% immaturity) and increased phospho-Cdc2 as compared with GFP-expressing control oocytes (55% maturity/45% immaturity) and with oocytes in which eIF5B depletion had been rescued with human eIF5B (77% maturity/23% immaturity) (Fig. 1D and Table S1). In contrast to eIF5B depletion, depletion of eIF2γ promoted maturation (81% maturity/19% immaturity) and decreased phospho-Cdc2 (Fig. S1 E and F and Table S1). These results indicate that the depletion of eIF5B, not any translation factor, impairs maturation.
To further test whether eIF5B promotes translation and maturation in late immature oocyte stages, we overexpressed either eIF5B or another translation factor, eukaryotic translation initiation factor 4 gamma, 3 (eIF4G3). Overexpression of eIF5B promoted translation in immature oocytes as compared with the expression of control GFP or eIF4G3 (Fig. 1 E and F). In contrast, eIF5B overexpression did not promote translation in matured oocytes, but eIF4G3 did promote translation in these conditions (Fig. S1B), indicating that eIF5B overexpression promotes translation specifically in immature oocytes. Overexpression of eIF5B demonstrated decreased phospho-Cdc2 (Fig. 1F), with 84% maturity/16% immaturity (Fig. 1G and Table S1) as compared with 55% maturity/45% immaturity in control oocytes expressing GFP. These findings support the hypothesis that eIF5B promotes maturation. In contrast, overexpression of eIF4G3 maintained immaturity (53% maturity/47% immaturity) (Fig. 1G and Table S1) at levels similar to those in GFP-expressing control oocytes, indicating that the overexpression of any general translation factor does not promote maturation. These data indicate that eIF5B transiently increases in late immature oocytes and can promote translation and maturation.
eIF5B Levels Are Increased in Mouse ES Cells Where It Is Required for Translation.
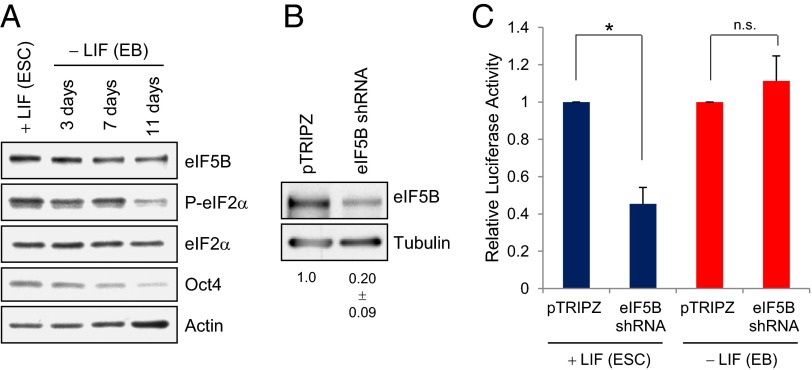
Mouse ES cells demonstrate an altered canonical translation mechanism with increased use of alternative start sites (7) until differentiation (6), yet translation ensues in ES cells (7), indicating that uncharacterized factors support translation in ES cells. We examined eIF5B in ES cells and in differentiating cells (embryoid bodies, EBs) formed upon the removal of the leukemia inhibitory factor (LIF) growth factor (24). eIF5B levels are increased in ES cells as compared with differentiating cells (Fig. 2A). ES cells were transfected with a doxycycline-inducible eIF5B-specific shRNA or with pTRIPZ control shRNA and a plasmid expressing Firefly luciferase reporter followed by doxycycline induction for 3 d. EBs were treated in the same way after differentiation. The cells were maintained as ES cells or differentiated into EBs (11 d with no LIF marked by the decrease in the ES cell marker Oct4 in Fig. 2A). eIF5B levels decreased significantly with eIF5B shRNA (Fig. 2B) and consistently decreased luciferase reporter translation (Fig. 2C) without altering mRNA levels in ES cells (Fig. S2A). The levels of endogenous ES cell proteins, such as Oct4, Nanog, and Sox2, decreased as compared with actin, a stable, abundant loading control (Fig. S2B), indicating that endogenous genes expressed in these conditions are also affected upon transient knockdown of eIF5B. In EBs, eIF5B shRNA did not alter reporter mRNA translation significantly (Fig. 2C). These data indicate that eIF5B contributes to translation in ES cells in which eIF5B levels are increased and translation was previously observed to be altered (7), similar to the results observed in late immature oocytes (Fig. 1 and Fig. S1).
Fig. 2.
eIF5B levels and eIF2α phosphorylation are increased in ES cells in which eIF5B depletion decreases general translation. (A) Western blot showing eIF5B levels and eIF2α phosphorylation in mouse ES cells [+LIF (ESC)] compared with differentiating EBs in the absence of LIF (24). Oct4 is an ES cell marker. Actin was used as a loading control. (B) Western blot of stem cells transfected with the luciferase reporter plasmid pTRIPZ control shRNA vector or with eIF5B-specific shRNA. shRNA was induced with doxycycline for 3 d in ES cells or for 3 d after differentiation into EBs (11 d −LIF). Pooled EBs and ES cells were checked for depletion. (C) Luciferase reporter assay normalized to total nucleic acid levels of ES cells (+LIF) and EBs (11 d −LIF) transfected with pTRIPZ control or eIF5B shRNA. Error bars indicate SEMs from three independent experiments. LIF, leukemia inhibitory factor. *P < 0.05.
EIF2α Phosphorylation Is Increased in Mouse ES Cells.
Increased eIF2α phosphorylation was observed in ES cells as compared with EBs (Fig. 2A), consistent with our results in late immature oocytes (Fig. 1). The effect of loss of eIF2 could not be determined as shRNA knockdown of eIF2γ did not decrease translation in either ES cells (Fig. S2 C and D) or EBs (Fig. S2 C and D). These findings indicate that the depletion is either insufficient or that ES cells and EBs compensate for the depletion of eIF2γ, as previously observed with eIF2B (25, 26) and other initiation factors (27, 28) in mammalian cells. EIF2 would continue to contribute to the translation in ES cells, depending on the relative levels of phosphorylated eIF2α and eIF2B (2, 21, 22), which could regulate the translation observed here, including the use of alternative start sites (7).
eIF5B Levels Increase Transiently Within One Day of Serum Starvation in THP1 Cells.
Serum deprivation induces an early transient stress that alters gene expression. Mammalian cells either adapt to these nonproliferative conditions and proceed further into G0 or alternatively, undergo cell death (9). One day (1 d) of serum starvation represents a transient and heterogeneous state and is distinct from prolonged (>1 d) serum starvation of cells that have entered G0 and late G0 (9). The altered gene expression involves a decrease in global translation, yet the translation of specific mRNAs is up-regulated (10), indicating that uncharacterized factors support translation in this condition, as in immature oocytes (Fig. 1). To test whether eIF5B is important for supporting general translation upon growth-factor deprivation (9, 10), as it is in immature oocytes and ES cells (Figs. 1 and 2), we examined eIF5B levels upon serum deprivation of THP1 cells. Interestingly, eIF5B is increased transiently in THP1 cells 1 d after serum starvation (SS1D, Western blot in Fig. 3A; a shorter form of eIF5B is also observed in serum-grown cells, Fig. S3A) when p27/KIP1, a marker of cell-cycle arrest, is increased (Fig. 3B, Right for quantitation). Increase in eIF5B is not observed in BJ fibroblasts (Fig. S3B). These data indicate that eIF5B may support translation transiently during early serum deprivation stress in specific cell types such as THP1.
Fig. 3.
eIF5B levels and eIF2α phosphorylation are transiently increased in THP1 cells serum-starved for 1 d in which eIF5B depletion promotes earlier G0 arrest. (A) Western blot analysis of eIF5B levels over days of serum starvation (SS1D, SS2D, SS4D, SS7D) compared with serum-grown cells (S+). (B, Left) Western blot analysis of eIF2α phosphorylation and eIF2α levels in THP1 cells that had been serum-starved for 1 d (SS1D) compared with serum-grown cells (S+). p27/KIP1 (p27) marks cell-cycle arrest. (Right) The phosphorylated form and total eIF2α levels from three experiments were quantitated. (C) By using a doxycycline-inducible lentiviral vector (which expresses eIF5B-specific shRNA, eIF5B shRNA, as in Fig. 2) that is stably transduced into THP1 cells, eIF5B was knocked down by 63–67% as observed by Western analysis. Actin was used as a loading control. (D) eIF5B shRNA was induced with doxycycline in serum-containing medium for 3 d; then one half of the cells were shifted to serum-free medium. Cell counts were plotted relative to day 0 cell counts to measure cell proliferation over 3 d. (Left) eIF5B depletion had no effect on proliferation rates in serum-grown cells. (Right) Upon serum starvation, G0 arrest was observed earlier in eIF5B-depleted cells than in undepleted cells. (E) MTS assay (Promega) to measure cell growth after 3 d in serum-grown and serum-starved eIF5B-depleted and control cells. (F, Left) Western blot analysis of eIF5B, p27, and p21CIP1 (p21) in eIF5B-depleted and control cells after 1 d of serum starvation. (Right) Quantitation from three experiments. Tubulin was used as a loading control. Graphs show the average of three independent experiments; error bars indicate SEM. *P < 0.05; **P < 0.01.
Depletion of eIF5B Causes Earlier G0 Arrest of Serum-Starved THP1 Cells.
Our results in oocytes, in which eIF5B depletion decreased maturation and promoted the G0-like, immature state (Fig. 1 C and D and Table S1), and in which eIF5B overexpression promoted maturation, decreasing the G0-like immature state (Fig. 1 F and G and Table S1), suggest that eIF5B promotes the transition away from G0-like (immature oocyte; refs. 8–10) states and could potentially prevent G0 arrest in mammalian cells as a stress response to early serum starvation. To investigate the effect of eIF5B upon serum starvation, a stable, inducible eIF5B knockdown cell line was created in THP1 cells. This eIF5B-specific shRNA, induced in THP1 cells by a doxycycline-inducible promoter, resulted in 63–67% eIF5B depletion (Western blot of eIF5B shRNA cells in Fig. 3C).
We investigated whether eIF5B regulates the transition from proliferation to G0 upon its increase during early serum starvation (Fig. 3D). eIF5B-specific shRNA was induced with doxycycline or, as a control, was not induced in serum-containing medium for 3 d; half of the cells were then shifted to serum-free medium to induce G0 for 3 d. Cell growth was measured by counting viable cells over time. We observed no effect of eIF5B depletion (Fig. 3D, Left, S+, eIF5B shRNA) in serum-containing medium where cell proliferation was similar to that of the undepleted control cells (Fig. 3D, Left, S+, control). However, serum-starved eIF5B-depleted cells (Fig. 3D, Right, SS, eIF5B shRNA) ceased proliferation more rapidly, exhibiting moderately earlier G0 arrest compared with uninduced control cells (Fig. 3D, Right, SS, control). These results were repeated with the MTS assay (Promega colorimetric assay for cell growth), in which a 25% decrease in absorbance (Fig. 3E) indicated decreased cell growth. Consistently, upon serum starvation, a 1.6-fold increase in the abundance of markers of cell-cycle arrest, p27/KIP1 and p21/CIP1, was observed in eIF5B-depleted cells as compared with control cells (Fig. 3F, Right for quantitation). The fact that cells depleted of eIF5B cease proliferation and enter arrest earlier upon serum starvation indicates that eIF5B is a transient inhibitor of G0.
Overexpression of eIF5B Causes Decreased Viability of Serum-Starved THP1 Cells.
If eIF5B is a transient inhibitor of G0 arrest as a stress response to serum starvation, then overexpression of eIF5B would block G0 entry and should cause the alternative fate of cell death upon serum starvation (9). eIF5B or GFP was expressed in serum-containing or serum-free medium over 2 d, and cell viability was measured. Some cell death is usually observed upon serum starvation and nucleofection; however, more GFP-expressing cells survived and remained arrested in G0 compared with cells expressing eIF5B, which showed a sharp, rapid decline in viability in 1 d, and very few cells survived (Fig. S3 C and D, SS1D GFP and SS1D eIF5B). In contrast, in serum-containing medium, eIF5B-expressing cells do not show rapid cell death, and proliferate similar to GFP-expressing cells (Fig. S3 C and D, S+GFP and S+eIF5B). EIF2α overexpression did not affect cell viability in serum-starved cells (Fig. S3 E and F), suggesting that overexpression of eIF5B, and not any general translation factor, decreases viability upon serum-starvation. These data support that eIF5B inhibits G0 arrest upon growth-factor deprivation.
eIF5B Depletion Affects Translation in Serum-Starved Cells.
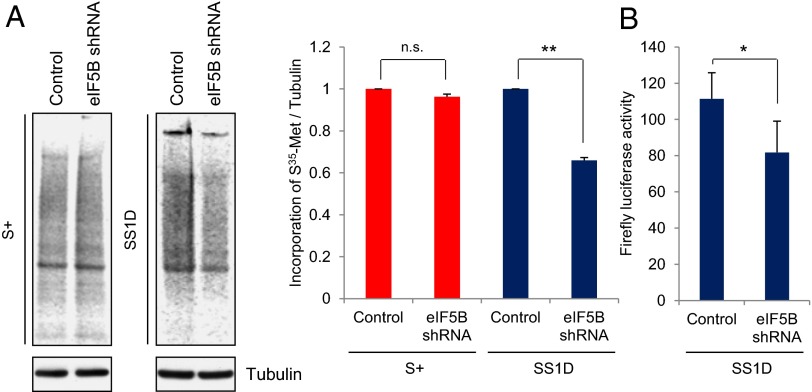
To investigate the quantitative effect of eIF5B on translation, we used the stable, inducible eIF5B knockdown THP1 cell line (Western blot, eIF5B shRNA cells, Fig. 3C). After 3 d of eIF5B shRNA induction, half of the cells were maintained in serum-containing medium, and the other half were shifted to serum-free medium for 1 d. To quantitate translation, the cells were incubated with 35S-methionine for incorporation into newly synthesized protein, followed by labeled protein analysis by PAGE and normalization to Western blot analysis of Tubulin (a stable, abundant protein) as a loading control. Similar 35S-methionine incorporation profiles were observed in cells grown in serum-containing medium with or without eIF5B depletion (Fig. 4A, Left, S+) (17). 35S-methionine incorporation into total protein decreased by one third (34%) in serum-starved eIF5B-depleted cells (Fig. 4A, Right for quantitation, SS1D) as compared with cells without eIF5B depletion. Since overexpression of eIF5B in serum-starved cells results in cell death (Fig. S3 C and D), and since the efficiency of transfection by nucleofection was low and variable, rescue of the shRNA-induced depletion with expression of a resistant form was difficult. To control for off-target effects, a second shRNA against eIF5B, eIF5B shRNA2, was used to generate a second inducible eIF5B-knockdown cell line (Fig. S4A, Lower Western blots, eIF5B shRNA2). Similar results with a 30% decrease in 35S-methionine incorporation into total protein was observed in eIF5B shRNA2 knockdown cells grown in serum-starved conditions for 1 d compared with undepleted cells. Serum-grown cells showed no difference in translation with or without eIF5B depletion (Fig. S4A, Right for quantitation). Consistently, eIF5B depletion moderately reduced the steady-state levels of general translation of a luciferase reporter when compared with control serum-starved cells that were not depleted of eIF5B (Fig. 4B). These results indicate that eIF5B has a general role in translation in serum-starved cells similar to its role in late immature oocytes (Fig. 1B) and in ES cells (Fig. 2C). The remaining translation observed in these eIF5B-depleted cells may be due to residual eIF5B, other factors, or heterogeneity in this transient serum-starved population (which includes cells that are at the 1 d serum-starved state, in which eIF5B is increased, and other cells that may have continued into G0 or cell death). In contrast, oocytes and ES cells, which show greater translation loss upon eIF5B depletion (Figs. 1 and 2), are homogenous, stable states that are clearly distinct from other states.
Fig. 4.
eIF5B depletion decreases translation in serum-starved cells. (A) 35S-methionine incorporation into newly synthesized protein without (Control) and with (eIF5B shRNA) eIF5B depletion in THP1 cells either grown in serum-containing medium (S+) or serum-starved for 1 d (SS1D). (Upper Left) Equal amounts of extracts, separated by SDS/PAGE, were exposed on the phosphorimager and normalized to Tubulin, a stable, abundant protein, for loading by Western analysis (Lower Left). (Right) 35S-methionine incorporation was quantitated. The graph shows the average of three independent experiments. Error bars indicate SEM. **P < 0.01. (B) Luciferase reporter assay in cells that had been serum-starved for 1 d with and without eIF5B depletion. The graph shows the average of five independent experiments. Error bars indicate SEM; *P < 0.03.
Increased eIF5B Levels Lead to Increased eIF5B Complexes with tRNA-Meti in Serum-Starved Cells.
eIF5B promotes 60S subunit joining and can indirectly support or stabilize tRNA-Meti association, as in the case of specific viral and stress-related mRNA translation (2, 11–13, 17–19). We, therefore, tested whether increased eIF5B expression leads to correspondingly increased complexes with tRNA-Meti in THP1 cells that had been serum-starved for 1 d. eIF5B immunoprecipitation (Fig. S4 B and C) from cells cross-linked in vivo with formaldehyde (to freeze in vivo direct and indirect interactions), followed by qRT-PCR analysis of coimmunoprecipitated RNAs, revealed increased eIF5B complexes with tRNA-Meti as compared with the IgG control in cells that had been serum-starved for 1 d (Fig. S4C, Right) and as compared with serum-grown cells (Fig. S4C, Left). The increased eIF5B–tRNA-Meti complexes reflect the 1.5-fold increase in eIF5B immunoprecipitated from these serum-starved cells as compared with serum-grown cells (P < 0.05) (Fig. S4B). (The eIF5B protein levels quantitated from three experiments and normalized to the input averaged 0.49 for eIF5B immunoprecipitated from SS1D cells, compared with levels of 0.32 for eIF5B immunoprecipitated from S+ cells, compared with the immunoprecipitation control levels of 0.088 for IgG immunoprecipitated from SS1D cells and 0.08 for IgG immunoprecipitated from S+ cells.) These eIF5B immunoprecipitates did not include ribosomal protein S3 or eIF4G3 (Fig. S4B); higher molecular weight complexes that were centrifuged and clarified out from the extract before immunoprecipitation would likely also contain eIF5B associated with ribosomal proteins. The eIF5B association with tRNA-Meti may be mediated indirectly via other proteins and complexes. These data indicate that the increased levels of eIF5B in cells that had been serum-starved for 1 d and the roles of eIF5B in translation, including 60S subunit joining, could increase eIF5B–tRNA-Meti complexes indirectly (2, 11–13, 17–19) and may contribute to or result from supporting translation.
EIF2α Is Phosphorylated in Serum-Starved THP1 Cells.
eIF5B enables the translation of a few viral and stress-related mRNAs in specific conditions in which eIF2α phosphorylation is also observed (12, 17–19). We find that eIF2α phosphorylation increases by twofold in cells that have been serum-starved for 1 d (Fig. 3B) as compared with cells grown in serum-containing medium. Consistently, previous studies showed that adding serum to cells leads to eIF2α dephosphorylation (29).
Additionally, in cells that had been serum-starved for 1 d, the critical component of eIF2 (2, 21, 22), eIF2γ (Fig. S5A, Right for quantitation), decreased by more than one third (35%) along with 27% decrease in eIF2α (Fig. 3B, Right for quantitation) and 22% decrease in eIF2β (Fig. S5A, Right for quantitation). The guanine nucleotide exchange factor eIF2B also decreased by one third (30%) in cells that had been serum-starved for 1 d (Fig. S5B, Right for quantitation). Knockdown of EIF2 (eIF2α, eIF2β, and eIF2γ) (2, 21, 22) was conducted similarly to eIF5B knockdown, with 39–86% depletion in stable, doxycycline-inducible cell lines (Fig. S5C). However, the effect of the loss of eIF2 could not be ascertained, as translation was not affected even in serum-grown cells (Fig. S5C); either depletion of eIF2 is insufficient or eIF2 loss is compensated for in serum-grown THP1 cells and transiently transfected serum-grown mouse ES cells and EBs (Fig. S2C). These results are similar to previous observations of eIF2B depletion in tumor cells (25, 26) and depletion of other initiation factors in mammalian serum-grown cells (27, 28). EIF2 would continue to contribute to translation in cells that had been serum-starved for 1 d, depending on the relative levels of eIF2B and phosphorylated eIF2α (2, 21, 22).
Discussion
Developmental stages, such as immature oocytes and ES cells, and cell-cycle transition states, such as arrest induced by growth-factor deprivation, alter and reduce general translation (3, 4, 6, 7, 10). Yet these conditions exhibit ongoing translation (3, 5–7, 10). Here we document that eIF5B (1, 2, 11, 13) increases (Figs. 1A, 2A, and 3A) and supports general translation in conditions known to have reduced translation: in late immature, G0-like oocytes (Fig. 1), in ES cells (Fig. 2), and transiently during the early stages of growth-factor deprivation in proliferating mammalian cells (Figs. 3 and 4). These conditions additionally exhibit increased phosphorylation of the general translation factor eIF2α (12). Importantly, these studies uncover the role of the translation factor eIF5B in regulating specific developmental stages and cell-state transitions. eIF5B is a transient antagonist of G0-like (immature oocyte; ref. 8) and G0-arrest states, promoting oocyte maturation and preventing G0 arrest upon serum starvation (Fig. 1 C, D, F, and G and Fig. S3 C–F).
eIF5B is increased in immature oocytes, ES cells, and THP1 cells that have been serum-starved for 1 d (Figs. 1A, 2A, and 3A) but not in all cell types (Fig. S3B). Overexpression of eIF5B is difficult to achieve with poor increase observed in eIF5B levels by Western blot analysis in ES cells, THP1 cells (Fig. S3D), and oocytes (Fig. 1F), indicating tight control over eIF5B levels. Similar control is also observed with other translation initiation factors, eIF5 and eIF1, in mammalian cells (30). Limited overexpression of eIF5B in immature oocytes led to increased translation (Fig. 1E). eIF5B depletion decreases general translation in immature oocytes, ES cells, and THP1 cells that have been serum-starved for 1 d (Figs. 1 B and C, 2 B and C, and 4 A and B and Fig. S4A); however, eIF5B depletion has no effect on mature oocytes, EBs, or serum-grown cells (Figs. 2 B and C and 4A and Figs. S1B and S4A), indicating that eIF5B’s role in translation becomes limiting in specific cell-cycle transition and developmental stages. Other specific conditions that alter and reduce general translation, such as hypoxia (17, 31), demonstrate transiently increased eIF5B (Fig. S6), indicating that eIF5B is increased in diverse situations (12, 17–20). Microarray analysis performed on polysome-associated mRNAs in THP1 cells that had been serum-starved for 1 d with or without eIF5B depletion (Fig. S7 A and B) showed that the degree of regulation by eIF5B was comparable for the majority of genes (Fig. S7C). Gene Ontology (GO) analysis showed marginally enriched categories with poor statistical significance (Benjamini P value 5.7E-2) in the 86 genes down-regulated upon eIF5B knockdown (Table S2); this is consistent with eIF5B mediating general translation (Figs. 1B, 2C, and 4 A and B and Fig. S4A).
eIF5B promotes 60S subunit joining in general translation (2, 13) and pre-40S proofreading (15, 16). No significant decrease in 80S (eIF5B’s function in 60S subunit joining) or 40S [eIF5B’s function in pre-40S proofreading (15, 16)] levels is observed with eIF5B depletion in serum-starved cells (Fig. S7B). However, these functions could contribute to the limiting role of eIF5B in translation in these specific conditions and indirectly might affect the formation of tRNA-Meti complexes with eIF5B. Reflecting the increased eIF5B levels, increased eIF5B complexes with tRNA-Meti are observed in cells that have been serum-starved for 1 d (Fig. S4 B and C). These results are consistent with eIF5B’s previously known roles in supporting or stabilizing tRNA-Meti association (2, 11–13) and in supporting the translation of viral, stress-associated, and other specific mRNAs in conditions with reduced translation (12, 17–20). Other functions of eIF5B could also be critical for supporting translation and may indirectly enable association with tRNA-Meti. The formation of the eIF5B–tRNA-Meti complex could be mediated indirectly by protein complexes and, along with eIF5B’s role in 60S subunit joining and pre-40S proofing, could contribute to or be a result of supporting general translation (2, 11–13).
eIF5B becomes limiting and supports translation in conditions (serum-starved cells, immature oocytes, and ES cells) that concomitantly show an increase in eIF2α phosphorylation (Figs. 1A, 2A, and 3B). However, the increased phosphorylation of eIF2α and increased eIF5B levels may be independent, parallel events. EIF2α phosphorylation may not be of a sufficient level to block translation, and eIF2 may continue to contribute to translation, depending on the relative levels of phosphorylated eIF2α and eIF2B (2, 21, 22). It is also possible that the reduction in eIF2 subunit levels (2, 21, 22) observed in immature oocytes (Fig. S1C) and serum-starved cells (Fig. 3B and Fig. S5A) could contribute to the reduced translation in these specific situations, independent of eIF2α phosphorylation. In THP1 cells that have been serum-starved for 1 d and in ES cells, eIF2 depletion analysis was inconclusive (Figs. S2 C and D and S5C), and eIF2 may continue to contribute to the altered translation observed in these cells. Depletion of eIF2γ did not impair translation in late immature oocytes (Fig. S1 D and E) but decreased translation in mature oocytes (Fig. S1 D and E). Therefore, eIF2 is not limiting for translation in late immature oocytes, in which eIF2 depletion analysis was conclusive. In the case of late immature oocytes alone, where eIF2 is not limiting for translation, one possible model for the function of the increased eIF5B could be to support eIF2-independent translation; this effect would be consistent with previous studies on viral and stress-related mRNAs in which eIF5B is required for eIF2-independent translation and supports or stabilizes tRNA-Meti association (12, 17–19).
The transition from immature (G0-like) to mature oocytes has been compared to the entry of G0 cells into the cell cycle (8), while serum starvation of proliferating mammalian cells induces an early phase of transient stress: Within 1 d of serum starvation, the cells either adapt to these nonproliferative conditions and enter G0 arrest or undergo cell death (9). Cells that have been serum-starved for 1 d represent a transient and heterogeneous state, with gene expression that is distinct from cells that have experienced prolonged (>1 d) serum starvation and cells that have entered G0 and late G0 (9). Our results reveal that eIF5B overexpression promotes maturation of oocytes (Fig. 1 F and G and Table S1) and cell death upon serum starvation of proliferating mammalian cells (Fig. S3 C and D). These data suggest that eIF5B negatively regulates G0 and G0-like (immature oocyte; ref. 8) states. Conversely, depletion of eIF5B promotes the G0-like, immature oocyte state (Fig. 1 C and D and Table S1) or earlier G0 arrest in serum-starved mammalian cells (Fig. 3 C–F), and eIF5B is decreased upon oocyte maturation or further entry into G0 (Figs. 1A and 3A). These data reveal eIF5B as a transient antagonist of G0 and a promoter of oocyte maturation, indicating a previously unidentified role for a general translation factor in specific cell-state transitions and developmental stages.
Methods
Oocytes, plasmids, RNAs, cross-linking, immunoprecipitation, and Western blot, polysome, and microarray analyses are described in SI Methods. Microarray results related to Figs. 3 and 4, Fig. S7, and Table S2 are provided in Dataset S1.
Human Cells.
Cells were maintained at 3–5 × 105 cells/mL in 10% FBS and Roswell Park Memorial Institute 1460 (THP1) and Dulbecco's modified Eagle medium (DMEM) (BJ) media and were serum-starved at a density of 3 × 105 cells/mL. Hypoxia was induced in a hypoxia incubator at 1% O2.
ES Cells.
Mouse ES cells were maintained in DMEM with 15% FBS, nonessential amino acids, 0.055 mM 2-mercaptoethanol, 2 mM glutamine, and 0.01 µg/mL LIF. EBs were prepared by the hanging-drop method in the absence of LIF (24).
Supplementary Material
Acknowledgments
We thank C. Wilusz for advice, A. Classon and V. Bhambhani for technical assistance, and the Partners Healthcare Center for Personalized Genetic Medicine Microarray facility for assistance with microarray analysis. This study was supported by the Cancer Research Institute, the D. and M.-E. Ryder, Smith Family Foundation, the Leukemia and Lymphoma Society Investigator awards, and by Massachusetts General Hospital Start-Up Award (to S.V.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1320477111/-/DCSupplemental.
References
- 1.Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol. 2010;11(2):113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell. 2009;136(4):731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray NK, Wickens M. Control of translation initiation in animals. Annu Rev Cell Dev Biol. 1998;14:399–458. doi: 10.1146/annurev.cellbio.14.1.399. [DOI] [PubMed] [Google Scholar]
- 4.Radford HE, Meijer HA, de Moor CH. Translational control by cytoplasmic polyadenylation in Xenopus oocytes. Biochim Biophys Acta. 2008;1779(4):217–229. doi: 10.1016/j.bbagrm.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mortensen RD, Serra M, Steitz JA, Vasudevan S. Posttranscriptional activation of gene expression in Xenopus laevis oocytes by microRNA-protein complexes (microRNPs) Proc Natl Acad Sci USA. 2011;108(20):8281–8286. doi: 10.1073/pnas.1105401108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sampath P, et al. A hierarchical network controls protein translation during murine embryonic stem cell self-renewal and differentiation. Cell Stem Cell. 2008;2(5):448–460. doi: 10.1016/j.stem.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Ingolia NT, Lareau LF, Weissman JS. Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell. 2011;147(4):789–802. doi: 10.1016/j.cell.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taieb F, Thibier C, Jessus C. On cyclins, oocytes, and eggs. Mol Reprod Dev. 1997;48(3):397–411. doi: 10.1002/(SICI)1098-2795(199711)48:3<397::AID-MRD14>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 9.Coller HA, Sang L, Roberts JM. A new description of cellular quiescence. PLoS Biol. 2006;4(3):e83. doi: 10.1371/journal.pbio.0040083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loayza-Puch F, et al. p53 induces transcriptional and translational programs to suppress cell proliferation and growth. Genome Biol. 2013;14(4):R32. doi: 10.1186/gb-2013-14-4-r32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen GS, Frank J. Structural insights on the translation initiation complex: Ghosts of a universal initiation complex. Mol Microbiol. 2007;63(4):941–950. doi: 10.1111/j.1365-2958.2006.05574.x. [DOI] [PubMed] [Google Scholar]
- 12.Terenin IM, Dmitriev SE, Andreev DE, Shatsky IN. Eukaryotic translation initiation machinery can operate in a bacterial-like mode without eIF2. Nat Struct Mol Biol. 2008;15(8):836–841. doi: 10.1038/nsmb.1445. [DOI] [PubMed] [Google Scholar]
- 13.Shin BS, et al. Structural integrity of α-helix H12 in translation initiation factor eIF5B is critical for 80S complex stability. RNA. 2011;17(4):687–696. doi: 10.1261/rna.2412511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pestova TV, et al. The joining of ribosomal subunits in eukaryotes requires eIF5B. Nature. 2000;403(6767):332–335. doi: 10.1038/35002118. [DOI] [PubMed] [Google Scholar]
- 15.Strunk BS, Novak MN, Young CL, Karbstein K. A translation-like cycle is a quality control checkpoint for maturing 40S ribosome subunits. Cell. 2012;150(1):111–121. doi: 10.1016/j.cell.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebaron S, et al. Proofreading of pre-40S ribosome maturation by a translation initiation factor and 60S subunits. Nat Struct Mol Biol. 2012;19(8):744–753. doi: 10.1038/nsmb.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thakor N, Holcik M. IRES-mediated translation of cellular messenger RNA operates in eIF2α- independent manner during stress. Nucleic Acids Res. 2012;40(2):541–552. doi: 10.1093/nar/gkr701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White JP, Reineke LC, Lloyd RE. Poliovirus switches to an eIF2-independent mode of translation during infection. J Virol. 2011;85(17):8884–8893. doi: 10.1128/JVI.00792-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pestova TV, de Breyne S, Pisarev AV, Abaeva IS, Hellen CU. eIF2-dependent and eIF2-independent modes of initiation on the CSFV IRES: A common role of domain II. EMBO J. 2008;27(7):1060–1072. doi: 10.1038/emboj.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrera P, et al. VASA mediates translation through interaction with a Drosophila yIF2 homolog. Mol Cell. 2000;5(1):181–187. doi: 10.1016/s1097-2765(00)80414-1. [DOI] [PubMed] [Google Scholar]
- 21.Zeenko VV, et al. An efficient in vitro translation system from mammalian cells lacking the translational inhibition caused by eIF2 phosphorylation. RNA. 2008;14(3):593–602. doi: 10.1261/rna.825008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin BS, et al. Initiation factor eIF2γ promotes eIF2-GTP-Met-tRNAi(Met) ternary complex binding to the 40S ribosome. Nat Struct Mol Biol. 2011;18(11):1227–1234. doi: 10.1038/nsmb.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Smedt V, et al. Thr-161 phosphorylation of monomeric Cdc2. Regulation by protein phosphatase 2C in Xenopus oocytes. J Biol Chem. 2002;277(32):28592–28600. doi: 10.1074/jbc.M202742200. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Yang P. In vitro differentiation of mouse embryonic stem (mES) cells using the hanging drop method. J Vis Exp. 2008;(17):825. doi: 10.3791/825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balachandran S, Barber GN. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell. 2004;5(1):51–65. doi: 10.1016/s1535-6108(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 26.Elsby R, et al. The alpha subunit of eukaryotic initiation factor 2B (eIF2B) is required for eIF2-mediated translational suppression of vesicular stomatitis virus. J Virol. 2011;85(19):9716–9725. doi: 10.1128/JVI.05146-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khaleghpour K, Pyronnet S, Gingras AC, Sonenberg N. Translational homeostasis: Eukaryotic translation initiation factor 4E control of 4E-binding protein 1 and p70 S6 kinase activities. Mol Cell Biol. 1999;19(6):4302–4310. doi: 10.1128/mcb.19.6.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galicia-Vázquez G, Cencic R, Robert F, Agenor AQ, Pelletier J. A cellular response linking eIF4AI activity to eIF4AII transcription. RNA. 2012;18(7):1373–1384. doi: 10.1261/rna.033209.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montine KS, Henshaw EC. Serum growth factors cause rapid stimulation of protein synthesis and dephosphorylation of eIF-2 in serum deprived Ehrlich cells Biochimica et Biophysica Acta (BBA) Molecular Cell Research. 1989;1014(3):282–288. doi: 10.1016/0167-4889(89)90224-3. [DOI] [PubMed] [Google Scholar]
- 30.Loughran G, Sachs MS, Atkins JF, Ivanov IP. Stringency of start codon selection modulates autoregulation of translation initiation factor eIF5. Nucleic Acids Res. 2012;40(7):2898–2906. doi: 10.1093/nar/gkr1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koritzinsky M, et al. Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J. 2006;25(5):1114–1125. doi: 10.1038/sj.emboj.7600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.